Effects of Interfacial Reactions on Microstructures and Mechanical Properties of 3003 Al/T2 Cu and 1035 Al/T2 Cu Brazed Joints
Abstract
1. Introduction
2. Materials and Methods
3. Result and Discussion
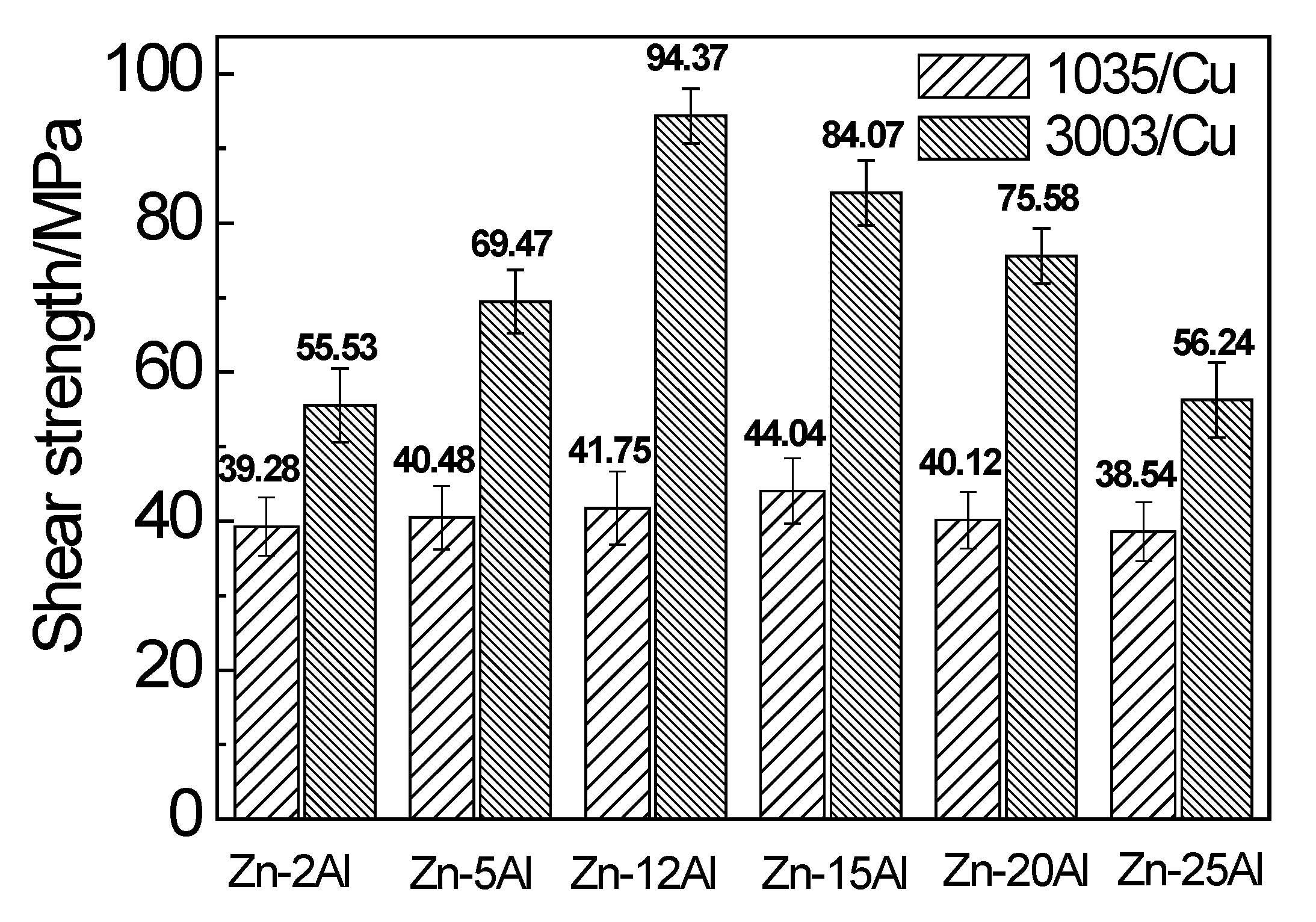
3.1. Joint Mechanical Property
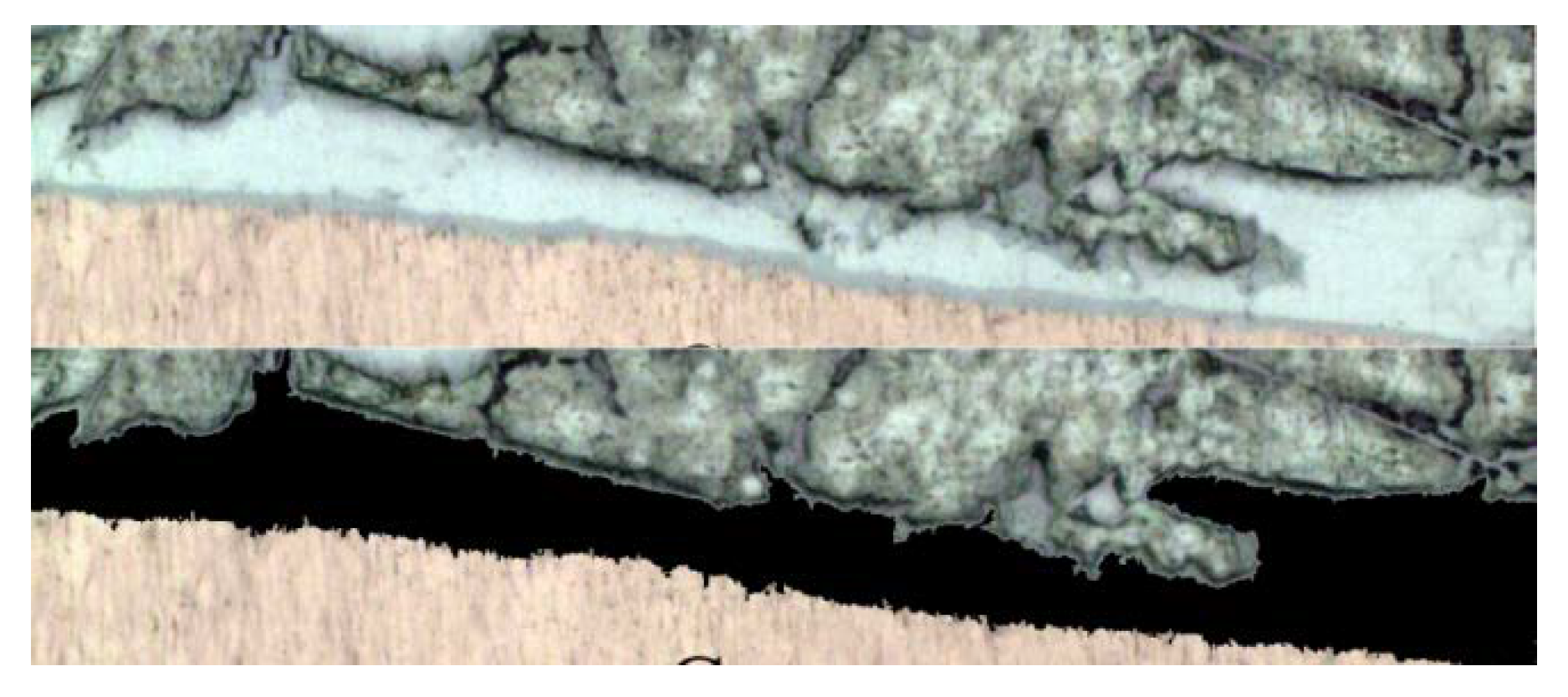
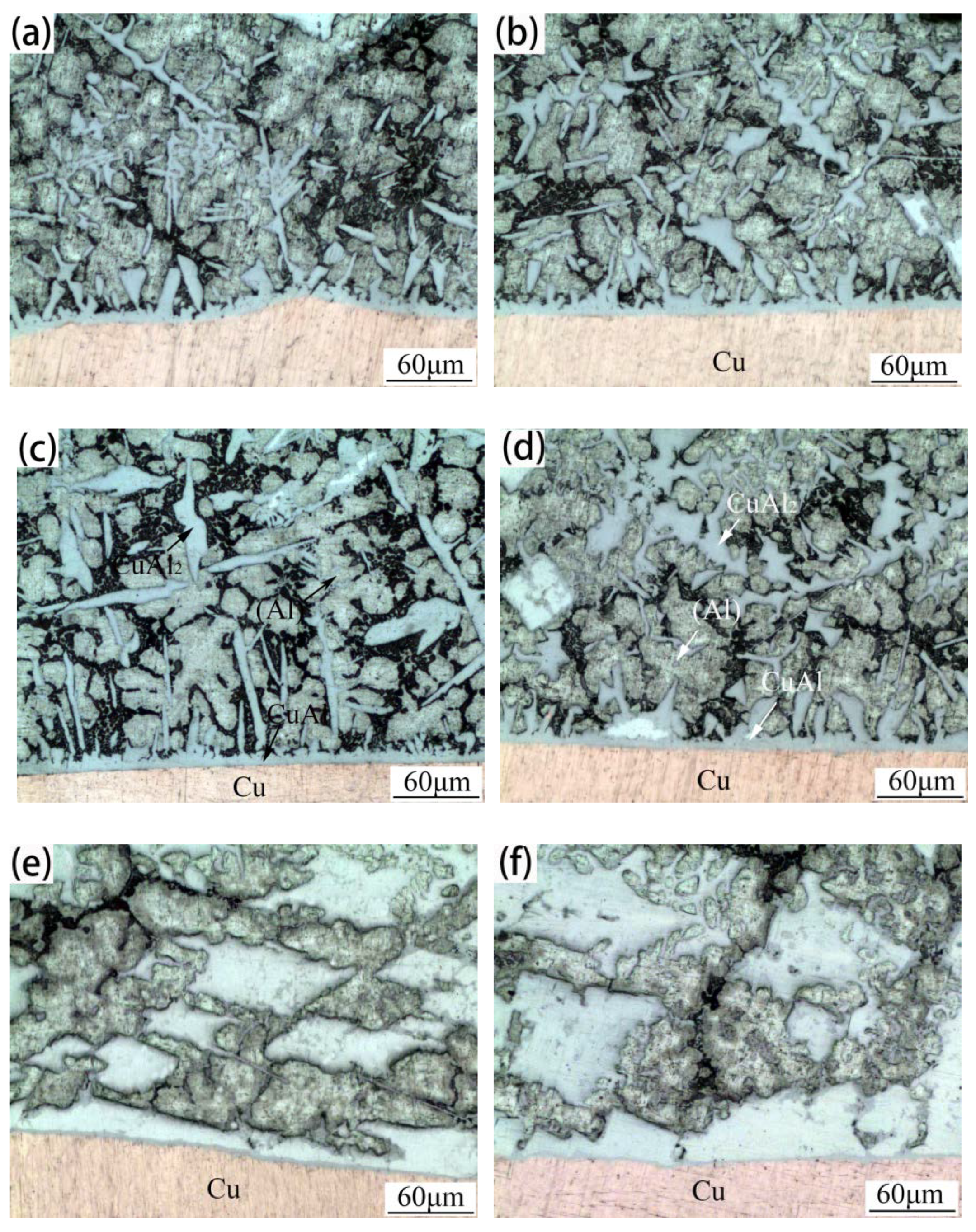
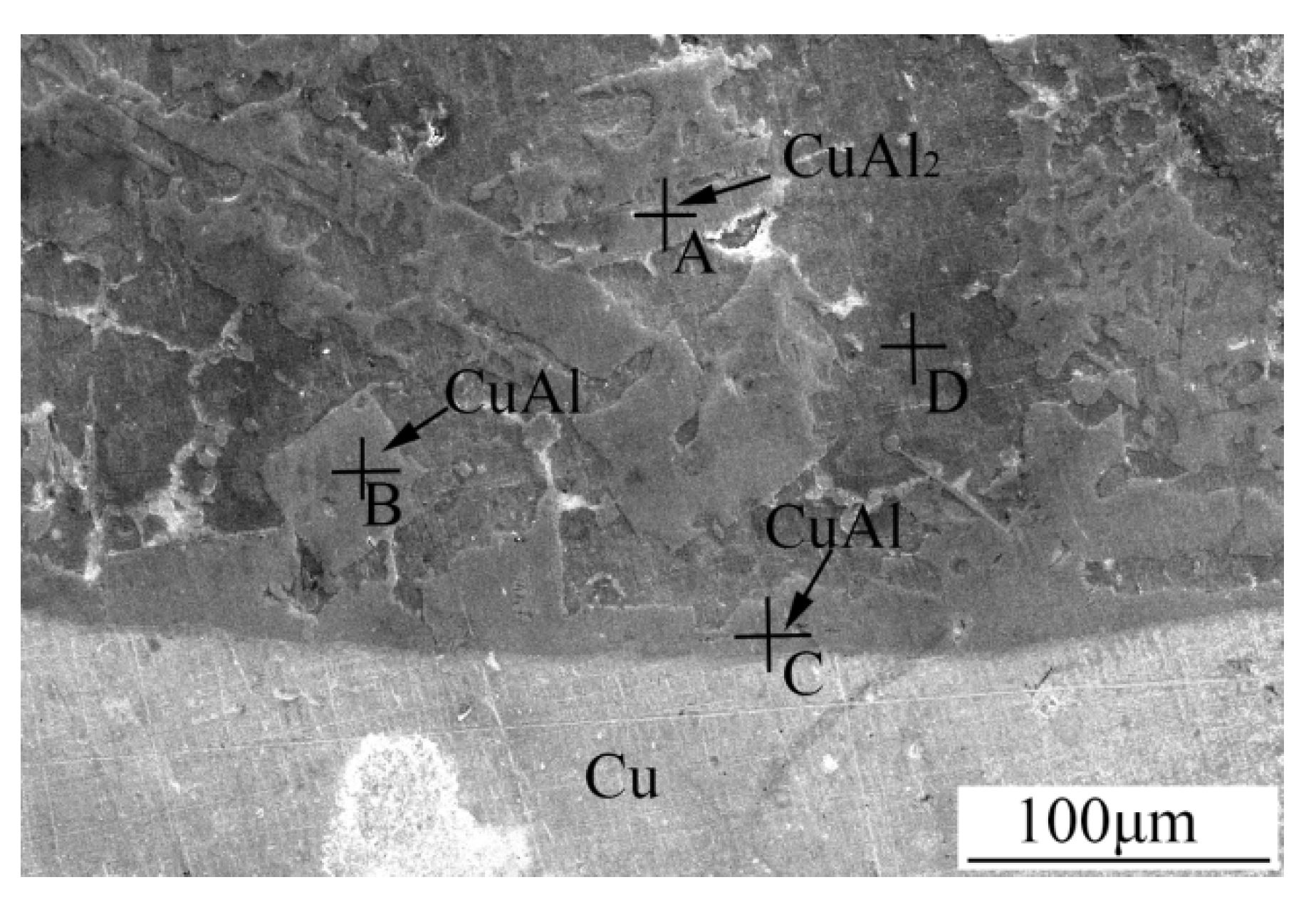
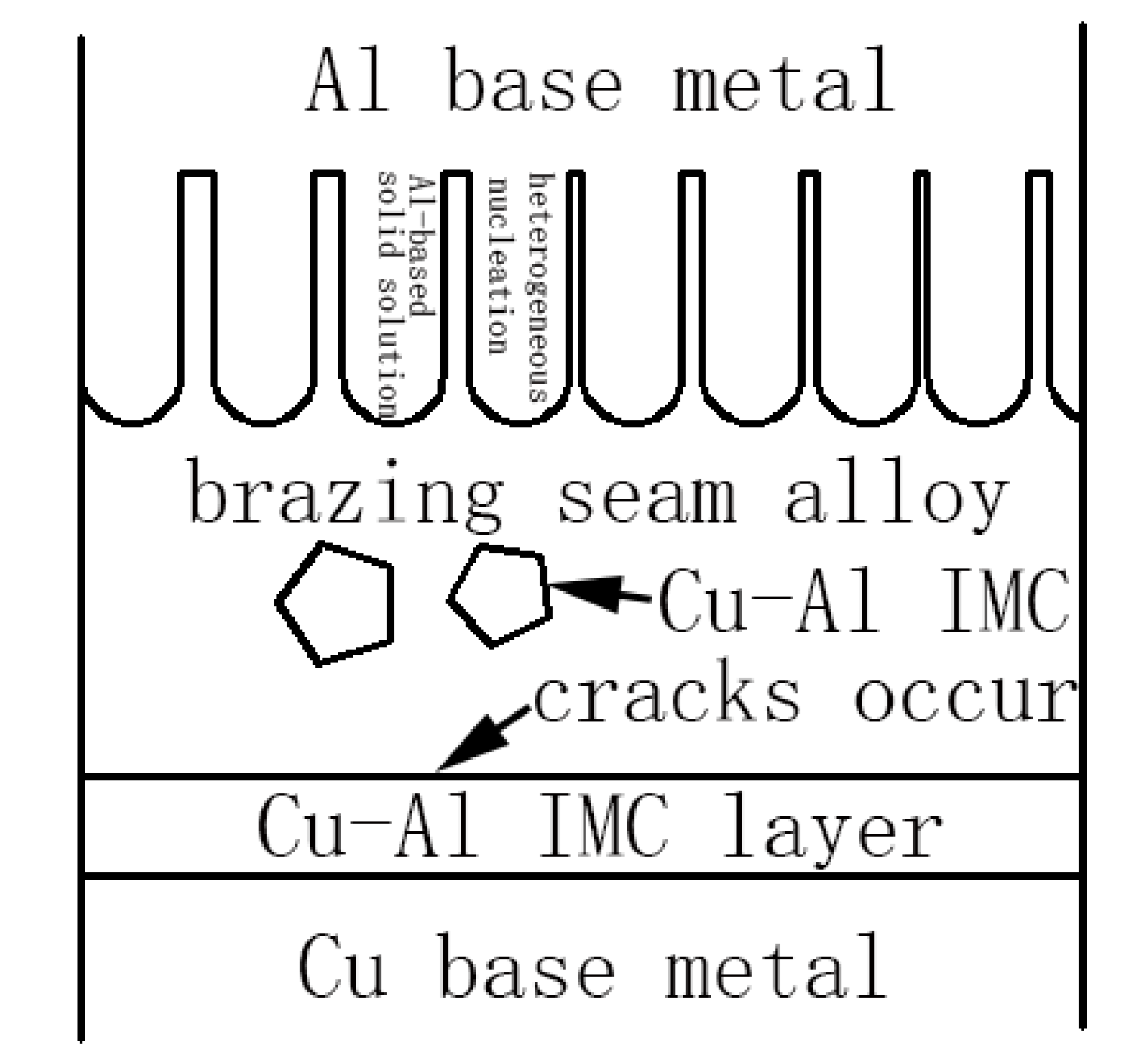
3.2. Microstructures of 3003 Al/Cu Brazed Joints
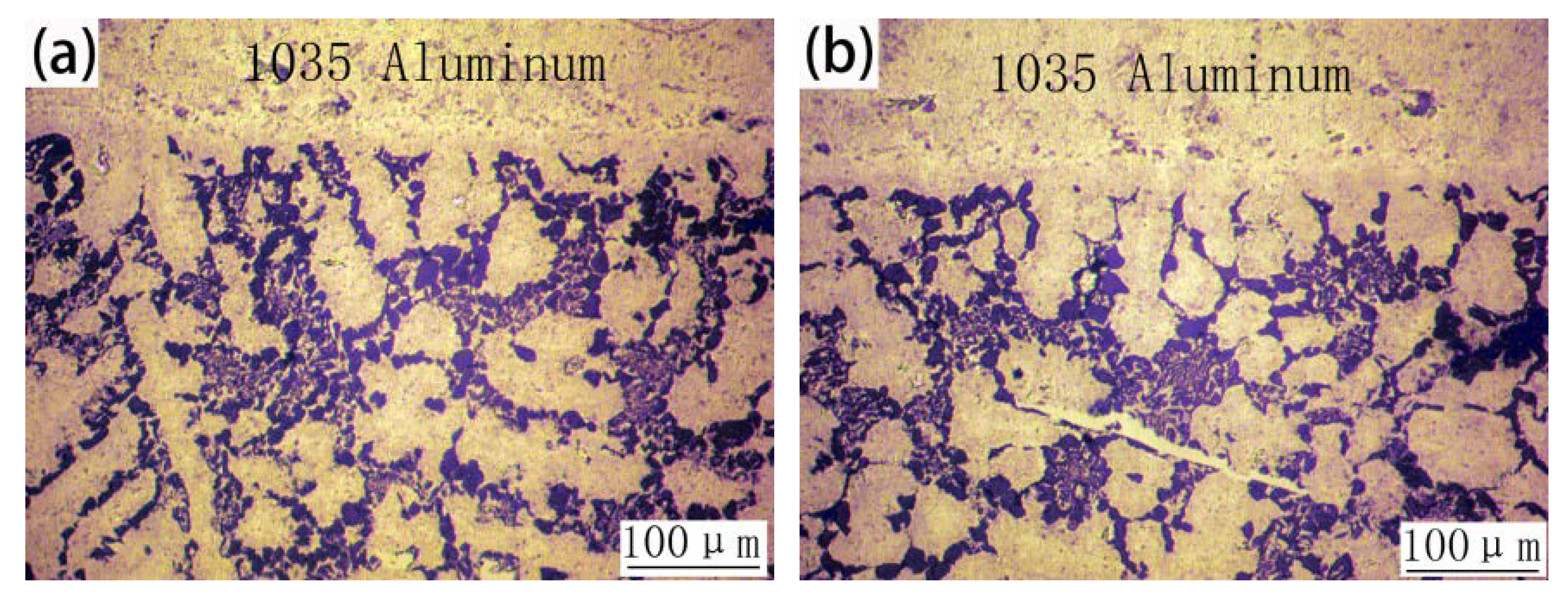
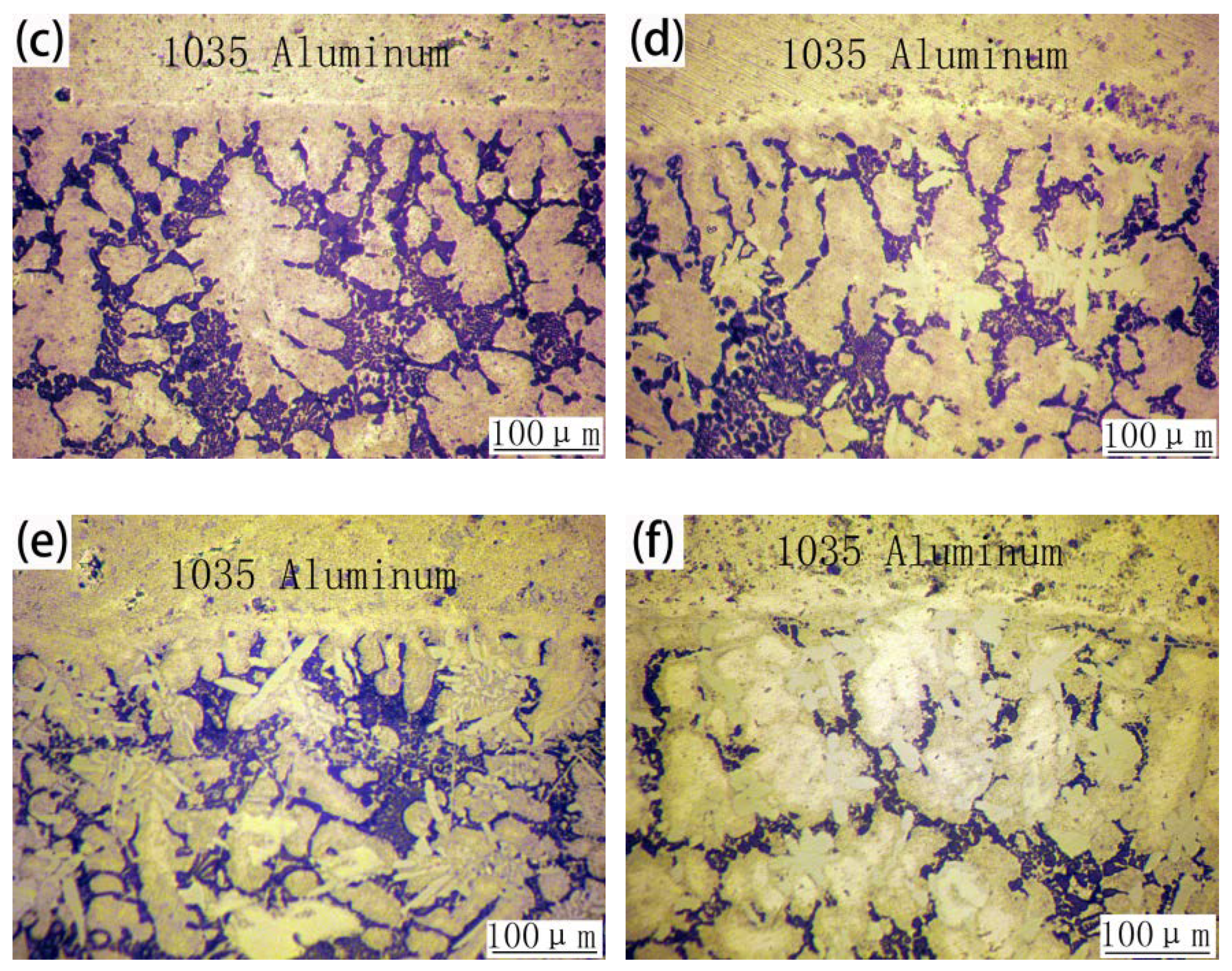
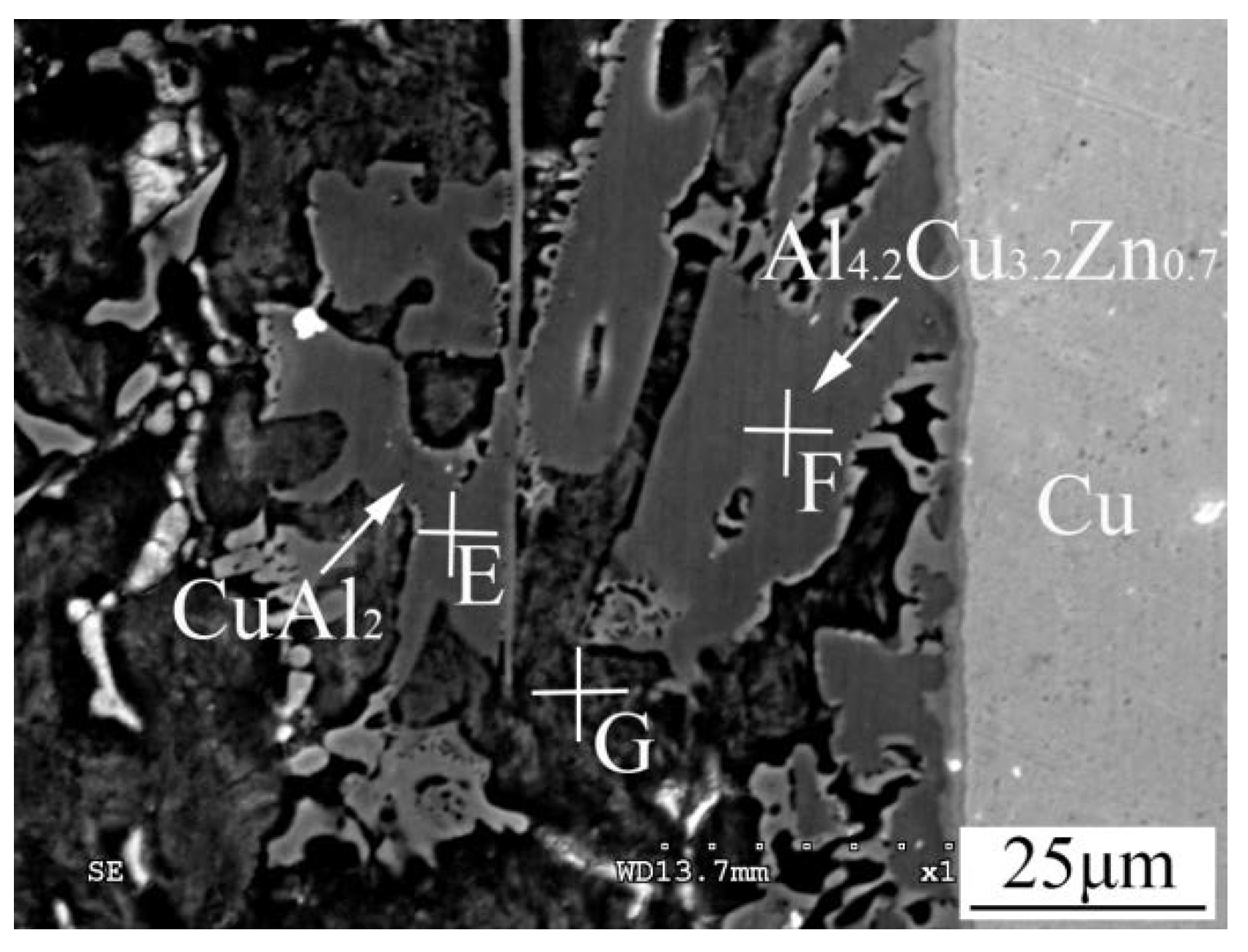
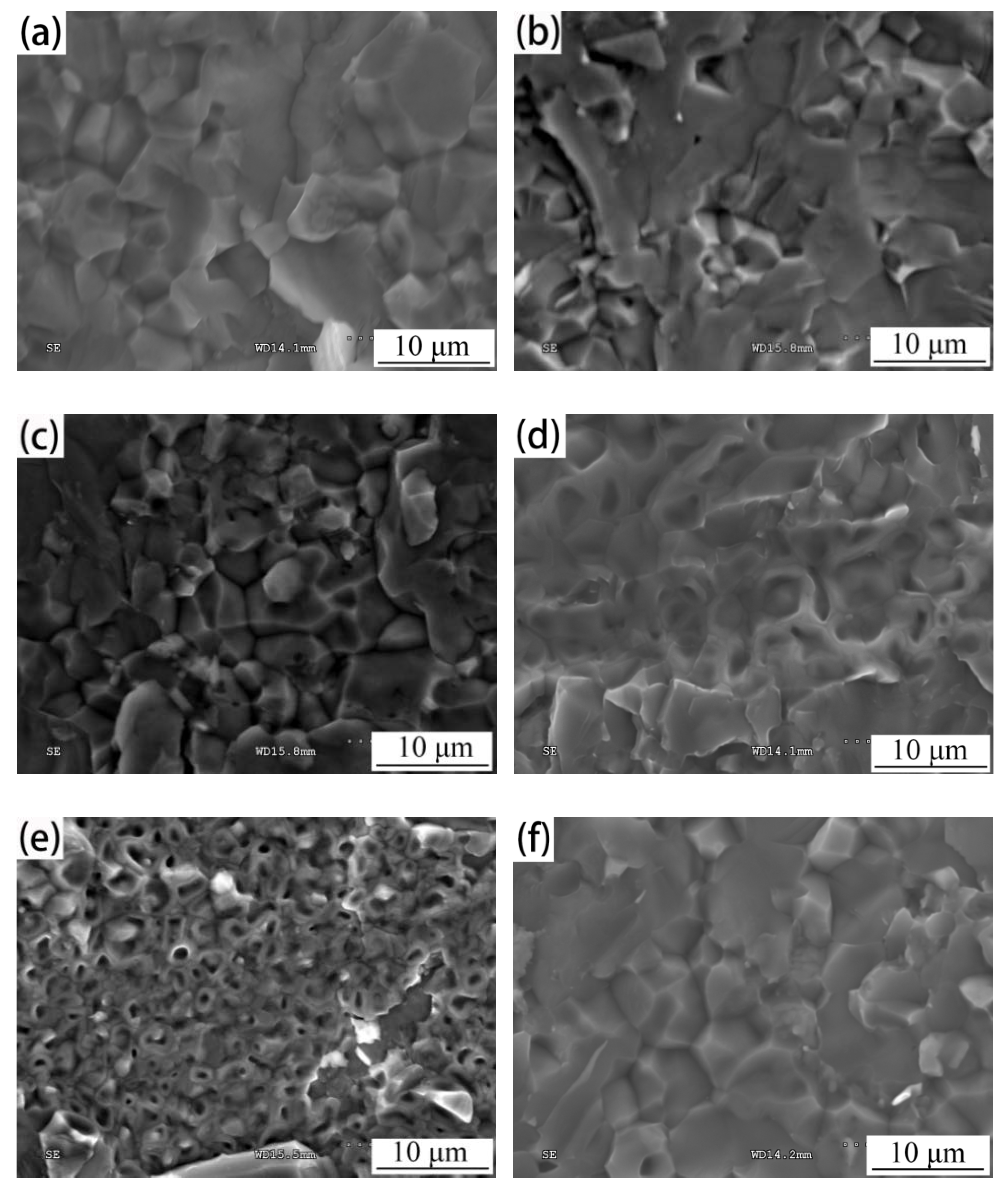
3.3. Microstructures of 1035 Al/Cu Brazed Joints
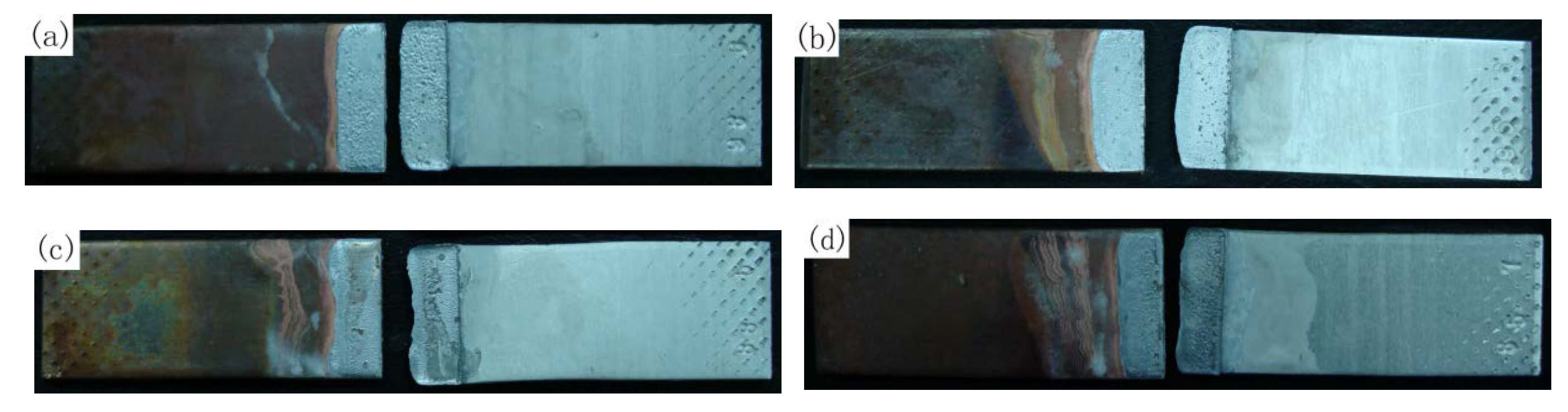
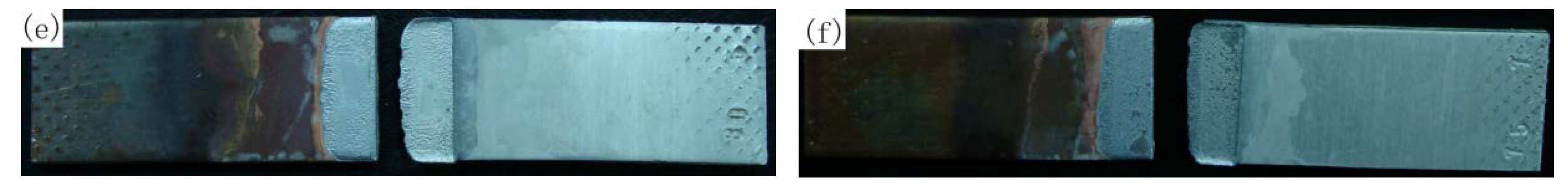
3.4. Fracture Analysis of 3003 Al/Cu Joints
3.5. Fracture Analysis of 1035 Al/Cu Joints
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eslami, N.; Hischer, Y.; Harms, A.; Lauterbach, D.; Böhm, S. Optimization of process parameters for friction stir welding of aluminum and copper using the Taguchi method. Metals 2019, 9, 63. [Google Scholar] [CrossRef]
- Mubiayi, M.P.; Akinlabi, E.T. Evolving properties of friction stir spot welds between AA1060 and commercially pure copper C11000. Trans. Nonferr. Metals Soc. 2016, 26, 1852–1862. [Google Scholar] [CrossRef]
- Ni, Z.L.; Ye, F.X. Effect of lap configuration on the microstructure and mechanical properties of dissimilar ultrasonic metal welded copper-aluminum joint. J. Mater. Process. Technol. 2017, 245, 180–192. [Google Scholar] [CrossRef]
- Balasundaram, R.; Patel, V.K.; Bhole, S.D.; Chen, D.L. Effect of zinc interlayer on ultrasonic spot welded aluminum-to-copper joints. Mater. Sci. Eng. A 2014, 607, 277–286. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Y.F.; Yan, Y.F.; Guo, R. Dissimilar friction stir welding of 6061 Al to T2 pure Cu adopting tooth-shaped joint configuration: Microstructure and mechanical properties. Mater. Sci. Eng. A 2017, 690, 355–364. [Google Scholar] [CrossRef]
- Reisgen, U.; Olschok, S.; Jakobs, S.; Holtum, N. Influence of the degree of dilution with laser beam vacuum-welded Cu-Al mixed joints on the electrical properties. Procedia CIRP 2018, 74, 23–26. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Shen, Y.F.; Yao, X.; Xu, H.S.; Li, B. Investigation on dissimilar underwater friction stir lap welding of 6061-T6 aluminum alloy to pure copper. Mater. Des. 2014, 64, 74–80. [Google Scholar] [CrossRef]
- Das, A.; Li, D.Z.; Williams, D.; Greenwood, D. Weldability and shear strength feasibility study for automotive electric vehicle battery tab interconnects. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 54. [Google Scholar] [CrossRef]
- Wei, Y.N.; Li, J.L.; Xiong, J.T.; Zhang, F.S. Investigation of interdiffusion and intermetallic compounds in Al–Cu joint produced by continuous drive friction welding. Eng. Sci. Technol. Int. J. 2017, 19, 90–95. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhang, G.; Shi, Y.; Zhu, M.; Yang, F.Q. Microstructures and mechanical behavior of aluminum-copper lap joints. Mater. Sci. Eng. A 2017, 705, 105–113. [Google Scholar] [CrossRef]
- Das, A.; Butterworth, I.; Masters, I.; Williams, D. Microstructure and mechanical properties of gap-bridged remote laser welded (RLW) automotive grade AA 5182 joints. Mater. Charact. 2018, 145, 697–712. [Google Scholar] [CrossRef]
- Das, A.; Masters, I.; Williams, D. Process robustness and strength analysis of multi-layered dissimilar joints using ultrasonic metal welding. Int. J. Adv. Manuf. Technol. 2019, 101, 881–900. [Google Scholar] [CrossRef]
- Weigla, M.; Alberta, F.; Schmidt, M. Enhancing the ductility of laser-welded copper-aluminum connections by using adapted filler materials. Phys. Procedia 2011, 12, 332–338. [Google Scholar] [CrossRef]
- Li, W.Y.; Wen, Q.; Yang, X.W.; Wang, Y.S.; Gao, D.L.; Wang, W.B. Interface microstructure evolution and mechanical properties of Al/Cu bimetallic tubes fabricated by a novel friction-based welding technology. Mater. Des. 2017, 134, 383–393. [Google Scholar] [CrossRef]
- Tan, C.W.; Jiang, Z.G.; Li, L.Q.; Chen, Y.B.; Chen, X.Y. Microstructural evolution and mechanical properties of dissimilar Al–Cu joints produced by friction stir welding. Mater. Des. 2013, 51, 466–473. [Google Scholar]
- Eslami, N.; Hischer, Y.; Harms, A.; Lauterbach, D.; Böhm, S. Influence of copper-sided tin coating on the weldability and formation of friction stir welded aluminum-copper-joints. Metals 2019, 9, 179. [Google Scholar] [CrossRef]
- Satpathya, M.P.; Sahoob, S.K. Mechanical performance and metallurgical characterization of ultrasonically welded dissimilar joints. J. Manuf. Process. 2017, 25, 443–451. [Google Scholar] [CrossRef]
- Shiran, M.K.G.; Khalaj, G.; Pouraliakbar, H.; Jandaghi, M.R.; Dehnavi, A.S.; Bakhtiari, H. Multilayer Cu/Al/Cu explosive welded joints: Characterizing heat treatment effect on the interface microstructure and mechanical properties. J. Manuf. Process. 2018, 35, 657–663. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Luo, Z.; Yuan, T.; Bi, J.; Wang, Z.M.; Wang, Z.P.; Chao, Y.J. Feasibility study of dissimilar joining of aluminum alloy 5052 to pure copper via thermo-compensated resistance spot welding. Mater. Des. 2016, 106, 235–246. [Google Scholar] [CrossRef]
- Wang, X.G.; Li, X.G.; Wang, C.G. Influence of diffusion brazing parameters on microstructure and properties of Cu/Al joints. J. Manuf. Process. 2018, 35, 343–350. [Google Scholar] [CrossRef]
- Xia, C.Z.; Li, Y.J.; Puchkov, U.A.; Gerasimov, S.A.; Wang, J. Microstructure and phase constitution near the interface of Cu/Al vacuum brazing using Al–Si filler metal. Vacuum 2008, 82, 799–804. [Google Scholar] [CrossRef]
- Huang, M.L.; Huang, Y.Z.; Ma, H.T.; Zhao, J. Mechanical properties and electrochemical corrosion behavior of Al/Sn-9Zn-xAg/Cu joints. J. Electron. Mater. 2011, 40, 315–323. [Google Scholar] [CrossRef]
- Niu, Z.W.; Ye, Z.; Huang, J.H.; Yang, H.; Yang, J.; Chen, S.H. Interfacial structure and properties of Cu/Al joints brazed with Zn-Al filler metals. Mater. Charact. 2018, 138, 78–88. [Google Scholar] [CrossRef]
- Ji, F.; Xue, S.B.; Dai, W. Effects of Ti on the Brazability of Zn-22AI-xTi Filler Metals as Well as Properties of Cu/Al Brazing Joints. Rare Metal Mater. Eng. 2013, 42, 2453–2457. [Google Scholar]
- Ji, F.; Xue, S.B. Growth behaviors of intermetallic compound layers in Cu/Al joints brazed with Zn–22Al and Zn–22Al–0.05Ce filler metals. Mater. Des. 2013, 51, 907–915. [Google Scholar]
- Xiao, Y.; Ji, H.J.; Li, M.Y.; Kim, J. Ultrasound-assisted brazing of Cu/Al dissimilar metals using a Zn–3Al filler metal. Mater. Des. 2013, 52, 740–747. [Google Scholar] [CrossRef]
- Zhang, H.W.; Cui, W.; He, J.S.; Yan, J.C.; Yang, S.Q. Formation and evolution of intermetallic compounds at interfaces of Cu/Al joints by ultrasonic-assisted soldering. J. Mater. Process. Technol. 2015, 223, 1–7. [Google Scholar]
- Ye, Z.; Yang, H.; Huang, J.H.; Yang, J.; Chen, S.H. A novel Zn-Al-Si corrosion resistant filler metal for Cu/Al brazing. Mater. Lett. 2017, 206, 201–204. [Google Scholar] [CrossRef]
- Liu, G.P.; Hu, X.W.; Fu, Y.S.; Li, Y.L. Microstructure and mechanical properties of ultrasonic welded joint of 1060 aluminum alloy and T2 pure copper. Metals 2017, 7, 361. [Google Scholar] [CrossRef]
- Solchenbach, T.; Plapper, P. Mechanical characteristics of laser braze-welded aluminium–copper connections. Opt. Laser Technol. 2013, 54, 249–256. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Zhuang, H.S. Brazing and Soldering Manual, 2nd ed.; China Machine Press: Beijing, China, 2005; pp. 66–120. [Google Scholar]
- Wang, Z.Z. Material Science Foundation, 1st ed.; China Machine Press: Beijing, China, 2005; p. 51. [Google Scholar]
- Wuhan University; Jilin University. Inorganic Chemistry, 3rd ed.; Higher Education Press: Beijing, China, 1994; p. 106. [Google Scholar]






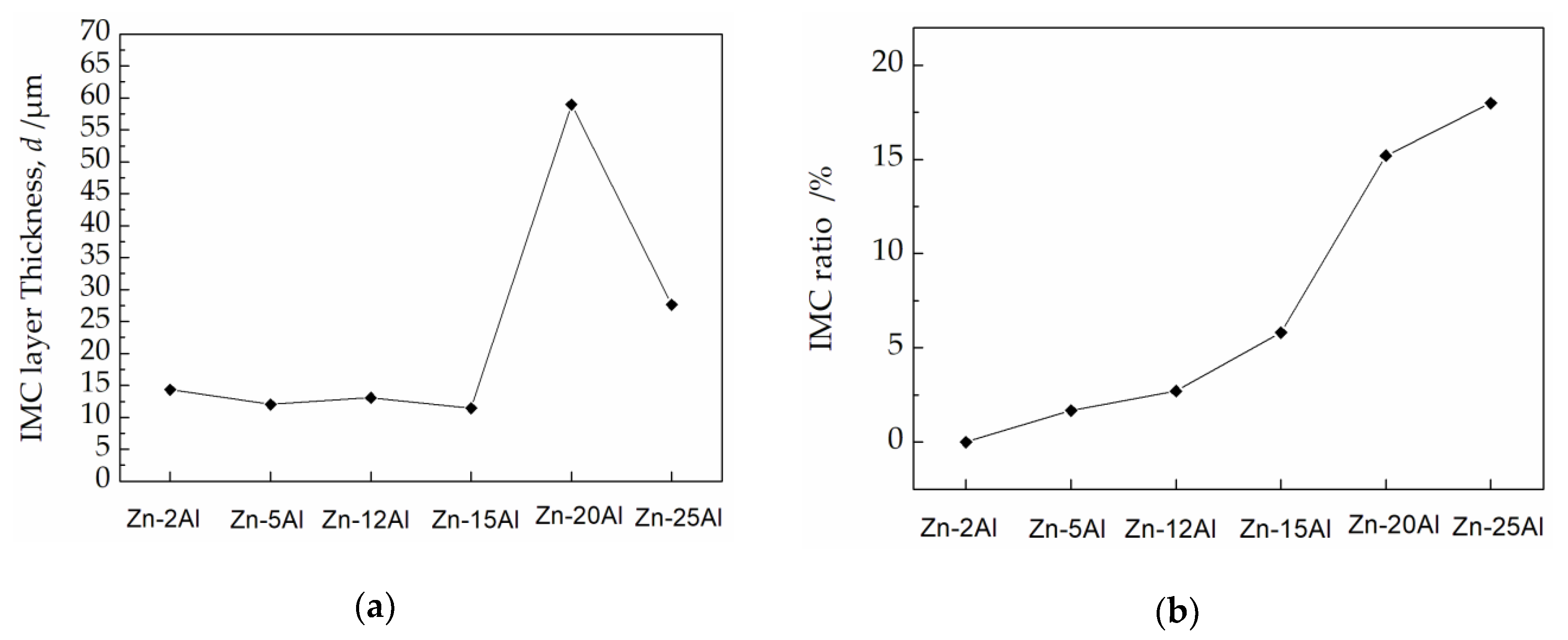

| Element | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Al | 2 | 5 | 12 | 15 | 20 | 25 |
| Zn | 98 | 95 | 88 | 85 | 80 | 75 |
| Material | Composition (wt.%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Zn | Cu | Fe | Sb | V | Si | Mg | Mn | As | Bi | Pb | Ti | S | |
| 1035 | Bal. | 0.1 | 0.1 | 0.6 | - | 0.05 | 0.35 | 0.05 | 0.05 | - | - | - | 0.03 | - |
| 3003 | Bal. | 0.1 | 0.05~0.2 | 0.7 | - | - | 0.6 | 1.0~1.5 | - | - | - | - | - | |
| T2Cu | - | - | Bal. | 0.005 | 0.002 | - | - | - | - | 0.002 | 0.001 | 0.005 | - | 0.005 |
| Point | Al | Cu | Zn | Mn |
|---|---|---|---|---|
| A | 63.16 | 36.84 | - | - |
| B | 57.08 | 42.92 | - | - |
| C | 52.97 | 47.03 | - | - |
| D | 81.29 | 6.52 | 11.22 | 0.97 |
| Point | Al | Cu | Zn |
|---|---|---|---|
| E | 67.42 | 30.27 | 2.31 |
| F | 56.26 | 35.98 | 7.76 |
| G | 75.91 | 5.34 | 18.75 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M. Effects of Interfacial Reactions on Microstructures and Mechanical Properties of 3003 Al/T2 Cu and 1035 Al/T2 Cu Brazed Joints. Crystals 2020, 10, 248. https://doi.org/10.3390/cryst10040248
Zhang M. Effects of Interfacial Reactions on Microstructures and Mechanical Properties of 3003 Al/T2 Cu and 1035 Al/T2 Cu Brazed Joints. Crystals. 2020; 10(4):248. https://doi.org/10.3390/cryst10040248
Chicago/Turabian StyleZhang, Man. 2020. "Effects of Interfacial Reactions on Microstructures and Mechanical Properties of 3003 Al/T2 Cu and 1035 Al/T2 Cu Brazed Joints" Crystals 10, no. 4: 248. https://doi.org/10.3390/cryst10040248
APA StyleZhang, M. (2020). Effects of Interfacial Reactions on Microstructures and Mechanical Properties of 3003 Al/T2 Cu and 1035 Al/T2 Cu Brazed Joints. Crystals, 10(4), 248. https://doi.org/10.3390/cryst10040248




