Inducing the Effect of a Ga2O3 Nano-Particle on the CsF-RbF-AlF3 Flux for Brazing Aluminum to Carbon Steels
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
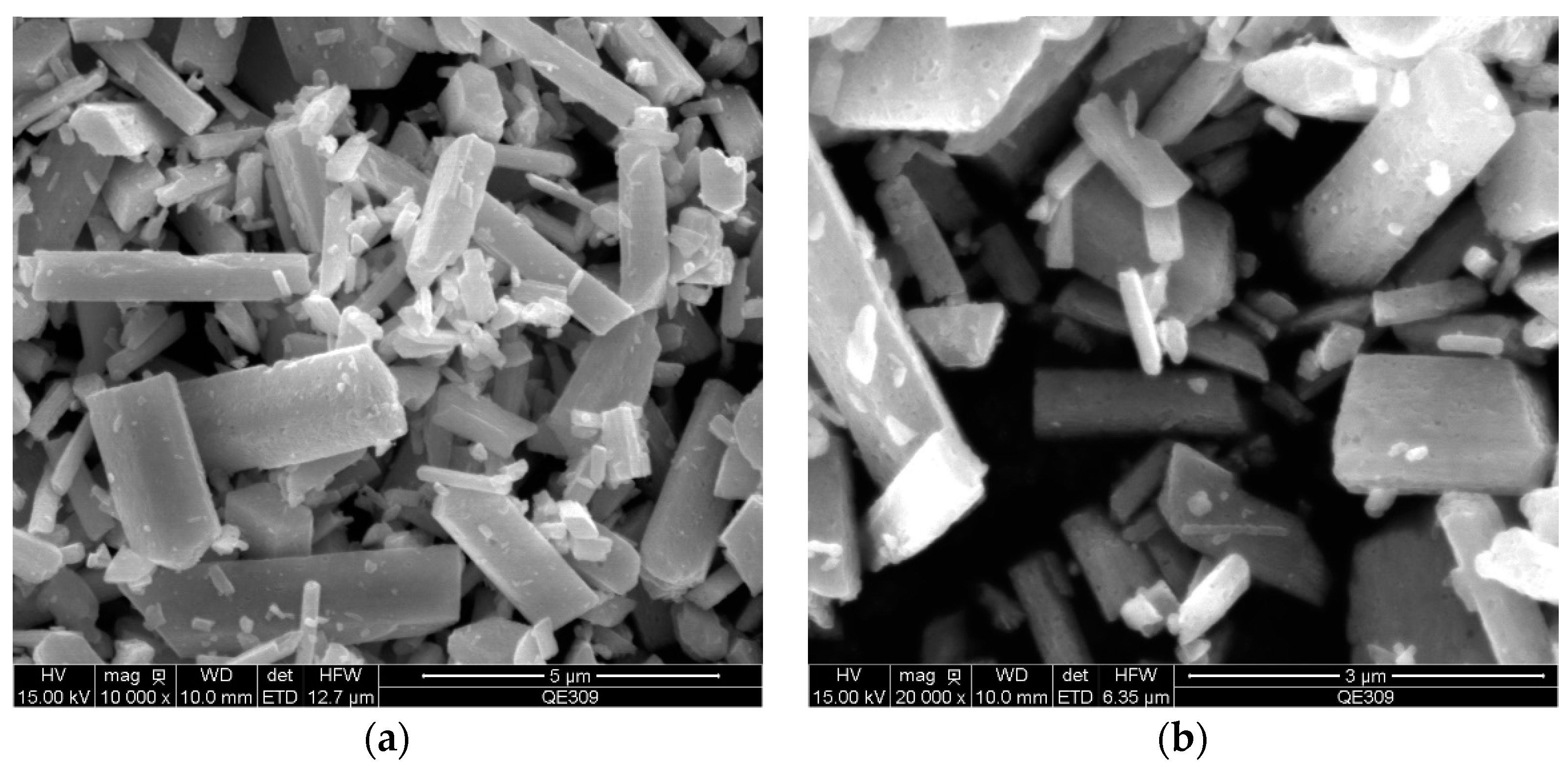
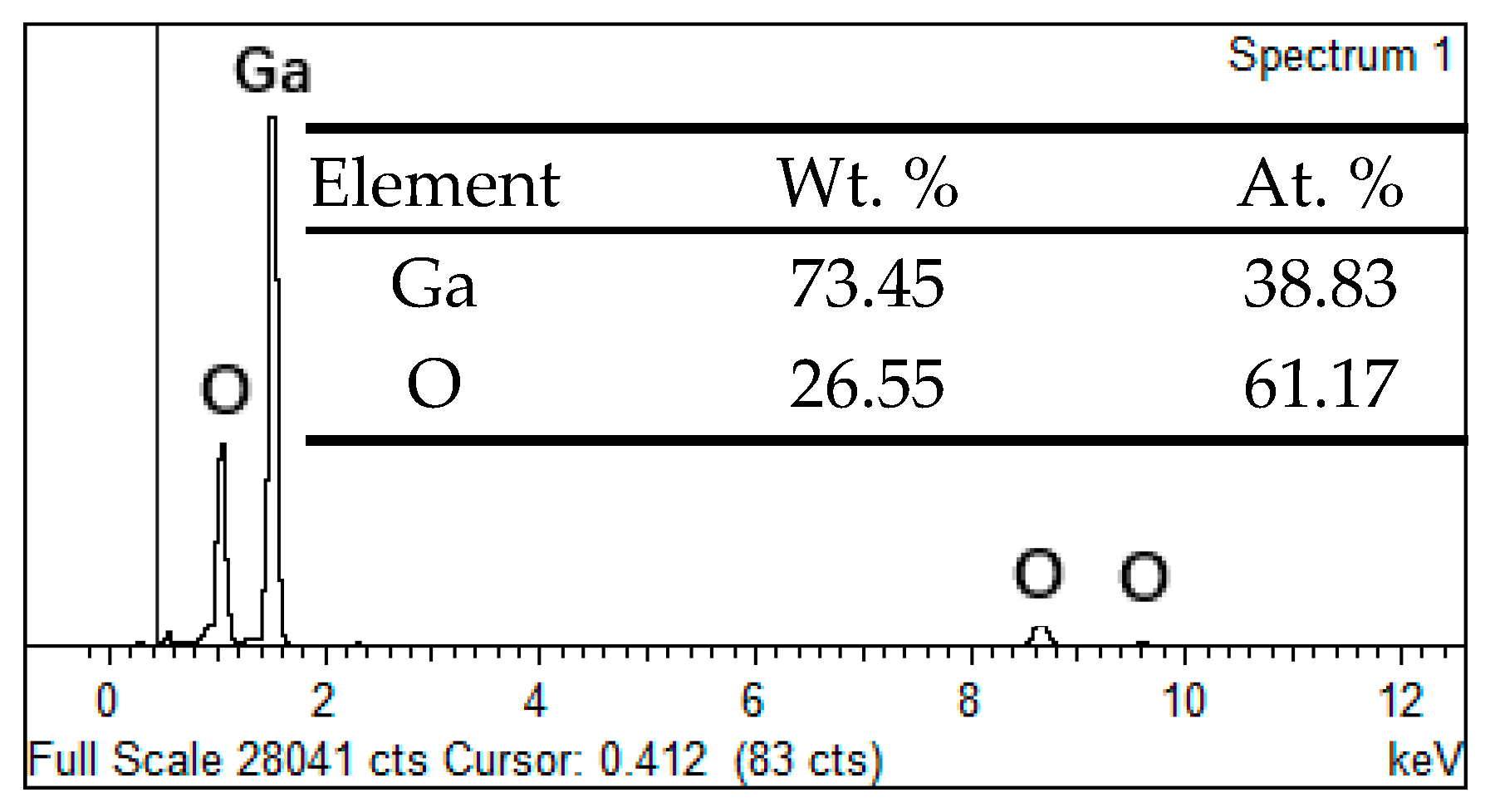
3.1. Morphology and Size of the Ga2O3 Micro-Nano Particle
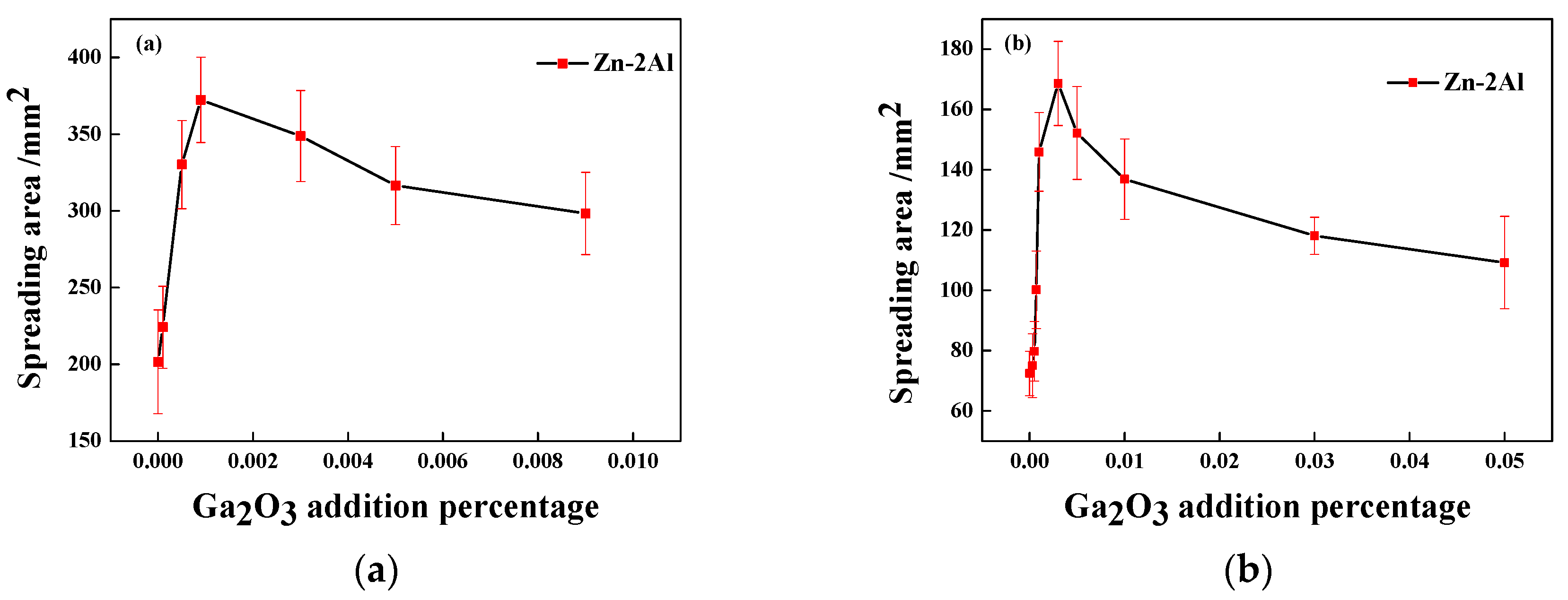
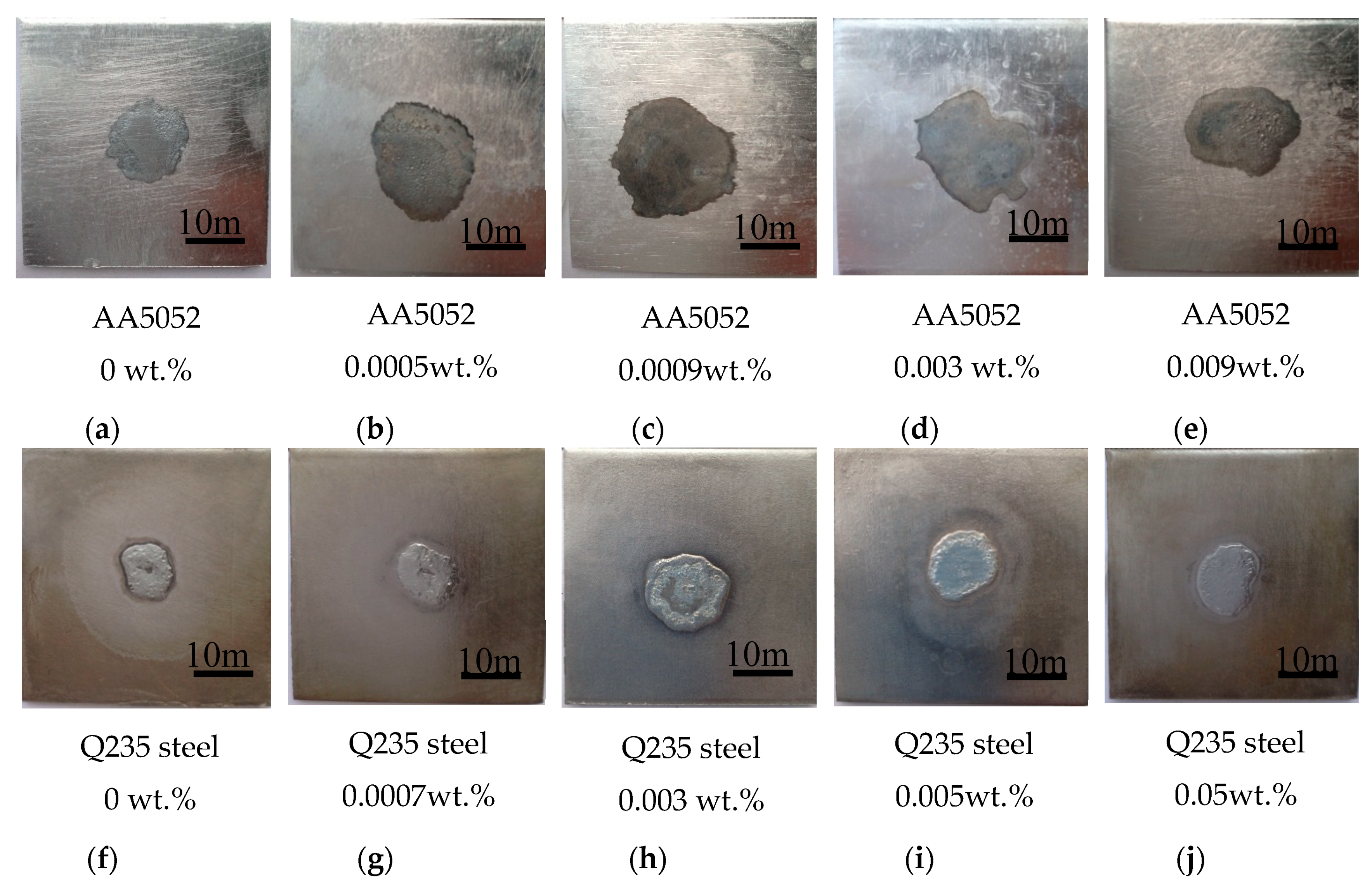
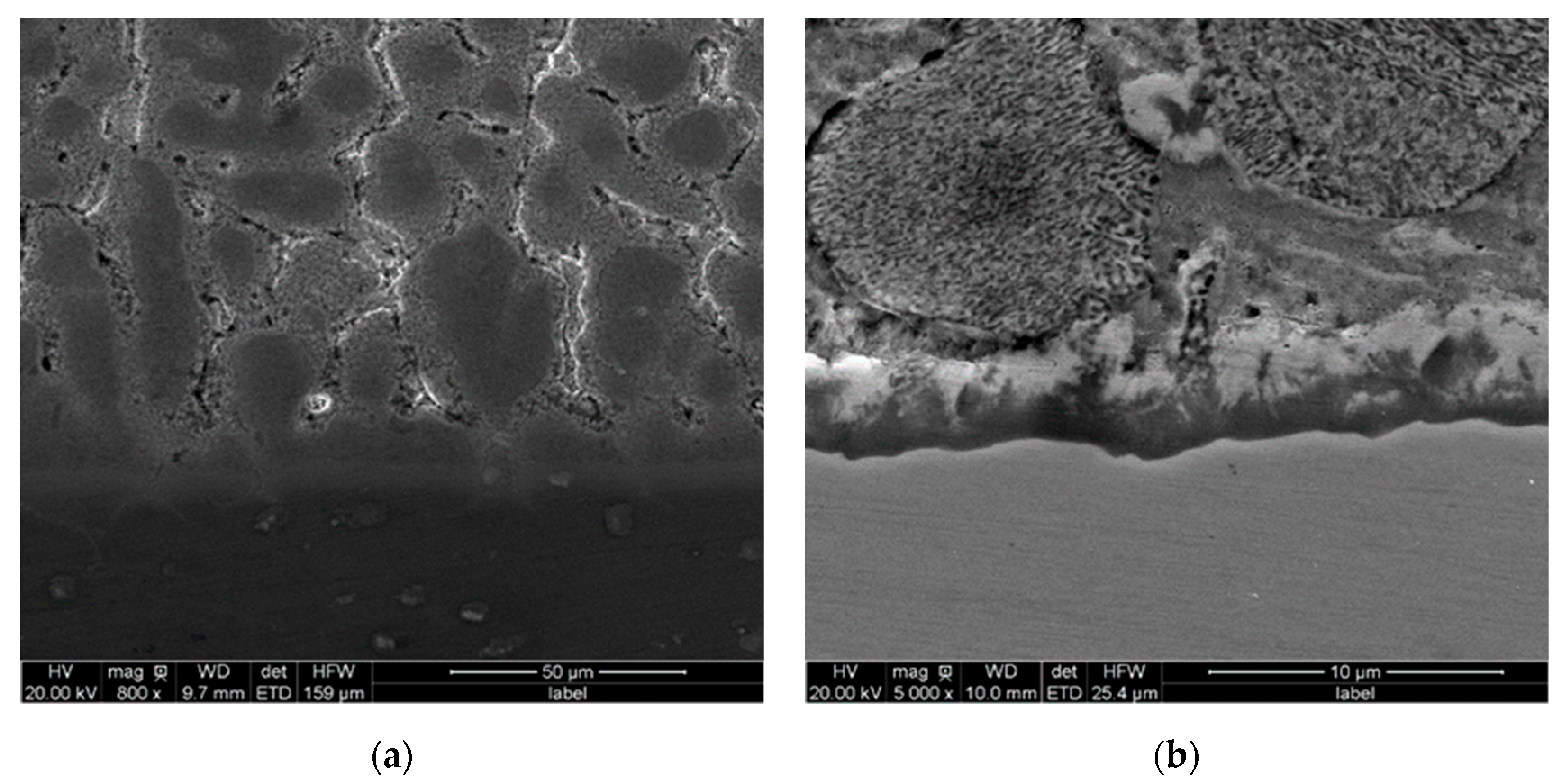
3.2. Application Properties of the Zn-2Al Filler Metal
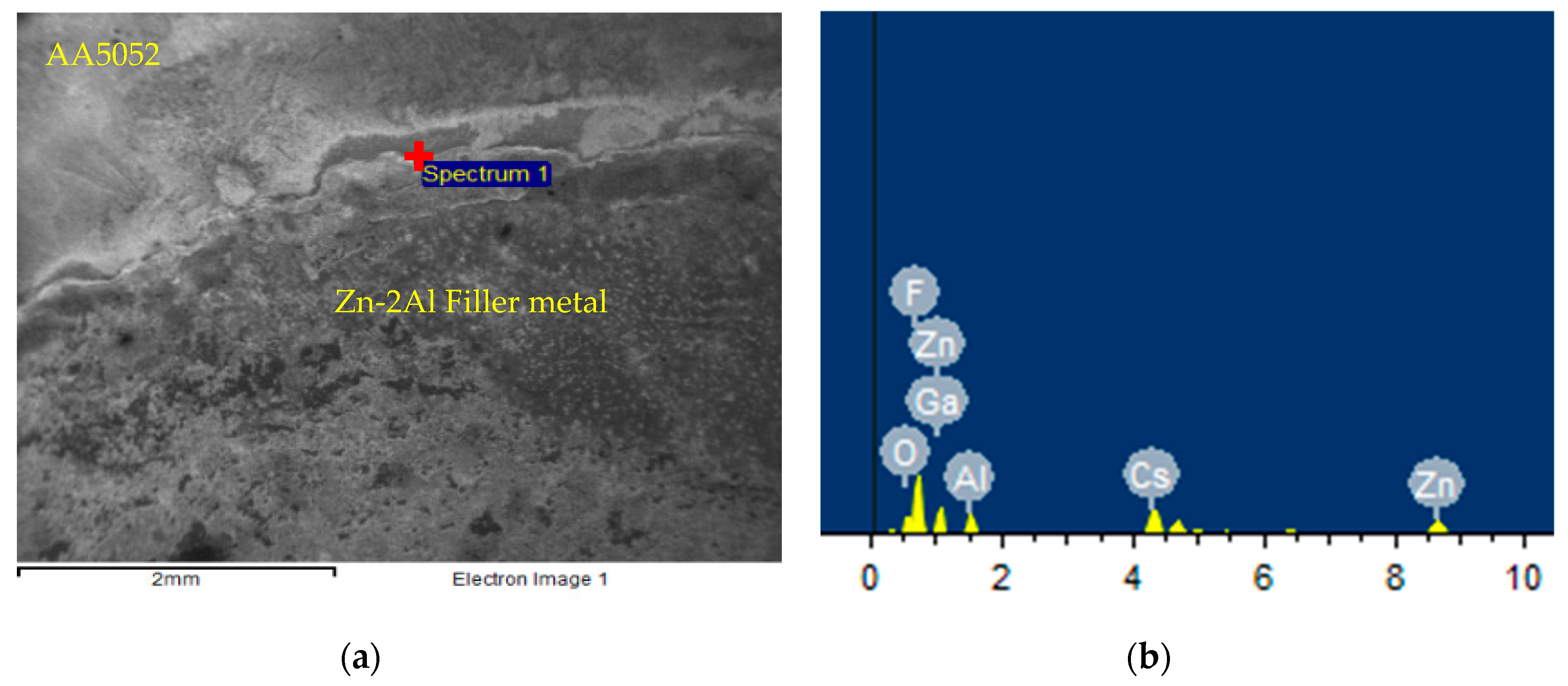
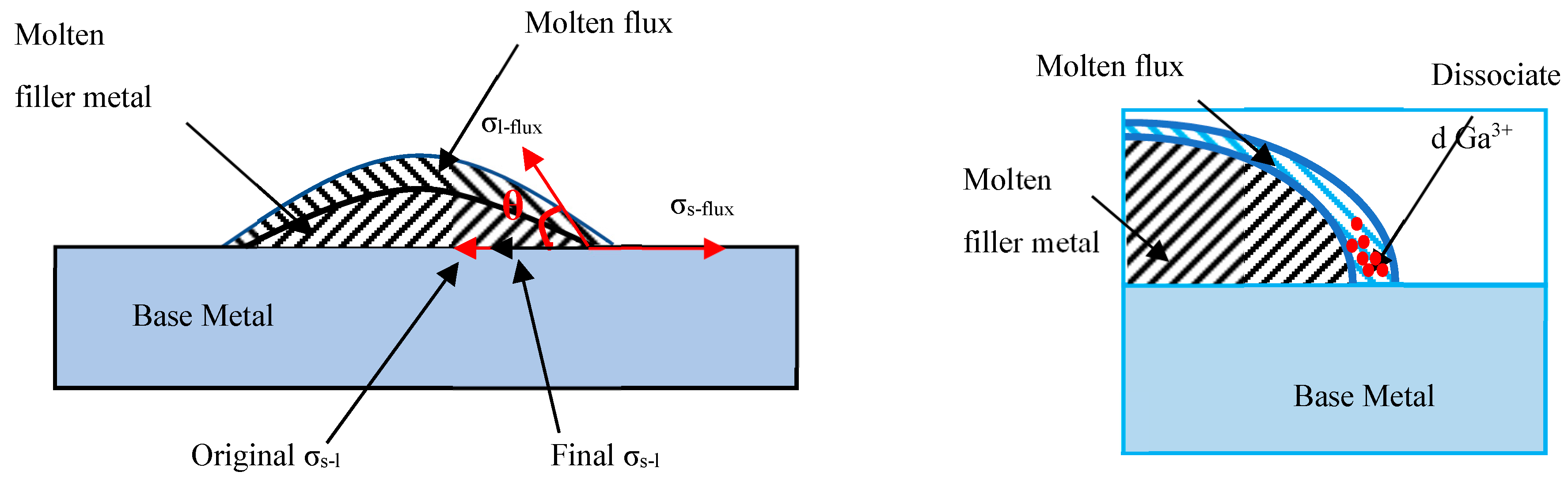
3.3. Interfacial Induction Effect of the Ga2O3 Particle
3.4. Analysis of the Flux Residue
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, F.J.; Yang, J.X.; Zhao, H.W.; Zhang, G.X.; Huang, J.L.; Long, W.M. Relationship between phase structure and melting characteristics of low cesium KF-CsF-AlF3 aluminum flux. Trans. China Weld. Inst. 2013, 34, 5–8. [Google Scholar]
- Cheng, F.J.; Jin, W.S.; Wang, Y.; Wang, D.P. The Chemical Reactions in Solution Synthesis of Aluminum Alloy Brazing Fluorate Flux. Adv. Mater. Res. 2011, 291, 924–928. [Google Scholar] [CrossRef]
- Xu, Z.W.; Yan, J.C.; Wang, C.; Yang, S.Q. Substrate oxide undermining by a Zn–Al alloy during wetting of alumina reinforced 6061 Al matrix composite. Mater. Chem. Phys. 2008, 112, 831–837. [Google Scholar] [CrossRef]
- Luo, W.; Wang, L.T.; Wang, Q.M.; Gong, H.L.; Yan, M. A new filler metal with low contents of Cu for high strength aluminum alloy brazed joints. Mater. Des. 2014, 63, 263–269. [Google Scholar] [CrossRef]
- Ji, F.; Xue, S.B.; Dai, W. Reliability studies of Cu/Al joints brazed with Zn–Al–Ce filler metals. Mater. Des. 2012, 42, 156–163. [Google Scholar]
- Yang, J.L.; Xue, S.B.; Xue, P.; Lv, Z.P.; Dai, W.; Zhang, J.X. Development of novel CsF–RbF–AlF3 flux for brazing aluminum to stainless steel with Zn–Al filler metal. Mater. Des. 2014, 64, 110–115. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, D.P.; Cheng, F.J.; Wang, Y. Oxide film on 5052 aluminium alloy: Its structure and removal mechanism by activated CsF–AlF3 flux in brazing. Appl. Surf. Sci. 2015, 337, 208–215. [Google Scholar] [CrossRef]
- Ma, F.; He, J.H.; Liang, C. Influence of Ga2O3 Morphology on Synthesis of YAGG: Ce3+ Phosphor Powders by Solid-state Reaction Method. Guangzhou Chem. Ind. Technol. 2018, 19, 36–38. [Google Scholar]
- Weber, C.; Stanjek, H. Energetic and entropic contributions to the work of adhesion in two-component, three-phase solid–liquid–vapour systems. Coll. Surf. A Physicochem. Eng. Asp. 2014, 441, 331–339. [Google Scholar] [CrossRef]
- Dezellus, O.; Eustathopoulos, N. Fundamental issues of reactive wetting by liquid metals. J. Mater. Sci. 2010, 45, 4256–4264. [Google Scholar] [CrossRef]
- Qian, H.; GAO, H.; Sun, B. Recent Researches and Development of Brazing Flux for Aluminum. Mater. Rev. 2007, 12, 76–78. [Google Scholar]
- Osório, W.R.; Garcia, A. Modeling dendritic structure and mechanical properties of Zn–Al alloys as a function of solidification conditions. Mater. Sci. Eng. A 2002, 325, 103–111. [Google Scholar] [CrossRef]
- Fan, R.H.; Lv, H.L.; Sun, K.N.; Wang, W.X.; Yi, X.B. Kinetics of thermite reaction in Al-Fe2O3 system. Thermochim. Acta 2006, 440, 129–131. [Google Scholar] [CrossRef]
- Dong, H.; Yang, L.; Dong, C.; Kou, S. Improving arc joining of Al to steel and Al to stainless steel. Mater. Sci. Eng. A 2012, 534, 424–435. [Google Scholar] [CrossRef]
- Zhao, H.T.; Shi, Y.Q.; Tian, M. Investigation on the Effect of the Substrate Temperature on the Photoelectric Properties of the Ga Doped ZnO Film. Mater. Sci. Forum 2016, 852, 1066–1069. [Google Scholar] [CrossRef]
- Guo, S.L.; Li, D.F.; Wu, X.P.; Xu, X.Q.; Du, P.; Hu, J. Characterization of hot deformation behavior of a Zn–10.2Al–2.1Cu alloy using processing maps. Mater. Des. 2012, 41, 158–166. [Google Scholar] [CrossRef]
- Zhang, J.X.; Xue, S.B.; Xue, P.; Liu, S. Thermodynamic reaction mechanism of the intermetallic compounds of SnxNdy and GaxNdy in soldered joint of Sn–9Zn–1Ga–0.5Nd. J. Mater. Sci. Mater. Electron. 2015, 26, 3064–3068. [Google Scholar] [CrossRef]
- Zhu, H.; Xue, S.B.; Sheng, Z. Effect of alloying elements on intermediate temperature filler metal in stepped welding of 6063 aluminum alloy. Trans. China Weld. Inst. 2009, 30, 33–36. [Google Scholar]
- Khan, T.I.; Kabir, M.H.; Bulpett, R. Effect of transient liquid-phase bonding variables on the properties of a micro-duplex stainless steel. Mater. Sci. Eng. A 2004, 372, 290–295. [Google Scholar] [CrossRef]
- Davies, G.B.; Krüger, T.; Coveney, P.V.; Harting, J.; Bresme, F. Interface deformations affect the orientation transition of magnetic ellipsoidal particles adsorbed at fluid-fluid interfaces. Soft Matter 2014, 10, 6742–6748. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, D.; Liu, H.Q.; Li, Y.J.; Hou, B.R. Influence of sulphate-reducing bacteria on environmental parameters and marine corrosion behavior of Q235 steel in aerobic conditions. Electrochim. Acta. 2010, 55, 1528–1534. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Q.; Li, X. Microstructure and properties of 20 steel pipe joints by transient liquid phase bonding and high temperature brazing. Trans. China Weld. Inst. 2005, 26, 56–60. [Google Scholar]
| Compounds | Inductions | ΔG/ kJ·mol−1 |
|---|---|---|
| FeO | Al | −259.98 |
| Fe2O3 | Al | −811.13 |
| Fe3O4 | Al | −1041.32 |
| Ga2O3 | Al | −575.18 |
| Compound | Density/g·mL−1 |
|---|---|
| Ga2O3 | 6.44 |
| CsAlF4 | 3.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Z.; Xue, S.; Yang, J.; Zhang, J. Inducing the Effect of a Ga2O3 Nano-Particle on the CsF-RbF-AlF3 Flux for Brazing Aluminum to Carbon Steels. Crystals 2020, 10, 183. https://doi.org/10.3390/cryst10030183
Yao Z, Xue S, Yang J, Zhang J. Inducing the Effect of a Ga2O3 Nano-Particle on the CsF-RbF-AlF3 Flux for Brazing Aluminum to Carbon Steels. Crystals. 2020; 10(3):183. https://doi.org/10.3390/cryst10030183
Chicago/Turabian StyleYao, Zhen, Songbai Xue, Jinlong Yang, and Junxiong Zhang. 2020. "Inducing the Effect of a Ga2O3 Nano-Particle on the CsF-RbF-AlF3 Flux for Brazing Aluminum to Carbon Steels" Crystals 10, no. 3: 183. https://doi.org/10.3390/cryst10030183
APA StyleYao, Z., Xue, S., Yang, J., & Zhang, J. (2020). Inducing the Effect of a Ga2O3 Nano-Particle on the CsF-RbF-AlF3 Flux for Brazing Aluminum to Carbon Steels. Crystals, 10(3), 183. https://doi.org/10.3390/cryst10030183






