High Refractive Index Inverse Vulcanized Polymers for Organic Photonic Crystals
Abstract
1. Introduction
2. Materials and Methods
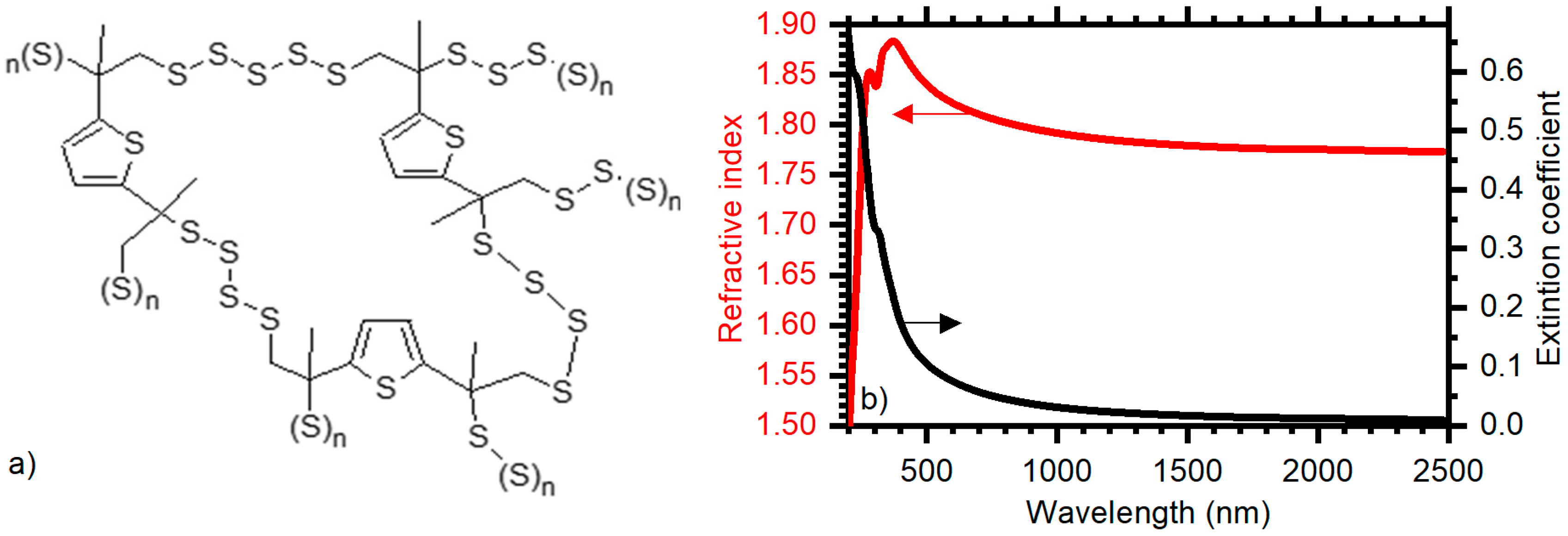
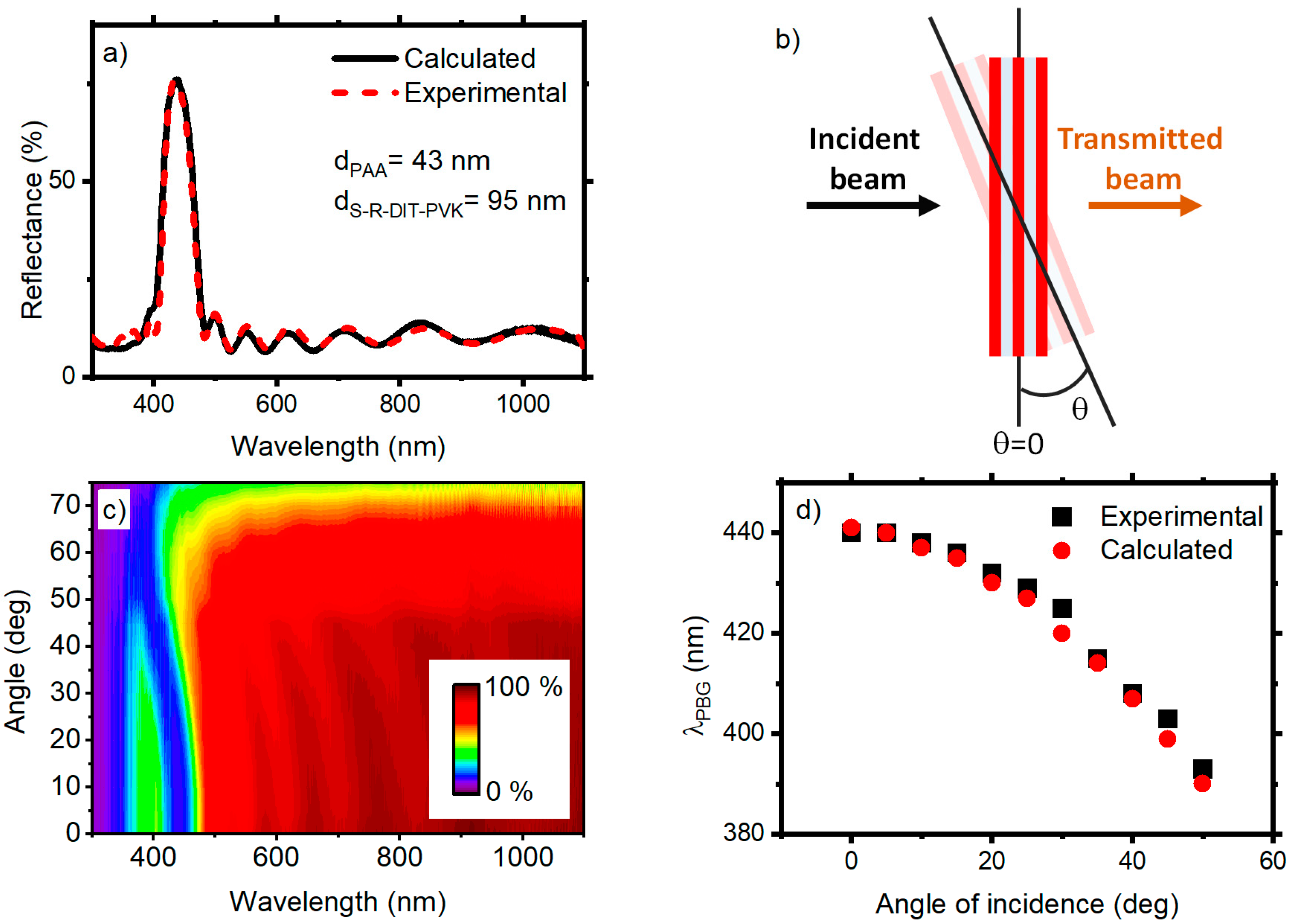
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Laussy, F.P. Microcavities; Oxford University Press: New York, NY, USA, 2017. [Google Scholar]
- Lova, P.; Manfredi, G.; Comoretto, D. Advances in functional solution processed planar one-dimensional photonic crystals. Adv. Opt. Mater. 2018, 6, 1800726–1800730. [Google Scholar] [CrossRef]
- Berti, L.; Cucini, M.; Di Stasio, F.; Comoretto, D.; Galli, M.; Marabelli, F.; Manfredi, N.; Marinzi, C.; Abbotto, A. Spectroscopic investigation of artificial opals infiltrated with a heteroaromatic quadrupolar dye. J. Phys. Chem. C 2010, 114, 2403–2413. [Google Scholar] [CrossRef]
- Dodero, A.; Vicini, S.; Alloisio, M.; Castellano, M. Sodium alginate solutions: Correlation between rheological properties and spinnability. J. Mater. Sci. 2019, 54, 8034–8046. [Google Scholar] [CrossRef]
- Vicini, S.; Mauri, M.; Vita, S.; Castellano, M. Alginate and alginate/hyaluronic acid membranes generated by electrospinning in wet conditions: Relationship between solution viscosity and spinnability. J. Appl. Polym. Sci. 2018, 135, 46390. [Google Scholar] [CrossRef]
- Zhou, J.; Singer, K.D.; Lott, J.; Song, H.; Wu, Y.; Andrews, J.; Baer, E.; Hiltner, A.; Weder, C. All-polymer distributed feedback and distributed Bragg-reflector lasers produced by roll-to-roll layer-multiplying co-extrusion. Nonlinear Opt. Quantum Opt. 2010, 41, 59–71. [Google Scholar]
- Ko, Y.-L.; Tsai, H.-P.; Lin, K.-Y.; Chen, Y.-C.; Yang, H. Reusable macroporous photonic crystal-based ethanol vapor detectors by doctor blade coating. J. Colloid Interface Sci. 2017, 487, 360–369. [Google Scholar] [CrossRef]
- Song, H.; Singer, K.; Lott, J.; Wu, Y.; Zhou, J.; Andrews, J.; Baer, E.; Hiltner, A.; Weder, C. Continuous melt processing of all-polymer distributed feedback lasers. J. Mater. Chem. 2009, 19, 7520–7524. [Google Scholar] [CrossRef]
- TORAY. Available online: http://www.toray.com/ (accessed on 27 January 2020).
- 3M DICHROIC. Available online: https://www.3m.com/3M/en_US/company-us/all-3m-products/~/3M-Dichroic-Films-for-Architectural-Laminated-Glass/?N=5002385+3291680356&rt=rud (accessed on 27 January 2020).
- Iasilli, G.; Francischello, R.; Lova, P.; Silvano, S.; Surace, A.; Pesce, G.; Alloisio, M.; Patrini, M.; Shimizu, M.; Comoretto, D.; et al. Luminescent solar concentrators: Boosted optical efficiency by polymer dielectric mirrors. Mater. Chem. Front. 2019, 3, 429–436. [Google Scholar] [CrossRef]
- Lova, P.; Giusto, P.; Stasio, F.D.; Manfredi, G.; Paternò, G.M.; Cortecchia, D.; Soci, C.; Comoretto, D. All-polymer methylammonium lead iodide perovskite microcavity. Nanoscale 2019, 11, 8978–8983. [Google Scholar] [CrossRef]
- Manfredi, G.; Lova, P.; Di Stasio, F.; Rastogi, P.; Krahne, R.; Comoretto, D. Lasing from dot-in-rod nanocrystals in planar polymer microcavities. RSC Adv. 2018, 8, 13026–13033. [Google Scholar] [CrossRef]
- Robbiano, V.; Paternò, G.M.; La Mattina, A.A.; Motti, S.G.; Lanzani, G.; Scotognella, F.; Barillaro, G. Room-temperature low-threshold lasing from monolithically integrated nanostructured porous silicon hybrid microcavities. ACS Nano 2018, 12, 4536–4544. [Google Scholar] [CrossRef]
- Liu, J.-G.; Ueda, M. High refractive index polymers: Fundamental research and practical applications. J. Mater. Chem. 2009, 19, 8907–8919. [Google Scholar] [CrossRef]
- Higashihara, T.; Ueda, M. Recent progress in high refractive index polymers. Macromolecules 2015, 48, 1915–1929. [Google Scholar] [CrossRef]
- Radice, S.V.; Gavezotti, P.; Simeone, G.; Albano, M.; Canazza, G.; Congiu, S. Photonic Crystals. WO PCT/EP2014/055590, 20 March 2014. [Google Scholar]
- Radice, S.V.; Srinivasan, P.; Comoretto, D.; Gazzo, S. One-Dimensional Planar Photonic Crystals Including Fluoropolymer Compositions and Corresponding Fabrication Methods. WO 2016/087439 A1, 9 June 2016. [Google Scholar]
- Russo, M.; Campoy-Quiles, M.; Lacharmoise, P.; Ferenczi, T.A.M.; Garriga, M.; Caseri, W.R.; Stingelin, N. One-pot synthesis of polymer/inorganic hybrids: Toward readily accessible, low-loss, and highly tunable refractive index materials and patterns. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 65–74. [Google Scholar] [CrossRef]
- Giusto, P.; Lova, P.; Manfredi, G.; Gazzo, S.; Srinivasan, B.; Radice, S.V.; Comoretto, D. Colorimetric detection of perfluorinated compounds by all-polymer photonic transducers. ACS Omega 2018, 3, 7517–7522. [Google Scholar] [CrossRef]
- Flaim, T.D.; Wang, Y.; Mercado, R. High-refractive-index polymer coatings for optoelectronics applications. In Proceedings of the Optical Systems Design, St. Etienne, France, 25 February 2004; p. 12. [Google Scholar]
- Shimizu, W.; Nakamura, S.; Sato, T.; Murakami, Y. Creation of high-refractive-index amorphous titanium oxide thin films from low-fractal-dimension polymeric precursors synthesized by a sol–gel technique with a hydrazine monohydrochloride catalyst. Langmuir 2012, 28, 12245–12255. [Google Scholar] [CrossRef]
- Lu, C.; Yang, B. High refractive index organic-inorganic nanocomposites: Design, synthesis and application. J. Mater. Chem. 2009, 19, 2884–2901. [Google Scholar] [CrossRef]
- Comoretto, D.; Dellepiane, G.; Cuniberti, C.; Rossi, L.; Borghesi, A.; LeMoigne, J. Photoinduced absorption of oriented poly 1,6-di(N-carbazolyl)-2,4-hexadiyne. Phys. Rev. B 1996, 53, 15653–15659. [Google Scholar] [CrossRef]
- Comoretto, D.; Cuniberti, C.; Musso, G.F.; Dellepiane, G.; Speroni, F.; Botta, C.; Luzzati, S. Optical properties and long-lived charged photoexcitations in polydiacetylenes. Phys. Rev. B 1994, 49, 8059–8066. [Google Scholar] [CrossRef]
- Ravnik, M.; Alexander, G.P.; Yeomans, J.M.; Žumer, S. Three-dimensional colloidal crystals in liquid crystalline blue phases. Proc. Natl. Acad. Sci. USA 2011, 108, 5188–5192. [Google Scholar] [CrossRef]
- Nussbaumer, R.J.; Caseri, W.R.; Smith, P.; Tervoort, T. Polymer-TiO2 nanocomposites: A route towards visually transparent broadband uv filters and high refractive index materials. Macromol. Chem. Phys. 2003, 288, 44–49. [Google Scholar] [CrossRef]
- Ogata, T.; Yagi, R.; Nakamura, N.; Kuwahara, Y.; Kurihara, S. Modulation of polymer refractive indices with diamond nanoparticles for metal-free multilayer film mirrors. ACS Appl. Mater. Interfaces 2012, 4, 3769–3772. [Google Scholar] [CrossRef] [PubMed]
- Gher, R.J.; Boyd, R.W. Optical properties of nanostructured optical materials. Chem. Mater. 1996, 8, 1807–1819. [Google Scholar] [CrossRef]
- Gaëtan, W.; Rolando, F.; Stefan, S.; Libero, Z. Nanoporous films with low refractive index for large-surface broad-band anti-reflection coatings. Macromol. Chem. Phys. 2010, 295, 628–636. [Google Scholar]
- Gazzo, S.; Manfredi, G.; Pötzsch, R.; Wei, Q.; Alloisio, M.; Voit, B.; Comoretto, D. High refractive index hyperbranched polyvinylsulfides for planar one-dimensional all-polymer photonic crystals. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 73–80. [Google Scholar] [CrossRef]
- Kleine, T.S.; Nguyen, N.A.; Anderson, L.E.; Namnabat, S.; LaVilla, E.A.; Showghi, S.A.; Dirlam, P.T.; Arrington, C.B.; Manchester, M.S.; Schwiegerling, J.; et al. High refractive index copolymers with improved thermomechanical properties via the inverse vulcanization of sulfur and 1,3,5-triisopropenylbenzene. ACS Macro Lett. 2016, 5, 1152–1156. [Google Scholar] [CrossRef]
- Anderson, L.E.; Kleine, T.S.; Zhang, Y.; Phan, D.D.; Namnabat, S.; LaVilla, E.A.; Konopka, K.M.; Ruiz Diaz, L.; Manchester, M.S.; Schwiegerling, J.; et al. Chalcogenide hybrid inorganic/organic polymers: Ultrahigh refractive index polymers for infrared imaging. ACS Macro Lett. 2017, 6, 500–504. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef]
- Griebel, J.J.; Glass, R.S.; Char, K.; Pyun, J. Polymerizations with elemental sulfur: A novel route to high sulfur content polymers for sustainability, energy and defense. Prog. Polym. Sci. 2016, 58, 90–125. [Google Scholar] [CrossRef]
- Griebel, J.J.; Nguyen, N.A.; Namnabat, S.; Anderson, L.E.; Glass, R.S.; Norwood, R.A.; Mackay, M.E.; Char, K.; Pyun, J. Dynamic covalent polymers via inverse vulcanization of elemental sulfur for healable infrared optical materials. ACS Macro Lett. 2015, 4, 862–866. [Google Scholar] [CrossRef]
- Ramarad, S.; Khalid, M.; Ratnam, C.T.; Chuah, A.L.; Rashmi, W. Waste tire rubber in polymer blends: A review on the evolution, properties and future. Prog. Mater. Sci. 2015, 72, 100–140. [Google Scholar] [CrossRef]
- Griebel, J.J.; Namnabat, S.; Kim, E.T.; Himmelhube, R.; Moronta, D.H.; Chung, W.J.; Simmonds Adam, G.; Kim Kyung, J.; Van der Laan, J.; Nguyen Ngoc, A.; et al. New infrared transmitting material via inverse vulcanization of elemental sulfur to prepare high refractive index polymers. Adv. Mater. 2014, 26, 3014–3018. [Google Scholar] [CrossRef] [PubMed]
- Concidian, D. Sulfur (in biological systems). In Van Nostrand’s Scientific Encyclopedia; D. Van Nostrand Company, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Boyd, D.A. Sulfur and its role in modern materials science. Angew. Chem. Int. Ed. 2016, 55, 15486–15502. [Google Scholar] [CrossRef] [PubMed]
- Lova, P.; Grande, V.; Manfredi, G.; Patrin, M.; Herbst, S.; Würthner, F.; Comoretto, D. All-polymer photonic microcavities doped with perylene bisimide J-aggregates. Adv. Opt. Mater. 2017, 5, 1700523. [Google Scholar] [CrossRef]
- Manfredi, G.; Lova, P.; Di Stasio, F.; Krahne, R.; Comoretto, D. Directional fluorescence shaping and lasing in all-polymer microcavities doped with CdSe/CdS dot-in-rod nanocrystals. In Proceedings of the 2017 European Conference on Lasers and Electro-Optics and European Quantum Electronics Conference, Munich, Germany, 25–29 June 2017. [Google Scholar]
- J.A. Woollam Co. WVASE32® Software; J.A. Woollam Co.: Lincoln, NE, USA, 2006. [Google Scholar]
- Lova, P.; Cortecchia, D.; Soci, C.; Comoretto, D. Solution processed polymer-ABX4 perovskite-like microcavities. Appl. Sci. 2019, 9, 5203. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst. J. Chem. Soc. Chem. Commun. 1979. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- American Chemical Society. Green chemical syntheses and processes. In Green Chemical Syntheses and Processes; American Chemical Society: Washington, DC, USA, 2000; Volume 767, pp. 1–5. [Google Scholar]
- Hooshmand, S.E.; Heidari, B.; Sedghi, R.; Varma, R.S. Recent advances in the Suzuki–Miyaura cross-coupling reaction using efficient catalysts in eco-friendly media. Green Chem. 2019, 21, 381–405. [Google Scholar] [CrossRef]
- Nobel Prize in Chemistry 2010. Available online: https://www.nobelprize.org/prizes/chemistry/2010/summary/ (accessed on 27 January 2020).
- Chen, J.; Liu, L.; Weng, L.; Lin, Y.; Liao, L.; Wang, C.; Yang, J.; Wu, Z. Synthesis and properties evolution of a family of tiara-like phenylethanethiolated palladium nanoclusters. Sci. Rep. 2015, 5, 16628. [Google Scholar] [CrossRef]
- Lova, P.; Cortecchia, D.S.; Krishnamoorthy, H.N.; Giusto, P.; Bastianini, C.; Bruno, A.; Comoretto, D.; Soci, C. Engineering the emission of broadband 2D perovskites by polymer distributed Bragg reflectors. ACS Photonics 2018, 5, 867–874. [Google Scholar] [CrossRef]
- Manfredi, G.; Lova, P.; Di Stasio, F.; Krahne, R.; Comoretto, D. Directional fluorescence spectral narrowing in all-polymer microcavities doped with CdSe/CdS dot-in-rod nanocrystals. ACS Photonics 2017, 4, 1761–1769. [Google Scholar] [CrossRef]
- Joannopulos, J.D.; Meade, R.D.; Win, J.N. Photonic Crystals: Molding the Flow of the Light; Princeton University Press: Princeton, NJ, USA, 1995. [Google Scholar]
- Unger, K.; Resel, R.; Czibula, C.; Ganser, C.; Teichert, C.; Jakopic, G.; Canazza, G.; Gazzo, S.; Comoretto, D. Distributed Bragg reflectors: Morphology of cellulose acetate and polystyrene multilayers. In Proceedings of the 2014 16th International Conference on Transparent Optical Networks (ICTON), Graz, Austria, 6–10 July 2014; pp. 1–4. [Google Scholar]
- Comoretto, D. Organic and Hybrid Photonic Crystals; Springer: Cham, Switzerland, 2015. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavella, C.; Lova, P.; Marsotto, M.; Luciano, G.; Patrini, M.; Stagnaro, P.; Comoretto, D. High Refractive Index Inverse Vulcanized Polymers for Organic Photonic Crystals. Crystals 2020, 10, 154. https://doi.org/10.3390/cryst10030154
Tavella C, Lova P, Marsotto M, Luciano G, Patrini M, Stagnaro P, Comoretto D. High Refractive Index Inverse Vulcanized Polymers for Organic Photonic Crystals. Crystals. 2020; 10(3):154. https://doi.org/10.3390/cryst10030154
Chicago/Turabian StyleTavella, Christian, Paola Lova, Martina Marsotto, Giorgio Luciano, Maddalena Patrini, Paola Stagnaro, and Davide Comoretto. 2020. "High Refractive Index Inverse Vulcanized Polymers for Organic Photonic Crystals" Crystals 10, no. 3: 154. https://doi.org/10.3390/cryst10030154
APA StyleTavella, C., Lova, P., Marsotto, M., Luciano, G., Patrini, M., Stagnaro, P., & Comoretto, D. (2020). High Refractive Index Inverse Vulcanized Polymers for Organic Photonic Crystals. Crystals, 10(3), 154. https://doi.org/10.3390/cryst10030154





