Crystal Structure and Spectroscopic Analysis of the Compatible Solute Nγ-Acetyl-L-2,4-Diaminobutyric Acid
Abstract
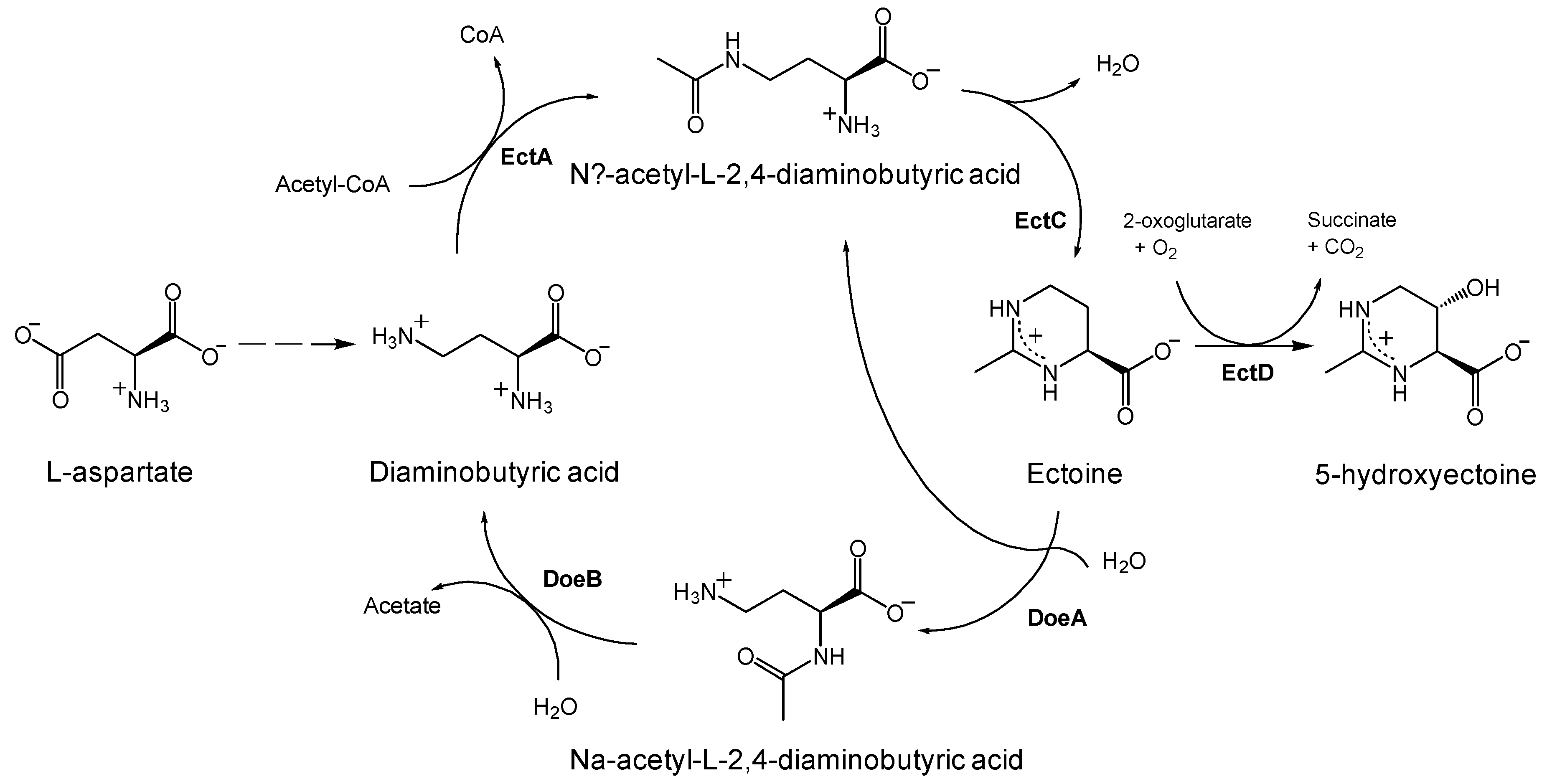
1. Introduction
2. Materials and Methods
3. Results
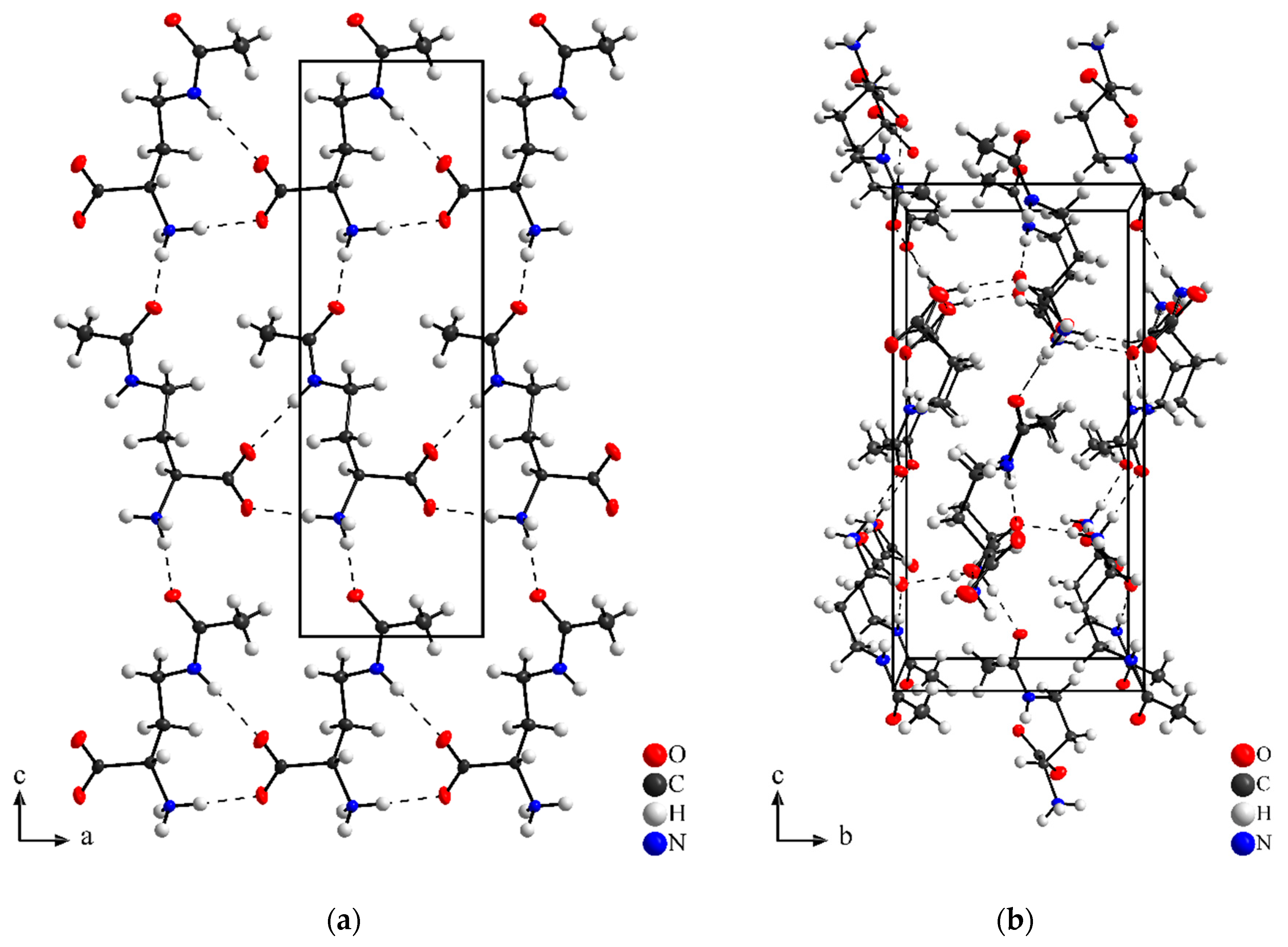
3.1. Crystal Shape and Structure
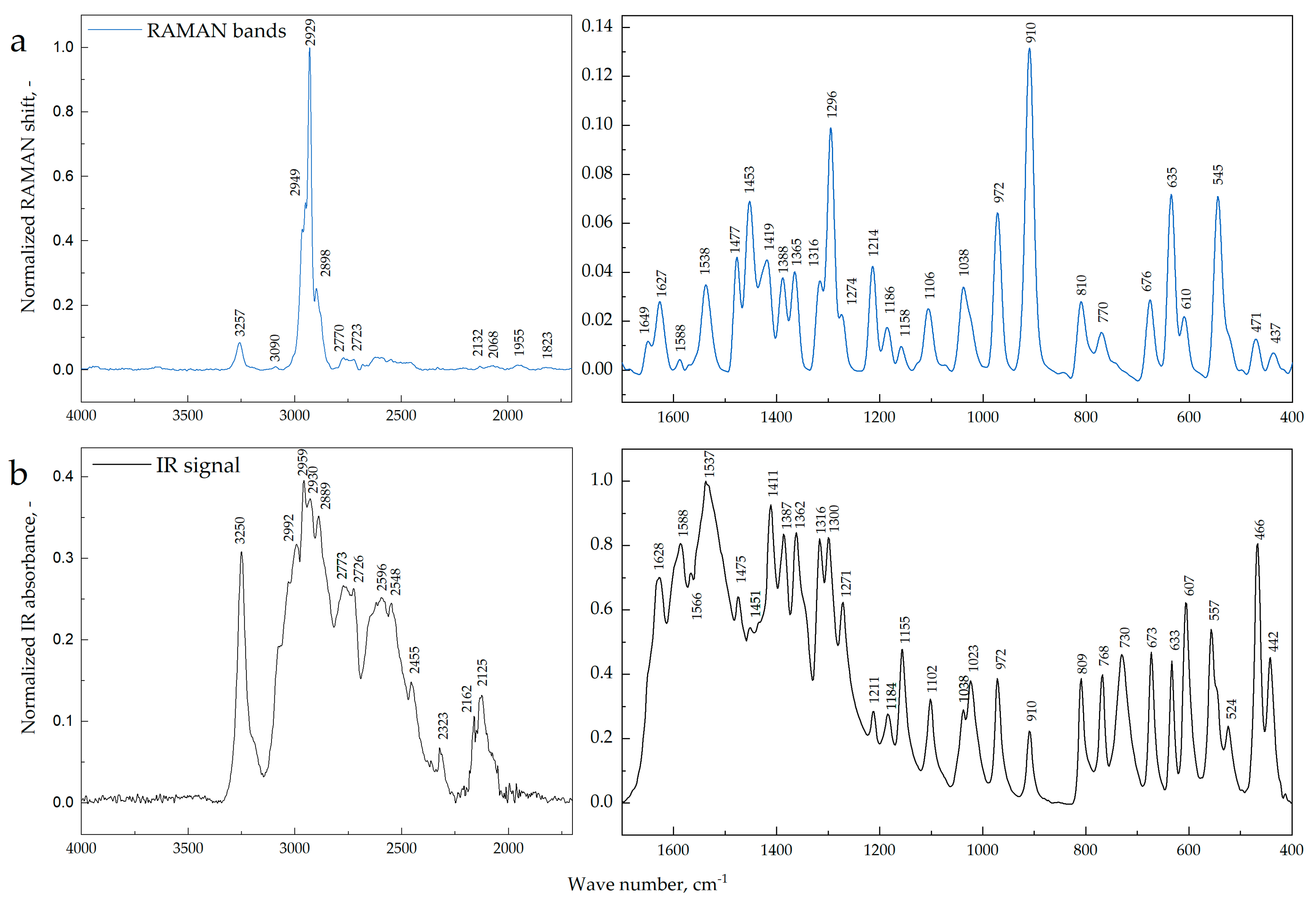
3.2. Raman and IR Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, A.D. Microbial water stress. Bacteriol. Rev. 1976, 40, 803–846. [Google Scholar] [CrossRef] [PubMed]
- Crowley, E. Compatible solute ectoine review: Protection mechanisms and production methods. J. Undergrad. Stud. Trent 2017, 5, 32–39. [Google Scholar]
- Sahle, C.J.; Schroer, M.A.; Jeffries, C.M.; Niskanen, J.; Salmannejad, F.; Nafissi-Varcheh, N.; Tran, B.H.; Dao, V.A.; Bilstein, A.; Unfried, K.; et al. Hydration in aqueous solutions of ectoine and hydroxyectoine. Phys. Chem. Chem. Phys. 2018, 20, 27917–27923. [Google Scholar] [CrossRef] [PubMed]
- Smiatek, J.; Harishchandra, R.K.; Rubner, O.; Galla, H.-J.; Heuer, A. Properties of compatible solutes in aqueous solution. Biophys. Chem. 2012, 160, 62–68. [Google Scholar] [CrossRef]
- Panuszko, A.; Bruździak, P.; Kaczkowska, E.; Stangret, J.; Rieckmann, T.; Gatzemeier, F.; Christiansen, S.; Rothkamm, K.; Münscher, A. General mechanism of osmolytes’ influence on protein stability irrespective of the type of osmolyte cosolvent. J. Phys. Chem. B 2016, 120, 11159–11169. [Google Scholar] [CrossRef]
- Zeman, J.; Holm, C.; Smiatek, J. The effect of small organic cosolutes on water structure and dynamics. J. Chem. Eng. Data 2019, 65, 1197–1210. [Google Scholar] [CrossRef]
- Galinski, E.A. Compatible solutes of halophilic eubacteria: Molecular principles, water-solute interaction, stress protection. Experientia 1993, 49, 487–496. [Google Scholar] [CrossRef]
- Galinski, E.A.; Gunde-Cimerman, N.; Plemenitaš, A.; Oren, A.; Kets, E.; Teunissen, P.; de Bont, J. Osmoadaptation in bacteria. Adv. Microb. Physiol. 1995, 37, 272–328. [Google Scholar] [CrossRef]
- Roberts, M.F. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst. 2005, 1, 5. [Google Scholar] [CrossRef]
- Kunte, H.J. Osmoregulation in bacteria: Compatible solute accumulation and osmosensing. Environ. Chem. 2006, 3, 94–99. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Plemenitaš, A.; Oren, A.; Kets, E.; Teunissen, P.; de Bont, J. Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiol. Rev. 2018, 42, 353–375. [Google Scholar] [CrossRef] [PubMed]
- da Costa, M.S.; Santos, H.; Galinski, E.A. An overview of the role and diversity of compatible solutes in Bacteria and Archaea. In Biotechnology of Extremophiles; Springer: Berlin/Heidelberg, Germany, 1998; pp. 117–153. [Google Scholar]
- Lippert, K.; Galinski, E.A. Enzyme stabilization be ectoine-type compatible solutes: Protection against heating, freezing and drying. Appl. Microbiol. Biotechnol. 1992, 37, 61–65. [Google Scholar] [CrossRef]
- Borges, N.; Ramos, A.; Raven, N.D.; Sharp, R.J.; Santos, H.; Brown, A.D. Comparative study of the thermostabilizing properties of mannosylglycerate and other compatible solutes on model enzymes. Extremophiles 2002, 6, 209–216. [Google Scholar] [CrossRef]
- Marini, A.; Reinelt, K.; Krutmann, J.; Bilstein, A.; Panuszko, A.; Bruździak, P.; Kaczkowska, E.; Stangret, J.; Rieckmann, T.; Gatzemeier, F.; et al. Ectoine-containing cream in the treatment of mild to moderate atopic dermatitis: A randomised, comparator-controlled, intra-individual double-blind, multi-center trial. Ski. Pharm. Physiol. 2014, 27, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Heider, L.; Hamasaki, T.; Masaki, H. Use of Compatible Solutes. U.S. Patent 2020/0030214 A1, 30 January 2020. [Google Scholar]
- Tran, B.H.; Dao, V.A.; Bilstein, A.; Unfried, K.; Shah-Hosseini, K.; Mösges, R. Ectoine-containing inhalation solution versus saline inhalation solution in the treatment of acute bronchitis and acute respiratory infections: A prospective, controlled, observational study. BioMed Res. Int. 2019, 2019, 7945091. [Google Scholar] [CrossRef] [PubMed]
- Unfried, K.; Krämer, U.; Sydlik, U.; Autengruber, A.; Bilstein, A.; Stolz, S.; Marini, A.; Schikowski, T.; Keymel, S.; Krutmann, J. Reduction of neutrophilic lung inflammation by inhalation of the compatible solute ectoine: A randomized trial with elderly individuals. Int. J. Chron. Obs. Pulmon. Dis. 2016, 11, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Rieckmann, T.; Gatzemeier, F.; Christiansen, S.; Rothkamm, K.; Münscher, A. The inflammation-reducing compatible solute ectoine does not impair the cytotoxic effect of ionizing radiation on head and neck cancer cells. Sci. Rep. 2019, 9, 6594. [Google Scholar] [CrossRef] [PubMed]
- Dao, V.A.; Bilstein, A.; Overhagen, S.; Géczi, L.; Baráth, Z.; Mösges, R. Effectiveness, tolerability, and safety of ectoine-containing mouthwash versus those of a calcium phosphate mouthwash for the treatment of chemotherapy-induced oral mucositis: A prospective, active-controlled, non-interventional study. Oncol. Ther. 2018, 6, 59–72. [Google Scholar] [CrossRef]
- Barth, S.; Huhn, M.; Matthey, B.; Klimka, A.; Galinski, E.A.; Engert, A. Compatible-solute-supported periplasmic expression of functional recombinant proteins under stress conditions. Appl. Environ. Microbiol. 2000, 66, 1572–1579. [Google Scholar] [CrossRef]
- Salmannejad, F.; Nafissi-Varcheh, N.; Tran, B.H.; Dao, V.A.; Bilstein, A.; Unfried, K.; Shah-Hosseini, K.; Mösges, R. Ectoine and hydroxyectoine inhibit thermal-induced aggregation and increase thermostability of recombinant human interferon Alfa2b. Eur. J. Pharm. Sci. 2017, 97, 200–207. [Google Scholar] [CrossRef]
- Barth, S. Pharmaceutical Formulation. U.S. Patent 7,147,849 B2, 12 December 2006. [Google Scholar]
- Kets, E.; Teunissen, P.; de Bont, J. Effect of compatible solutes on survival of lactic Acid bacteria subjected to drying. Appl. Environ. Microbiol. 1996, 62, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Weinbuch, D.; Hawe, A.; Jiskoot, W.; Friess, W. Introduction into formulation development of biologics. In Challenges in Protein Product Development; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–22. [Google Scholar]
- Wang, W.; Ohtake, S. Science and art of protein formulation development. Int. J. Pharm. 2019, 568, 118505. [Google Scholar] [CrossRef] [PubMed]
- Barnett, G.V.; Razinkov, V.I.; Kerwin, B.A.; Blake, S.; Qi, W.; Curtis, R.A.; Roberts, C.J. Osmolyte effects on monoclonal antibody stability and concentration-dependent protein interactions with water and common osmolytes. J. Phys. Chem. B 2016, 120, 3318–3330. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, O.; Dozen, M.; Nomura, K. Thermodynamic analysis of osmolyte effect on thermal stability of ribonuclease A in terms of water activity. Biophys. Chem. 2014, 185, 19–24. [Google Scholar] [CrossRef]
- Kunte, H.J.; Lentzen, G.; Galinski, E. Industrial production of the cell protectant ectoine: Protection mechanisms, processes, and products. Curr. Biotechnol. 2014, 3, 10–25. [Google Scholar] [CrossRef]
- Vreeland, R.H.; Litchfield, C.; Martin, E.; Elliot, E. Halomonas elongata, a new genus and species of extremely salt-tolerant bacteria. Int. J. Syst. Evol. Microbiol. 1980, 30, 485–495. [Google Scholar] [CrossRef]
- Ono, H.; Sawada, K.; Khunajakr, N.; Tao, T.; Yamamoto, M.; Hiramoto, M.; Shinmyo, A.; Takano, M.; Murooka, Y. Characterization of biosynthetic enzymes for ectoine as a compatible solute in a moderately halophilic eubacterium, Halomonas elongata. J. Bacteriol. 1999, 181, 91–99. [Google Scholar] [CrossRef]
- Schwibbert, K.; Marin-Sanguino, A.; Bagyan, I.; Heidrich, G.; Lentzen, G.; Seitz, H.; Rampp, M.; Schuster, S.C.; Klenk, H.P.; Pfeiffer, F.; et al. A blueprint of ectoine metabolism from the genome of the industrial producer Halomonas elongata DSM 2581 T. Environ. Microbiol. 2011, 13, 1973–1994. [Google Scholar] [CrossRef]
- Liss, I. N-Acetyldiaminobuttersäure, eine neue Aminosäure aus dem Latex von Euphorbia pulcherrima Willd ex Klotzsch. Phytochemistry 1962, 1, 87–88. [Google Scholar] [CrossRef]
- Cánovas, D.; Vargas, C.; Iglesias-Guerra, F.; Csonka, L.N.; Rhodes, D.; Ventosa, A.; Nieto, J.J.; Cánovas, D.; Borges, N.; Vargas, C.; et al. Isolation and characterization of salt-sensitive mutants of the moderate halophile Halomonas elongata and cloning of the ectoine synthesis genes. J. Biol. Chem. 1997, 272, 25794–25801. [Google Scholar] [CrossRef]
- García-Estepa, R.; Cánovas, D.; Iglesias-Guerra, F.; Ventosa, A.; Csonka, L.N.; Nieto, J.J.; Vargas, C. Osmoprotection of Salmonella enterica serovar Typhimurium by Ngamma-acetyldiaminobutyrate, the precursor of the compatible solute ectoine. Syst. Appl. Microbiol. 2006, 29, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Cánovas, D.; Borges, N.; Vargas, C.; Ventosa, A.; Nieto, J.J.; Santos, H. Role of Ngamma-acetyldiaminobutyrate as an enzyme stabilizer and an intermediate in the biosynthesis of hydroxyectoine. Appl. Environ. Microbiol. 1999, 65, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Myerson, A.S. Handbook of Industrial Crystallization; Butterworth-Heinemann: Boston, MA, USA, 2002. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Variankaval, N.; Cote, A.S.; Doherty, M.F. From form to function: Crystallization of active pharmaceutical ingredients. AICHE J. 2008, 54, 1682–1688. [Google Scholar] [CrossRef]
- Kirwan, D.J.; Orella, C.J. Crystallization in the pharmaceutical and bioprocessing industries. In Handbook of Industrial Crystallization, 2nd ed.; Myerson, A.S., Ed.; Butterworth-Heinemann: Boston, MA, USA, 2002; pp. 249–266. [Google Scholar]
- Wibowo, C.; Chang, W.C.; Ng, K.M. Design of integrated crystallization systems. AICHE J. 2001, 47, 2474–2492. [Google Scholar] [CrossRef]
- Perini, G.; Salvatori, F.; Ochsenbein, D.R.; Mazzotti, M.; Vetter, T. Filterability prediction of needle-like crystals based on particle size and shape distribution data. Sep. Purif. Technol. 2019, 211, 768–781. [Google Scholar] [CrossRef]
- Wilson, D.; Bunker, M.; Milne, D.; Jawor-Baczynska, A.; Powell, A.; Blyth, J.; Streather, D. Particle engineering of needle shaped crystals by wet milling and temperature cycling: Optimisation for roller compaction. Powder Technol. 2018, 339, 641–650. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. D Biol. Cryst. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Görbitz, C.H. Crystal structures of amino acids: From bond lengths in glycine to metal complexes and high-pressure polymorphs. Crystallogr. Rev. 2015, 21, 160–212. [Google Scholar] [CrossRef]
- Schuh, W.; Puff, H.; Galinski, E.A.; Trüper, H.G. Die Kristallstruktur des Ectoin, einer neuen osmoregulatorisch wirksamen Aminosäure/the crystal structure of ectoine, a novel amino acid of potential osmoregulatory function. Z. Für Nat. C 1985, 40, 780–784. [Google Scholar] [CrossRef]
- Derissen, J.L.; Endeman, H.J.; Peerdeman, A.F. The crystal and molecular structure of L-aspartic acid. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1968, 24, 1349–1354. [Google Scholar] [CrossRef]
- Schütte, H.R.; Schütz, W. Synthese von Nγ-Acetyl-ornithin und Nδ-Acetyl-Diaminobuttersäure. Justus Liebigs Ann. Chem. 1963, 665, 203–204. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Larsen, R.A.; Williams, T.B.; Howell, N.K.; Arteaga, G.; Nakai, S.; Li-Chan, E.C. Characterization of amino acids using Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Larkin, P.J. General outline for IR and raman spectral interpretation. In Infrared and Raman Spectroscopy, 2nd ed.; Larkin, P.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 135–151. [Google Scholar]
- Morhardt, C.; Ketterer, B.; Heißler, S.; Franzreb, M. Direct quantification of immobilized enzymes by means of FTIR ATR spectroscopy—A process analytics tool for biotransformations applying non-porous magnetic enzyme carriers. J. Mol. Catal. B Enzym. 2014, 107, 55–63. [Google Scholar] [CrossRef]
- Howell, N.K.; Arteaga, G.; Nakai, S.; Li-Chan, E.C. Raman spectral analysis in the C-H stretching region of proteins and amino acids for investigation of hydrophobic interactions. J. Agric. Food Chem. 1999, 47, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Fraga García, P.; Merck, G.K.; Bodensteiner, F.A.; Heissler, S.; Günther, S.; Berensmeier, S. Nature of interactions of amino acids with bare magnetite nanoparticles. J. Phys. Chem. C 2015, 119, 23032–23041. [Google Scholar] [CrossRef]
- Larkin, P.J. IR and Raman spectra-structure correlations: Characteristic group frequencies. In Infrared and Raman Spectroscopy, 2nd ed.; Larkin, P.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 85–134. [Google Scholar]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Vasi, S.; Vasi, C.; Dugo, G. The role of water in protein’s behavior: The two dynamical crossovers studied by NMR and FTIR techniques. Comput. Struct. Biotechnol. J. 2015, 13, 33–37. [Google Scholar] [CrossRef]
- Lambert, J.B.; Shurvell, H.F.; Lightner, D.A.; Cooks, R.G. Introduction to Organic Spectroscopy; Macmillan: New York, NY, USA, 1987. [Google Scholar]


| Compound | C6H12N2O3 |
|---|---|
| crystal system | orthorhombic |
| space group | P212121 (No. 19) |
| temperature | 100(2) K |
| a | 5.36470(10) Å |
| b | 8.3652(2) Å |
| c | 16.9149(5) Å |
| cell volume | 759.09(3) Å3 |
| formula units | 4 |
| molar mass | 160.18 g mol−1 |
| X-ray density | 1.402 g·cm−3 |
| wavelength | Mo-Kα |
| absorption coefficient | 0.112 mm−1 |
| measured reflections | 39,700 |
| unique reflections | 5250 |
| Rint | 0.0273 |
| 2θ region | 5.43°–84.10° |
| hkl range | −10 ≤ h ≤ 10 |
| −11 ≤ k ≤ 15 | |
| −31 ≤ l ≤ 31 | |
| absorption correction | empirical |
| reflections > 2σ | 4776 |
| number of parameters | 136 |
| R(F)(obs), R(F)(all) | 0.0293, 0.0348 |
| Rw(F2)(obs), Rw(F2)(all) | 0.0711, 0.0733 |
| goodness of fit | 1.025 |
| max./min. difference density | 0.408/−0.230 e·Å−3 |
| depository no. | CCDC 2024475 |
| Atoms | Distance | Atoms | Distance |
|---|---|---|---|
| C1–O1 | 1.2637(8) | C3–C4 | 1.5251(8) |
| C1–O2 | 1.2551(8) | C4–H4A | 0.979(14) |
| C1–C2 | 1.5352(8) | C4–H4B | 0.979(14) |
| C2–H2A | 0.960(13) | C4–N2 | 1.4588(8) |
| C2–N1 | 1.4906(7) | N2–H2B | 0.846(14) |
| N1–H1A | 0.882(14) | N2–C5 | 1.3390(8) |
| N1–H1B | 0.905(14) | C5–O3 | 1.2470(8) |
| N1–H1C | 0.918(13) | C5–C6 | 1.5070(9) |
| C2–C3 | 1.5391(8) | C6–H6A | 0.929(16) |
| C3–H3A | 0.949(14) | C6–H6B | 0.939(15) |
| C3–H3B | 1.000(14) | C6–H6C | 0.953(16) |
| Atoms | Angle | Atoms | Angle |
|---|---|---|---|
| O2–C1–O1 | 126.72(6) | H3A–C3–H3B | 108.2(12) |
| O2–C1–C2 | 116.49(5) | N2–C4–C3 | 110.08(5) |
| O1–C1–C2 | 116.75(6) | N2–C4–H4A | 108.5(8) |
| N1–C2–C1 | 109.79(5) | C3–C4–H4A | 113.7(8) |
| N1–C2–C3 | 108.09(5) | N2–C4–H4B | 108.9(8) |
| C1–C2–C3 | 110.26(5) | C3–C4–H4B | 109.5(8) |
| N1–C2–H2A | 108.1(8) | H4A–C4–H4B | 106.1(12) |
| C1–C2–H2A | 110.5(9) | C5–N2–C4 | 120.80(5) |
| C3–C2–H2A | 110.1(8) | C5–N2–H2B | 119.3(10) |
| C2–N1–H1A | 110.7(8) | C4–N2–H2B | 119.1(10) |
| C2–N1–H1B | 108.7(8) | O3–C5–N2 | 121.31(6) |
| H1A–N1–H1B | 106.6(12) | O3–C5–C6 | 122.31(6) |
| C2–N1–H1C | 111.4(8) | N2–C5–C6 | 116.35(5) |
| H1A–N1–H1C | 111.0(12) | C5–C6–H6A | 109.9(10) |
| H1B–N1–H1C | 108.3(12) | C5–C6–H6B | 112.3(9) |
| C4–C3–C2 | 113.38(5) | H6A–C6–H6B | 106.3(14) |
| C4–C3–H3A | 109.9(8) | C5–C6–H6C | 109.5(11) |
| C2–C3–H3A | 108.2(8) | H6A–C6–H6C | 110.1(14) |
| C4–C3–H3B | 109.1(8) | H6B–C6–H6C | 108.7(12) |
| C2–C3–H3B | 107.9(8) |
| Atoms | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|
| N1–H1A···O1 i | 0.882(14) | 1.968(14) | 2.8323(8) | 166.2(13) |
| N1–H1B···O2 ii | 0.905(14) | 1.836(14) | 2.7389(7) | 176.4(12) |
| N1–H1C···O3 iii | 0.918(13) | 1.879(13) | 2.7898(7) | 171.8(13) |
| N2–H2B···O1 ii | 0.846(14) | 2.042(14) | 2.8876(7) | 176.7(14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, L.; Klein, W.; Schwaminger, S.P.; Fässler, T.F.; Berensmeier, S. Crystal Structure and Spectroscopic Analysis of the Compatible Solute Nγ-Acetyl-L-2,4-Diaminobutyric Acid. Crystals 2020, 10, 1136. https://doi.org/10.3390/cryst10121136
Martin L, Klein W, Schwaminger SP, Fässler TF, Berensmeier S. Crystal Structure and Spectroscopic Analysis of the Compatible Solute Nγ-Acetyl-L-2,4-Diaminobutyric Acid. Crystals. 2020; 10(12):1136. https://doi.org/10.3390/cryst10121136
Chicago/Turabian StyleMartin, Lea, Wilhelm Klein, Sebastian P. Schwaminger, Thomas F. Fässler, and Sonja Berensmeier. 2020. "Crystal Structure and Spectroscopic Analysis of the Compatible Solute Nγ-Acetyl-L-2,4-Diaminobutyric Acid" Crystals 10, no. 12: 1136. https://doi.org/10.3390/cryst10121136
APA StyleMartin, L., Klein, W., Schwaminger, S. P., Fässler, T. F., & Berensmeier, S. (2020). Crystal Structure and Spectroscopic Analysis of the Compatible Solute Nγ-Acetyl-L-2,4-Diaminobutyric Acid. Crystals, 10(12), 1136. https://doi.org/10.3390/cryst10121136






