Abstract
A novel heteroleptic paddlewheel-type dirhodium (Rh2) complex [Rh2(O2CCH3)3(PABC)] (1; PABC = para-aminobenzenecarboxylate), which has an amino group as a potential donor site for coordination with the metal ion, was synthesized and characterized by 1H NMR, ESI-TOF-MS, infrared spectra, and elemental analysis. The slow evaporation of N,N-dimethylformamide (DMF)-dissolved 1 produces the purple-colored crystalline polymeric species [Rh2(O2CCH3)3 (PABC)(DMF)]n (1P). Single-crystal and powder X-ray diffraction analyses, as well as thermo-gravimetric analysis, clarified that 1P formed a one-dimensional polymeric structure, in which the two axial sites of the Rh2 ion in 1P are coordinated by a DMF molecule and an amino group of the PABC ligand of the neighboring molecule 1, by coordination-induced self-assembly (polymerization) with an Rh-amino bond. The reversible structural change (self-assembly and disassembly transformations) between the discrete species [Rh2(O2CCH3)3(PABC)(DMF)2] (1D; green solution) and the polymeric species 1P (purple solid) was accompanied by a color change, which easily occurred by the dissolution and evaporation procedures with DMF.
1. Introduction
Paddlewheel-type dirhodium (Rh2) complexes have been attracting considerable interest owing to the basic understanding of their molecular geometries and electronic structures with metal-metal bonds [1] as well as their intriguing properties as antitumor agents [2,3,4], chemical sensors [5,6,7], and catalysts for various types of organic reactions [8,9,10,11] and photochemical and electrochemical hydrogen evolution from aqueous solutions [12,13,14]. A considerable number of experimental and theoretical studies have adequately evaluated the synthesis, molecular geometries, and electronic structures of homoleptic Rh2 complexes with the same bridging carboxylate ligands, [Rh2(O2CR)4L2] (O2CR = bridging carboxylate ligand, L = axial coordinated ligand) [1,15,16,17,18,19,20,21]. However, heteroleptic Rh2 complexes with mixed bridging carboxylate ligands, [Rh2(O2CR)n (O2CR’)4-nL2] (n = 1–3), are very limited [22,23]; thus, related research is also insufficient.
Recently, paddlewheel-type Rh2 complexes have been utilized as secondary building units (SBUs) for supra-molecular complexes [24,25], such as coordination polymers (CPs) [26,27,28,29,30] and metal-organic frameworks (MOFs) [31,32,33,34,35], owing to their water and thermal stabilities. There are two conventional synthetic strategies for the production of Rh2-SBU-based CPs and MOFs. The first strategy is the ligand-exchange reaction between the equatorial carboxylate ligands of the Rh2 complex precursor and organic poly-carboxylic acids at high temperature [14,31,32,33,34]. The second strategy is the coordination-induced self-assembly reaction, in which polydentate imine ligands, such as pyradine and 4,4’-bipyridine [26,27,28,29,35], are coordinated with the axial sites of Rh2 complexes. In general, two synthetic strategies are applied to homoleptic Rh2-SBUs with the same bridging carboxylate ligands. To our knowledge, CPs and MOFs constructed from heteroleptic Rh2-SBUs with mixed carboxylate ligands have not been reported.
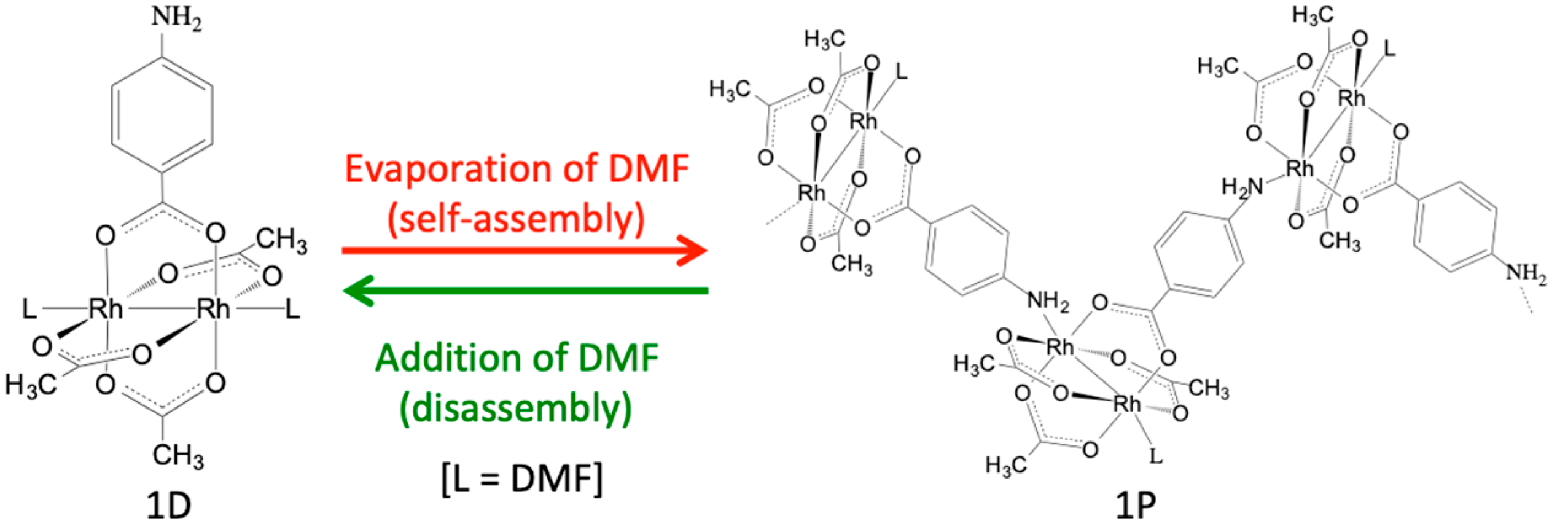
With this background in mind, this study integrates the two abovementioned synthetic strategies to establish a new synthetic strategy for the synthesis of Rh2-SBU-based CPs and MOFs. Specifically, we prepared a new heteroleptic Rh2 complex [Rh2(O2CCH3)3(PABC)] (1; PABC = para-aminobenzenecarboxylate), in which the amino group of the PABC ligand is coordinated with the axial sites of the Rh2 unit. As shown in Figure 1, although complex 1 exists as a discrete molecular structure [Rh2(O2CCH3)3(PABC)(DMF)2] (1D) in N,N-dimethylformamide (DMF), it was easily self-assembled to form a one-dimensional polymeric structure [Rh2(O2CCH3)3(PABC)(DMF)]n (1P) by the evaporation of the DMF solvent. Evidently, the polymeric species 1P can be re-dissolved in DMF, which indicates that the self-assembly and disassembly transformations between 1D and 1P easily occurred by the dissolution and evaporation procedures with DMF. We believe that this study affords a new strategy for the construction of self-assembled CPs with paddlewheel-type Rh2-SBUs.
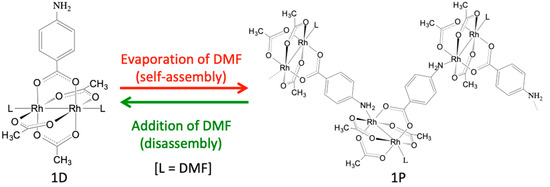
Figure 1.
Self-assembly and disassembly transformations between [Rh2(O2CCH3)3(PABC)(DMF)2] (1D) and [Rh2(O2CCH3)3(PABC)(DMF)]n (1P).
2. Materials and Methods
2.1. General
All materials, solvents, and gases used in this study were purchased from commercial suppliers and were used without further purifications. [Rh2(O2CCH3)4(H2O)2] was prepared according to the literature [36]. Proton nuclear magnetic resonance (1H NMR) spectra were performed with a JEOL-500SS (500 MHz) spectrometer (Tokyo, Japan) with DMF-d7 as the solvent. Chemical shifts were referenced to residual DMF (δ = 2.75 ppm). Electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS) was performed with a Bruker micrO-TOF II analyzer (Billerica, MS, USA). The instrument was calibrated with a solution of sodium formate. Infrared spectra were obtained with a JASCO FT/IR 6300 spectrometer equipped with a JASCO ATR PRO ONE module (Tokyo, Japan). Powder X-ray diffraction analysis was performed on a Rigaku SmartLab X-ray diffractometer with Cu Kα radiation (Tokyo, Japan). Thermo-gravimetric (TG) analysis was conducted with a MAC Science TG-DTA 2000S (Billerica, MS, USA) with a heating rate of 4 K/min under a nitrogen atmosphere. The UV-visible absorption spectrum in DMF was recorded with a JASCO V-670 spectrometer (Tokyo, Japan). The diffuse reflectance spectrum was measured with a JASCO V-670 spectrometer equipped with a JASCO ISN-923 integrating sphere (Tokyo, Japan). Elemental analysis was conducted on a Yanaco CHN CORDER MT-6 installed at Shimane University, Japan.
2.2. Synthesis of [Rh2(O2CCH3)3(PABC)] (1)
A mixture of [Rh2(O2CCH3)4(H2O)2] (191.2 mg, 0.40 mmol), HPABC (54.8 mg, 0.40 mmol), and CH3CN (20.0 mL) was placed into the autoclave and sealed under an Ar atmosphere. Then, the reaction mixture was heated at 413 K for 6 h. The resulting solution was evaporated until dry, and the residue was purified by silica-gel column chromatography (eluent: 5% MeOH in CHCl3). After the evaporation of the solvent, the green-colored residue was collected and dried for 3 h at 353 K. Yield: 32.9 mg (0.0634 mmol, 15.9%). Anal. calc. for Rh2C13H15NO8: C, 30.08%; H, 2.91%; N, 2.70%. Found: C, 29.64%; H, 3.26%; N, 2.81%. 1H NMR (500 MHz, DMF-d7, 300 K): δ = 7.55 (d, 2H, PABC-CH), 6.54 (d, 2H, PABC-CH), 5.78 (s, 2H, PABC-NH2), 1.79 (s, 3H, trans-O2CCH3), 1.71 (s, 6H, cis-O2CCH3). ESI-TOF-MS: calc. for [M+Na]+: 541.8800 m/z; found 541.8783 m/z. Infrared (ATR, cm−1): 3273(w), 3184(w), 2985(w), 1596(s), 1575(s), 1500(w), 1402(s), 1345(m), 1253(m), 1176(m), 1097(w), 1020(m), 1003(m), 893(m), 862(m), 848(w), 806(w), 773(m), 696(s), 659(m), 626(w), 591(w).
2.3. Single-Crystal X-Ray Diffraction Analysis
Single crystals of 1P suitable for single-crystal X-ray diffraction analysis were obtained by the slow evaporation of the DMF solution of 1. The X-ray diffraction data of 1P were collected at 150 K using a RIGAKU Mercury system equipped with a rotating-anode X-ray generator with Mo Kα radiation (λ = 0.71075 Å) installed at the Institute of Molecular Science and were processed using the RIGAKU CrystalClear-SM Expert 2.0 program. The structure of 1P was solved by the direct method SIR-2011 [37] and was refined using the full-matrix least-squares technique F2 with SHELXL2014 [38] equipped in the RIGAKU CrystalStructure 4.3 software. Non-hydrogen atoms were refined with anisotropic displacement, and hydrogen atoms were located at the calculated positions and were refined as riding models. The crystal data and details of the data collection and refinement of 1P are summarized in Table 1 and can be obtained as a CIF from the Cambridge Crystallographic Data Center (CCDC). The deposition number of 1P is CCDC-1973889.

Table 1.
Crystallographic and refinement data of 1P.
2.4. Theoretical Calculation Method
All density functional theory (DFT) calculations applied in this study were performed with B3LYP functional with the LANL08(f) basis set for Rh atoms and cc-pVDZ for other atoms using the Gaussian 09 (C.02) program package [39]. The geometry optimizations of [Rh2(O2CCH3)3(PABC)], [Rh2(O2CCH3)3(PABC)(DMF)], and 1D were carried out without symmetry constraints (see Supplementary Materials Figure S1 and Tables S1–S3), and the resulting optimized structures were confirmed to be energy minima by the frequency analyses.
3. Results
3.1. Synthesis and Characterizations
Complex 1 was synthesized by the solvothermal reaction of [Rh2(O2CCH3)4(H2O)2] and HPABC (in 1:1 molar ratio) in CH3CN at 413 K for 6 h with a Teflon-lined steel autoclave. The pure product of 1 can be purified by silica-gel column chromatography (Eluent: 5% MeOH in CHCl3) with a 15.9% yield. The obtained 1 is stable in air (even in moist air) and can be dissolved in DMF. Complex 1 was characterized by 1H NMR, ESI-TOF-MS, infrared spectroscopy, and elemental analysis. In the 1H NMR spectrum measured in DMF-d7 (see Figure S2), the two doublet signals, which were assigned as aromatic protons in the PABC ligand, were detected at 7.55 and 6.54 ppm. Moreover, a singlet signal that was derived from the amino protons appeared at 5.78 ppm. The signals for methyl protons in O2CCH3 ligands were divided into two signals (1.71 and 1.79 ppm) with the integral intensities of 2:1, which correspond to the cis- and trans-positions, respectively, relative to the PABC ligand. As depicted in Figure S3, the 1H NMR spectral features of 1 measured in DMSO-d6 were similar to those measured in DMF-d7. In the positive-ion mode ESI-TOF-MS, the signal was observed at 541.8783 m/z, which corresponds to the [M+Na]+ value of complex 1 (541.8800 m/z). As depicted in Figure S4, the observed shape and pattern of the isotope pattern also fit well with the simulated isotope distribution of complex 1. Moreover, no signal assignable to unreacted [Rh2(O2CCH3)4(H2O)2] was not detected. In the infrared spectrum (ATR method), the weak absorption bands of the amino group were observed at 3273 and 3184 cm−1. The asymmetric [ν(CO2−)asymm] and symmetric [ν(CO2−)symm] vibrations of the bridging carboxylate groups were observed at 1596–1575 and 1402 cm−1, respectively, which are close to those of homoleptic paddlewheel-type Rh2 complexes such as [Rh2(O2CCH3)4(H2O)2] [ν(CO2−)asymm = 1587 cm−1, ν(CO2−)symm = 1437 cm−1] [36]. The vibration of the carboxylic acid group owing to the presence of the unreacted HPABC was not observed in the spectrum. The purity of complex 1 was further confirmed by CHN elemental analysis; the observed ratio of carbon, hydrogen, and nitrogen of the obtained powder was in good agreement with the calculated ratio of [Rh2(O2CCH3)3(PABC)] (Rh2C13H15NO8), as shown in the experimental section.
3.2. Crystal Structure
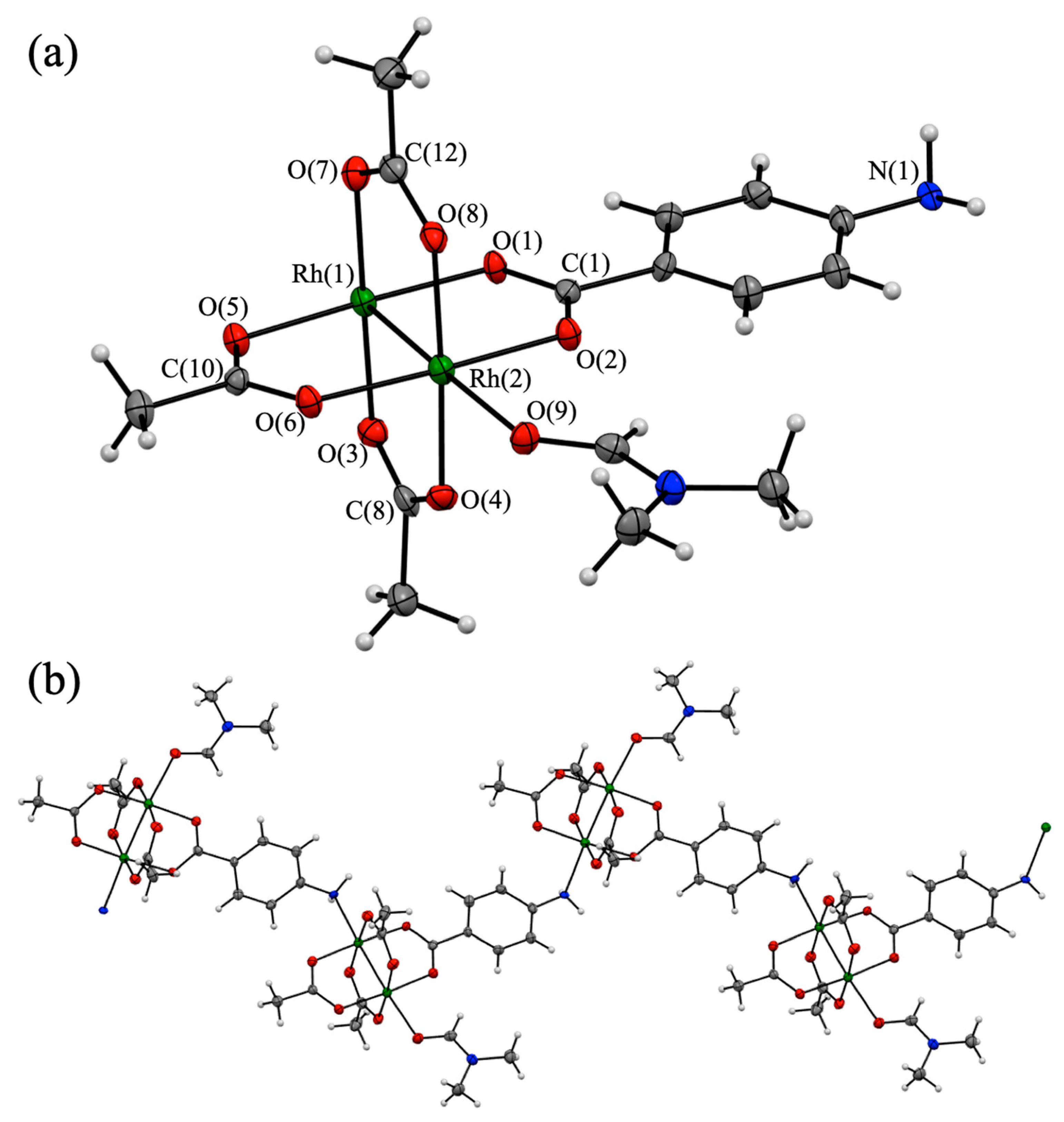
Small purple-colored single crystals of 1P, which are suitable for single-crystal X-ray diffraction analysis, can be obtained by the slow evaporation of the DMF solution of 1. The X-ray diffraction pattern revealed that this complex crystallized in the monoclinic system with the space group P 21/c. The final refined model of 1P is shown in Figure 2, and the crystallographic data and structural parameters of 1P are summarized in Table 1 and Table 2, respectively.
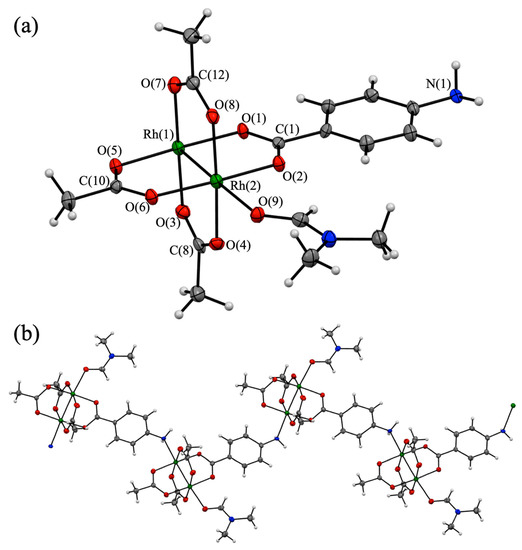
Figure 2.
(a) Asymmetric unit of [Rh2(O2CCH3)3(PABC)(DMF)]n (1P) (30% thermal ellipsoid probability). (b) One-dimensional coordination structure of 1P (Rh: green, O: red, N: blue, C: gray, and H: white).

Table 2.
Structural parameters of 1P (bond length: Å, bond angle: °).
The asymmetric unit of 1P contains two Rh ions, three O2CCH3 ligands, one PABC ligand, and one DMF molecule (Figure 2a) and adopts a typical paddlewheel-type structure in which the Rh2 core is coordinated by the bridging carboxylates of three O2CCH3 ligands and one PABC ligand at the equatorial positions. Figure 2b shows that the structural unit of 1P was self-assembled to form the one-dimensional chain (polymeric) structure using the rhodium-amine coordination bond. Therefore, the axial position of the Rh2 core was coordinated by one O atom of the DMF molecule and one N atom of the PABC ligand. Thus, both Rh ions in 1P have a distorted octahedral coordination environment. The Rh-Rh bond length of 1P is estimated as 2.3840(8) Å, which is within the typical range of the Rh-Rh bond length of homoleptic paddlewheel-type Rh2 complexes such as [Rh2(O2CCH3)4(DMF)2] (2.383 Å) [40] and [Rh2(O2CCH3)4(NHEt)2] (2.402 Å) [41]. The differences in the Rh-O(carboxylate) bond lengths in 1P are negligible and less than 0.024 Å. The O-C-O angles of the bridging carboxylates of 1P are in the range of 126.2(7)°–128.1(8)°, which are also close to those of the paddlewheel-type Rh2 complex, such as [Rh2(O2CCH3)4(DMF)2] (125.1°–126.2°) [40]. However, the Rh-N(amine) and Rh-O(DMF) bond lengths in 1P are 2.293(5) and 2.308(5) Å, respectively, which are clearly longer than those of Rh-O(carboxylate). These results indicate that the coordination bonds in the axial sites of 1P were weaker than those in the equatorial sites. The phenyl rings of the PABC ligand in 1P are almost coplanar (2.87°) relative to the Rh2-carboxylate(PABC) plane. This result suggests the formation of the transition dipole moment between the PABC ligand and the Rh2 moiety [25]. As shown in Figure S5, the one-dimensional chains of 1P were closely assembled in the crystal without non-coordinated DMF molecules.
3.3. Self-Assembly and Disassembly Phenomena
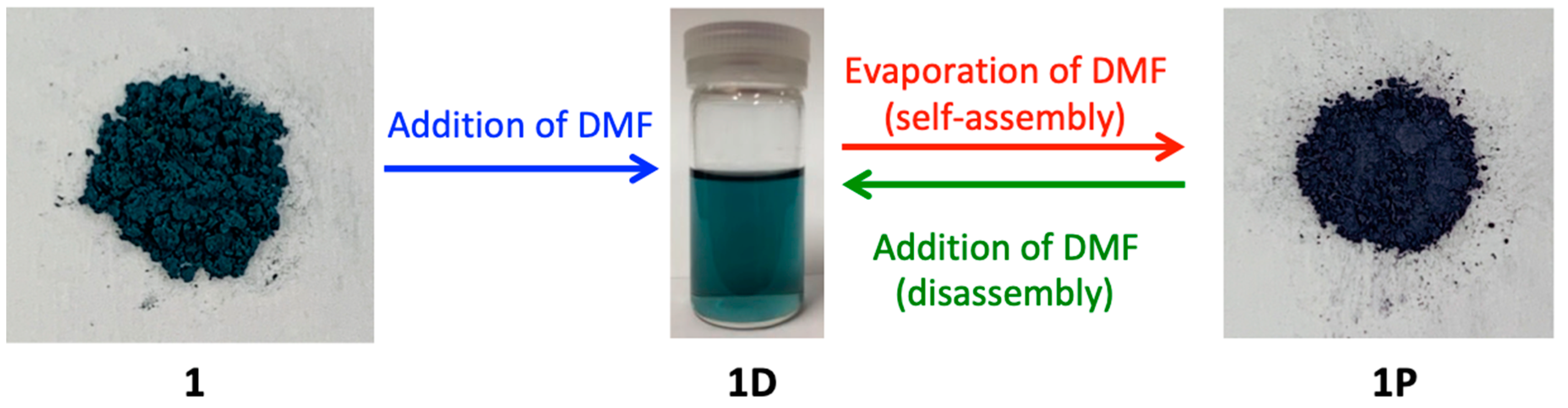
As shown in Figure 3, a green solution was obtained when 1 was dissolved in DMF, and the evaporation of DMF from the green solution produced a purple-colored crystalline product 1P. On the basis of the color of the solution and the knowledge of previously reported paddlewheel-type Rh2 complexes [7], it is speculated that [Rh2(O2CCH3)3(PABC)(DMF)2] (1D), in which two DMF molecules are coordinated with the axial sites of Rh2 ions, is formed in the DMF solution because the solution and solids of the paddlewheel-type Rh2 complexes with axial O-donor ligands typically have a green color while those with axial N-donor ligands have a purple (or red) color [7]. Remarkably, we confirmed that the polymeric species 1P can be easily re-dissolved in DMF and afford 1D, which results in the green color of the solution. This indicates that the self-assembly and disassembly transformations between 1D and 1P easily occur by the dissolution and evaporation procedures with DMF.
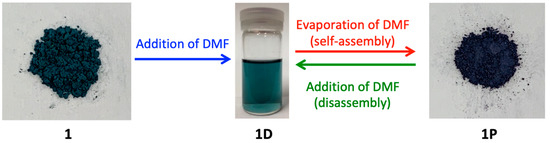
Figure 3.
Pictures of 1 (green powder), 1D in DMF (green solution), and 1P in solid state (purple powder).
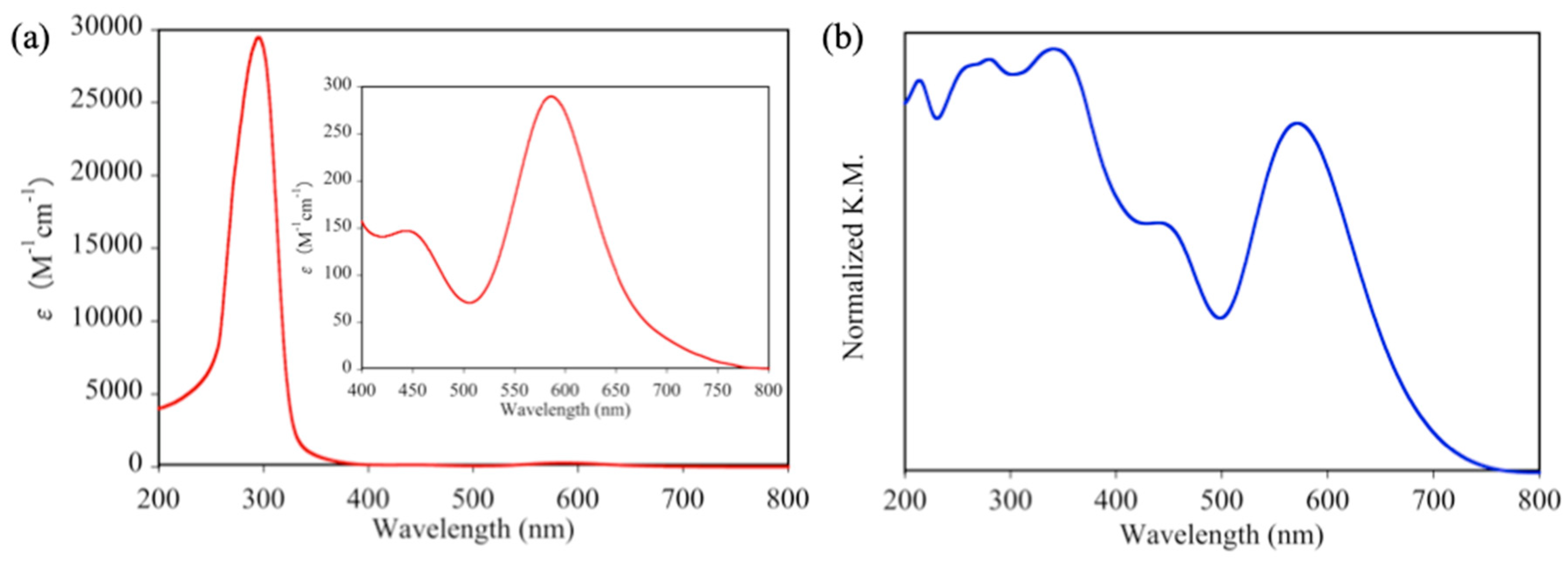
To investigate the absorption properties of 1P and 1D, the absorption spectrum of 1D in DMF and the diffuse reflectance spectrum of 1P were measured at room temperature (see Figure 4). In the visible light region, both 1D and 1P show two absorption maxima. The lower energy absorption band of 1P was observed at 572 nm, which was slightly blue-shifted compared to that of 1D (586 nm), while the higher energy absorption bands of 1D (444 nm) and 1P (441 nm) were located at approximately the same position. Hence, the origin of the color difference between 1D and 1P is primarily due to the difference in the location of their lower energy absorption bands. The other difference in the absorption bands between 1D and 1P was also confirmed in the UV region; 1P shows an intense absorption band at 341 nm, which is not detected in the absorption spectrum of 1D. Since this intense band of 1P arises from the visible light region (~441 nm), it is confirmed that this absorption band edge slightly influences the color of 1P. Although 1D shows an intense absorption band at 295 nm (ε = 29,472 M−1cm−1) in the UV region, 1P shows three continuous absorption bands at 281, 260, and 214 nm.
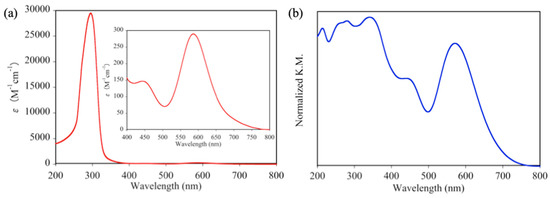
Figure 4.
(a) Absorption spectrum of 1D in DMF and (b) diffuse reflectance spectrum of 1P.
The coordination nature of paddlewheel-type Rh2 complexes can be easily discerned from their absorption spectra. In detail, Cotton et al. confirmed that paddlewheel-type Rh2 complexes without axial donor ligands show an intense d-d(Rh2) absorption band at approximately 760 nm [42]. In contrast, the absorption spectrum of 1D shows no related band in the same region. This result indicates that two DMF molecules are coordinated at the two axial sites of Rh2 ions of 1D, which is similar to [Rh2(O2CCH3)4(DMF)2] [40].
To further confirm the molecular structure of 1D in DMF, DFT calculations were performed using the model structures of 1D, [Rh2(O2CCH3)3(PABC)(DMF)], and [Rh2(O2CCH3)3(PABC)], in which Rh2 ions are coordinated by two, one, and no DMF molecules, respectively (their molecular structures are shown in Figure S1, and selected geometrical parameters are summarized in Table S4). The fully optimized structures of 1D, [Rh2(O2CCH3)3(PABC)(DMF)], and [Rh2(O2CCH3)3(PABC)] commonly had paddlewheel-type structures with Rh-Rh bond lengths of 2.418, 2.407, and 2.381 Å, respectively, which is similar to the crystal structure of 1P. As expected, the obtained sum of electronic and thermal free energy of the optimized geometry of 1D is approximately 1.41 and 7.31 kcal/mol more stable than those of ([Rh2(O2CCH3)3(PABC)(DMF)] + DMF) and ([Rh2(O2CCH3)3(PABC)] + two DMF), respectively, which indicates that 1D should be a thermodynamically favorable species in DMF (see Table S5).
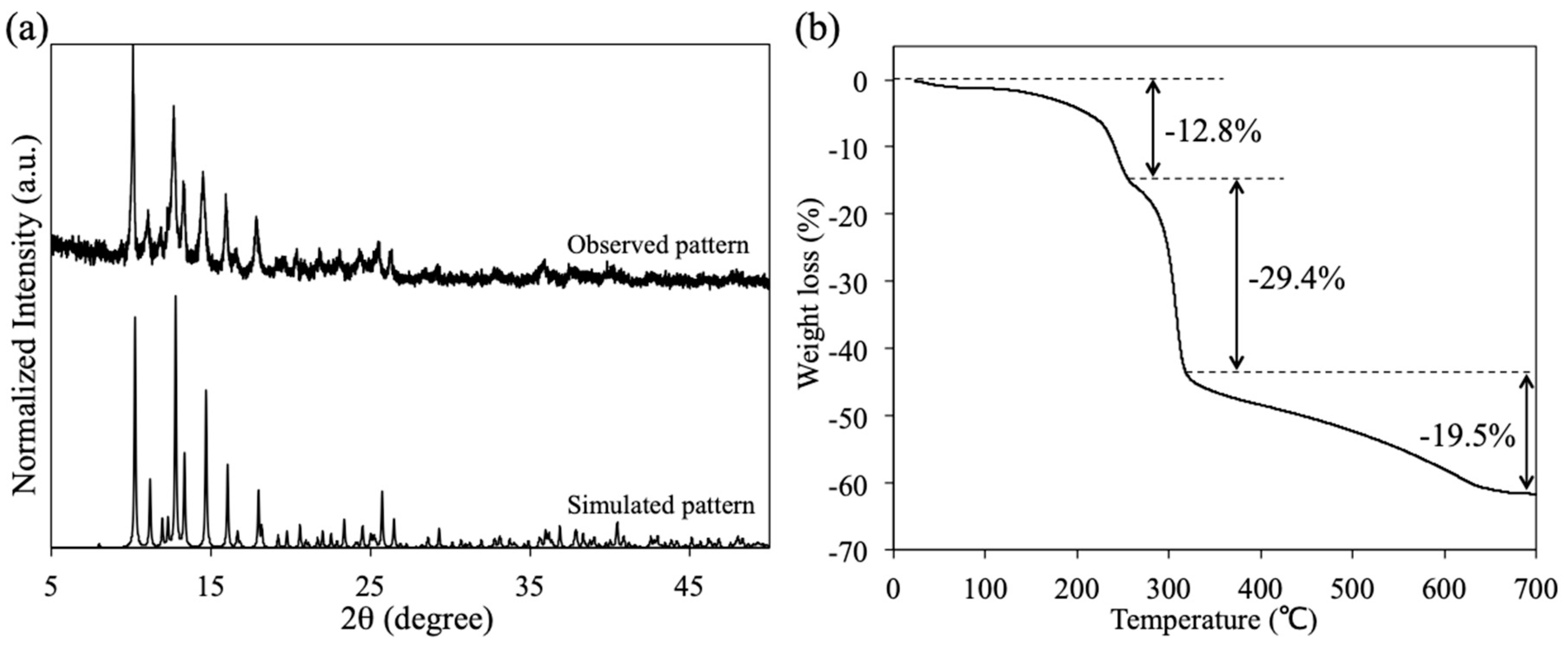
3.4. Powder X-Ray Diffraction and TG Analyses of 1P
To investigate the phase purity and thermal stability of 1P, powder X-ray diffraction (XRD) and TG analyses were performed. As depicted in Figure 5a, the observed XRD pattern of 1P is in good agreement with the simulated pattern of the crystal structure of 1P. This result indicates that the obtained 1P has a crystalline single phase. The TG analysis of 1P shows a distinct three-step weight loss process (see Figure 5b). The initial step in the temperature range of 27–249 °C corresponds to the loss of the DMF molecule (Obs. –12.8%, Calcd. –12.3%), which is coordinated to the axial site of Rh2 ions in 1P, whereas the second (249–315 °C) and third (315–700 °C) steps are attributed to the decomposition of the O2CCH3 and PABC ligands of 1P, respectively. This TG result is also consistent with the chemical formula of the crystal structure of 1P.
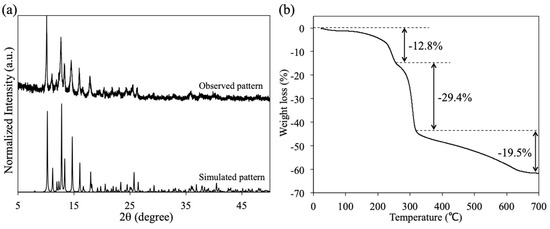
Figure 5.
(a) Observed and simulated X-ray diffraction (XRD) patterns of 1P and (b) thermo-gravimetric (TG) curve of 1P.
4. Conclusions
In this study, we successfully developed a new synthetic strategy for CPs with heteroleptic paddlewheel-type Rh2-SBUs using coordination-induced self-assembly with an amino group of the PABC ligand. Single-crystal X-ray diffraction analyses clarified that 1P, which is obtained by the slow evaporation of DMF-dissolved 1, forms a one-dimensional polymeric structure in which two axial sites of the Rh2 ion in 1P are coordinated by a DMF molecule and an amino group of the PABC ligand of the neighboring molecule 1. It is also confirmed that polymeric species 1P can be easily re-dissolved in DMF, which indicates that the self-assembly and disassembly transformations between 1D and 1P easily occurred by dissolution and evaporation procedures with DMF. As previously reported, the types of organic solvents in the synthetic processes of CPs and MOFs affect their molecular and self-assembly structures [43,44,45]. We speculate that weak O-donor solvents are preferable in the synthetic strategy with Rh2-SBUs. It is expected that this synthetic strategy can be applied to the synthesis of various novel CPs with heteroleptic Rh2-SBUs using similar ligands with potential coordination donor sites.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/10/2/85/s1, Figure S1: Optimized geometries of (a) [Rh2(O2CCH3)3(PABC)], (b) [Rh2(O2CCH3)3(PABC)(DMF)], and (c) [Rh2(O2CCH3)3(PABC)(DMF)2] (1D); Figure S2: 1H NMR spectrum of [Rh2(O2CCH3)3(PABC)] (1) in DMF-d7; Figure S3: 1H NMR spectrum of [Rh2(O2CCH3)3(PABC)] (1) in DMSO-d6; Figure S4: Observed (top) and simulated (down) ESI-TOF-MS spectra of [Rh2(O2CCH3)3(PABC)] (1); Figure S5: Packing view (along an axis) of the crystal structure of [Rh2(O2CCH3)3(PABC)(DMF)]n (1P). Hydrogen atoms are omitted for clarity; Table S1: Coordinate of optimized geometry of [Rh2(O2CCH3)3(PABC)]; Table S2: Coordinate of optimized geometry of [Rh2(O2CCH3)3(PABC)(DMF)]; Table S3: Coordinate of optimized geometry of [Rh2(O2CCH3)3(PABC)(DMF)2]; Table S4: Selected geometrical parameters of optimized geometries of [Rh2(O2CCH3)3(PABC)], [Rh2(O2CCH3)3(PABC)(DMF)], and [Rh2(O2CCH3)3(PABC)(DMF)2] (1D); Table S5: Sum of electronic and thermal free energies of optimized geometries of [Rh2(O2CCH3)3(PABC)], [Rh2(O2CCH3)3(PABC)(DMF)], [Rh2(O2CCH3)3(PABC)(DMF)2] (1D), and DMF.
Author Contributions
Conceptualization, Y.K. (Yusuke Kataoka) and M.H.; formal analysis, K.A., N.Y., N.I., Y. K., D.Y., and D.A.; investigation, K.A., N.Y., N.I., Y.K. (Yoshihiro Kohara), and Y.K. (Yusuke Kataoka); writing—original draft preparation, Y.K. (Yusuke Kataoka); writing—review and editing, K.A., N.Y., N.I., Y.K. (Yoshihiro Kohara), D.Y., D.A., M.H., and Y.K. (Yusuke Kataoka); supervision, Y.K. (Yusuke Kataoka). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI Grant Numbers 19K15588 and 18H05166.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cotton, F.A.; Murillo, C.A.; Walton, R.A. Multiple Bonds between Metal Atoms, 3rd ed.; Springer Science and Business Media: New York, NY, USA, 2005. [Google Scholar]
- Chifotides, H.T.; Dunber, K.R. Interactions of Metal−Metal-Bonded Antitumor Active Complexes with DNA Fragments and DNA. Acc. Chem. Res. 2005, 38, 146–156. [Google Scholar] [CrossRef]
- Jalilehvand, F.; Garcia, A.E.; Niksirat, P. Reactions of Antitumor Active Dirhodium(II) Tetraacetate Rh2(CH3COO)4 with Cysteine and Its Derivatives. ACS Omega 2017, 2, 6174–6186. [Google Scholar] [CrossRef]
- Wong, D.L.; Stillman, M.J. Destructive interactions of dirhodium(II) tetraacetate with b metallothionein rh1a. Chem. Commun. 2016, 52, 5698–5701. [Google Scholar]
- Hilderbrand, S.A.; Lim, M.H.; Lippard, S.J. Dirhodium Tetracarboxylate Scaffolds as Reversible Fluorescence-Based Nitric Oxide Sensors. J. Am. Chem. Soc. 2004, 126, 4972–4978. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, S.L.; Piraino, P.; Bonavita, A.; Micali, G.; Rizzo, G.; Neri, G. A dirhodium(II,II) molecular species as a candidate material for resistive carbon monoxide gas sensors. Sens. Actuator B Chem. 2008, 129, 772–778. [Google Scholar] [CrossRef]
- Warzacha, E.; Berto, T.C.; Wilkinson, C.C.; Berry, F. Rhodium Rainbow: A Colorful Laboratory Experiment Highlighting Ligand Field Effects of Dirhodium Tetraacetate. J. Chem. Educ. 2019, 96, 571–576. [Google Scholar] [CrossRef]
- Hansen, J.; Davies, H.M.L. High Symmetry Dirhodium(II) Paddlewheel Complexes as Chiral Catalysts. Coord. Chem. Rev. 2008, 252, 545–555. [Google Scholar] [CrossRef]
- Srivastava, P.; Yang, H.; Ellis-Guardiola, K.; Lewis, J.C. Engineering a dirhodium artificial metalloenzyme for selective olefin cyclopropanation. Nat Commun. 2015, 6, 7789. [Google Scholar] [CrossRef] [PubMed]
- Fiori, K.W.; Du Bois, J. Catalytic Intermolecular Amination of C−H Bonds: Method Development and Mechanistic Insights. J. Am. Chem. Soc. 2007, 129, 562–568. [Google Scholar] [CrossRef]
- Anada, M.; Washio, T.; Shimada, N.; Kitagaki, S.; Nakajima, M.; Shiro, M.; Hashimoto, S. A New Dirhodium(II) Carboxamidate Complex as a Chiral Lewis Acid Catalyst for Enantioselective Hetero-Diels-Alder Reactions. Angew. Chem. Int. Ed. 2004, 43, 2665–2668. [Google Scholar] [CrossRef]
- Kataoka, Y.; Yano, N.; Handa, M.; Kawamoto, T. Intrinsic Hydrogen Evolution Capability and Theoretically Supported Reaction Mechanism of Paddlewheel-type Dirhodium Complex. Dalton Trans. 2019, 48, 7302–7312. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Yano, N.; Kohara, Y.; Tsuji, T.; Inoue, S.; Kawamoto, T. Experimental and Theoretical Study of Photochemical Hydrogen Evolution Catalyzed by Paddlewheel-type Dirhodium Complexes with Electron Withdrawing Carboxylate Ligands. ChemCatChem 2019, 11, 15720–15725. [Google Scholar] [CrossRef]
- Kataoka, Y.; Sato, K.; Miyazaki, Y.; Suzuki, Y.; Tanaka, H.; Kitagawa, Y.; Kawakami, T.; Okumura, M.; Mori, W. Photocatalytic Hydrogen Production from Water Using Heterogeneous Two-dimensional Rhodium Coordination Polymer [Rh2(p-BDC)2]n. Chem. Lett. 2010, 39, 358–359. [Google Scholar] [CrossRef]
- Norman, J.G.; Kolari, H.J. Strength and trans influence of the rhodium-rhodium bond in rhodium(II) carboxylate dimers. J. Am. Chem. Soc. 1978, 100, 791–799. [Google Scholar] [CrossRef]
- Nakatsuji, H.; Ushio, J.; Kanda, K.; Onishi, Y.; Kawamura, T.; Yonezawa, T. Electronic structure of dirhodium tetracarboxylate complexes by the AB initio SCF MO method. Chem. Phys. Lett. 1981, 79, 299–304. [Google Scholar]
- Sizova, O.V.; Sokolov, A.Y.; Skripnikov, L.V.; Baranovsko, V.I. Quantum chemical study of the bond orders in the ruthenium, dirutheniu, and dirhodium nitrosyl complexes. Polyhedron 2007, 26, 4680–4690. [Google Scholar] [CrossRef]
- Kataoka, Y.; Kitagawa, Y.; Saito, T.; Nakanishi, Y.; Matsui, T.; Sato, K.; Miyazaki, Y.; Kawakami, T.; Okumura, M.; Mori, W.; et al. Theoretical Study on the Electronic Configurations and Nature of Chemical Bonds of Dirhodium Tetraacetato Complexes [Rh2(CH3COO)4(L)2] (L = H2O, Free): Broken Symmetry Approach. Bull. Chem. Soc. Jpn. 2010, 83, 1481–1488. [Google Scholar] [CrossRef]
- Das, K.; Kadish, K.M.; Bear, J.L. Substituent and solvent effects on the electrochemical propertyes of tetra-μ-carboxylato-dirhodium(II). Inorg. Chem. 1978, 17, 930–934. [Google Scholar] [CrossRef]
- Cotton, F.A.; Dikarev, E.V.; Feng, X. Unligated dirhodium tetra(trifleoroacetate): Preparation, crystal structure and electronic structure. Inorg. Chimica Acta. 1995, 237, 19–26. [Google Scholar] [CrossRef]
- Kataoka, Y.; Fukumoto, R.; Yano, N.; Atarashi, D.; Tanaka, H.; Kawamoto, T.; Handa, M. Synthesis, Characterization, Absorption Properties, and Electronic Structures of Paddlewheel-type Dirhodium(II) Tetra-μ-(n-naphthoate) Complexes: An Experimental and Theoretical Study. Molecules 2019, 24, 447. [Google Scholar] [CrossRef]
- Ebihara, M.; Nomura, M.; Sakai, S.; Kawamura, T. Synthesis, structure and properties of TTF-carboxylate bridged paddlewheel dirhodium complexes, Rh2(ButCO2)3(TTFCO2) and Rh2(ButCO2)2(TTFCO2)2. Inorg. Chimica Acta 2007, 360, 2345–2352. [Google Scholar] [CrossRef]
- Tong, L.H.; Clifford, S.; Gomila, A.; Duval, S.; Guenee, L.; Williams, A.F. Supramolecular squares of dirhodium(II) tetracarboxylate: Combining carboxylate-exchange and metal-ligand coordination for self-assembly. Chem. Commun. 2012, 48, 9891–9893. [Google Scholar] [CrossRef] [PubMed]
- Cotton, F.A.; Murillo, C.A.; Stiriba, S.-E.; Wang, X.; Yu, R. Chiral Organometallic Triangles with Rh−Rh Bonds. 2. Compounds Prepared from Enantiopure cis-Rh2(C6H4PPh2)2(OAc)2(HOAc)2 and Their Catalytic Potentials. Inorg. Chem. 2005, 44, 8223–8233. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Arakawa, K.; Ueda, H.; Yano, N.; Kawamoto, T.; Handa, M. Experimental and Theoretical Study for Dimer-of-Dimers-type Tetrarhodium(II) Complexes Bridged by 1,4-Benzenedicarboxylate Linkers. Dalton Trans. 2018, 47, 17233–17242. [Google Scholar] [CrossRef]
- Takamizawa, S.; Nakata, E.; Yokoyama, H.; Mochizuki, K.; Mori, W. Carbon Dioxide Inclusion Phases of a Transformable 1D Coordination Polymer Host [Rh2(O2CPh)4(pyz)]n. Angew. Chem. Int. Ed. 2003, 42, 4331–4334. [Google Scholar] [CrossRef]
- Handa, M.; Watanabe, M.; Yoshioka, D.; Kawabata, S.; Nukada, R.; Mikuriya, M.; Azuma, H.; Kasuga, K. Adduct Polymers and Dimers of Rhodium(II) Pivalate with Pyradine, 4,4’-Bipyridine, 1,4-Diazabicyclo[2.2.2]octane, Triethylamine, and Pyridine. Bull.Chem.Soc.Jpn. 1999, 72, 12, 2681–2686. [Google Scholar] [CrossRef]
- Cotton, F.A.; Felthouse, T.R. Molecular and Chain Structures of four Tetrakis(μ-propionato) -dirhodium(II) Complexes with Axial Nitrogen-Donor Ligands. Inorg. Chem. 1981, 20, 600–608. [Google Scholar]
- Kosaka, W.; Yamagishi, K.; Hori, A.; Sato, H.; Matsuda, R.; Kitagawa, S.; Takata, M.; Miyasaka, H. Selective NO Trapping in the Pores of Chain-Type Complex Assemblies Based on Electronically Activated Paddlewheel-Type [Ru2II,II]/[Rh2II,II] Dimers. J. Am. Chem. Soc. 2013, 135, 18469–18480. [Google Scholar] [CrossRef]
- Kataoka, Y.; Yano, N.; Shimodaira, T.; Yan, Y.-N.; Yamasaki, M.; Tanaka, H.; Omata, K.; Kawamoto, T.; Handa, M. Paddlewheel-Type Dirhodium Tetrapivalate Based Coordination Polymer: Synthesis, Characterization, and Self-Assembly and Disassembly Transformation Properties. Eur. J. Inorg. Chem. 2016, 17, 2810–2815. [Google Scholar] [CrossRef]
- Naito, S.; Tanibe, T.; Saito, E.; Miyao, T.; Mori, W. A Novel Reaction Pathway in Olefin-Deuterium Exchange Reaction inside the Micropores of Rh(II) Dicarboxylate Polymer Complexes. Chem. Lett. 2001, 30, 1178–1179. [Google Scholar] [CrossRef]
- Sato, T.; Mori, W.; Kato, C.N.; Yanaoka, E.; Kuribayashi, T.; Ohtera, R.; Shiraishi, Y. Novel microporous rhodium(II) carboxylate polymer complexes containing metaloporphyrin: Syntheses and catalytic performances in hydrogenation of olefins. J. Catal. 2005, 232, 186–198. [Google Scholar] [CrossRef]
- Nickerl, G.; Stoeck, U.; Burkhardt, U.; Senkovska, I.; Kaskel, S. A catalytically active porous coordination polymer based on a dinuclear rhodium paddle-wheel unit. J. Mater. Chem. A. 2014, 2, 144–148. [Google Scholar] [CrossRef]
- Kataoka, Y.; Kataoka, K.S.; Murata, H.; Handa, M.; Mori, W.; Kawamoto, T. Synthesis and characterizations of a paddlewheel-type dirhodium-based photoactive porous metal-organic framework. Inorg. Chem. Commun. 2016, 68, 37–41. [Google Scholar] [CrossRef]
- Yano, N.; Kataoka, Y.; Tanaka, H.; Kawamoto, T.; Handa, M. A New Paddlewheel-Type Dirhodium- Based Metal-Organic Framework with Deprotonated 2,6-Bis(2-benzimidazolyl)pyridine. Chem. Sel. 2016, 1, 2571–2575. [Google Scholar] [CrossRef]
- Kataoka, Y.; Yano, N.; Kawamoto, T.; Handa, M. Isolation of the Intermediate in the Synthesis of Paddlewheel-type Dirhodium Tetraacetate. Eur. J. Inorg. Chem. 2015, 34, 5650–5655. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G.; Spagna. R. SIR2011: A new package for crystal structure determination and refinement. J. Appl. Cryst. 2012, 45, 357–361. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision C.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Moszner, M.; Głowiak, T.; Ziółowski, J.J. The crystal and moleculer structure of Rh2(CH3CO2)4[HCON(CH3)]2-effect of ligands on metal-metal bonding. Polyhedron 1985, 4, 1413–1417. [Google Scholar] [CrossRef]
- Koh, Y.B.; Christoph, G.G. Metal-metal bonding in dirhodium tetracarboxylates. Structure of bis(diethylamine) adduct of tetra-μ-acetato dirhodium(II) and systematics of the bonding in tetracarboxylate-bridged metal dimers. Inorg. Chem. 1979, 18, 1122–1128. [Google Scholar]
- Cotton, F.A.; Hillard, E.A.; Murillo, C.A. The First Dirhodium Tetracarboxylate Molecule without Axial Ligation: New Insight into the Electronic Structures of Molecules with Importance in Catalysis and Other Reactions. J. Am. Chem. Soc. 2002, 124, 5658–5660. [Google Scholar] [CrossRef] [PubMed]
- Rancan, M.; Armelao, L. Exploiting dimensional variability in coordination polymers: Solvent promotes reversible conversion between 3D and chiral 1D architectures. Chem. Commun. 2015, 51, 12947–12949. [Google Scholar] [CrossRef] [PubMed]
- Truccolo, G.; Tessari, Z.; Tessarolo, J.; Quici, S.; Armelao, L.; Rancan, M. A Cu(II) metalocycle for the reversible self-assembly of coordination-driven polyrotaxane-like architectures. Dalton Trans. 2018, 47, 12079–12084. [Google Scholar] [CrossRef] [PubMed]
- Rancan, M.; Carlotto, A.; Bottaro, G.; Armelao, L. Effects if Coordinating Solvents on the Structure of Cu(II)-4,4’-bipyridine Coordination Polymers. Inorganics 2019, 7, 103. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).