Abstract
Anti-counterfeiting technologies with the features of easy distinguishability, high cost performance, and good processability are needed to meet the demands of a market during the consumption upgrading moment. A series of side-chain liquid crystal co-polymers (SCLCPs) are designed, synthesized, and blended, and the preparation of a series of angular photochromic materials that have different center reflection wavelengths in the visible and near infra-red region is reported in this article. Differential scanning calorimetry and polarized optical microscopy were utilized to characterize the phase transition behaviors and self-assembling structures of the SCLCPs. The selective reflection properties were characterized with a UV/VIS/IR spectrum study and further verified by scanning electron microscopy. The results showed that the SCLCPs had the desired reflection wavelengths and thermal stability. The SCLCPs could easily form a planar texture of cholesteric liquid crystal and, depending on the good processability, anti-counterfeiting powders and fibers with angular photochromic features were prepared and characterized to prove the potential applications of the SCLCPs in anti-counterfeiting labels.
1. Introduction
Consumption upgrading is happening with the development of the global economy. The demand for new anti-counterfeiting technologies has begun to emerge and new ones have been invented to meet the demand. According to the mechanism, anti-counterfeiting technologies could be categorized into four aspects: colorimetric approaches [1,2,3], fluorometric approaches [4,5], mass-spectrometry encryption [6,7], and organic electronic approaches [8,9].
The first-line anti-counterfeiting technology is a kind of anti-counterfeiting technology that could show obvious optical features through direct observation. It has characters such as a simple identification method, no need for any instrument, and wide application scenarios. The existing anti-counterfeiting materials are mainly divided into thermochromism, photochromism, and structural color [10]. Huang et al reported a kind of color tunable composite film of carbon nanotubes (CNTs) and anodic aluminum oxide (AAO) [11]. By tuning the etching duration from 0 to 17.5 min, a shift from 632 to 498 nm of the interference band with the maximum reflectance in the visible region could be achieved. Sun et al reported a method by performing electrochemical oxidation of aluminum to prepare porous anodic alumina thin films with the structural color [12], and the samples showed application potentials in anti-counterfeiting devices.
The existing methods can achieve certain optical anti-counterfeiting characteristics, but the preparation process is complex, the material cost is high, and the intuitiveness of the distinguished methods needs to be improved. In terms of the first-line anti-counterfeiting technology, at present, the monetary manufacturing institutions of various countries have carefully designed anti-counterfeiting measures in materials; printing technology; and other aspects such as watermark, safety line, anti-counterfeiting fiber in paper, fluorescent ink, magnetic ink, infrared ink, manual engraving intaglio, counter printing, micro character printing, invisible pattern, and so on.
As a first-line anti-counterfeiting material, it is necessary to have anti-counterfeiting characteristics that are easy to identify. Because of the special optical, stimuli response, and self-assembled properties of liquid crystal (LC) materials, the above problems can be solved [13,14,15]. The molecular arrangement of cholesteric LC has a periodic structure, so the cholesteric LC could selectively reflect circularly polarized incident light whose handedness is identical to the helical axis [16]. The selective reflection color could be easily observed by the human eye, and could be precisely measured by instruments. Moreover, the refection wavelength could be tuned using different methods as well as circular polarization properties [17,18]. λ = nPsinθ is the equation of wavelength; λ and n are the average of the ordinary and extraordinary refractive indices, respectively, of the ChLC; P is the cholesteric pitch (the length of a 2π molecular rotation); and θ is the viewing angle. On the basis of the equation, for a certain cholesteric LC material, the reflection colors are proportional to the viewing angle and could be observed by the naked eye if the wavelengths are in the visible region, which means it could be used for anti-counterfeiting purposes.
The anti-counterfeiting film prepared by cholesteric LC can show obvious reflection color, and the reflection color has the characteristics of changing with the observation angle. However, the application of LC in the first-line anti-counterfeiting materials has higher requirements on the thermal stability, optical properties, and processing properties of the materials. It is necessary to prepare the LC materials suitable for anti-counterfeiting films through reasonable molecular design and material synthesis. It is also necessary for the LC materials to form a better planar orientation in the process of preparing composite films, so as to present the characteristics of angular discoloration. Side-chain liquid crystal co-polymer (SCLCP) not only has the characteristics of periodic arrangement structure of small molecular LC, but also has the good processability and mechanical properties of polymer materials [19,20,21]. It is a good choice for anti-counterfeiting applications.
To be used for anti-counterfeiting materials, LC has to meet the requirements including angular photochromic property, easy distinguishability, high cost performance, and good processability. In this paper, the SCLCPs suitable for anti-counterfeiting film were prepared through reasonable molecular design and material synthesis. The SCLCPs could easily form a planar orientation to show the angular photochromic property and had good processability and mechanical properties, which was a good choice for anti-counterfeiting applications. Moreover, the synthetic SCLCPs could be mixed to prepare materials with different reflective wavelengths to meet the needs of different application scenarios.
2. Results and Discussion
2.1. Mesomorphic Property of the SCLCPs
Figure 1a shows the chemical structure of the polymers. For 3HG8020, the molar ratio of cholesteric and nematic monomers was 1:4 and the ratio for 3HG2080 was 4:1. Table 1 shows the characterization data of the SCLCPs. By adjusting the proportion between cholesteric and nematic liquid crystalline monomers, two polymers 3HG8020 and 3HG2080 were prepared. The 5% weight loss temperatures (Td′s) of the samples were all above 300 °C, thus proving their high thermal stabilities, using thermogravimetric analysis.
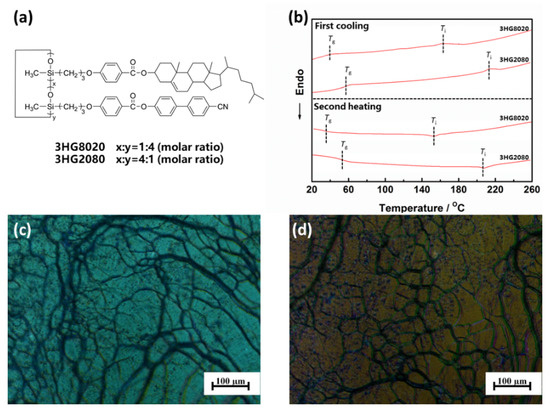
Figure 1.
(a) The chemical structures of the side-chain liquid crystal co-polymers; (b) differential scanning calorimetry curves of samples 3HG8020 and 3HG2080 during the first cooling and second heating scans at a rate of 20 °C min−1; (c) polarized optical microscopic image of sample 3HG8020 at 80 °C; (d) polarized optical microscopic image of sample 3HG2080 at 80 °C.

Table 1.
The characterization data of the side-chain liquid crystal co-polymers (SCLCPs).
The phase transition behaviours of the polymers 3HG8020 and 3HG2080 were characterized using differential scanning calorimetry (DSC, PerkinElmer DSC8000, PerkinElmer, Waltham, MA, USA) and polarized optical microscopy (POM, Carl Zeiss Axio Vision SE64, Carl Zeiss AG, Jena, Germany) measurement. All of the DSC samples were prepared with synthesized or blended polymers of powders states and characterizations were conducted at temperatures below Td in order to avoid thermal degradation. Figure 1b shows the DSC curves for samples 3HG8020 and 3HG2080. Two exothermic peaks at about 41.60 °C and about 163.47 °C were detected in the DSC curve of sample 3HG8020 during the first cooling scan, and no other peaks shown indicated that the polymer only had one LC phase. The former could be the glass transition temperature and the latter could be the isotropic temperature, which needed to be confirmed by POM. The DSC curve of sample 3HG2080 was similar, but with two exothermic peaks at about 58.96 °C and about 215.49 °C.
The POM technique was utilized to further confirm the phase behaviours and structures of the samples. The two polymers were filled into the LC cells whose inner surfaces had been planar orientated respectively by capillary action, and polyethylene terephthalate films of 20 μm thickness were used as cell spacers. Figure 1c,d show the typical LC textures of samples 3HG8020 and 3HG2080, respectively, at 80 °C and the POM pictures show the oily streak texture, which is a characteristic of the cholesteric phase. Upon heating, the polymers developed the LC phase and show the oily streak texture immediately after the glass transition temperatures and the texture disappeared after the isotropic temperatures. Taking sample 3HG8020 as an example, Figure 2a–d shows the POM images during the heating and cooling process. The oily streak texture developed again while cooling from the isotropic state, and the sample showed the texture during the whole cooling process. Interestingly, further cooling to room temperature, which was below Tg, the oily streak textures could be maintained. Combining the results of DSC and POM, it could be confirmed that samples 3HG8020 and 3HG2080 developed a wide Ch phase structure during the heating and cooling process, and the oily streak texture could be kept under Tg at room temperature, which was useful for the applications in anti-counterfeiting powder and fiber.
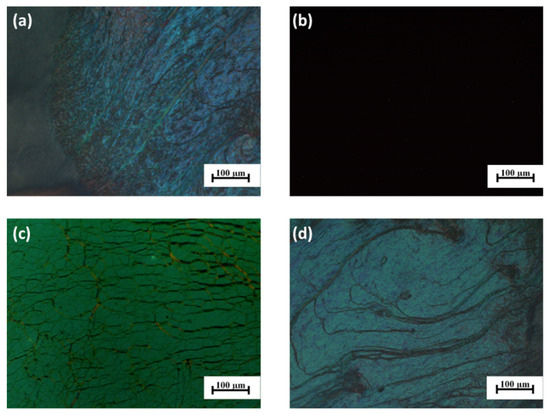
Figure 2.
Polarized optical microscopic images of sample 3HG8020: (a) at 45 °C; (b) at 165 °C; (c) at 150 °C; (d) at 25 °C.
Samples HG1, HG2, and HG3 were prepared according to the composition listed in Table 2 by stirring SCLCPs 3HG8020 and 3HG2080 homogeneously at 100 °C for 20 min. The phase transition behaviours of samples HG1, HG2, and HG3 were examined by DSC and POM measurements. From the DSC results showed in Figure 3a, it could be found that all the samples had glass transition temperatures and isotropic temperatures, but no other phase transition temperatures. Combining the POM pictures shown in Figure 3b–d, which were all an oily streak texture, it could be concluded that all the samples showed the cholesteric LC phase. The molar ratio of cholesteric mesogen in 3HG8020 was lower than that of 3HG2080, and the glass transition temperature and isotropic temperature of 3HG8020 were lower that those of 3HG2080. This was because the SCLCP with a lower molar ratio of cholesteric mesogen had a lower average molecular weight, thus showing lower phase transition temperatures. Samples HG1, HG2, and HG3 prepared with SCLCPs 3HG8020 and 3HG2080 had rising molar ratios of cholesteric mesogen and rising average molecular weights, resulting in the ascending glass transition temperatures and isotropic temperatures. All the samples had an LC temperature region wider than 150 °C and could meet the demand of manufacturing anti-counterfeiting products.

Table 2.
The compositions and selective reflection properties of all the samples.
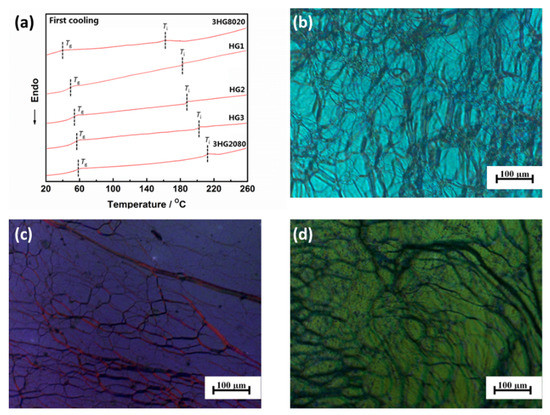
Figure 3.
(a) Differential scanning calorimetry curves of samples 3HG8020, HG1, HG2, HG3, and 3HG2080 during the first cooling scan at a rate of 20 °C min−1. Polarized optical microscopic images of the samples at 90 °C: (b) HG1; (c) HG2; and (d) HG3.
2.2. Optical Property and Morphology of the SCLCPs
It has been found that the center reflection wavelength of chiral compounds was mainly dependent on the content of the chiral component in previous studies [22,23,24]. The increasing content of the chiral groups leads to higher helical twisting power in the molecules, and thus the center reflection wavelength blue shifts. As listed in Table 2, 3HG8020 with the lowest molar ratio of cholesteric mesogen showed the largest center reflection wavelength of 1130 nm, while 3HG2080 had the highest molar ratio of cholesteric mesogen and the shortest center reflection wavelength of 560 nm. By tuning the ratios of 3HG8020 and 3HG2080, the contents of cholesteric mesogen could be controlled and samples HG1, HG2, and HG3 showed center reflection wavelengths of 1015 nm, 794 nm, and 588 nm, respectively. The center reflection wavelengths of the SCLCPs could be controlled by changing the compositions of 3HG8020 and 3HG2080 and the wavelengths could cover the visible and near infra-red region.
Scanning electron microscopy (SEM, HITACHI S-4800, HITACHI, Tokyo, Japan) was utilized to further confirm the planar texture of the cholesteric phase and the variation trend of the center reflection wavelengths of the samples. Figure 4b–f show the SEM images of the fractured surface of samples 3HG8020, HG1, HG2, HG3, and 3HG2080, respectively. Corresponding to a 2π cholesteric molecular rotation, the pitch is the dimension of the three bands in the fine structure on the fractured plane. It could be seen clearly that, from Figure 4b to Figure 4f, the piths gradually descended, which corresponded to the blue shifting of the center reflection wavelengths of samples 3HG8020, HG1, HG2, HG3, and 3HG2080. LC angular photochromic materials will face a wide range of application scenarios in the future, and have requirements for various reflection colors. Through the molecular design and synthesis and the research on the mixing of SCLCPs, the preparation method of LC materials meeting the specific selective wavelength needs by adjusting the ratio of 3HG8020 and 3HG2080 is found, which provides a way to prepare angular photochromic films with different reflection wavelengths.
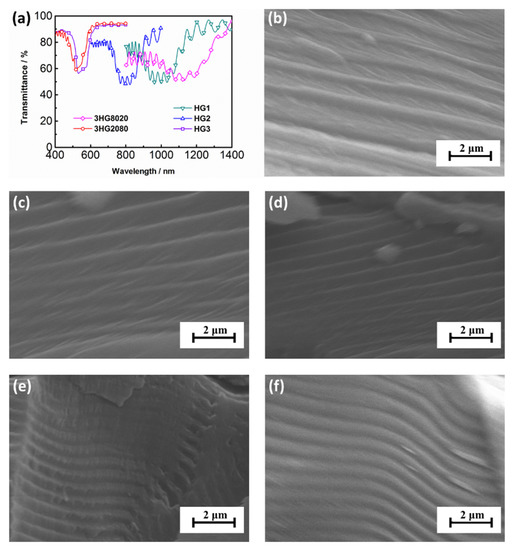
Figure 4.
(a) The transmission spectra of the samples. Scanning electron microscopy micrographs of the polymers: (b) 3HG8020; (c) HG1; (d) HG2; (e) HG3; and (f) 3HG2080.
2.3. Device Applications of the SCLCP for Anti-Counterfeiting Powder and Fiber
On the basis of the results above, the SCLCPs showed three advantages for applications in anti-counterfeiting powder and fiber: (1) the samples could perform selective reflection of the cholesteric phase of LC and the center reflection wavelengths could be tuned in visible and near infra-red region by changing the compositions of 3HG8020 and 3HG2080; (2) the planar texture of cholesteric phase of the samples could be maintained under Tg till room temperature and the films showed photochromic properties; and (3) the SCLCPs retained the mechanical properties of polymer materials and could be made into films and fibers. Figure 5a–d show the optical microscopic images of the powders made from randomly crushed 3HG2080 film. The powders had different shapes and sizes under microscopy and the planar texture of cholesteric phase remained, thus the powders could show selective reflection property. The powders were then randomly scattered on the substrate, from Figure 5e and Figure 5f, it could be seen that the same powder shows different colors when viewed from different angles, which could be easily distinguished by the naked eye. Furthermore, the shapes, sizes, and locations of the powders could be recorded by high-resolution digital camera for comparison when using them for anti-counterfeiting labels.
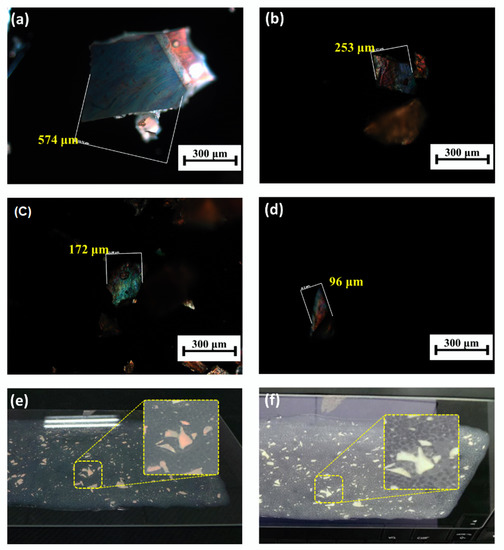
Figure 5.
(a–d) Optical microscopic images of the powders made from the side-chain liquid crystal co-polymer 3HG2080; (e,f) photographs of the powder film from different viewing angles with enlarged pictures of the same area.
The SCLCPs also showed desirable fiber forming property and could be manufactured into fibers by using the pulling method in the LC phase temperature region. Figure 6a–d show the optical microscopic images of the fibers made from 3HG2080 at 90 °C with different pulling speed and natural cooling. The fiber thickness could be controlled by the pulling speed and fibers with different thicknesses of 10 µm, 37 µm, 180 µm, and 237 µm were observed. The fibers were then adherent to the surface of a ticket with aqua-solvable glue. As shown in Figure 6e and Figure 6f, the fiber appears and disappears when viewed from different angles. Typical anti-counterfeiting fibers are color fibers and show the same color when viewed at different angles, but the SCLCP fibers could perform the angular photochromic property, which is of higher anti-counterfeiting level.
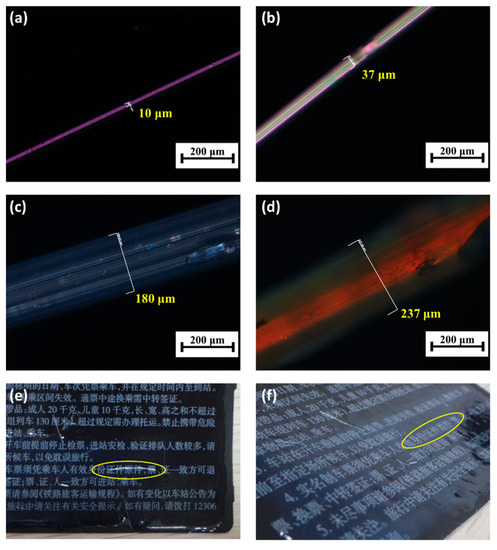
Figure 6.
(a–d) Optical microscopic image of the fibers made from the side-chain liquid crystal co-polymer 3HG2080; (e,f) photographs of the fiber from different viewing angles.
3. Experimental Section
3.1. Materials
The polymers were obtained via a radical-initiated thiol-ene addition polymerization of cholesteric and nematic liquid crystalline monomers in different ratios; the details of the synthetic route of the monomers and polymers can be found in our previous work [25,26]. Tetrahydrofuran (THF, Beijing Chemical Works, Beijing, China) was refluxed over sodium and distilled.
3.2. Measurements
A gel permeation chromatography equipped with a Waters 2410 (Waters, Millford, MA, USA) instrument equipped with a Waters 2410 RI detector and three Waters μ-Styragel columns (103, 104, and 105 Å) was used to determine the molecular weights and molecular weight distributions of the polymers, using THF as the eluent at a flow rate of 1.0 mL min−1 at 35 °C. The calibration curve was obtained using polystyrene as the standard. Thermogravimetry was performed on a TA Q50 (TA instruments, New Castle, PA, USA) instrument under nitrogen at a heating rate of 10 °C min−1. The DSC results were obtained with a PerkinElmer DSC8000 (PerkinElmer, Waltham, MA, USA) under dry nitrogen at a heating and cooling rate of 20 °C min−1. The temperature and heat-flow scale were calibrated using zinc and indium as the standard. POM was performed on a Carl Zeiss Axio Vision SE64 (Carl Zeiss AG, Jena, Germany) polarized optical microscopy with a Linkam LTS420 hot stage (Linkam, London, UK). Spectral characterization was carried out by an unpolarized UV/VIS/IR spectrophotometer (PerkinElmer Lambda 950, PerkinElmer, Waltham, MA, USA) in transmission mode at normal incidence. The SEM morphology of the cross sections of the samples was observed on a HITACHI S-4800 (HITACHI, Tokyo, Japan) instrument.
3.3. Preparation of the SCLCP Anti-Counterfeiting Powder and Fiber
The SCLCP 3HG2080 (5 g) was first added into a 50 mL single-necked round-bottom flask, stirred, and heated at 90 °C for 20 min. Then, 0.5 g of 3HG2080 was dropped on a glass substrate and naturally cooled to room temperature to form the film. The film was peeled from the glass substrate and made into powders of random sizes in the mortar with a pestle. The fibers were prepared based on the pulling method using a tweezer attached with an electromotor to control the pulling speed. After natural cooling to room temperature, the fibers were prepared.
4. Conclusions
In summary, based on the design and synthesis of two SCLCPs and the investigation of their blending properties, a series of SCLCPs with different center reflection wavelengths in the visible and near infra-red region was prepared with the advantages of various angular photochromic properties and good processability. A detailed investigation found that the SCLCPs all had good thermal stability and a wide cholesteric liquid crystal temperature region and their center reflection wavelengths could cover the visible and near infra-red region. Utilizing the prepared SCLCPs, powders and fibers were manufactured and characterized and corresponding devices were fabricated to prove the practical applications of the materials in anti-counterfeiting labels.
Author Contributions
Writing—original draft preparation, Y.G.; project administration, K.F.; visualization, J.Z.; supervision, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation, grant number 51573003, and the Joint Fund of the Ministry of Education for Equipment Pre-Research, grant number 6141A02033238.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boudeta, B.; Binet, C.; Mitov, M.; Bourgerette, C.; Boucher, E. Microstructure of variable pitch cholesteric films and its relationship with the optical properties. Eur. Phys. J. E 2000, 2, 247–253. [Google Scholar] [CrossRef]
- Broer, D.J. Deformed chiral-nematic networks obtained by polarized excitation of a dichroic photoinitiator. Curr. Opin. Solid State Mater. 2002, 6, 553–561. [Google Scholar] [CrossRef]
- Bartolino, R.; Scaramuzza, N.; Lucchetta, D.E.; Barna, E.S.; Ionescu, A.T.; Blinov, L.M. Polarity sensitive electrooptical response in a nematic liquid crystal-polymer mixture. J. Appl. Phys. 1999, 85, 2870–2874. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, S.; Yuan, W.; Zhao, Z.; Duan, A.; Xu, C.; Jiang, L.; Song, Y.; Zhu, D. Photo- and Proton-Dual-Responsive Fluorescence Switch Based on a Bisthienylethene-Bridged Naphthalimide Dimer and Its Application in Security Data Storage. Eur. J. Org. Chem. 2007, 2064–2067. [Google Scholar] [CrossRef]
- Xuan, R.; Ge, J. Invisible photonic prints shown by water. J. Mater. Chem. 2012, 22, 367–372. [Google Scholar] [CrossRef]
- Ifa, D.R.; Gumaelius, L.M.; Eberlin, L.S.; Manicke, N.E.; Cooks, R.G. Forensic analysis of inks by imaging desorption electrospray ionization (DESI) mass spectrometry. Analyst 2007, 132, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, X.; Lin, Z.; He, M.; Han, G.; Yang, C.; Xing, Z.; Zhang, S.; Zhang, X. Imaging Mass Spectrometry with a Low-Temperature Plasma Probe for the Analysis of Works of Art. Angew. Chem. Int. Ed. 2010, 49, 4435–4437. [Google Scholar] [CrossRef] [PubMed]
- Zschieschang, U.; Yamamoto, T.; Takimiya, K.; Kuwabara, H.; Ikeda, M.; Sekitani, T.; Someya, T.; Klauk, H. Organic electronics on banknotes. Adv. Mater. 2011, 23, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Bhansali, U.S.; Alshareef, H.N. High-performance non-volatile organic ferroelectric memory on banknotes. Adv. Mater. 2012, 24, 2165–2170. [Google Scholar] [CrossRef]
- Yoon, B.; Lee, J.; Park, I.S.; Jeon, S.; Lee, J.; Kim, J. Recent functional material based approaches to prevent and detect counterfeiting. J. Mater. Chem. C 2013, 1, 2388–2403. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, G.; Xu, Q.; Han, F.; Huang, Q. Color Fine-Tuning of CNTs@AAO Composite Thin Films via Isotropically Etching Porous AAO Before CNT Growth and Color Modification by Water Infusion. Adv. Mater. 2010, 22, 2637–2641. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yang, Y.; Gu, J.; Li, Z.; Sun, H. Influence of Al substrate on the optical properties of porous anodic alumina films. Mater. Lett. 2012, 74, 137–139. [Google Scholar]
- Nakayama, K. Ohtsubo, Optical security device providing fingerprint and designed pattern indicator using fingerprint texture in liquid crystal. J. Opt. Eng. 2012, 51, 040506. [Google Scholar] [CrossRef]
- Li, W.S.; Shen, Y.; Chen, Z.J.; Cui, Q.; Li, S.S.; Chen, L.J. Demonstration of patterned polymer-stabilized cholesteric liquid crystal textures for anti-counterfeiting two-dimensional barcodes. Appl. Opt. 2017, 56, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Ohtsubo, J. Optical security devices using nonuniform schlieren texture of UV-curable nematic liquid crystal. Appl. Opt. 2016, 55, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Vartak, S.; Eakin, J.N.; Faris, S.M. Surface anchoring effects on spectral broadening of cholesteric liquid crystal films. J. Appl. Phys. 2008, 104, 023108. Available online: https://aip.scitation.org/doi/full/10.1063/1.2957079 (accessed on 26 February 2020). [CrossRef]
- Lin, Y.; Yang, Y.; Shan, Y.; Gong, L.; Chen, J.; Li, S.; Chen, L. Magnetic Nanoparticle-Assisted Tunable Optical Patterns from Spherical Cholesteric Liquid Crystal Bragg Reflectors. Nanomaterials 2017, 7, 376. [Google Scholar] [CrossRef]
- Lee, K.M.; Tondiglia, V.; Godman, N.; Middleton, C.; White, T. Blue-shifting Tuning of the Selective Reflection of Polymer Stabilized Cholesteric Liquid Crystals. Soft Matter 2017, 13, 5842–5848. [Google Scholar]
- Ji, Y.; Huang, Y.Y.; Terentjev, E.M. Dissolving and Aligning Carbon Nanotubes in Thermotropic Liquid Crystals. Langmuir 2011, 27, 13254–13260. [Google Scholar] [CrossRef]
- Yamazaki, H.; Takeda, M.; Kohno, Y.; Ando, H.; Urayama, K.; Takigawa, T. Dynamic Viscoelasticity of Poly(butyl acrylate) Elastomers Containing Dangling Chains with Controlled Lengths. Macromolecules 2011, 44, 8829–8834. [Google Scholar] [CrossRef]
- Hijnen, N.; Wood, T.A.; Wilson, D.; Clegg, P.S. Self-Organization of Particles with Planar Surface Anchoring in a Cholesteric Liquid Crystal. Langmuir 2010, 26, 13502–13510. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.S.; Zhang, B.Y.; Wang, Y.; Meng, F.B. Synthesis and phase behavior of side-chain liquid-crystalline polymers containing malachite green lactone groups. J. Polym. Sci. Pol. Chem. 2004, 42, 3870–3878. [Google Scholar] [CrossRef]
- Jana, R.N.; Cho, J.W. Synthesis and characterization of polyurethane–based side–chain cholesteric liquid crystal co-polymers. Fiber. Polym. 2009, 10, 569–575. [Google Scholar] [CrossRef]
- Meng, F.B.; Cheng, C.S.; Zhang, B.Y.; He, X.Z. Synthesis and characterization of a novel liquid crystal-bearing ionic mesogen. Liq. Cryst. 2005, 32, 1161–1167. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Cao, H.; Zhao, D.Y.; Hu, W.; He, W.L.; Yuan, X.T.; Xiao, J.M.; Zhang, H.Q.; Yang, H. Chiral nematic liquid crystals with helix inversion from (R)-1,1′-binaphthyl and cholesteryl ester moieties. Liq. Cryst. 2011, 38, 9–15. [Google Scholar] [CrossRef]
- Yao, W.H.; Gao, Y.Z.; Yuan, X.; He, B.F.; Yu, H.F.; Zhang, L.Y.; Zhi, Z.H.; He, W.L.; Yang, Z.; Yang, H.; et al. Synthesis and self-assembly behaviours of side-chain smectic thiol–ene polymers based on the polysiloxane backbone. J. Mater. Chem. C 2016, 4, 1425–1440. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).