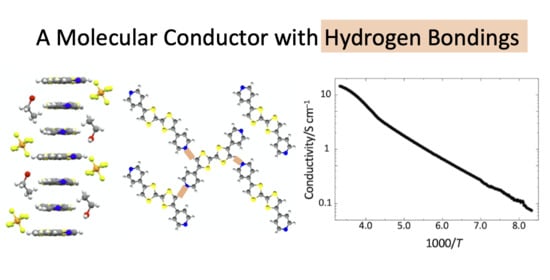
Synthesis, Structure and Physical Properties of (trans-TTF-py2)1.5(PF6)·EtOH: A Molecular Conductor with Weak CH∙∙∙N Hydrogen Bondings
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
2.1.1. Single X-ray Diffraction
2.1.2. Computational Methods
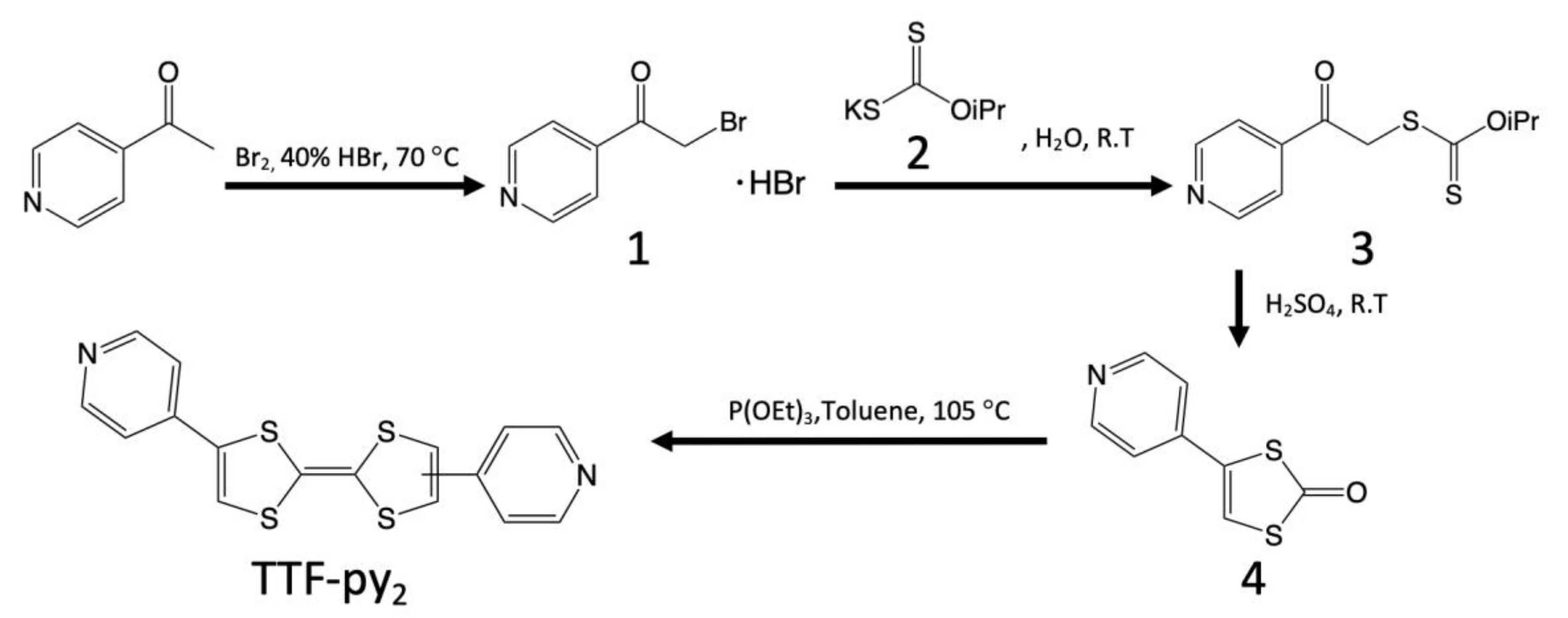
2.2. Syntheses
2.2.1. Synthesis of 4-(2-bromoacetyl)pyridine Hydrobromide (1)
2.2.2. Synthesis of Potassium Isopropylxanthate (2)
2.2.3. Synthesis of O-(1-methylethyl)S-[2-oxo-2-(4-pyridinyl)ethyl]carbonodithioate (3)
2.2.4. Synthesis of 4-(4-pyridyl)-1,3-dithiol-2-one (4)
2.2.5. Synthesis of TTF-py2 (the Mixture of cis and trans Isomers)
2.2.6. Synthesis of (trans-TTF-py2)1.5(PF6)·EtOH (TTF-py2_PF6)
3. Results
3.1. Crystal Structure
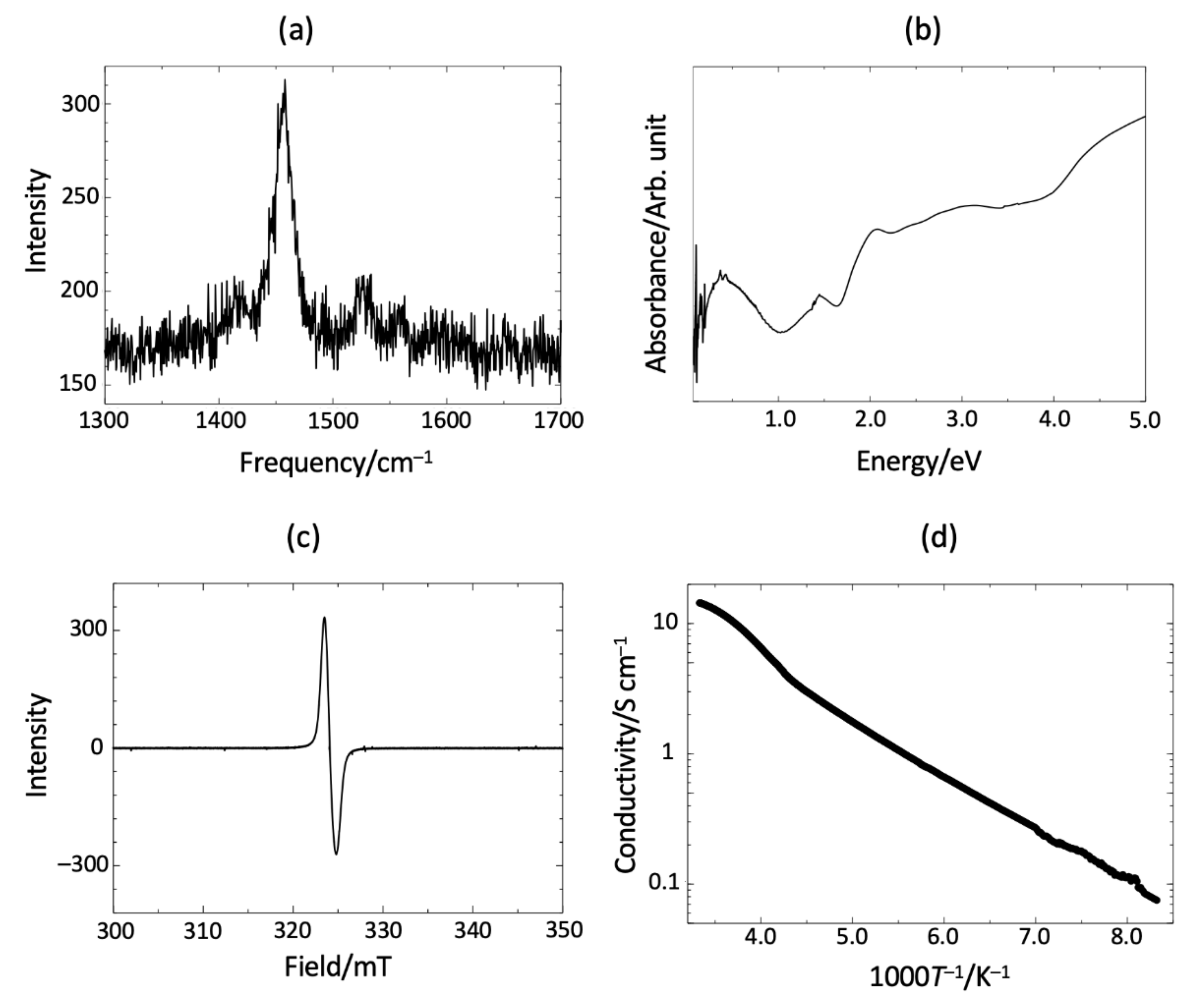
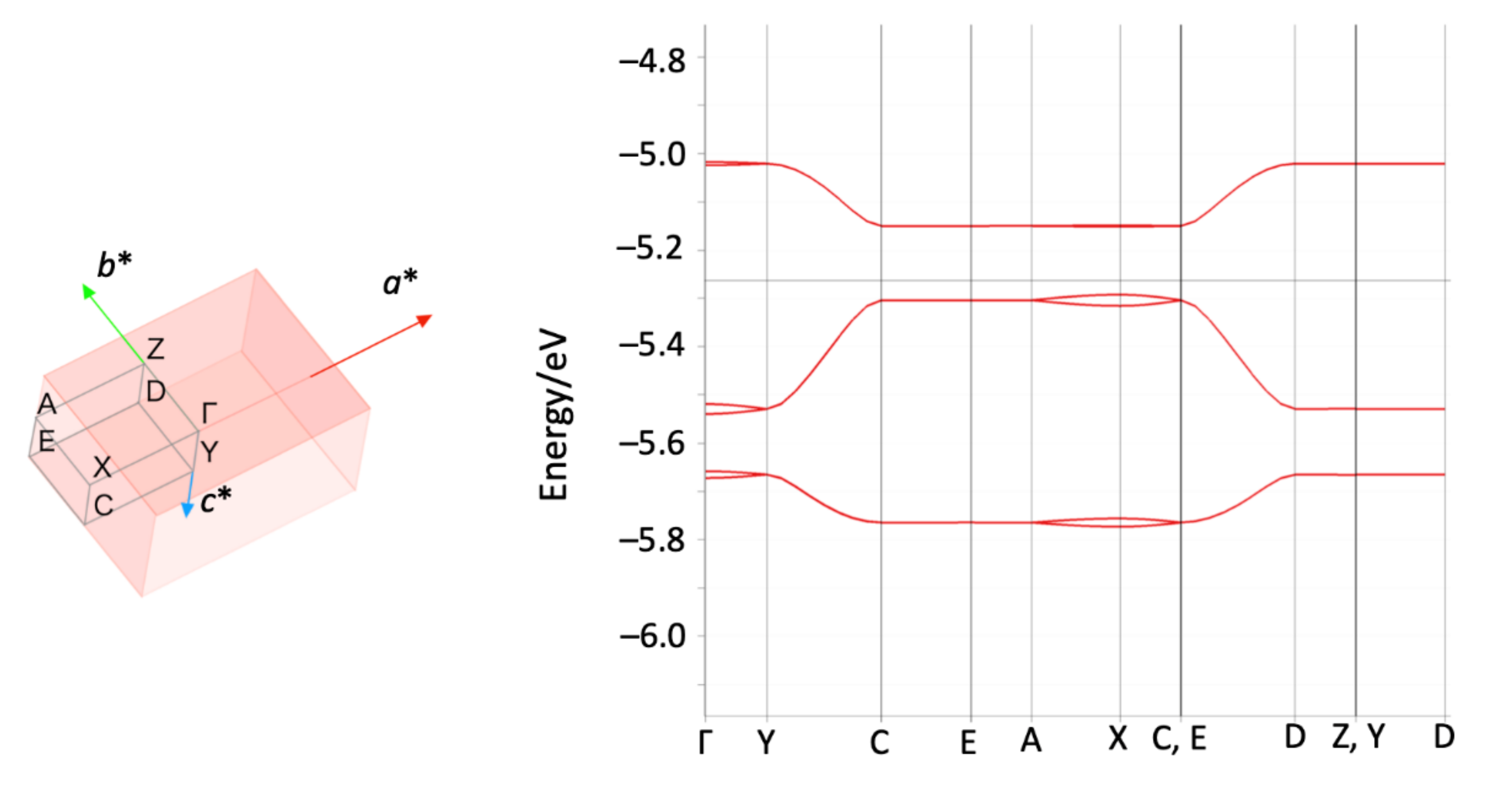
3.2. Electronic States and Conductivity of TTF-py2_PF6
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferraris, J.; Cowan, D.O.; Walatka, V.; Perlstein, J.H. Electron transfer in a new highly conducting donor-acceptor complex. J. Am. Chem. Soc. 1973, 95, 948–949. [Google Scholar] [CrossRef]
- Frère, P.; Skabara, P.J. Salts of extended tetrathiafulvalene analogues: Relationships between molecular structure, electrochemical properties and solid state organisation. Chem. Soc. Rev. 2005, 34, 69–98. [Google Scholar] [CrossRef] [PubMed]
- Otsubo, T.; Takimiya, K. Recent Synthetic Advances of Tetrathiafulvalene-Based Organic Conductors. Bull. Chem. Soc. Jpn. 2004, 77, 43–58. [Google Scholar] [CrossRef]
- Yamochi, H.; Koshihara, S. Organic metal (EDO-TTF)2PF6 with multi-instability. Sci. Technol. Adv. Mater. 2009, 10, 024305. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A. Development of Novel Functional Organic Crystals by Utilizing Proton- and π-Electron-Donating/Accepting Abilities. Bull. Chem. Soc. Jpn. 2017, 90, 1181–1188. [Google Scholar] [CrossRef]
- Fourmigué, M.; Batail, P. Activation of Hydrogen- and Halogen-Bonding Interactions in Tetrathiafulvalene-Based Crystalline Molecular Conductors. Chem. Rev. 2004, 104, 5379–5418. [Google Scholar] [CrossRef]
- Mroweh, N.; Pop, F.; Mézière, C.; Allain, M.; Auban-Senzier, P.; Vanthuyne, N.; Alemany, P.; Canadell, E.; Avarvari, N. Combining Chirality and Hydrogen Bonding in Methylated Ethylenedithio-Tetrathiafulvalene Primary Diamide Precursors and Radical Cation Salts. Cryst. Growth Des. 2020, 20, 2516–2526. [Google Scholar] [CrossRef]
- Sahadevan, S.A.; Monni, N.; Abhervé, A.; Auban-Senzier, P.; Canadell, E.; Mercuri, M.L.; Avarvari, N. Synthesis and Physical Properties of Purely Organic BEDT-TTF-Based Conductors Containing Hetero-/Homosubstituted Cl/CN-Anilate Derivatives. Inorg. Chem. 2017, 56, 12564–12571. [Google Scholar] [CrossRef]
- Lopes, G.; Gama, V.; Belo, D.; Simão, D.; Santos, I.C.; Almeida, M.; Rabaça, S. Cyanobenzene–Ethylenedithio–Tetrathiafulvalene Salts with ClO4–: Bilayer Polymorphs and Different Stoichiometries. Cryst. Growth Des. 2017, 17, 2801–2808. [Google Scholar] [CrossRef]
- Baudron, S.A.; Batail, P.; Coulon, C.; Clérac, R.; Canadell, E.; Laukhin, V.; Melzi, R.; Wzietek, P.; Jérome, D.; Auban-Senzier, P.; et al. (EDT-TTF-CONH2)6[Re6Se8(CN)6], a Metallic Kagome-Type Organic−Inorganic Hybrid Compound: Electronic Instability, Molecular Motion, and Charge Localization. J. Am. Chem. Soc. 2005, 127, 11785–11797. [Google Scholar] [CrossRef]
- Rabaça, S.; Oliveira, S.; Santos, I.C.; Gama, V.; Belo, D.; Lopes, E.B.; Canadell, E.; Almeida, M. Polymorphism and Superconductivity in Bilayer Molecular Metals (CNB-EDT-TTF)4I3. Inorg. Chem. 2016, 55, 10343–10350. [Google Scholar] [CrossRef] [PubMed]
- Baudron, S.A.; Avarvari, N.; Batail, P.; Coulon, C.; Clérac, R.; Canadell, E.; Auban-Senzier, P. Singular Crystalline β‘-Layered Topologies Directed by Ribbons of Self-Complementary Amide···Amide Ring Motifs in [EDT-TTF-(CONH2)2]2X (X = HSO4−, ClO4−, ReO4−, AsF6−): Coupled Activation of Ribbon Curvature, Electron Interactions, and Magnetic Susceptibility. J. Am. Chem. Soc. 2003, 125, 11583–11590. [Google Scholar] [CrossRef] [PubMed]
- Lieffrig, J.; Le Pennec, R.; Jeannin, O.; Auban-Senzier, P.; Fourmigué, M. Toward chiral conductors: Combining halogen bonding ability and chirality within a single tetrathiafulvalene molecule. CrystEngComm 2013, 15, 4408–4412. [Google Scholar] [CrossRef]
- Shin, K.S.; Brezgunova, M.; Jeannin, O.; Roisnel, T.; Camerel, F.; Auban-Senzier, P.; Fourmigué, M. Strong Iodine···Oxygen Interactions in Molecular Conductors Incorporating Sulfonate Anions. Cryst. Growth Des. 2011, 11, 5337–5345. [Google Scholar] [CrossRef]
- Nakano, Y.; Takahashi, Y.; Ishida, K.; Ishikawa, M.; Yamochi, H.; Uruichi, M. Crystal structure and physical properties of radical cation salt based on 4,5-ethylenedioxy-4′-iodotetrathiafulvalene (EDO-TTF-I) with iodine bonding ability. Mater. Chem. Front. 2018, 2, 752–759. [Google Scholar] [CrossRef]
- Shin, K.-S.; Jeannin, O.; Brezgunova, M.; Dahaoui, S.; Aubert, E.; Espinosa, E.; Auban-Senzier, P.; Świetlik, R.; Frąckowiak, A.; Fourmigué, M. Inter-layer charge disproportionation in the dual-layer organic metal (tTTF-I)2ClO4with unsymmetrical I⋯O halogen bond interactions. Dalton Trans. 2014, 43, 5280–5291. [Google Scholar] [CrossRef]
- Steiner, T. The hydrogen bond in solid state. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Kanetou, T.; Tsunashima, R.; Hoshino, N.; Akutagawa, T. Solid-state structure and electronic states of hydrogen-bonded dimer of pyridyl-substituted tetrathiafulvalene salted with PF 6? RSC Adv. 2017, 7, 6236–6241. [Google Scholar] [CrossRef]
- Lee, S.C.; Ueda, A.; Kamo, H.; Takahashi, K.; Uruichi, M.; Yamamoto, K.; Yakushi, K.; Nakao, A.; Kumai, R.; Kobayashi, K.; et al. Charge-order driven proton arrangement in a hydrogen-bonded charge-transfer complex based on a pyridyl-substituted TTF derivative. Chem. Commun. 2012, 48, 8673–8675. [Google Scholar] [CrossRef]
- Liu, S.-X.; Dolder, S.; Franz, P.; Neels, A.; Stoeckli-Evans, H.; Decurtins, S. Structural studies of transition metal complexes with 4,5-Bis(2-pyridylmethylsulfanyl)-4′,5′-ethylenedithiotetrathiafulvalene: Probing their potential for the construction of multifunctional molecular assemblies. Inorg. Chem. 2003, 42, 4801–4803. [Google Scholar] [CrossRef]
- Jia, C.; Liu, S.-X.; Ambrus, C.; Neels, A.; Labat, G.; Decurtins, S. One-Dimensional μ-Chloromanganese(II)−Tetrathiafulvalene (TTF) Coordination Compound. Inorg. Chem. 2006, 45, 3152–3154. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, Y.; Yorimitsu, H.; Oshima, K.; Osuka, A. Straightforward access to aryl-substituted tetrathiafulvalenes by palladium-catalysed direct C–H arylation and their photophysical and electrochemical properties. Chem. Sci. 2011, 2, 2017–2021. [Google Scholar] [CrossRef]
- Han, Y.-F.; Zhang, J.-S.; Lin, Y.-J.; Dai, J.; Jin, G.-X. Synthesis and characterization of half-sandwich iridium complexes containing 2,6(7)-bis(4-pyridyl)-1,4,5,8-tetrathiafulvalene and ancillary ortho-carborane-1,2-dichalcogenolato ligands. J. Organomet. Chem. 2007, 692, 4545–4550. [Google Scholar] [CrossRef]
- Wang, R.; Kang, L.-C.; Xiong, J.; Dou, X.; Chen, X.-Y.; Zuo, J.-L.; You, X.-Z. Structures and physical properties of oligomeric and polymeric metal complexes based on bis(pyridyl)-substituted TTF ligands and an inorganic analogue. Dalton Trans. 2011, 40, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-Z.; Weng, Y.-G.; Yin, W.-Y.; Jiang, M.; Zhu, Q.-Y.; Dai, J. A Potential Hybrid Hole-Transport Material Incorporating a Redox-Active Tetrathiafulvalene Derivative with CuSCN. Inorg. Chem. 2019, 58, 15824–15831. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.-N.; Li, Y.-H.; Sun, Y.-G.; Chen, T.; Xu, J.; Zhu, Q.-Y.; Dai, J. 3D Copper Tetrathiafulvalene Redox-Active Network with 8-Fold Interpenetrating Diamond-like Topology. Inorg. Chem. 2016, 55, 9154–9157. [Google Scholar] [CrossRef] [PubMed]
- Zappe, L.; Schönfeld, S.; Hoerner, G.; Zenere, K.A.; Leong, C.F.; Kepert, C.J.; D’Alessandro, D.M.; Weber, B.; Neville, S.M. Spin crossover modulation in a coordination polymer with the redox-active bis-pyridyltetrathiafulvalene (py2TTF) ligand. Chem. Commun. 2020, 56, 10469–10472. [Google Scholar] [CrossRef]
- Sherman, D.A.; Murase, R.; Duyker, S.G.; Gu, Q.; Lewis, W.; Lu, T.; Liu, Y.; D’Alessandro, D.M. Reversible single crystal-to-single crystal double [2+2] cycloaddition induces multifunctional photo-mechano-electrochemical properties in framework materials. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Cerrada, E.; Laguna, M.; Bartolomé, J.; Campo, J.; Orera, V.; Jones, P.G. Cation-radical salts with organometallic gold anions. X-ray structure of [TTFPh]2[Au(C6F5)2]. Synth. Met. 1998, 92, 245–251. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT– Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Boys, S.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree–Fock and local density—Functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr. Electron affinities of the first--row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Del Bene, J.E.; Person, W.B.; Szczepaniak, K. Properties of Hydrogen-Bonded Complexes Obtained from the B3LYP Functional with 6-31G(d,p) and 6-31+G(d,p) Basis Sets: Comparison with MP2/6-31+G(d,p) Results and Experimental Data. J. Phys. Chem. 1995, 99, 10705–10707. [Google Scholar] [CrossRef]
- Shultz, M.J.; Vu, T.H. Hydrogen Bonding between Water and Tetrahydrofuran Relevant to Clathrate Formation. J. Phys. Chem. B 2015, 119, 9167–9172. [Google Scholar] [CrossRef]
- Joshi, R.; Ghanty, T.K. Hydrogen bonding interaction between HO2 radical and selected organic acids, RCOOH (R=CH3, H, Cl and F). Chem. Phys. Lett. 2013, 584, 43–48. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Y.; Wang, H.-J.; Liu, J.; Chen, J. Theoretical Study on the Structure and Cation–Anion Interaction of Amino Acid Cation Based Amino Acid Ionic Liquid [Pro]+[NO3]−. J. Phys. Chem. A 2010, 114, 10243–10252. [Google Scholar] [CrossRef] [PubMed]
- Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Guerra, C.F.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- ADF 2019.3, SCM, Theoretical Chemistry; Vrije Universiteit: Amsterdam, The Netherlands; Available online: https://www.scm.com/product/adf/ (accessed on 26 November 2020).
- AMS DFTB 2019.3, SCM, Theoretical Chemistry; Vrije Universiteit: Amsterdam, The Netherlands; Available online: https://www.scm.com/product/ams/ (accessed on 26 November 2020).
- Levi, O.P.-T.; Becker, J.Y.; Ellern, A.; Khodorkovsky, V. Synthesis of 2,3-dimethylthio-6-pyridyl tetrathiafulvalene: A precursor for a new system involving a direct linkage between a strong donor (D) and a strong acceptor (A). Tetrahedron Lett. 2001, 42, 1571–1573. [Google Scholar] [CrossRef]
- Crystallographic data for TTF-py2_PF6: C52H42F12N6O2P2S12, M = 1457.57, T = 120 K, Monoclinic, Space group P21/c, Z = 2, a = 10.5747(10) Å, b = 13.6826(7) Å, c = 19.9351(11) Å, β = 90.256(6)°, V = 2884.4(3) Å3, Dc = 1.678 mg cm–3, 16917 reflections collected, final R1 and wR2 (I > 2σ(I)) = 0.0910 and 0.2264. CCDC deposition number 2040487. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 26 November 2020).
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Moriyama, T.; Toyoda, K. Raman spectra of mixed valent TTF salts; relation between Raman frequency and formal charge. Solid State Commun. 1980, 34, 857–859. [Google Scholar] [CrossRef]
- Rosokha, S.V.; Kochi, J.K. Molecular and Electronic Structures of the Long-Bonded π-Dimers of Tetrathiafulvalene Cation-Radical in Intermolecular Electron Transfer and in (Solid-State) Conductivity. J. Am. Chem. Soc. 2007, 129, 828–838. [Google Scholar] [CrossRef]
- Scott, B.A.; La Placa, S.J.; Torrance, J.B.; Silverman, B.D.; Welber, B. The crystal chemistry of organic metals. Composition, structure, and stability in the tetrathiafulvalinium-halide systems. J. Am. Chem. Soc. 1977, 99, 6631–6639. [Google Scholar] [CrossRef]
- Torrance, J.B.; Scott, B.A.; Welber, B.; Kaufman, F.B.; Seiden, P.E. Optical properties of the radical cation tetrathiafulvalenium (TT F+ ) in its mixed-valence and monovalence halide salts. Phys. Rev. B 1979, 19, 730–741. [Google Scholar] [CrossRef]
- Jacobsen, C.S. Optical Properties in Semiconductors and Semimetals; Conwell, E., Ed.; Academic Press: Boston, MA, USA, 1988; Volume 27, pp. 293–384. [Google Scholar]
- Kittel, C. Introduction to Solid State Physics; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Wood, P.A.; Allen, F.H.; Pidcock, E. Hydrogen-bond directionality at the donor H atom—Analysis of interaction energies and database statistics. CrystEngComm 2009, 11, 1563–1571. [Google Scholar] [CrossRef]
- Qu, L.; Iguchi, H.; Takaishi, S.; Habib, F.; Leong, C.F.; D’Alessandro, D.M.; Yoshida, T.; Abe, H.; Nishibori, E.; Yamashita, M. Porous Molecular Conductor: Electrochemical Fabrication of Through-Space Conduction Pathways among Linear Coordination Polymers. J. Am. Chem. Soc. 2019, 141, 6802–6806. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Tanabe, T.; Takaishi, S.; Yamashita, M.; Iguchi, H. Preliminary chemical reduction for synthesizing a stable porous molecular conductor with neutral metal nodes. Chem. Commun. 2020, 56, 13109–13112. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.Y.; Yakiyama, Y.; Lee, G.R.; Lee, J.; Choi, H.C.; Morita, Y.; Kawano, M. Selective Formation of Conductive Network by Radical-Induced Oxidation. J. Am. Chem. Soc. 2016, 138, 1776–1779. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xing, H.; Su, Z.-M.; Wang, C. Electrical conductivity and electroluminescence of a new anthracene-based metal–organic framework with π-conjugated zigzag chains. Chem. Commun. 2016, 52, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Hontz, E.R.; Sun, L.; Hendon, C.H.; Walsh, A.; Van Voorhis, T.; Dincă, M. Cation-Dependent Intrinsic Electrical Conductivity in Isostructural Tetrathiafulvalene-Based Microporous Metal–Organic Frameworks. J. Am. Chem. Soc. 2015, 137, 1774–1777. [Google Scholar] [CrossRef] [PubMed]
- Skorupskii, G.; Trump, B.A.; Kasel, T.W.; Brown, C.M.; Hendon, C.H.; Dincă, M. Efficient and tunable one-dimensional charge transport in layered lanthanide metal-organic framworks. Nat. Chem. 2020, 12, 131–136. [Google Scholar] [CrossRef]
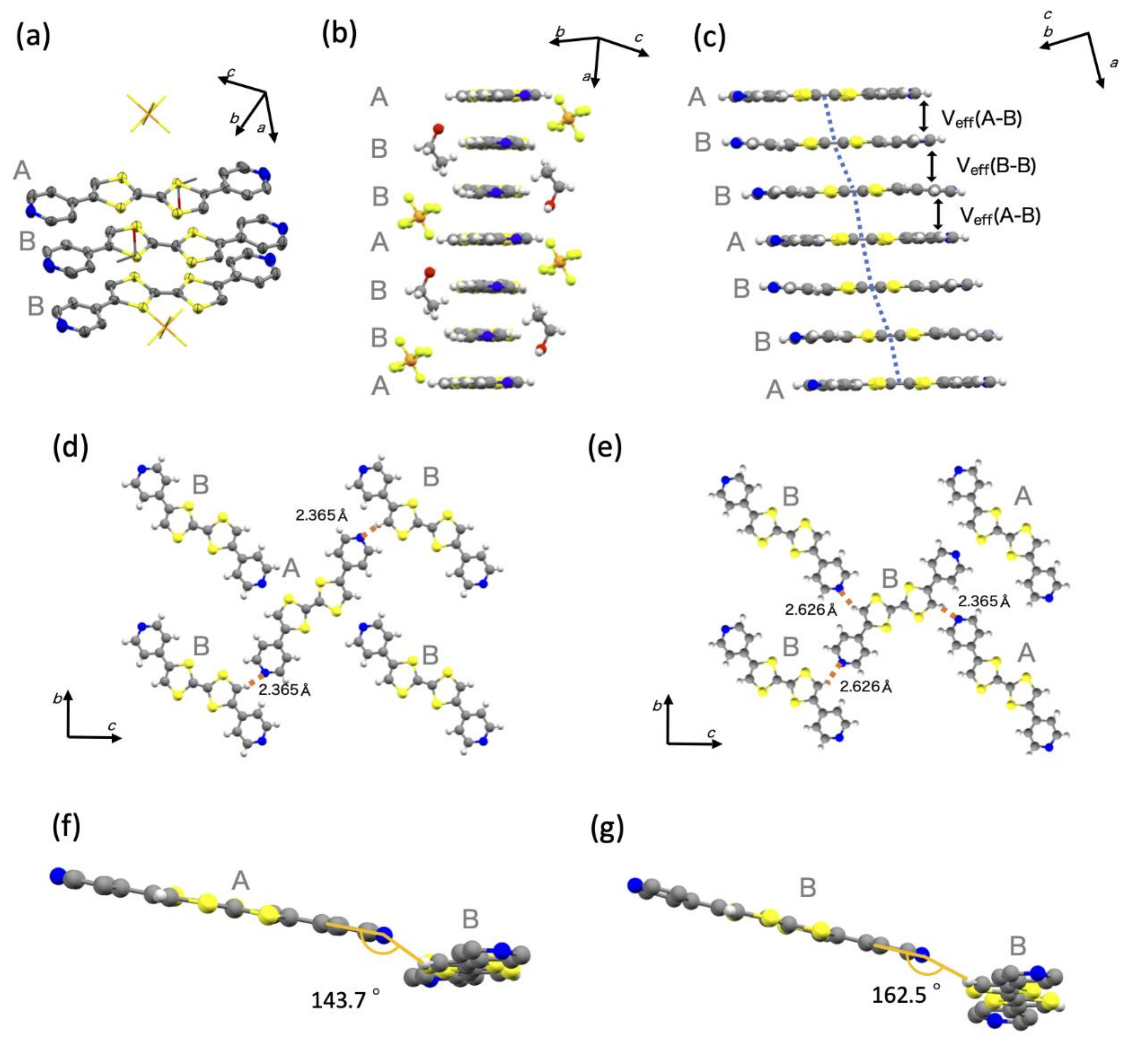
| A–B | B–B | |
|---|---|---|
| Intracolumnar S∙∙∙S distance/Å | 3.584 3.481 3.493 3.408 | 3.701 3.666 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koyama, S.; Kawai, M.; Takaishi, S.; Yamashita, M.; Hoshino, N.; Akutagawa, T.; Kanno, M.; Iguchi, H. Synthesis, Structure and Physical Properties of (trans-TTF-py2)1.5(PF6)·EtOH: A Molecular Conductor with Weak CH∙∙∙N Hydrogen Bondings. Crystals 2020, 10, 1081. https://doi.org/10.3390/cryst10121081
Koyama S, Kawai M, Takaishi S, Yamashita M, Hoshino N, Akutagawa T, Kanno M, Iguchi H. Synthesis, Structure and Physical Properties of (trans-TTF-py2)1.5(PF6)·EtOH: A Molecular Conductor with Weak CH∙∙∙N Hydrogen Bondings. Crystals. 2020; 10(12):1081. https://doi.org/10.3390/cryst10121081
Chicago/Turabian StyleKoyama, Shohei, Morio Kawai, Shinya Takaishi, Masahiro Yamashita, Norihisa Hoshino, Tomoyuki Akutagawa, Manabu Kanno, and Hiroaki Iguchi. 2020. "Synthesis, Structure and Physical Properties of (trans-TTF-py2)1.5(PF6)·EtOH: A Molecular Conductor with Weak CH∙∙∙N Hydrogen Bondings" Crystals 10, no. 12: 1081. https://doi.org/10.3390/cryst10121081
APA StyleKoyama, S., Kawai, M., Takaishi, S., Yamashita, M., Hoshino, N., Akutagawa, T., Kanno, M., & Iguchi, H. (2020). Synthesis, Structure and Physical Properties of (trans-TTF-py2)1.5(PF6)·EtOH: A Molecular Conductor with Weak CH∙∙∙N Hydrogen Bondings. Crystals, 10(12), 1081. https://doi.org/10.3390/cryst10121081