Synthesis by Hydrothermal Treatment of ZnO-Based Varistors Doped with Rare Earth Oxides and Their Characterization by Impedance Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
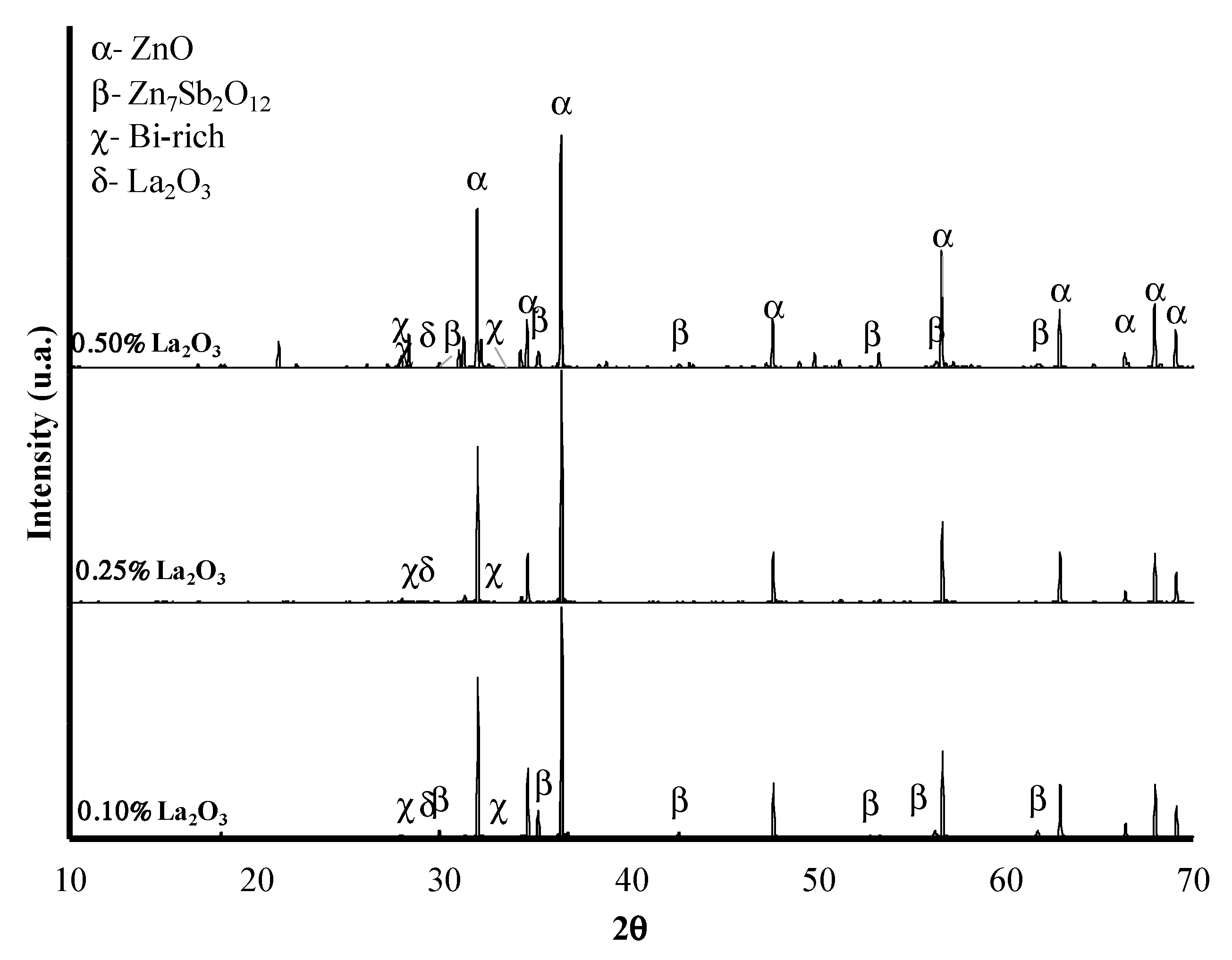
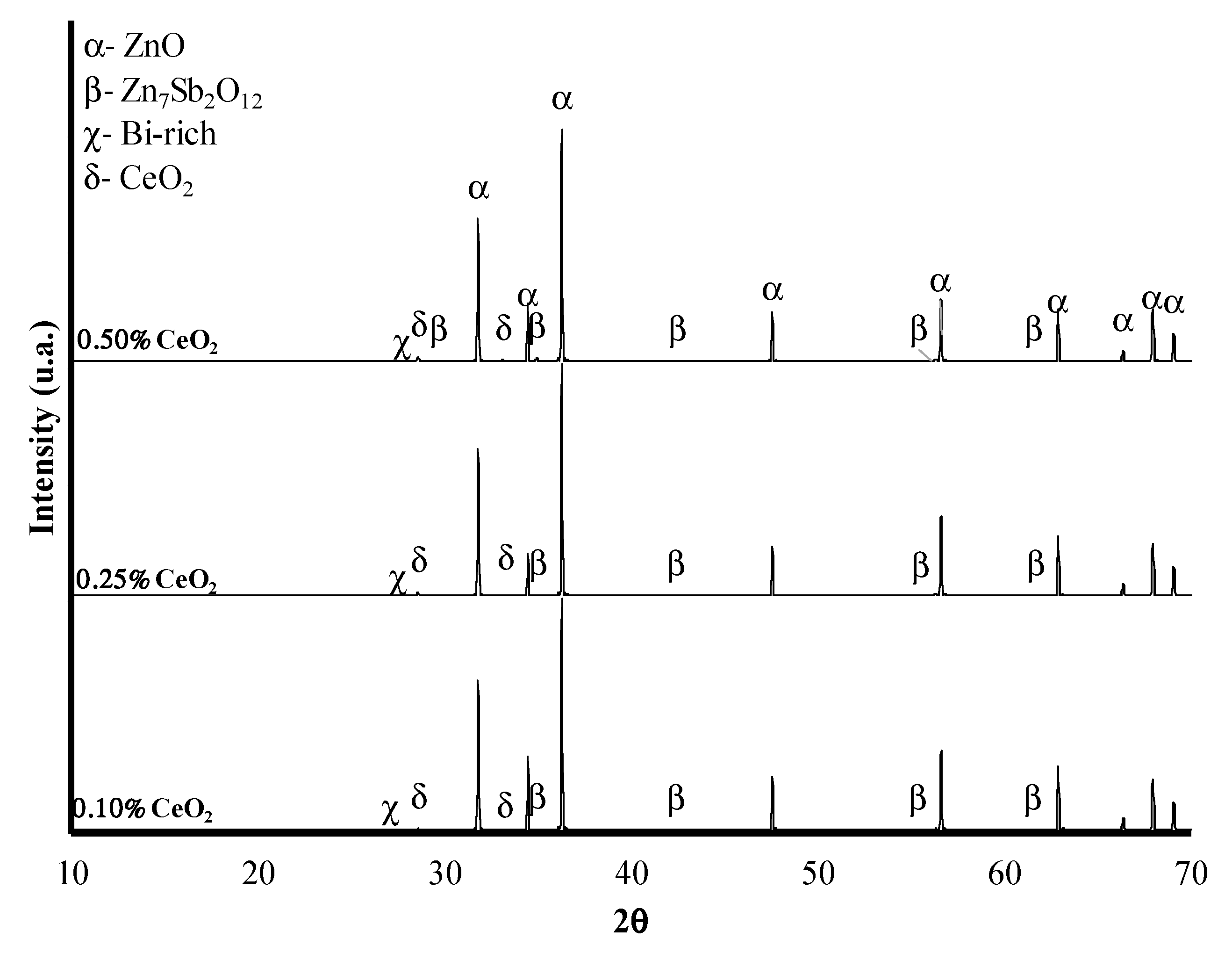
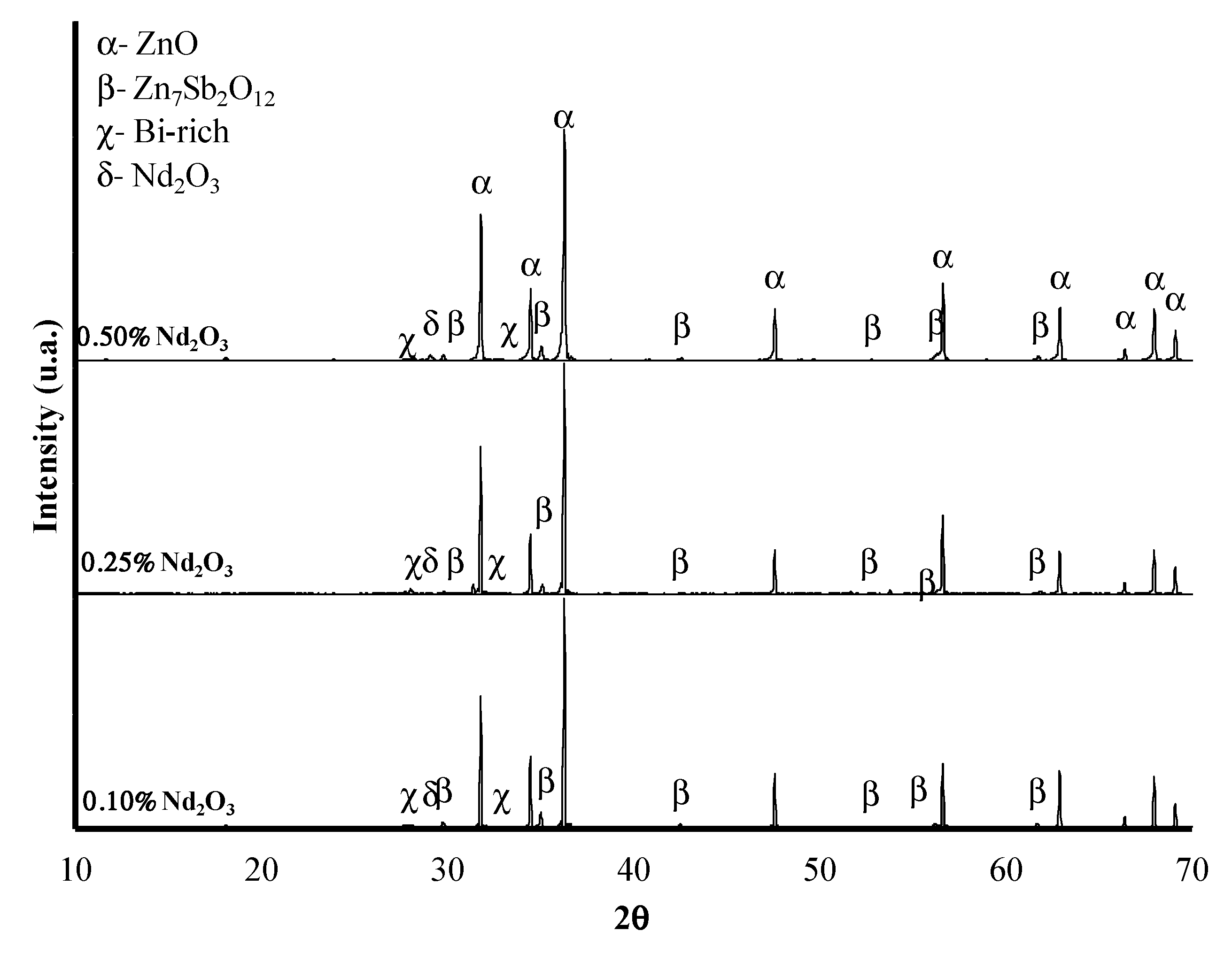
3.1. XRD Analysis
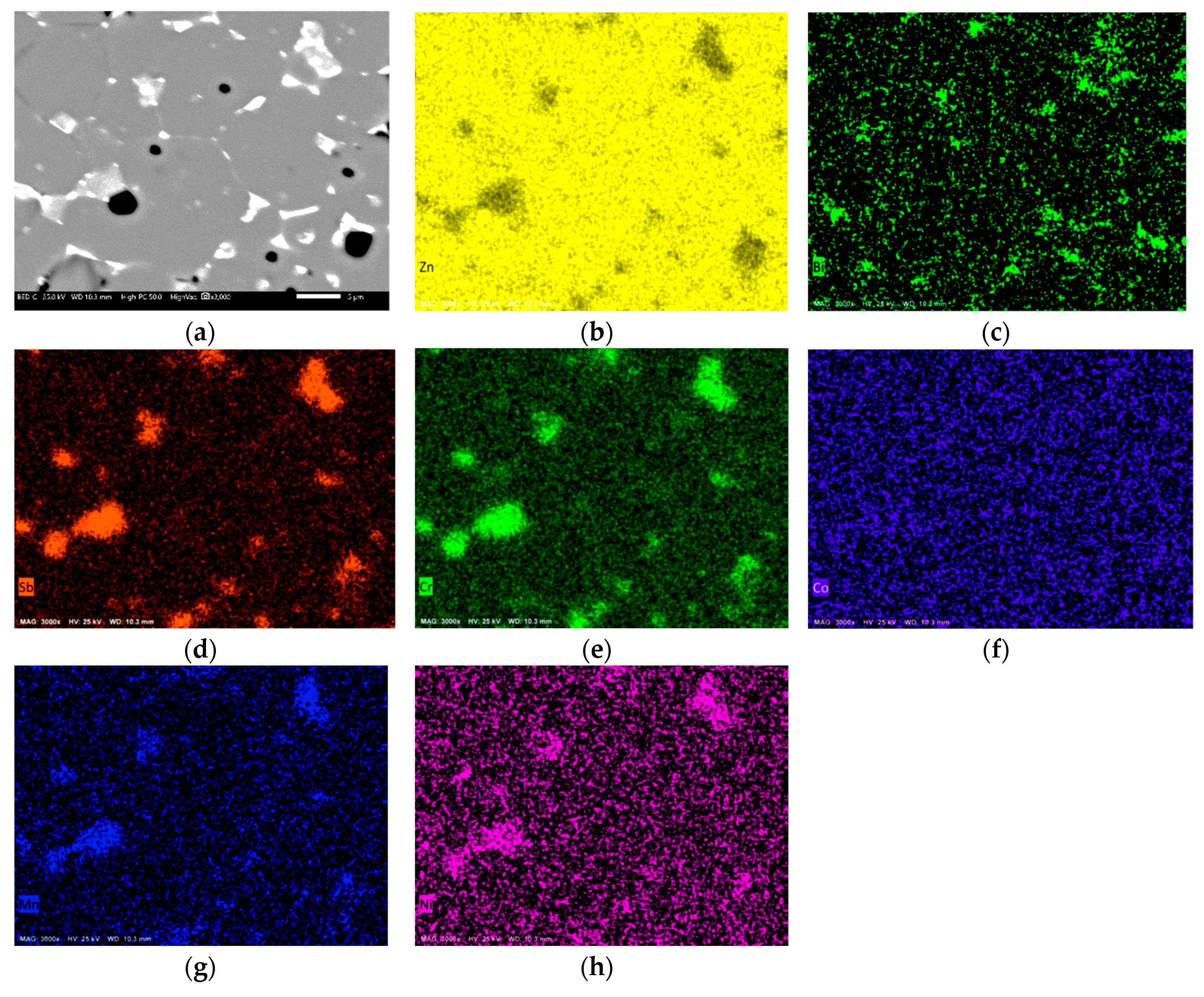
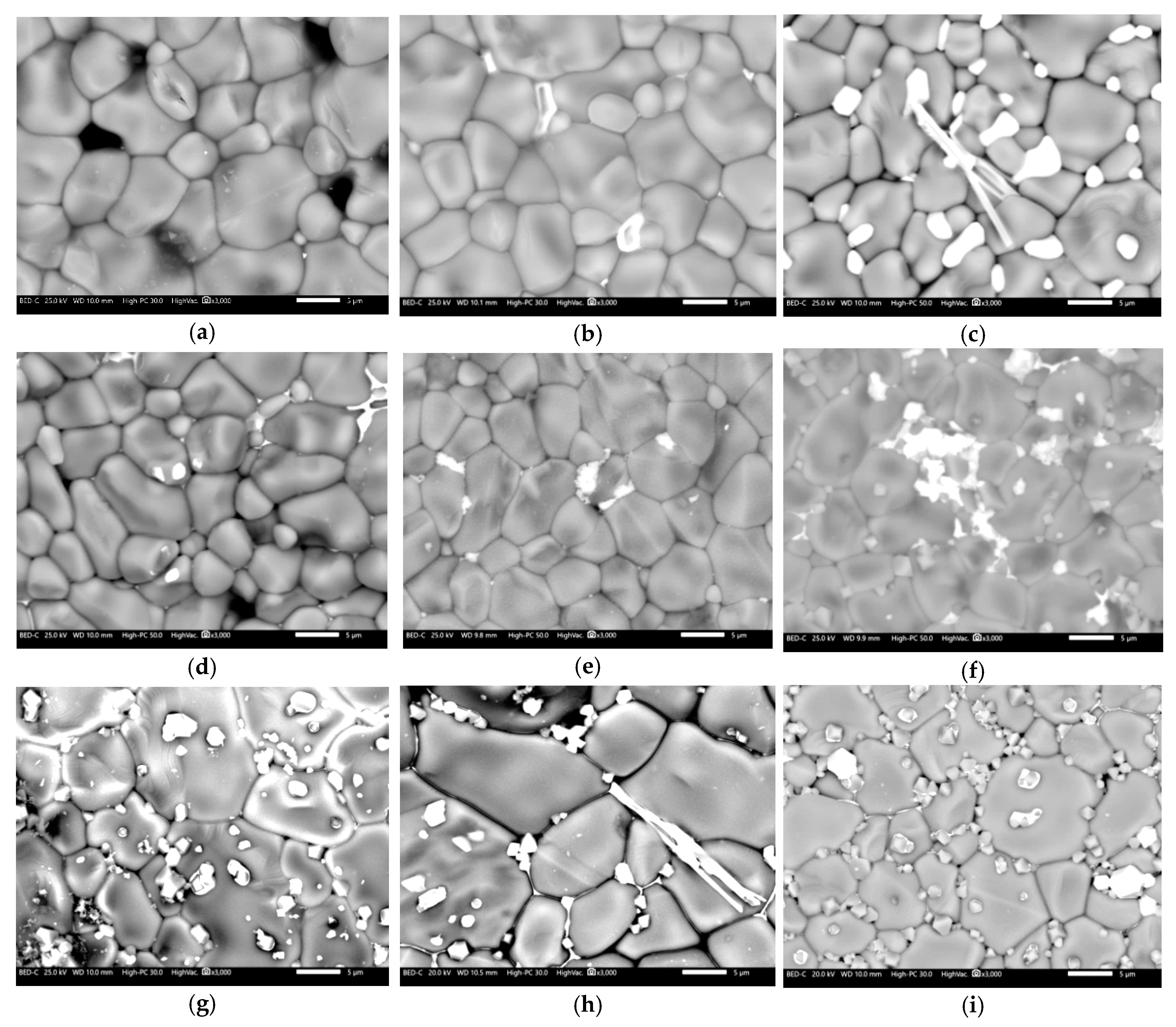
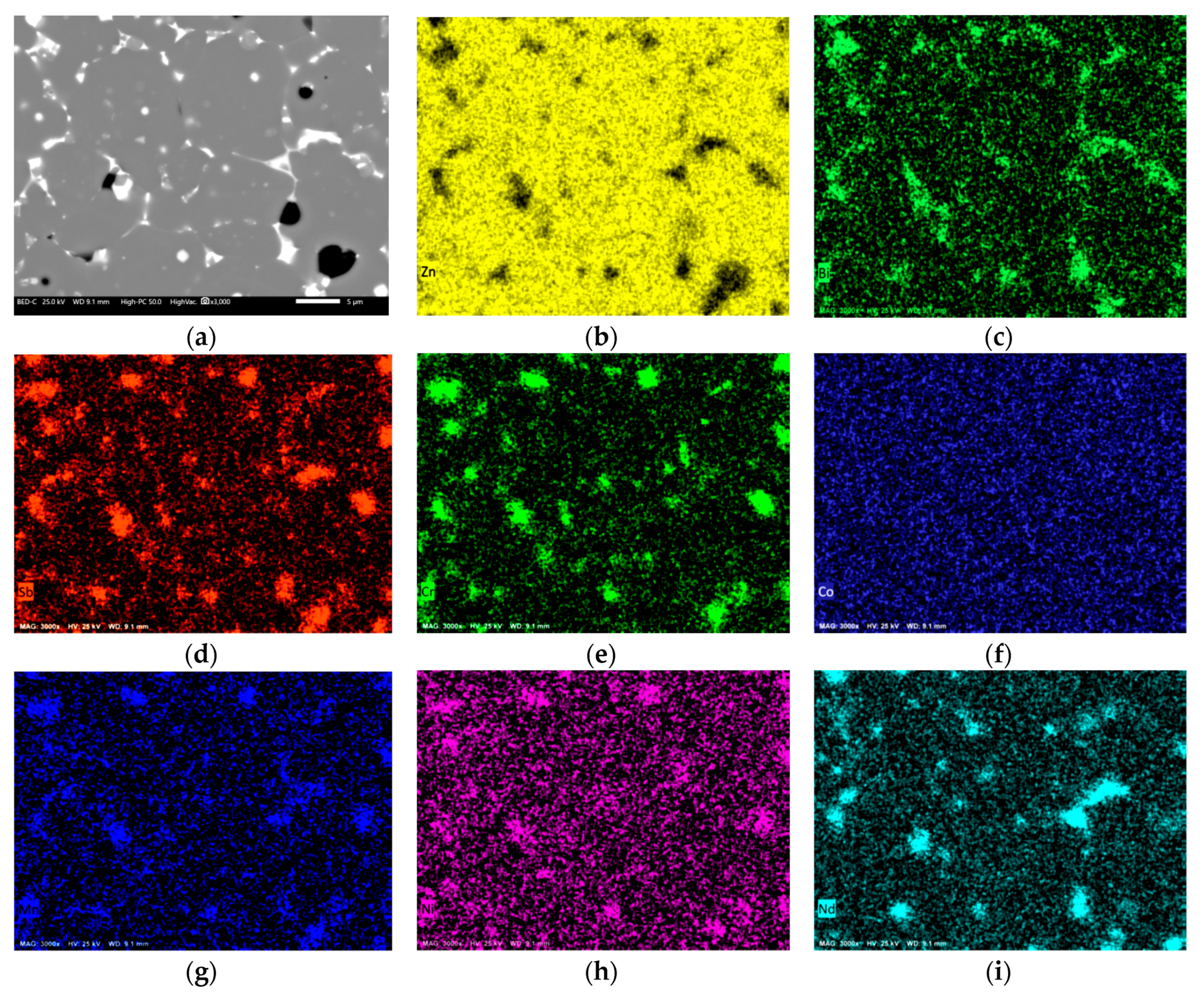
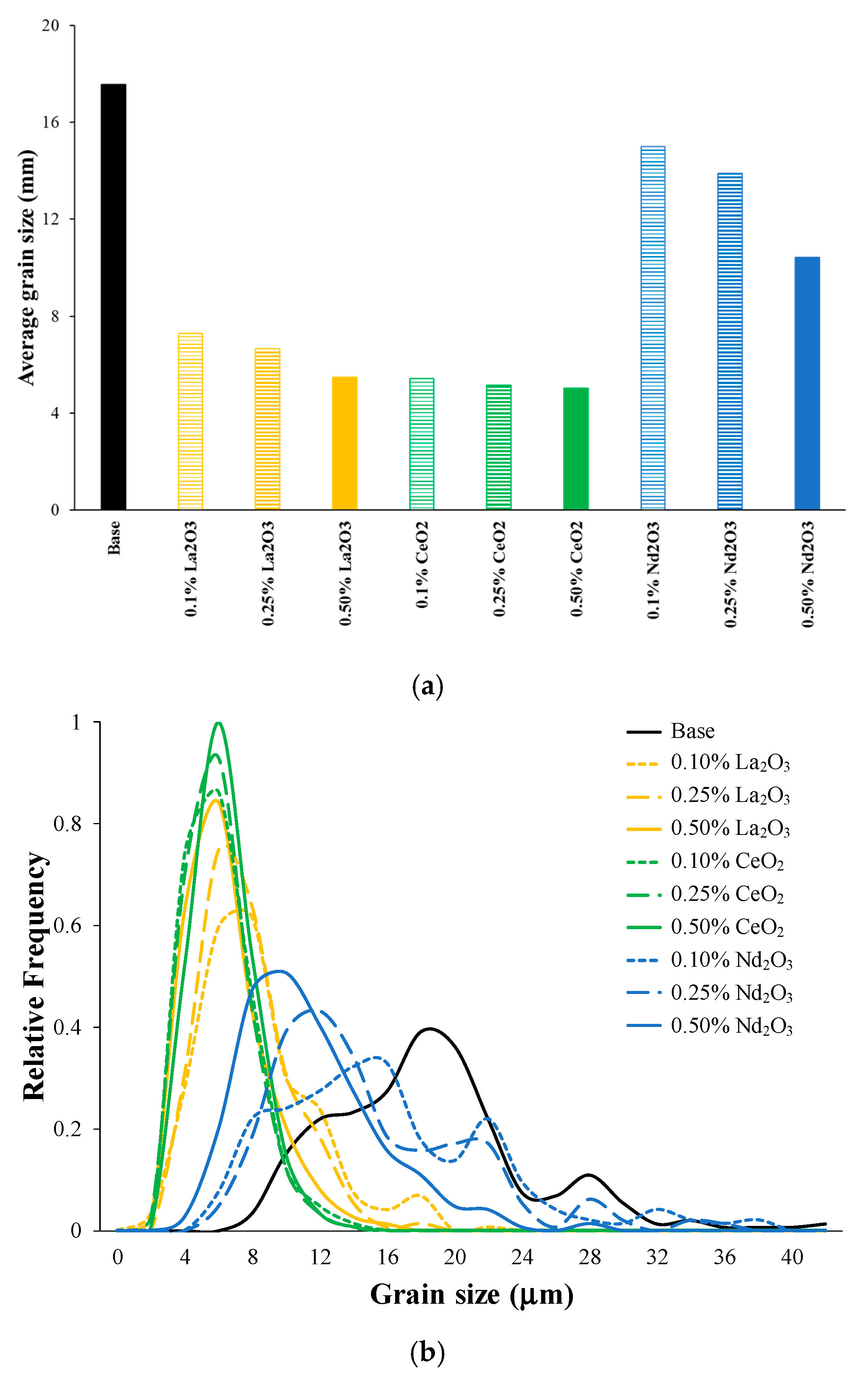
3.2. Microstructural Analysis
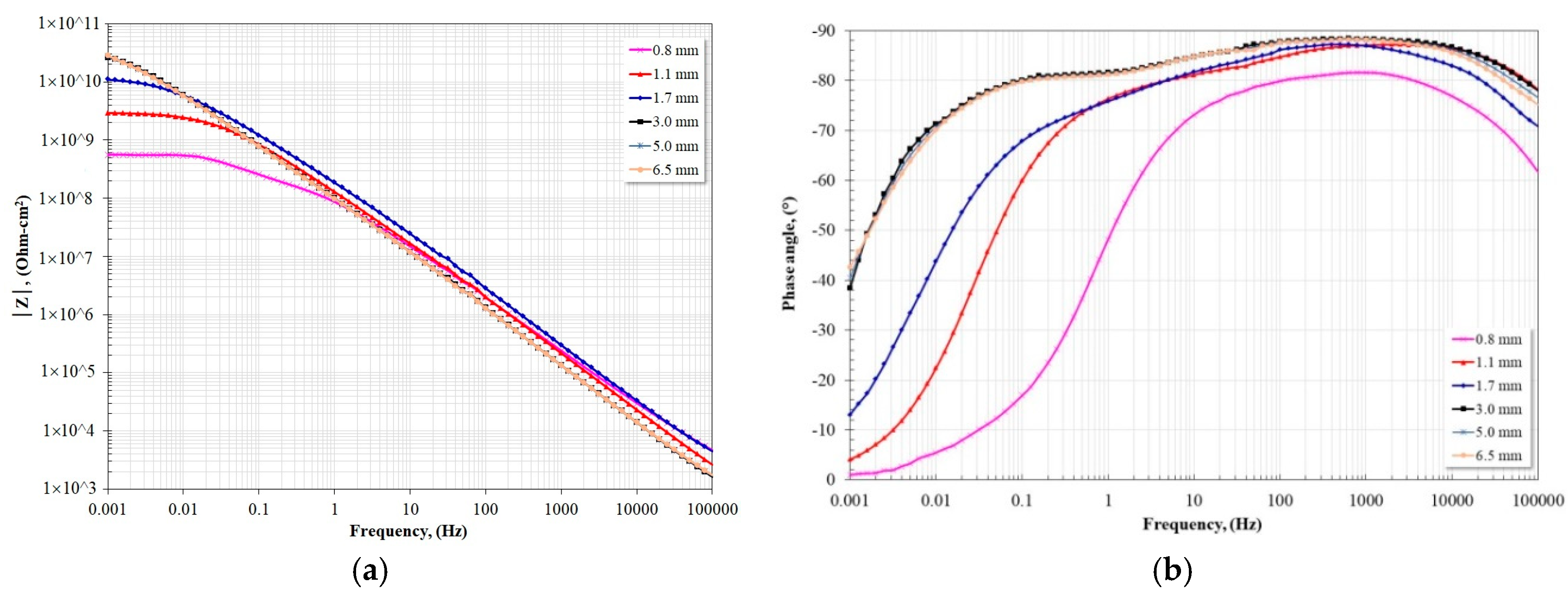
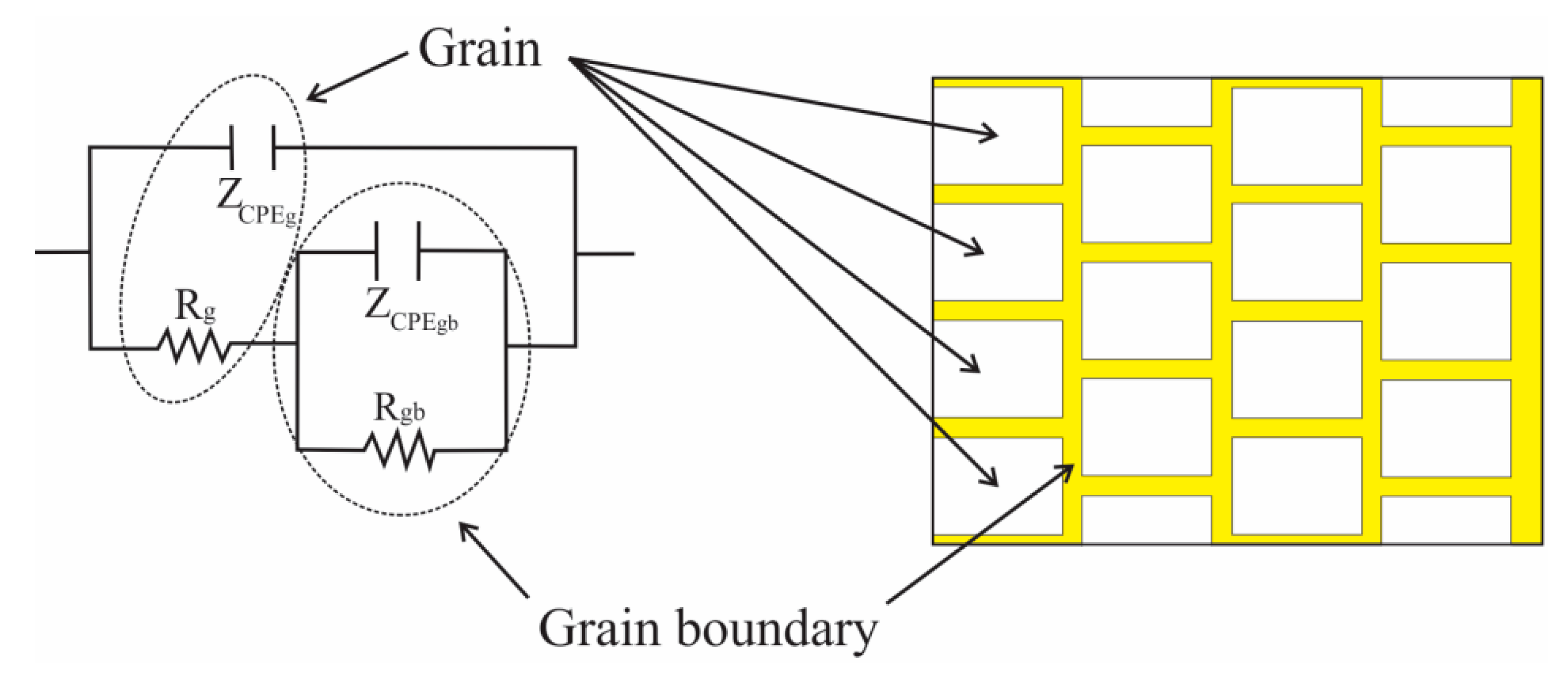
3.3. Impedance Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ben Belgacem, R.; Chaari, M.; Braña, A.F.; Garcia, B.J.; Matoussi, A. Structural, electric modulus and complex impedance analysis of ZnO/TiO2 composite ceramics. J. Am. Ceram. Soc. 2017, 100, 2045–2058. [Google Scholar] [CrossRef]
- Levinson, L.M.; Philipp, H.R. The physics of metal oxide varistors. J. Appl. Phys. 1975, 46, 1332–1341. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.-L.; Chen, X.; Kang, X.-L.; Yuan, C.-L. Sintering temperature dependence of varistor properties and impedance spectroscopy behavior in ZnO based varistor ceramics. J. Mater. Sci. Mater. Electron. 2015, 26, 2389–2396. [Google Scholar] [CrossRef]
- Guo, M.; Wu, T.; Liu, T.; Wang, S.-X.; Zhao, X. Characterization of CaCu3Ti4O12 varistor-capacitor ceramics by impedance spectroscopy. J. Appl. Phys. 2006, 99, 124113. [Google Scholar] [CrossRef]
- Lustosa, G.M.; Jacomaci, N.; Costa, J.P.; Gasparotto, G.; Perazolli, L.A.; Zaghete, M.A. New Approaches to Preparation of SnO2-Based Varistors—Chemical Synthesis, Dopants, and Microwave Sintering. In Advanced Ceramic Processing; Mohamed, A., Ed.; IntechOpen: Rijeka, Croatia, 2015; p. 25. [Google Scholar] [CrossRef]
- West, A.; Andres-Verges, M. Impedance and Modulus Spectroscopy of ZnO Varistors. J. Electroceramics 1997, 1, 125–132. [Google Scholar] [CrossRef]
- Nahm, C.W. Effect of La2O3 addition on microstructure and electrical properties of ZnO-Pr6O11-based varistor ceramics. J. Mater. Sci. Mater. Electron. 2005, 16, 345–349. [Google Scholar] [CrossRef]
- Nahm, C.-W. Effect of sintering temperature on nonlinear electrical properties and stability against DC accelerated aging stress of (CoO, Cr2O3, La2O3)-doped ZnO–Pr6O11-based varistors. Mater. Lett. 2006, 60, 3311–3314. [Google Scholar] [CrossRef]
- Al-Abdullah, K. Elaboration of ZnO Based Varistors and the Effect of the Rare-Earths on their Electrical Behaviour. Energy Procedia 2012, 19, 116–127. [Google Scholar] [CrossRef]
- Houabes, M.; Metz, R. Rare earth oxides effects on both the threshold voltage and energy absorption capability of ZnO varistors. Ceram. Int. 2007, 33, 1191–1197. [Google Scholar] [CrossRef]
- Gupta, T.K. Application of Zinc Oxide Varistors. J. Am. Ceram. Soc. 1990, 73, 1817–1840. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, Y.; Li, M.-Y.; Bao, R.; Shen, M.; Huang, C.; Zhang, G.; Ke, Y.; Li, H.; Jiang, S. Effects of Fe and Al co-doping on the leakage current density and clamp voltage ratio of ZnO varistor. J. Alloys Compd. 2018, 747, 1018–1026. [Google Scholar] [CrossRef]
- Nahm, C.W. Sintering effect on ageing behavior of rare earths (Pr6O11-Er2O3-Y2O3)-doped ZnO varistor ceramics. J. Rare Earths 2012, 30, 1028–1033. [Google Scholar] [CrossRef]
- Cai, J.; Lin, Y.; Li, M.; Nan, C.-W.; He, J.; Yuan, F. Sintering Temperature Dependence of Grain Boundary Resistivity in a Rare-Earth-Doped ZnO Varistor. J. Am. Ceram. Soc. 2007, 90, 291–294. [Google Scholar] [CrossRef]
- Li, J.L.; Chen, G.-H.; Yuan, C.-L. Microstructure and electrical properties of rare earth doped ZnO-based varistor ceramics. Ceram. Int. 2013, 39, 2231–2237. [Google Scholar] [CrossRef]
- Xu, N.; Xu, D.; Zhao, G.; Yang, J.; Shi, L. Microstructure and electrical properties of Sc2O3-doped ZnO–Bi2O3-based varistor ceramics. Ceram. Int. 2011, 37, 701–706. [Google Scholar] [CrossRef]
- Nahm, C.-W. Microstructure and electrical properties of Tb-doped zinc oxide-based ceramics. J. Non-Cryst. Solids 2007, 353, 2954–2957. [Google Scholar] [CrossRef]
- Bernik, S.; Maček, S.; Ai, B. Microstructural and electrical characteristics of Y2O3-doped ZnO–Bi2O3-based varistor ceramics. J. Eur. Ceram. Soc. 2001, 21, 1875–1878. [Google Scholar] [CrossRef]
- Nahm, C.-W.; Shin, B.-C.; Min, B.-H. Microstructure and electrical properties of Y2O3-doped ZnO–Pr6O11-based varistor ceramics. Mater. Chem. Phys. 2003, 82, 157–164. [Google Scholar] [CrossRef]
- Keil, P.; Gehringer, M.; Frömling, T.; Novak, N.; Rödel, J. ZnO-based single crystal-polycrystal structures for piezotronic applications. J. Am. Ceram. Soc. 2019, 102, 2640–2647. [Google Scholar] [CrossRef]
- Cavenaghi, J.M.; Molisani, A.L.; Yoshimura, H.N.; De Sousa, V.C. Influence of compaction manufacturing process on the physical and electrical characteristics of high-voltage varistor. J. Mater. Sci. Mater. Electron. 2007, 18, 957–962. [Google Scholar] [CrossRef]
- Lee, W.; Chen, W.; Lee, Y.; Yang, T.; Su, C.; Hu, C. Influence of sintering on microstructure and electrical properties of ZnO-based multilayer varistor (MLV). Ceram. Int. 2007, 33, 1001–1005. [Google Scholar] [CrossRef]
- Xia, W.-S.; Zhang, G.-Y.; Shi, L.; Zhang, M.-M. Enhanced microwave dielectric properties of ZnTa2O6 ceramics with Sb5+ ion substitution. Mater. Lett. 2014, 124, 64–66. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Sendi, R. A comparative study on degradation characteristics of ZnO nanoparticles-Bi2O3-Mn2O3varistors at various ambient sintering processes. Chin. J. Phys. 2017, 55, 2605–2613. [Google Scholar] [CrossRef]
- Nahm, C.W. Varistor characteristics of ZnO/V2O5/MnO2/Nb2O5 semiconducting ceramics with Tb4O7 addition. J. Mater. Sci. Mater. Electron. 2015, 26, 4144–4151. [Google Scholar] [CrossRef]
- Leite, E.R.; Nobre, M.A.L.; Longo, E.; Varela, J.A. Microstructural development of ZnO varistor during reactive liquid phase sintering. J. Mater. Sci. 1996, 31, 5391–5398. [Google Scholar] [CrossRef]
- Lin, W.; Xu, Z.; Wang, Z.; Yang, J.; Zhu, C.; Chu, R. Influence of Bi3Zn2Sb3O14 pre-synthesis phase on electrical properties of the ZnO-Bi2O3 based varistor ceramics. J. Alloy. Compd. 2020, 834, 155070. [Google Scholar] [CrossRef]
- Gutknecht, T.; Gustafsson, A.; Forsgren, C.; Ekberg, C.; Steenari, B.M. Investigations into recycling zinc from used metal oxide varistors via pH selective leaching: Characterization, leaching, and residue analysis. Sci. World J. 2015, 2015, 653219. [Google Scholar] [CrossRef]
- Drache, M.; Roussel, P.; Wignacourt, J.-P. Structures and Oxide Mobility in Bi−Ln−O Materials: Heritage of Bi2O3. Chem. Rev. 2007, 107, 80–96. [Google Scholar] [CrossRef]
- Xu, D.; Cheng, X.; Yuan, H.; Yang, J.; Lin, Y. Microstructure and electrical properties of Y(NO3)3·6H2O-doped ZnO–Bi2O3-based varistor ceramics. J. Alloys Compd. 2011, 509, 9312–9317. [Google Scholar] [CrossRef]
- Peiteado, M. Varistores cerámicos basados en óxido de cinc. Bol. Soc. Esp. Ceram. Vidr. 2005, 44, 77–87. [Google Scholar] [CrossRef]
- Hung, N.T.; Quang, N.D.; Bernik, S. Electrical and microstructural characteristics of ZnO–Bi2O3-based varistors doped with rare-earth oxides. J. Mater. Res. 2001, 16, 2817–2823. [Google Scholar] [CrossRef]
- Nobre, M.A.L.; Lanfredi, S. New evidence of grain boundary phenomenon in Zn7Sb2O12 ceramic: An analysis by impedance spectroscopy. Mater. Lett. 2001, 50, 322–327. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, K.; Xing, Z.; Zhang, C.; Hu, X.; Guo, P.; Zhang, J.; Li, J. Understanding the validity of impedance and modulus spectroscopy on exploring electrical heterogeneity in dielectric ceramics. J. Appl. Phys. 2019, 125, 084103. [Google Scholar] [CrossRef]
- Pillai, S.C.; Kelly, J.M.; Ramesh, R.; McCormack, D.E. Advances in the synthesis of ZnO nanomaterials for varistor devices. J. Mater. Chem. C 2013, 1, 3268–3281. [Google Scholar] [CrossRef]
- Al Abdullah, K.; Bui, A.; Loubière, A. Low frequency and low temperature behavior of ZnO-based varistor by ac impedance measurements. J. Appl. Phys. 1991, 69, 4046–4052. [Google Scholar] [CrossRef]





| [REO] | La2O3 | CeO2 | Nd2O3 | |||
|---|---|---|---|---|---|---|
| Rgb [Ω-cm2] | Cgb [F-cm2] | Rgb [Ω-cm2] | Cgb [F-cm2] | Rgb [Ω-cm2] | Cgb [F-cm2] | |
| 0.00 | 1.79 × 1011 | 3.51 × 10−14 | 1.79 × 1011 | 3.51 × 10−14 | 1.79 × 1011 | 3.51 × 10−14 |
| 0.10 | 1.43 × 1011 | 4.40 × 10−14 | 7.62 × 1010 | 1.04 × 10−13 | 3.96 × 1010 | 5.02 × 10−13 |
| 0.25 | 4.77 × 1010 | 1.66 × 10−13 | 5.06 × 1010 | 2.47 × 10−13 | 1.49 × 1010 | 3.34 × 10−12 |
| 0.50 | 1.50 × 1010 | 3.33 × 10−12 | 2.12 × 1010 | 1.48 × 10−12 | 7.88 × 1009 | 1.26 × 10−11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcayo-Calderon, J.; Ramos-Hernandez, J.J.; Arrieta-Gonzalez, C.D.; Chacon-Nava, J.G.; Gonzalez-Rodriguez, J.G.; Porcayo-Palafox, E.; Sanchez-Carrillo, M.; Flores-De los Rios, J.P.; Martinez-Gomez, L. Synthesis by Hydrothermal Treatment of ZnO-Based Varistors Doped with Rare Earth Oxides and Their Characterization by Impedance Spectroscopy. Crystals 2020, 10, 1134. https://doi.org/10.3390/cryst10121134
Porcayo-Calderon J, Ramos-Hernandez JJ, Arrieta-Gonzalez CD, Chacon-Nava JG, Gonzalez-Rodriguez JG, Porcayo-Palafox E, Sanchez-Carrillo M, Flores-De los Rios JP, Martinez-Gomez L. Synthesis by Hydrothermal Treatment of ZnO-Based Varistors Doped with Rare Earth Oxides and Their Characterization by Impedance Spectroscopy. Crystals. 2020; 10(12):1134. https://doi.org/10.3390/cryst10121134
Chicago/Turabian StylePorcayo-Calderon, J., J.J. Ramos-Hernandez, C.D. Arrieta-Gonzalez, J.G. Chacon-Nava, J.G. Gonzalez-Rodriguez, E. Porcayo-Palafox, M. Sanchez-Carrillo, J.P. Flores-De los Rios, and L. Martinez-Gomez. 2020. "Synthesis by Hydrothermal Treatment of ZnO-Based Varistors Doped with Rare Earth Oxides and Their Characterization by Impedance Spectroscopy" Crystals 10, no. 12: 1134. https://doi.org/10.3390/cryst10121134
APA StylePorcayo-Calderon, J., Ramos-Hernandez, J. J., Arrieta-Gonzalez, C. D., Chacon-Nava, J. G., Gonzalez-Rodriguez, J. G., Porcayo-Palafox, E., Sanchez-Carrillo, M., Flores-De los Rios, J. P., & Martinez-Gomez, L. (2020). Synthesis by Hydrothermal Treatment of ZnO-Based Varistors Doped with Rare Earth Oxides and Their Characterization by Impedance Spectroscopy. Crystals, 10(12), 1134. https://doi.org/10.3390/cryst10121134







