Recent Advancements of N-Doped Graphene for Rechargeable Batteries: A Review
Abstract
1. Introduction
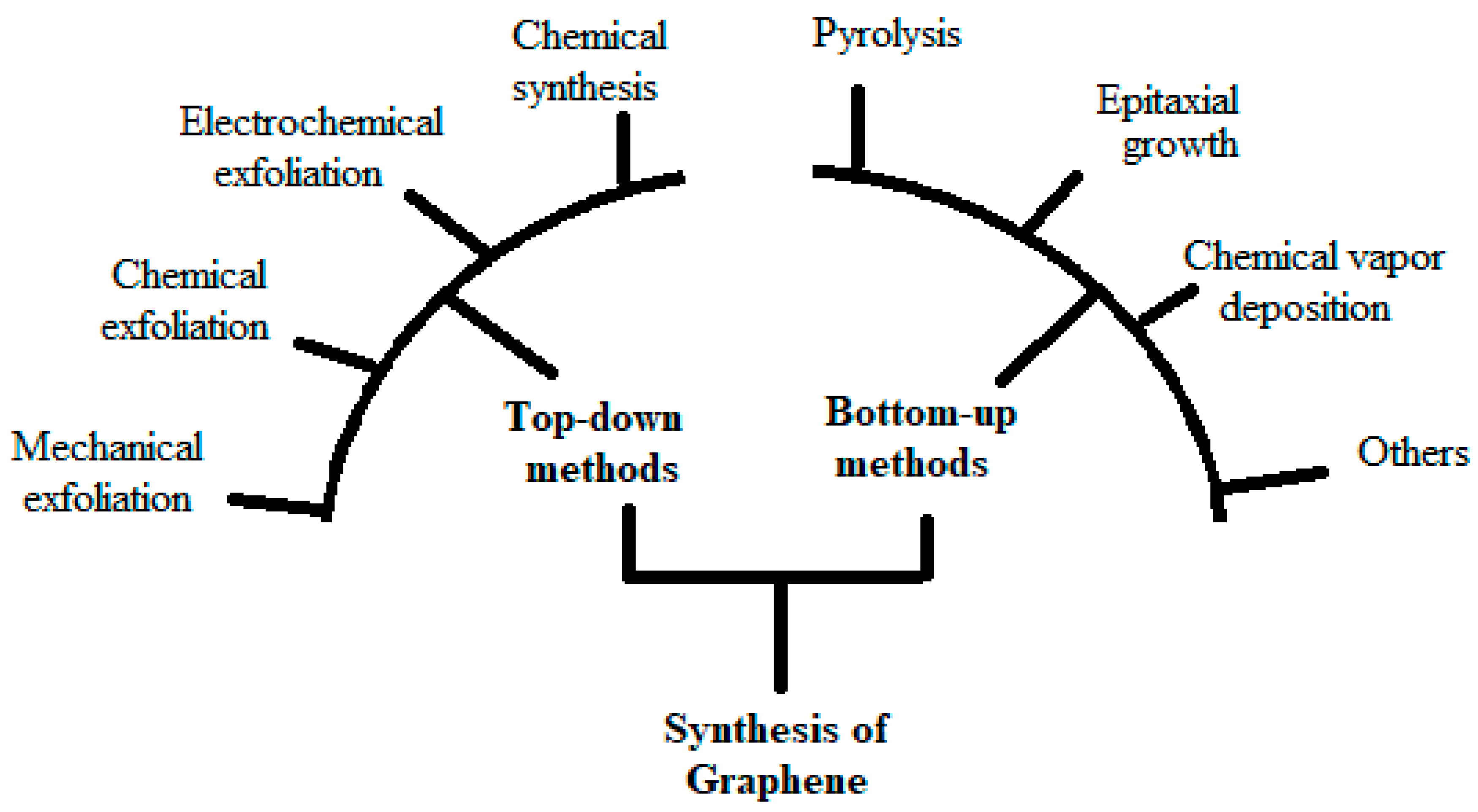
2. Gp Synthesis Routes for Battery Application
3. Characterization Tools for Gp Materials
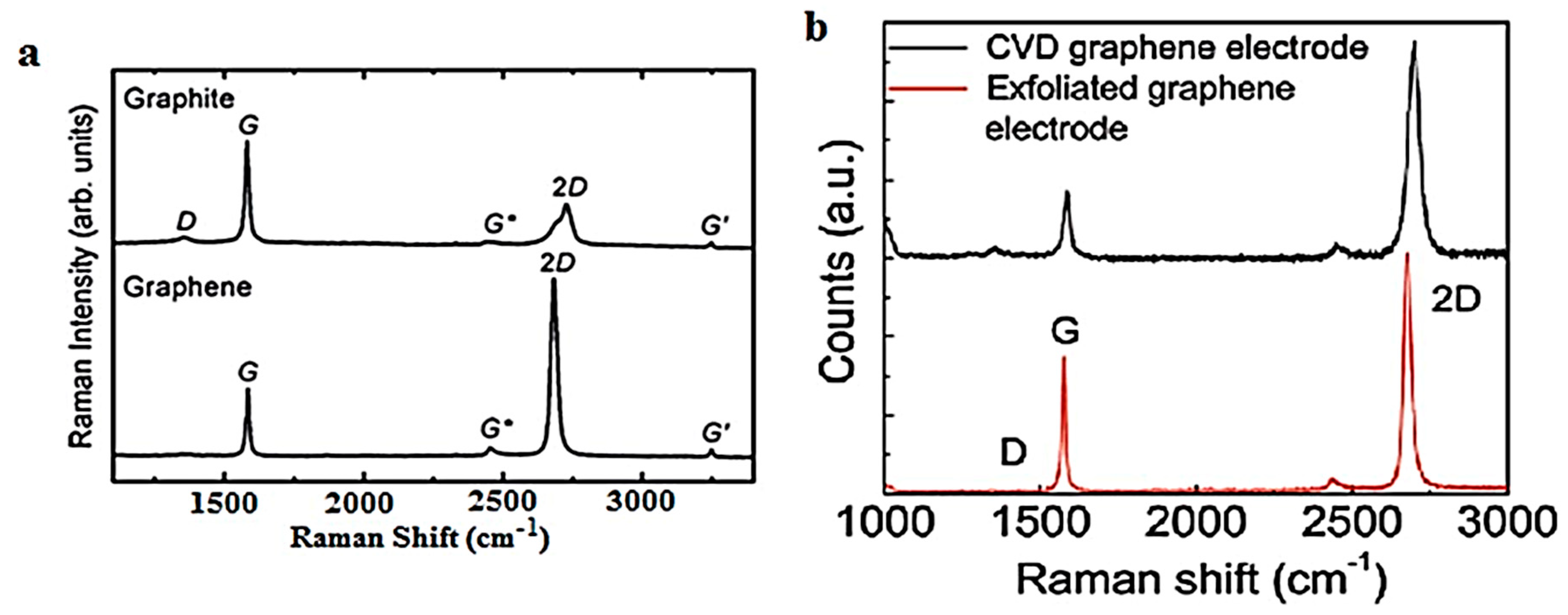
3.1. Raman Spectroscopy (RS)
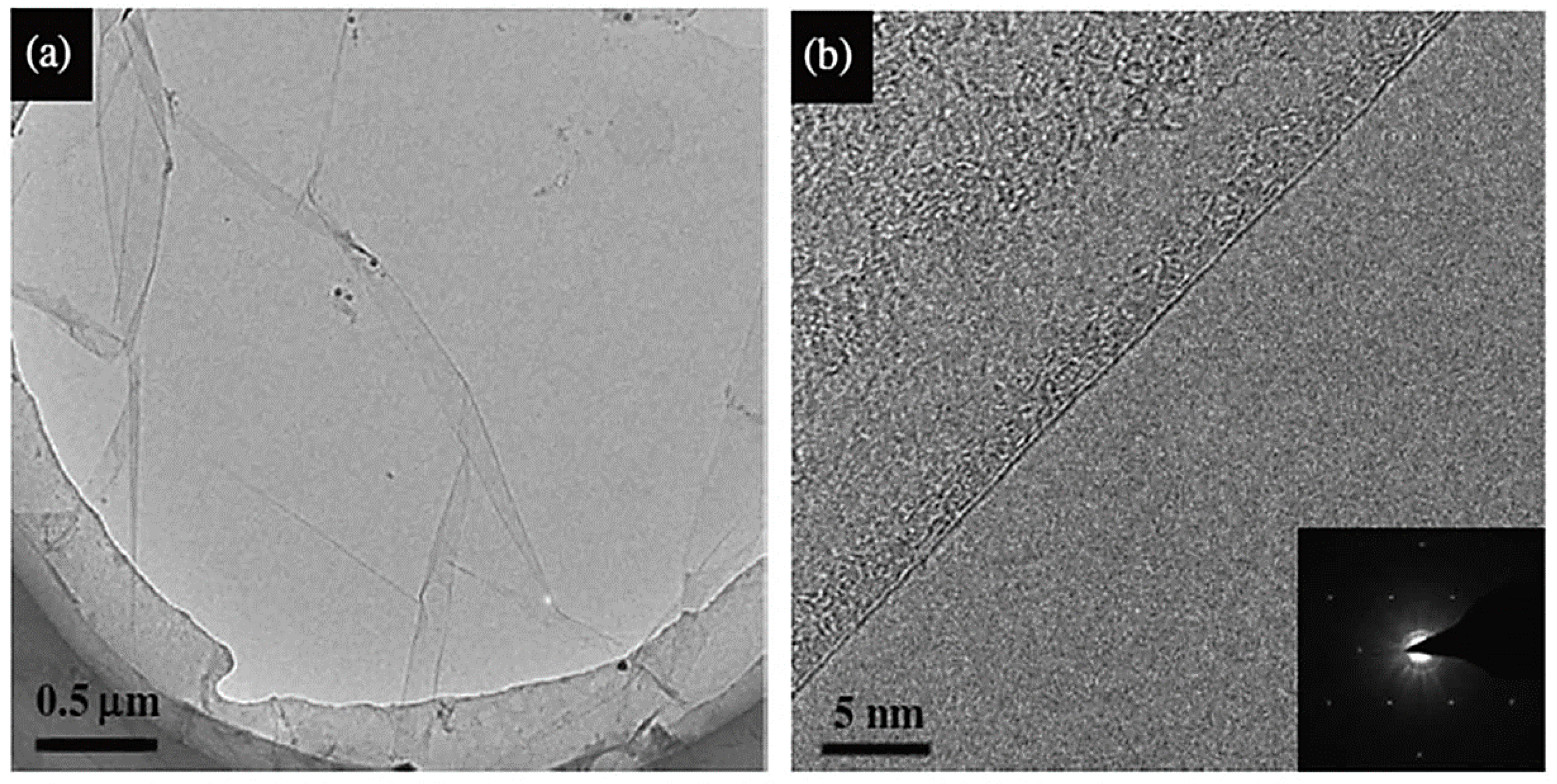
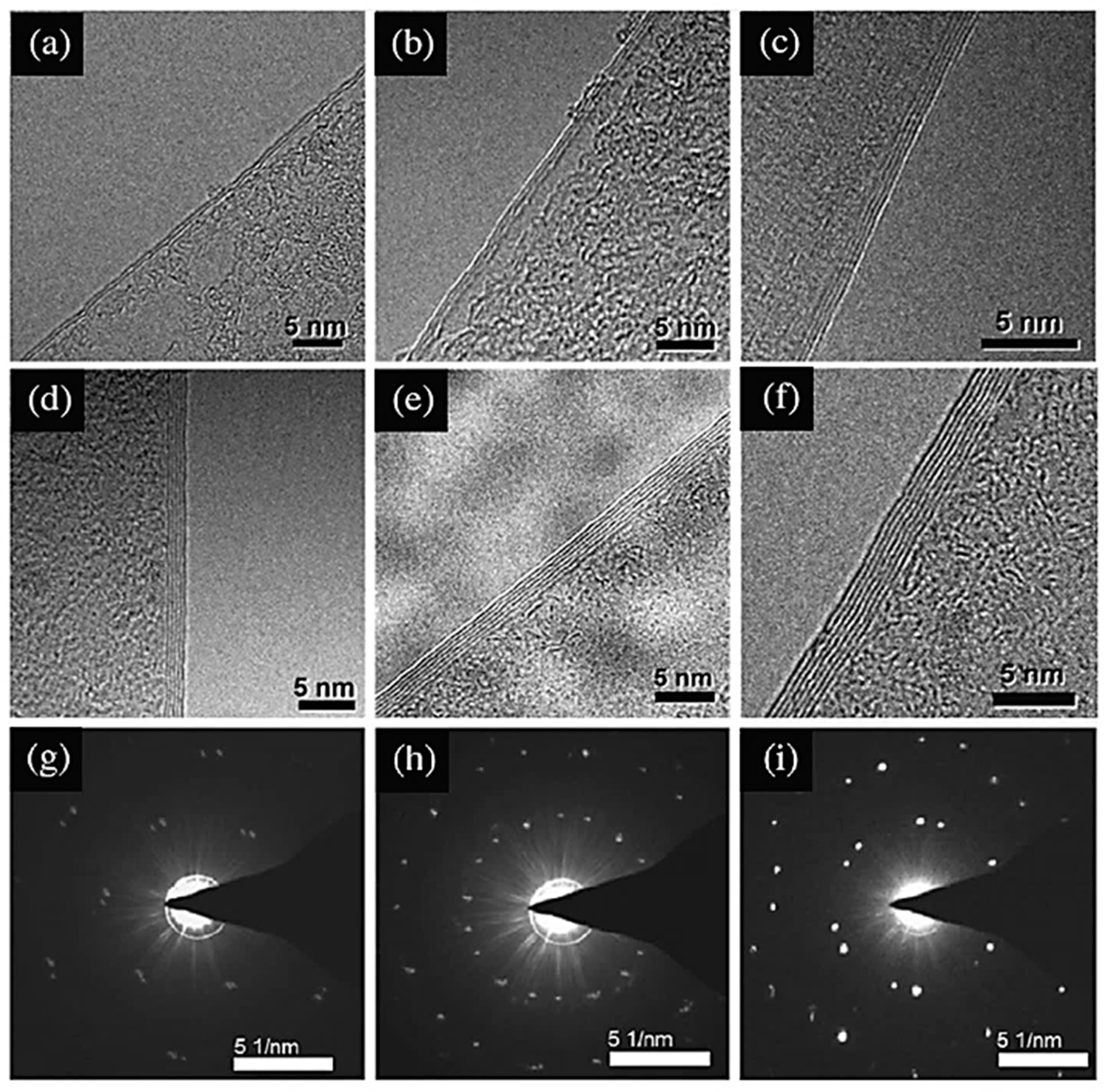
3.2. Transmission Electron Microscopy (TEM)
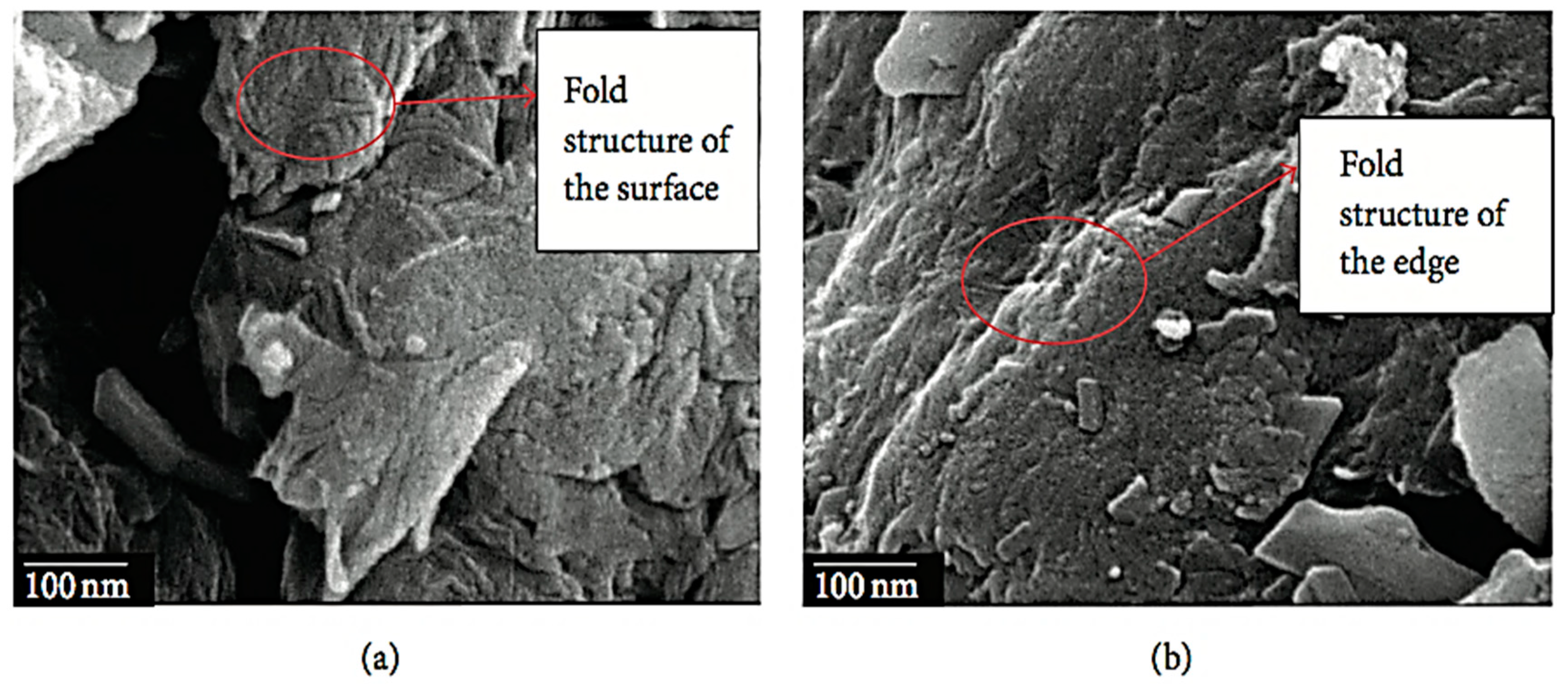
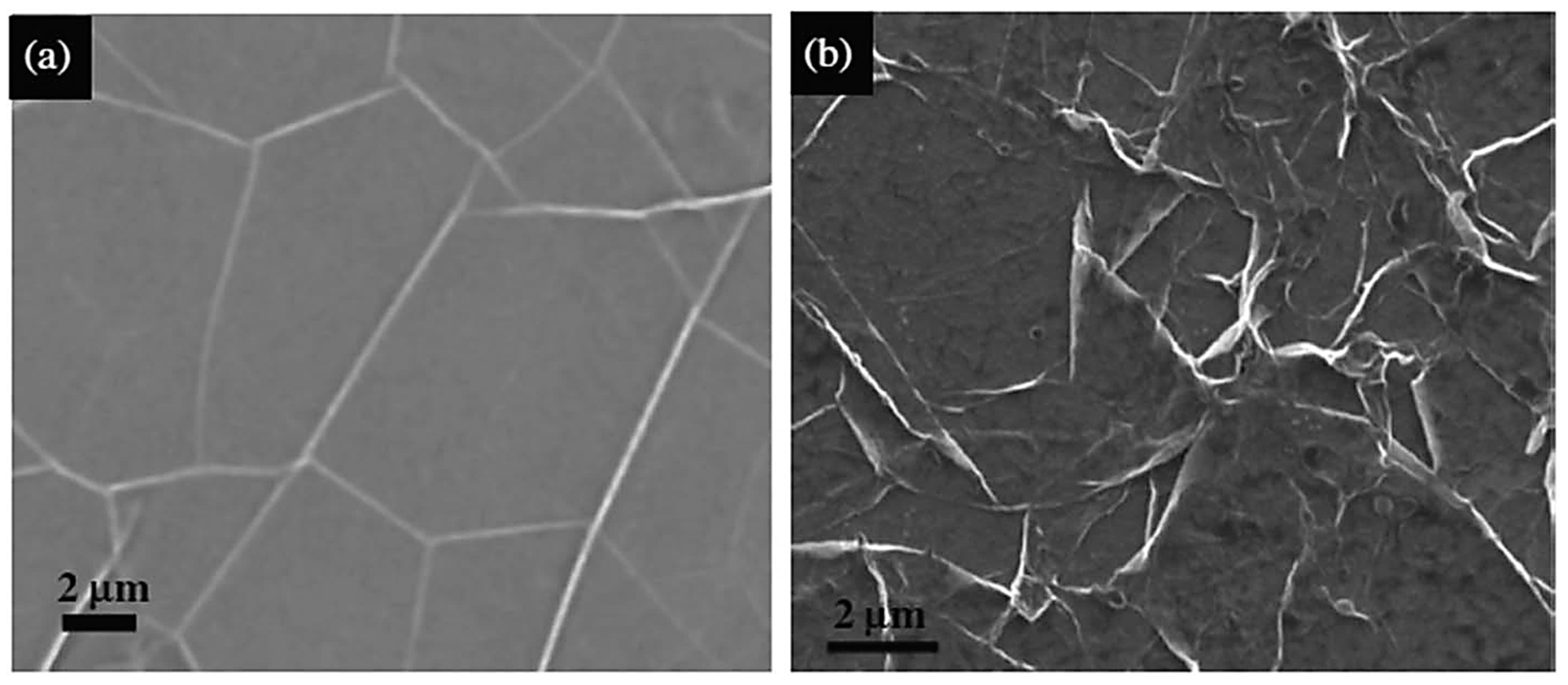
3.3. Scanning Electron Microscopy (SEM)
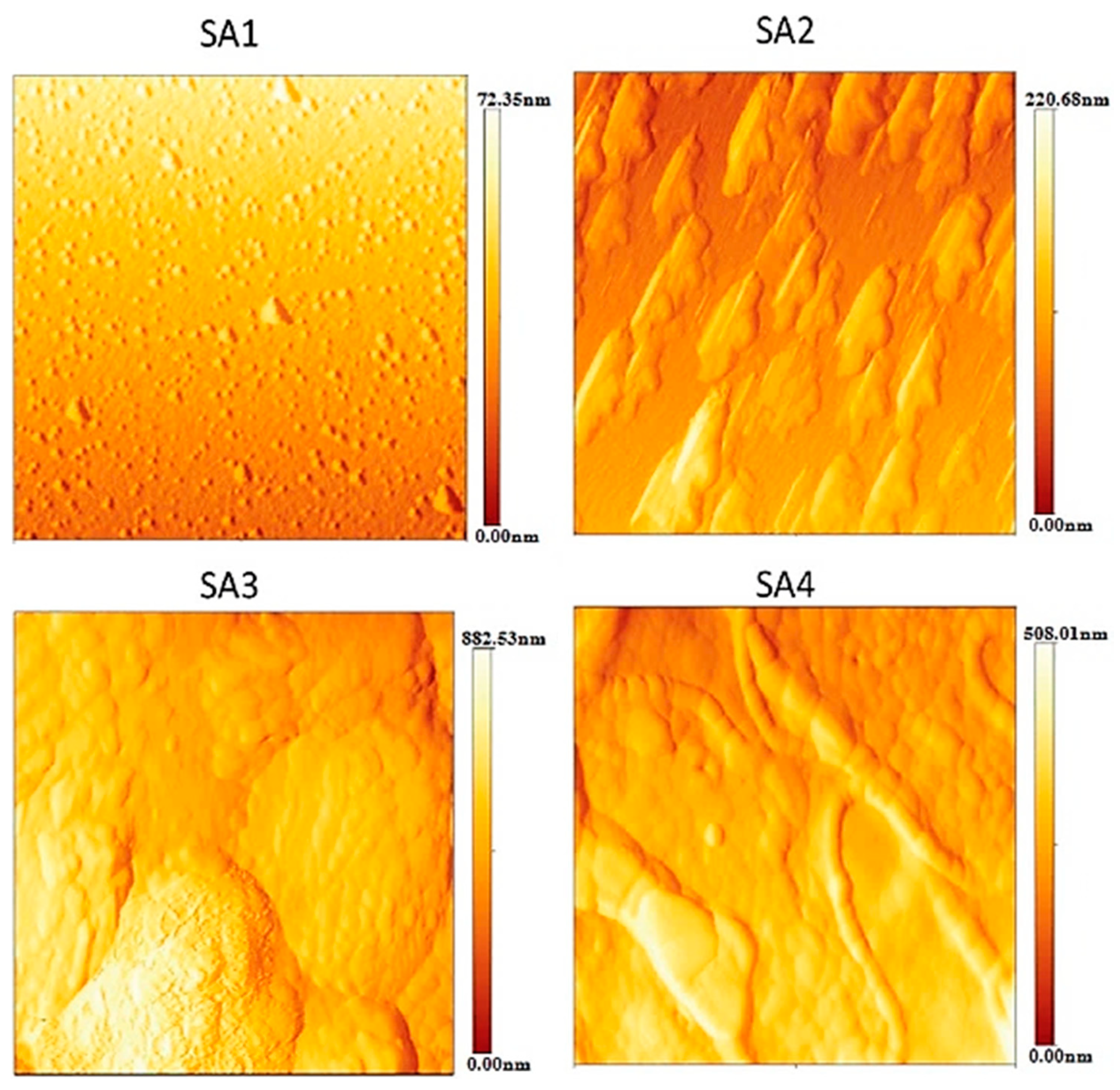
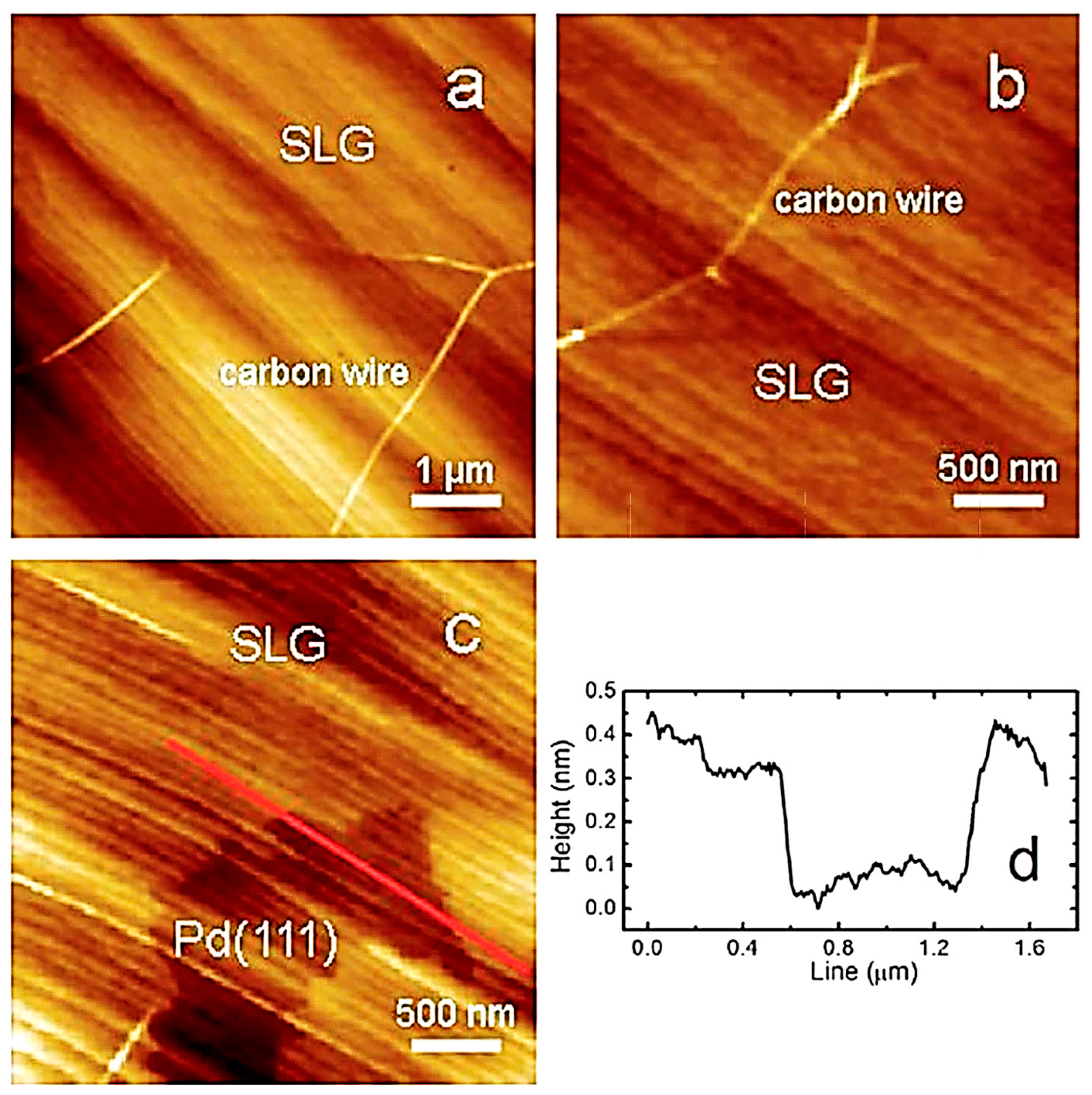
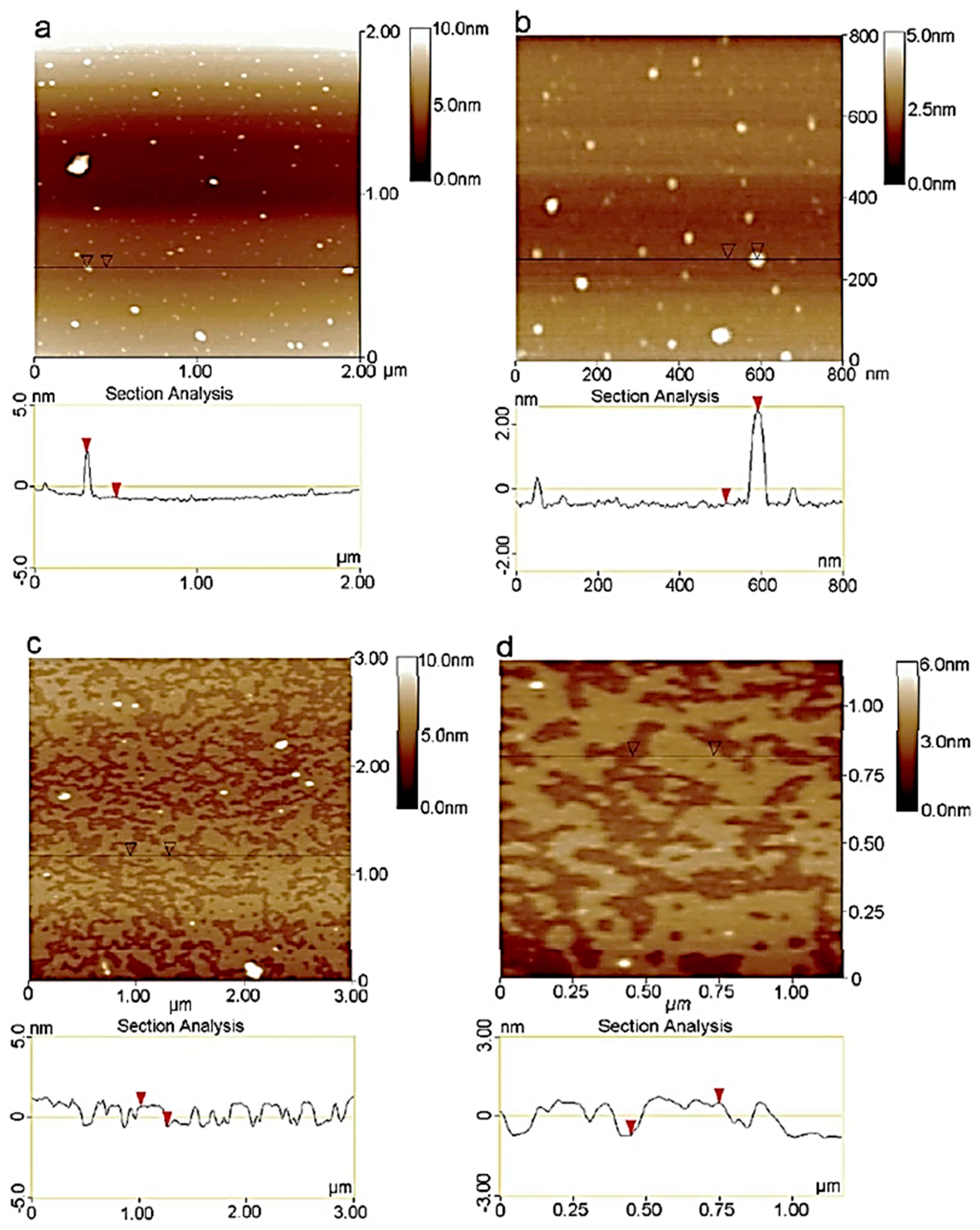
3.4. Atomic Force Microscopy (AFM)
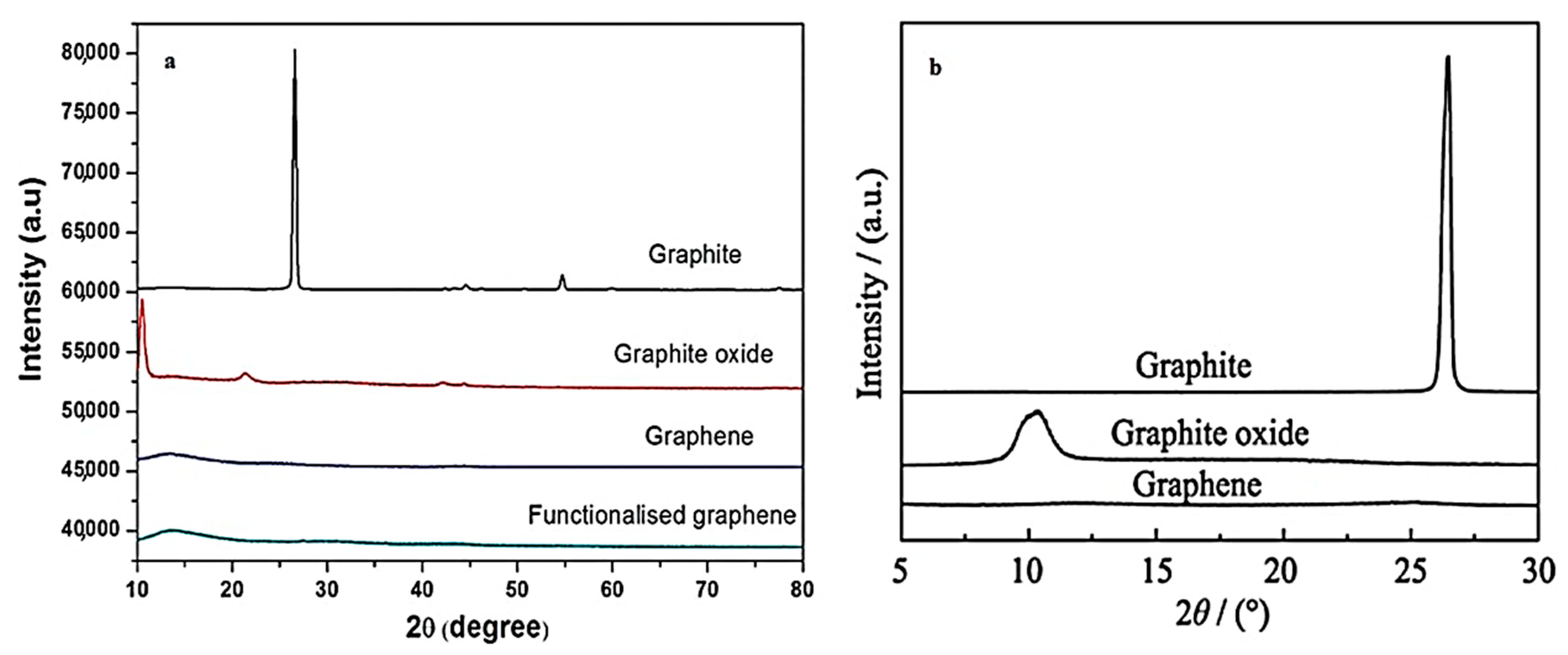
3.5. X-ray Diffraction (XRD)
4. Significance of N-Doped Gp for Energy Storage Systems
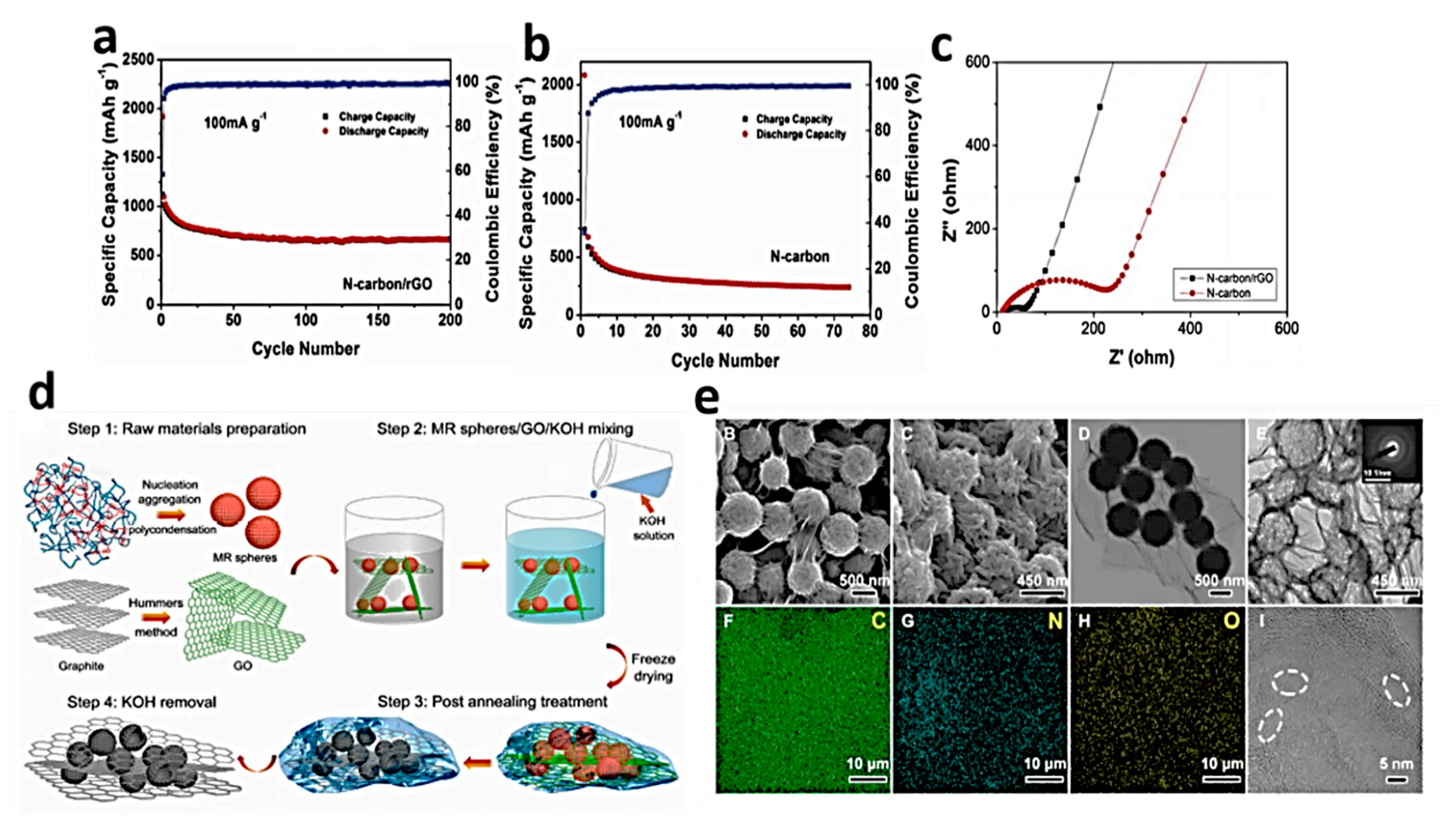
4.1. N-Doped Gp in Conventional Li-Ion Batteries
4.2. Role of Gp-Based Materials in LSBs
4.2.1. Li-Sulfur Batteries
4.2.2. Significance of Lithium-Sulfur Batteries
4.2.3. Role of N-Doped Gp in LSBs
4.3. N-Doped Gp Materials for Metal-Air Chemistries
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jakob, M.; Hilaire, J. Climate science: Unburnable fossil-fuel reserves. Nature 2015, 517, 150–152. [Google Scholar] [CrossRef]
- Nunes, L.; Causer, T.; Ciolkosz, D. Biomass for energy: A review on supply chain management models. Renew. Sustain. Energy Rev. 2020, 120, 109658. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, S.M.; Spinks, G.M.; Kim, S.J. Carbon Nanotube Yarn for Fiber-Shaped Electrical Sensors, Actuators, and Energy Storage for Smart Systems. Adv. Mater. 2020, 32, 1902670. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Mueller, T.; Lin, Y.-M.; Valdes-Garcia, A.; Avouris, P. Ultrafast graphene photodetector. Nat. Nanotechnol. 2009, 4, 839. [Google Scholar] [CrossRef]
- Gao, W.; Alemany, L.B.; Ci, L.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, T.; Zhao, G.; An, L.; Zeng, L. A high-performance carbon nanoparticle-decorated graphite felt electrode for vanadium redox flow batteries. Appl. Energy 2016, 176, 74–79. [Google Scholar] [CrossRef]
- Kim, H.; Miura, Y.; Macosko, C.W. Graphene/polyurethane nanocomposites for improved gas barrier and electrical conductivity. Chem. Mater. 2010, 22, 3441–3450. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical properties of monolayer graphene oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef]
- Mariyappan, S.; Marchandier, T.; Rabuel, F.; Iadecola, A.; Rousse, G.; Morozov, A.V.; Abakumov, A.M.; Tarascon, J.-M. The role of divalent (Zn2+/Mg2+/Cu2+) substituents in achieving full capacity of sodium layered oxides for Na-ion battery applications. Chem. Mater. 2020, 32, 1657–1666. [Google Scholar] [CrossRef]
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-liquid-based polymer electrolytes for battery applications. Angew. Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Yahia, M.B.; Vergnet, J.; Saubanère, M.; Doublet, M.-L. Unified picture of anionic redox in Li/Na-ion batteries. Nat. Mater. 2019, 18, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Pervez, S.A.; Kim, D.; Lee, S.M.; Doh, C.H.; Lee, S.; Farooq, U.; Saleem, M. Study of tin-sulphur-carbon nanocomposites based on electrically exploded tin as anode for sodium battery. J. Power Sources 2016, 315, 218–223. [Google Scholar] [CrossRef]
- Kaveevivitchai, W.; Manthiram, A. High-capacity zinc-ion storage in an open-tunnel oxide for aqueous and nonaqueous Zn-ion batteries. J. Mater. Chem. A 2016, 4, 18737–18741. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous hollow carbon@ sulfur composites for high-power lithium–sulfur batteries. Angew. Chem. Int. Ed. 2011, 50, 5904–5908. [Google Scholar] [CrossRef] [PubMed]
- Pervez, S.A.; Vinayan, B.P.; Cambaz, M.A.; Melinte, G.; Diemant, T.; Braun, T.; Karkera, G.; Behm, R.J.; Fichtner, M. Electrochemical and compositional characterization of solid interphase layers in an interface-modified solid-state Li–sulfur battery. J. Mater. Chem. A 2020, 8, 16451–16462. [Google Scholar] [CrossRef]
- Jian, Z.; Luo, W.; Ji, X. Carbon electrodes for K-ion batteries. J. Am. Chem. Soc. 2015, 137, 11566–11569. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: London, UK, 2010; pp. 11–19. [Google Scholar]
- Du, J.; Pei, S.; Ma, L.; Cheng, H.M. 25th anniversary article: Carbon nanotube-and graphene-based transparent conductive films for optoelectronic devices. Adv. Mater. 2014, 26, 1958–1991. [Google Scholar] [CrossRef]
- Jang, S.; Hwang, E.; Lee, Y.; Lee, S.; Cho, J.H. Multifunctional graphene optoelectronic devices capable of detecting and storing photonic signals. Nano Lett. 2015, 15, 2542–2547. [Google Scholar] [CrossRef]
- Polat, E.O.; Balci, O.; Kakenov, N.; Uzlu, H.B.; Kocabas, C.; Dahiya, R. Synthesis of large area graphene for high performance in flexible optoelectronic devices. Sci. Rep. 2015, 5, 16744. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, H.; Lai, H.; Li, Z.; Dong, X.; Cai, S.; Chu, X.; Gao, C. Capacitive charge storage enables an ultrahigh cathode capacity in aluminum-graphene battery. J. Energy Chem. 2020, 45, 40–44. [Google Scholar] [CrossRef]
- Simanjuntak, C.; Siburian, R.; Marpaung, H. Properties of Mg/graphite and Mg/graphene as cathode electrode on primary cell battery. Heliyon 2020, 6, e03118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Hou, Z.; Zhang, L.; Li, C. Uniform SiOx/graphene composite materials for lithium ion battery anodes. J. Alloys Compd. 2019, 809, 151798. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, J.; Liu, M.; Ming, H.; Zhang, H.; Li, M.; Zhang, S.; Zhang, T. A surface multiple effect on the ZnO anode induced by graphene for a high energy lithium-ion full battery. J. Alloys Compd. 2020, 824, 153945. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kavalsky, L.; Chattopadhyay, K.; Singh, C.V. Dramatic improvement in the performance of graphene as Li/Na battery anodes with suitable electrolytic solvents. Carbon 2020, 161, 570–576. [Google Scholar] [CrossRef]
- Yue, L.; Jayapal, M.; Cheng, X.; Zhang, T.; Chen, J.; Ma, X.; Dai, X.; Lu, H.; Guan, R.; Zhang, W. Highly dispersed ultra-small nano Sn-SnSb nanoparticles anchored on N-doped graphene sheets as high performance anode for sodium ion batteries. Appl. Surf. Sci. 2020, 512, 145686. [Google Scholar] [CrossRef]
- Dada, O.J. Higher capacity utilization and rate performance of lead acid battery electrodes using graphene additives. J. Energy Storage 2019, 23, 579–589. [Google Scholar] [CrossRef]
- Luan, Y.; Yin, J.; Zhu, K.; Cheng, K.; Yan, J.; Ye, K.; Wang, G.; Cao, D. Arc-discharge production of high-quality fluorine-modified graphene as anode for Li-ion battery. Chem. Eng. J. 2019, 392, 123668. [Google Scholar] [CrossRef]
- Na, R.; Liu, Y.; Wu, Z.-P.; Cheng, X.; Shan, Z.; Zhong, C.-J.; Tian, J. Nano-Silicon composite materials with N-doped graphene of controllable and optimal pyridinic-to-pyrrolic structural ratios for lithium ion battery. Electrochim. Acta 2019, 321, 134742. [Google Scholar] [CrossRef]
- Lang, B. A LEED study of the deposition of carbon on platinum crystal surfaces. Surf. Sci. 1975, 53, 317–329. [Google Scholar] [CrossRef]
- Ajayan, P.; Nugent, J.; Siegel, R.; Wei, B.; Kohler-Redlich, P. Growth of carbon micro-trees. Nature 2000, 404, 243. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.; Dubonos, S.; Zhang, Y.; Jiang, D. Room-temperature electric field effect and carrier-type inversion in graphene films. arXiv 2004, arXiv:cond-mat/0410631. [Google Scholar]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Huang, Y.; Ma, Y.; Wan, X.; Chen, Y. Controlled synthesis of few-layered graphene sheets on a large scale using chemical exfoliation. Carbon 2010, 48, 2367–2371. [Google Scholar] [CrossRef]
- Park, J.-U.; Nam, S.; Lee, M.-S.; Lieber, C.M. Synthesis of monolithic graphene–graphite integrated electronics. Nat. Mater. 2012, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Sharif, F.; Zeraati, A.S.; Ganjeh-Anzabi, P.; Yasri, N.; Perez-Page, M.; Holmes, S.M.; Sundararaj, U.; Trifkovic, M.; Roberts, E.P.L. Synthesis of a high-temperature stable electrochemically exfoliated graphene. Carbon 2020, 157, 681–692. [Google Scholar] [CrossRef]
- Obraztsov, A.N. Chemical vapour deposition: Making graphene on a large scale. Nat. Nanotechnol. 2009, 4, 212. [Google Scholar] [CrossRef]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef]
- Wu, W.; Liu, M.; Gu, Y.; Guo, B.; Ma, H.; Wang, P.; Wang, X.; Zhang, R. Fast chemical exfoliation of graphite to few-layer graphene with high quality and large size via a two-step microwave-assisted process. Chem. Eng. J. 2020, 381, 122592. [Google Scholar] [CrossRef]
- Tsyganov, D.; Bundaleska, N.; Dias, A.; Henriques, J.; Felizardo, E.; Abrashev, M.; Kissovski, J.; do Rego, A.B.; Ferraria, A.; Tatarova, E. Microwave plasma-based direct synthesis of free-standing N-graphene. Phys. Chem. Chem. Phys. 2020, 22, 4772–4787. [Google Scholar] [CrossRef] [PubMed]
- Lockett, M.; Sarmiento, V.; Balingit, M.; Oropeza-Guzmán, M.T.; Vázquez-Mena, O. Direct chemical conversion of continuous CVD graphene/graphite films to graphene oxide without exfoliation. Carbon 2020, 158, 202–209. [Google Scholar] [CrossRef]
- Lee, S.; Park, W.K.; Yoon, Y.; Baek, B.; Yoo, J.S.; Kwon, S.B.; Kim, D.H.; Hong, Y.J.; Kang, B.K.; Yoon, D.H. Quality improvement of fast-synthesized graphene films by rapid thermal chemical vapor deposition for mass production. Mater. Sci. Eng. B 2019, 242, 63–68. [Google Scholar] [CrossRef]
- Li, M.; Liu, D.; Wei, D.; Song, X.; Wei, D.; Wee, A.T.S. Controllable synthesis of graphene by plasma-enhanced chemical vapor deposition and its related applications. Adv. Sci. 2016, 3, 1600003. [Google Scholar] [CrossRef] [PubMed]
- Shiraz, H.G.; Tavakoli, O. Investigation of graphene-based systems for hydrogen storage. Renew. Sustain. Energy Rev. 2017, 74, 104–109. [Google Scholar] [CrossRef]
- Petnikota, S.; Rotte, N.K.; Srikanth, V.V.; Kota, B.S.; Reddy, M.; Loh, K.P.; Chowdari, B. Electrochemical studies of few-layered graphene as an anode material for Li ion batteries. J. Solid State Electrochem. 2014, 18, 941–949. [Google Scholar] [CrossRef]
- Petnikota, S.; Rotte, N.K.; Reddy, M.V.; Srikanth, V.V.S.S.; Chowdari, B.V.R. MgO-Decorated Few-Layered Graphene as an Anode for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 2301–2309. [Google Scholar] [CrossRef]
- Goh, B.-M.; Wang, Y.; Reddy, M.; Ding, Y.L.; Lu, L.; Bunker, C.; Loh, K.P. Filling the voids of graphene foam with graphene “eggshell” for improved lithium-ion storage. ACS Appl. Mater. Interfaces 2014, 6, 9835–9841. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Huang, H.; Xuan, J.; Wang, H.; Leung, D. Graphene materials in green energy applications: Recent development and future perspective. Renew. Sustain. Energy Rev. 2020, 120, 109656. [Google Scholar] [CrossRef]
- Kruskopf, M.; Pakdehi, D.M.; Pierz, K.; Wundrack, S.; Stosch, R.; Dziomba, T.; Götz, M.; Baringhaus, J.; Aprojanz, J.; Tegenkamp, C. Comeback of epitaxial graphene for electronics: Large-area growth of bilayer-free graphene on SiC. 2D Materials 2016, 3, 041002. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Q.; Wang, S.; Liu, X.; Li, L. Poly(vinyl alcohol)-Assisted Fabrication of Hollow Carbon Spheres/Reduced Graphene Oxide Nanocomposites for High-Performance Lithium-Ion Battery Anodes. ACS Nano 2018, 12, 4824–4834. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.S.; Kim, M.J.; Yeom, K.S.; An, S.S.A.; Ju, H.; Yi, D.K. Raman spectrum of graphene with its versatile future perspectives. TrAC Trends Anal. Chem. 2016, 80, 125–131. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Zabel, J.; Nair, R.R.; Ott, A.; Georgiou, T.; Geim, A.K.; Novoselov, K.S.; Casiraghi, C. Raman spectroscopy of graphene and bilayer under biaxial strain: Bubbles and balloons. Nano Lett. 2012, 12, 617–621. [Google Scholar] [CrossRef]
- Han, C.; Zhang, H.; Zhang, D.; Deng, Y.; Shen, J.; Zeng, G. Ultrafine FeNi3 Nanocrystals Embedded in 3D Honeycomb-Like Carbon Matrix for High-Performance Microwave Absorption. Nanomaterials 2020, 10, 598. [Google Scholar] [CrossRef]
- Chen, J.; Dai, Y.; Ma, Y.; Dai, X.; Ho, W.; Xie, M. Ultrathin β-tellurium layers grown on highly oriented pyrolytic graphite by molecular-beam epitaxy. Nanoscale 2017, 9, 15945–15948. [Google Scholar] [CrossRef]
- Casimir, D.; Alghamdi, H.; Ahmed, I.Y.; Garcia-Sanchez, R.; Misra, P. Raman Spectroscopy of Graphene, Graphite and Graphene Nanoplatelets. In 2D Materials; IntechOpen: London, UK, 2019. [Google Scholar]
- Ye, X.; Zhou, Q.; Jia, C.; Tang, Z.; Zhu, Y.; Wan, Z. Producing large-area, foldable graphene paper from graphite oxide suspensions by in-situ chemical reduction process. Carbon 2017, 114, 424–434. [Google Scholar] [CrossRef]
- Lee, H.C.; Liu, W.-W.; Chai, S.-P.; Mohamed, A.R.; Aziz, A.; Khe, C.-S.; Hidayah, N.M.; Hashim, U. Review of the synthesis, transfer, characterization and growth mechanisms of single and multilayer graphene. RSC Adv. 2017, 7, 15644–15693. [Google Scholar] [CrossRef]
- Yoon, D.; Cheong, H. Raman spectroscopy for characterization of graphene. In Raman Spectroscopy for Nanomaterials Characterization; Springer: Berlin/Heidelberg, Germany, 2012; pp. 191–214. [Google Scholar]
- Luo, L.; Wu, J.; Luo, J.; Huang, J.; Dravid, V.P. Dynamics of electrochemical lithiation/delithiation of graphene-encapsulated silicon nanoparticles studied by in-situ TEM. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Singh, D.P. Natural and waste hydrocarbon precursors for the synthesis of carbon based nanomaterials: Graphene and CNTs. Renew. Sustain. Energy Rev. 2016, 58, 976–1006. [Google Scholar] [CrossRef]
- Lo, K.S.K.; Leung, W.W.F. Dye-sensitized solar cells with shear-exfoliated graphene. Sol. Energy 2019, 180, 16–24. [Google Scholar] [CrossRef]
- Cao, N.; Zhang, Y. Study of reduced graphene oxide preparation by Hummers’ method and related characterization. J. Nanomater. 2015, 2015, 168125. [Google Scholar] [CrossRef]
- Van Khai, T.; Kwak, D.S.; Kwon, Y.J.; Cho, H.Y.; Huan, T.N.; Chung, H.; Ham, H.; Lee, C.; Van Dan, N.; Tung, N.T. Direct production of highly conductive graphene with a low oxygen content by a microwave-assisted solvothermal method. Chem. Eng. J. 2013, 232, 346–355. [Google Scholar] [CrossRef]
- Gui, Y.; Yuan, J.; Wang, W.; Zhao, J.; Tian, J.; Xie, B. Facile solvothermal synthesis and gas sensitivity of graphene/WO3 nanocomposites. Materials 2014, 7, 4587–4600. [Google Scholar] [CrossRef]
- Meng, L.; Li, Y.; Liu, T.S.; Zhu, C.; Li, Q.Y.; Chen, X.; Zhang, S.; Zhang, X.; Bao, L.; Huang, Y.; et al. Wrinkle networks in exfoliated multilayer graphene and other layered materials. Carbon 2020, 156, 24–30. [Google Scholar] [CrossRef]
- Hawaldar, R.; Merino, P.; Correia, M.R.; Bdikin, I.; Grácio, J.; Méndez, J.; Martín-Gago, J.A.; Singh, M.K. Large-area high-throughput synthesis of monolayer graphene sheet by Hot Filament Thermal Chemical Vapor Deposition. Sci. Rep. 2012, 2, 682. [Google Scholar] [CrossRef]
- Dang, C.; Che, Q.L.; Gao, B.L.; Li, L.; Wang, B.B. Growth, photoluminescence and thermal conductance of graphene-like nanoflakes grown on copper foils in methane environment. Mater. Sci. Semicond. Process. 2014, 27, 97–102. [Google Scholar] [CrossRef]
- Tu, Z.; Liu, Z.; Li, Y.; Yang, F.; Zhang, L.; Zhao, Z.; Xu, C.; Wu, S.; Liu, H.; Yang, H.; et al. Controllable growth of 1–7 layers of graphene by chemical vapour deposition. Carbon 2014, 73, 252–258. [Google Scholar] [CrossRef]
- Martin-Olmos, C.; Rasool, H.I.; Weiller, B.H.; Gimzewski, J.K. Graphene MEMS: AFM Probe Performance Improvement. ACS Nano 2013, 7, 4164–4170. [Google Scholar] [CrossRef] [PubMed]
- Lui, C.H.; Liu, L.; Mak, K.F.; Flynn, G.W.; Heinz, T.F. Ultraflat graphene. Nature 2009, 462, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-H.; Ishida, N.; Scott, I.; Fujita, D. Controllable growth of single-layer graphene on a Pd(111) substrate. Carbon 2012, 50, 1674–1680. [Google Scholar] [CrossRef]
- Liu, C.; Hu, G.; Gao, H. Preparation of few-layer and single-layer graphene by exfoliation of expandable graphite in supercritical N,N-dimethylformamide. J. Supercrit. Fluids 2012, 63, 99–104. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Indra Mahlia, T.M.; Cornelis Metselaar, H.S. Preparation and properties of highly conductive palmitic acid/graphene oxide composites as thermal energy storage materials. Energy 2013, 58, 628–634. [Google Scholar] [CrossRef]
- Pool, V.L.; Dou, B.; Van Campen, D.G.; Klein-Stockert, T.R.; Barnes, F.S.; Shaheen, S.E.; Ahmad, M.I.; van Hest, M.F.A.M.; Toney, M.F. Thermal engineering of FAPbI3 perovskite material via radiative thermal annealing and in situ XRD. Nat. Commun. 2017, 8, 14075. [Google Scholar] [CrossRef] [PubMed]
- Naebe, M.; Wang, J.; Amini, A.; Khayyam, H.; Hameed, N.; Li, L.H.; Chen, Y.; Fox, B. Mechanical Property and Structure of Covalent Functionalised Graphene/Epoxy Nanocomposites. Sci. Rep. 2014, 4, 4375. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Z.; Zhang, Y.; Wang, B.; Peng, H. The recent progress of nitrogen-doped carbon nanomaterials for electrochemical batteries. J. Mater. Chem. A 2018, 6, 12932–12944. [Google Scholar] [CrossRef]
- Rana, M.; Luo, B.; Kaiser, M.R.; Gentle, I.; Knibbe, R. The role of functional materials to produce high areal capacity lithium sulfur battery. J. Energy Chem. 2019, 42, 195–209. [Google Scholar] [CrossRef]
- Sui, Z.-Y.; Wang, C.; Yang, Q.-S.; Shu, K.; Liu, Y.-W.; Han, B.-H.; Wallace, G.G. A highly nitrogen-doped porous graphene—An anode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 18229–18237. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Bruce, P.G. Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries. Nature 1996, 381, 499–500. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis of nitrogen-doped graphene films for lithium battery application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Guo, S.; Pinna, N. Graphene/N-doped carbon sandwiched nanosheets with ultrahigh nitrogen doping for boosting lithium-ion batteries. J. Mater. Chem. A 2016, 4, 1423–1431. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Okotrub, A.V.; Kurenya, A.G.; Zhang, H.; Zhang, H.; Chen, X.; Song, H. Electrochemical properties of nitrogen-doped carbon nanotube anode in Li-ion batteries. Carbon 2011, 49, 4013–4023. [Google Scholar] [CrossRef]
- Zhou, X.; Wan, L.-J.; Guo, Y.-G. Binding SnO2 Nanocrystals in Nitrogen-Doped Graphene Sheets as Anode Materials for Lithium-Ion Batteries. Adv. Mater. 2013, 25, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.N.; Subramaniyam, C.M.; Haque, E.; Islam, M.M.; Noorbehesht, N.; Roy, A.K.; Islam, M.S.; Liu, H.K.; Dou, S.X.; Harris, A.T.; et al. Nanoarchitectured Nitrogen-Doped Graphene/Carbon Nanotube as High Performance Electrodes for Solid State Supercapacitors, Capacitive Deionization, Li-Ion Battery, and Metal-Free Bifunctional Electrocatalysis. ACS Appl. Energy Mater. 2018, 1, 5211–5223. [Google Scholar] [CrossRef]
- Puttapati, S.K.; Gedela, V.; Srikanth, V.V.; Reddy, M.; Adams, S.; Chowdari, B. Unique reduced graphene oxide as efficient anode material in Li ion battery. Bull. Mater. Sci. 2018, 41, 53. [Google Scholar] [CrossRef]
- Fang, R.; Chen, K.; Yin, L.; Sun, Z.; Li, F.; Cheng, H.M. The regulating role of carbon nanotubes and graphene in lithium-ion and lithium–sulfur batteries. Adv. Mater. 2019, 31, 1800863. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Qu, L.; Liu, P.; Yi, Y.; Wang, T.; Yang, P.; Tian, X.; Li, M.; Yang, B.; Dai, S. Enhanced Cycling Performance for Lithium–Sulfur Batteries by a Laminated 2D g-C3N4/Graphene Cathode Interlayer. ChemSusChem 2019, 12, 213–223. [Google Scholar] [CrossRef]
- Jin, J.; Cai, W.; Cai, J.; Shao, Y.; Song, Y.; Xia, Z.; Zhang, Q.; Sun, J. MOF-derived hierarchical CoP nanoflakes anchored on vertically erected graphene scaffolds as self-supported and flexible hosts for lithium–sulfur batteries. J. Mater. Chem. A 2020, 8, 3027–3034. [Google Scholar] [CrossRef]
- Chiochan, P.; Kosasang, S.; Ma, N.; Duangdangchote, S.; Suktha, P.; Sawangphruk, M. Confining Li2S6 catholyte in 3D graphene sponge with ultrahigh total pore volume and oxygen-containing groups for lithium-sulfur batteries. Carbon 2020, 158, 244–255. [Google Scholar] [CrossRef]
- Lu, L.; Pei, F.; Abeln, T.; Pei, Y. Tailoring three-dimensional interconnected nanoporous graphene micro/nano-foams for lithium-sulfur batteries. Carbon 2020, 157, 437–447. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, J.; Deng, Y.; Zhou, G.; Wang, R.; He, Y.-B.; Lv, W.; Yang, Q.-H. Graphene-Templated Growth of WS2 Nanoclusters for Catalytic Conversion of Polysulfides in Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2020, 3, 4923–4930. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Z.; Tian, W.; Chen, W.; Xi, B.; Li, H.; Feng, J.; Xiong, S. Ni12P5 nanoparticles bound on graphene sheets for advanced lithium–sulfur batteries. Nanoscale 2020, 12, 10760–10770. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W.; Lei, T.; Jiao, Y.; Wang, H.; Wang, X.; Rao, G.; Wang, X.; Chen, B.; Xiong, J. Graphene quantum dots as the nucleation sites and interfacial regulator to suppress lithium dendrites for high-loading lithium-sulfur battery. Nano Energy 2020, 68, 104373. [Google Scholar] [CrossRef]
- Wang, B.; Jin, F.; Xie, Y.; Luo, H.; Wang, F.; Ruan, T.; Wang, D.; Zhou, Y.; Dou, S. Holey graphene modified LiFePO4 hollow microsphere as an efficient binary sulfur host for high-performance lithium-sulfur batteries. Energy Storage Mater. 2020, 26, 433–442. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.-W.; Cheng, H.-M.; Li, F. More Reliable Lithium-Sulfur Batteries: Status, Solutions and Prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Wang, C. Sulfur-Impregnated Disordered Carbon Nanotubes Cathode for Lithium–Sulfur Batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef]
- Song, J.; Gordin, M.L.; Xu, T.; Chen, S.; Yu, Z.; Sohn, H.; Lu, J.; Ren, Y.; Duan, Y.; Wang, D. Strong Lithium Polysulfide Chemisorption on Electroactive Sites of Nitrogen-Doped Carbon Composites For High-Performance Lithium–Sulfur Battery Cathodes. Angew. Chem. Int. Ed. 2015, 54, 4325–4329. [Google Scholar] [CrossRef]
- Wen, X.; Xiang, K.; Zhu, Y.; Xiao, L.; Liao, H.; Chen, W.; Chen, X.; Chen, H. 3D hierarchical nitrogen-doped graphene/CNTs microspheres as a sulfur host for high-performance lithium-sulfur batteries. J. Alloys Compd. 2020, 815, 152350. [Google Scholar] [CrossRef]
- Li, H.; Liu, D.; Zhu, X.; Qu, D.; Xie, Z.; Li, J.; Tang, H.; Zheng, D.; Qu, D. Integrated 3D electrodes based on metal-nitrogen-doped graphitic ordered mesoporous carbon and carbon paper for high-loading lithium-sulfur batteries. Nano Energy 2020, 73, 104763. [Google Scholar] [CrossRef]
- Cheng, D.; Wu, P.; Wang, J.; Tang, X.; An, T.; Zhou, H.; Zhang, D.; Fan, T. Synergetic pore structure optimization and nitrogen doping of 3D porous graphene for high performance lithium sulfur battery. Carbon 2019, 143, 869–877. [Google Scholar] [CrossRef]
- Du, Z.; Chen, X.; Hu, W.; Chuang, C.; Xie, S.; Hu, A.; Yan, W.; Kong, X.; Wu, X.; Ji, H. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium–sulfur batteries. J. Am. Chem. Soc. 2019, 141, 3977–3985. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Su, K.; Wan, W.; Guo, H.; Zhou, H.; Chen, J.; Zhang, X.; Huang, Y. High sulfur loading composite wrapped by 3D nitrogen-doped graphene as a cathode material for lithium–sulfur batteries. J. Mater. Chem. A 2014, 2, 5018–5023. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Q.; Zhao, M.-Q.; Huang, J.-Q.; Cheng, X.-B.; Tian, G.-L.; Peng, H.-J.; Wei, F. Nitrogen-Doped Aligned Carbon Nanotube/Graphene Sandwiches: Facile Catalytic Growth on Bifunctional Natural Catalysts and Their Applications as Scaffolds for High-Rate Lithium-Sulfur Batteries. Adv. Mater. 2014, 26, 6100–6105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, D.; Muhammad, Z.; Wan, F.; Xie, W.; Wang, Y.; Song, L.; Niu, Z.; Chen, J. Single Nickel Atoms on Nitrogen-Doped Graphene Enabling Enhanced Kinetics of Lithium–Sulfur Batteries. Adv. Mater. 2019, 31, 1903955. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Zhen, M.; Wang, C. Ni@N-doped graphene nanosheets and CNTs hybrids modified separator as efficient polysulfide barrier for high-performance lithium sulfur batteries. Nano Res. 2019, 12, 829–836. [Google Scholar] [CrossRef]
- Li, F.; Liu, Q.; Hu, J.; Feng, Y.; He, P.; Ma, J. Recent advances in cathode materials for rechargeable lithium–sulfur batteries. Nanoscale 2019, 11, 15418–15439. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable lithium–sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Su, D.; Zhou, D.; Wang, C.; Wang, G. Lithium-Sulfur Batteries: Toward High Performance Lithium–Sulfur Batteries Based on Li2S Cathodes and Beyond: Status, Challenges, and Perspectives (Adv. Funct. Mater. 38/2018). Adv. Funct. Mater. 2018, 28, 1870273. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief History of Early Lithium-Battery Development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Subba Rao, G.; Chowdari, B. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Julien, C.M.; Mauger, A.; Zaghib, K. Sulfide and oxide inorganic solid electrolytes for all-solid-state Li batteries: A Review. Nanomaterials 2020, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Wang, C.; Pei, F.; Cui, J.; Fang, X.; Zheng, N. Recent advances in hollow porous carbon materials for lithium–sulfur batteries. Small 2019, 15, 1804786. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Niu, Z.; Chen, J. Advanced nanostructured carbon-based materials for rechargeable lithium-sulfur batteries. Carbon 2019, 141, 400–416. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, P.; Yan, C.; Dong, X.; Zhang, X. Recent progress in polymer materials for advanced lithium-sulfur batteries. Prog. Polym. Sci. 2019, 90, 118–163. [Google Scholar] [CrossRef]
- Ren, W.; Ma, W.; Zhang, S.; Tang, B. Recent advances in shuttle effect inhibition for lithium sulfur batteries. Energy Storage Mater. 2019, 23, 707–732. [Google Scholar] [CrossRef]
- Wang, C.; Yi, Y.; Li, H.; Wu, P.; Li, M.; Jiang, W.; Chen, Z.; Li, H.; Zhu, W.; Dai, S. Rapid gas-assisted exfoliation promises V2O5 nanosheets for high performance lithium-sulfur batteries. Nano Energy 2020, 67, 104253. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, D.; Su, C.; Tang, W.; Ju, H.; Zhang, J.; Tian, B. Hermetically encapsulating sulfur by FePS3 flakes for high-performance lithium sulfur batteries. Chem. Commun. 2020, 56, 810–813. [Google Scholar] [CrossRef]
- Qi, W.; Jiang, W.; Xu, F.; Jia, J.; Yang, C.; Cao, B. Improving confinement and redox kinetics of polysufides through hollow NC@ CeO2 nanospheres for high-performance lithium-sulfur batteries. Chem. Eng. J. 2020, 382, 122852. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Zhong, W.; Zheng, F.; Hu, J.; Ji, X.; Liu, W.; Yang, C.; Lin, Z.; Liu, M. MOFs-derived porous Mo2C–C nano-octahedrons enable high-performance lithium–sulfur batteries. Energy Storage Mater. 2020, 25, 547–554. [Google Scholar] [CrossRef]
- Hao, Q.; Cui, G.; Zhang, Y.; Li, J.; Zhang, Z. Novel MoSe2/MoO2 heterostructure as an effective sulfur host for high-performance lithium/sulfur batteries. Chem. Eng. J. 2020, 381, 122672. [Google Scholar] [CrossRef]
- Chen, X.; Hou, T.; Persson, K.A.; Zhang, Q. Combining theory and experiment in lithium–sulfur batteries: Current progress and future perspectives. Mater. Today 2019, 22, 142–158. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, J.; Cheng, M.; Wu, J.; Zhang, Q.; Liu, X.; Wang, C.; Wang, J.; Li, K.; Wang, J. N-doped porous carbon-graphene cables synthesized for self-standing cathode and anode hosts of Li–S batteries. Electrochim. Acta 2020, 349, 136231. [Google Scholar] [CrossRef]
- Wang, D.; Liu, H.; Shan, Z.; Xia, D.; Na, R.; Liu, H.; Chen, Z.; Wang, B.; Tian, J. Nitrogen, Sulfur Co-doped Porous Graphene Boosts Li4Ti5O12 Anode Performance for High-Rate and Long-Life Lithium Ion Batteries. Energy Storage Mater. 2020, 27, 387–395. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Liu, Z.; Wang, L.; Han, P.; Xu, H.; Zhang, K.; Dong, S.; Yao, J.; Cui, G. Nitrogen-doped graphene nanosheets with excellent lithium storage properties. J. Mater. Chem. 2011, 21, 5430–5434. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.M.; Wang, Z.H.; Shao, Q.G.; Li, X.; Yuan, L.X.; Hu, X.L.; Zhang, W.X.; Huang, Y.H. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Tan, X.; Wang, H.; Holt, C.M.; Stephenson, T.; Olsen, B.C.; Mitlin, D. Mesoporous nitrogen-rich carbons derived from protein for ultra-high capacity battery anodes and supercapacitors. Energy Environ. Sci. 2013, 6, 871–878. [Google Scholar] [CrossRef]
- Ou, J.; Zhang, Y.; Chen, L.; Zhao, Q.; Meng, Y.; Guo, Y.; Xiao, D. Nitrogen-rich porous carbon derived from biomass as a high performance anode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 6534–6541. [Google Scholar] [CrossRef]
- Hou, J.; Cao, C.; Idrees, F.; Ma, X. Hierarchical porous nitrogen-doped carbon nanosheets derived from silk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Dong, Y.; Wang, Z.; Zhao, Z.; Qiu, J. Nitrogen-doped graphene nanoribbons for high-performance lithium ion batteries. J. Mater. Chem. A 2014, 2, 16832–16835. [Google Scholar] [CrossRef]
- Tian, L.-L.; Wei, X.-Y.; Zhuang, Q.-C.; Jiang, C.-H.; Wu, C.; Ma, G.-Y.; Zhao, X.; Zong, Z.-M.; Sun, S.-G. Bottom-up synthesis of nitrogen-doped graphene sheets for ultrafast lithium storage. Nanoscale 2014, 6, 6075–6083. [Google Scholar] [CrossRef]
- Wang, S.; Yang, L.; Stubbs, L.; Li, X.; He, C.; Fibrous, L.-D.F.E.C. Mats as High Performance Anode Materials for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 12275–12282. [Google Scholar] [CrossRef]
- Li, X.; Zhu, X.; Zhu, Y.; Yuan, Z.; Si, L.; Qian, Y. Porous nitrogen-doped carbon vegetable-sponges with enhanced lithium storage performance. Carbon 2014, 69, 515–524. [Google Scholar] [CrossRef]
- Li, D.; Ding, L.-X.; Chen, H.; Wang, S.; Li, Z.; Zhu, M.; Wang, H. Novel nitrogen-rich porous carbon spheres as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 16617–16622. [Google Scholar] [CrossRef]
- Hu, C.; Xiao, Y.; Zhao, Y.; Chen, N.; Zhang, Z.; Cao, M.; Qu, L. Highly nitrogen-doped carbon capsules: Scalable preparation and high-performance applications in fuel cells and lithium ion batteries. Nanoscale 2013, 5, 2726–2733. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, G.; Wang, T.; Zhu, X.; Yang, F.; Li, Y.; Lu, Y.; Wei, F. Monolithic nitrogen-doped graphene frameworks as ultrahigh-rate anodes for lithium ion batteries. J. Mater. Chem. A 2015, 3, 15738–15744. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, Z.-J.; Yang, L.; Cheng, S.; Liu, M. A high-performance anode for lithium ion batteries: Fe3O4 microspheres encapsulated in hollow graphene shells. J. Mater. Chem. A 2015, 3, 11847–11856. [Google Scholar] [CrossRef]
- He, C.; Wang, R.; Fu, H.; Shen, P.K. Nitrogen-self-doped graphene as a high capacity anode material for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 14586–14591. [Google Scholar] [CrossRef]
- Freunberger, S.A.; Chen, Y.; Peng, Z.; Griffin, J.M.; Hardwick, L.J.; Bardé, F.; Novák, P.; Bruce, P.G. Reactions in the Rechargeable Lithium–O2 Battery with Alkyl Carbonate Electrolytes. J. Am. Chem. Soc. 2011, 133, 8040–8047. [Google Scholar] [CrossRef]
- Lee, J.-S.; Tai Kim, S.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K.T.; Cho, J. Metal–Air Batteries with High Energy Density: Li–Air versus Zn–Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Yang, S.; Knickle, H. Design and analysis of aluminum/air battery system for electric vehicles. J. Power Sources 2002, 112, 162–173. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Shan, H.; Fan, L.; Wu, C.; Li, D.; Lu, S. Superior Cathode Performance of Nitrogen-Doped Graphene Frameworks for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 10643–10651. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Nie, H.; Yang, Z.; Zhang, J.; Jin, Z.; Lu, Y.; Xiao, Z.; Huang, S. Sulfur–nitrogen co-doped three-dimensional carbon foams with hierarchical pore structures as efficient metal-free electrocatalysts for oxygen reduction reactions. Nanoscale 2013, 5, 3283–3288. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Bai, Z.; Wu, M.; Qiao, J.; Chen, Z. 3-Dimensional porous N-doped graphene foam as a non-precious catalyst for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 3343–3350. [Google Scholar] [CrossRef]
- Yun, X.; Li, J.; Chen, X.; Chen, H.; Xiao, L.; Xiang, K.; Chen, W.; Liao, H.; Zhu, Y. Porous Fe2O3 Modified by Nitrogen-Doped Carbon Quantum Dots/Reduced Graphene Oxide Composite Aerogel as a High-Capacity and High-Rate Anode Material for Alkaline Aqueous Batteries. ACS Appl. Mater. Interfaces 2019, 11, 36970–36984. [Google Scholar] [CrossRef]
- Yang, J.; Hu, J.; Weng, M.; Tan, R.; Tian, L.; Yang, J.; Amine, J.; Zheng, J.; Chen, H.; Pan, F. Fe-Cluster Pushing Electrons to N-Doped Graphitic Layers with Fe3C(Fe) Hybrid Nanostructure to Enhance O2 Reduction Catalysis of Zn-Air Batteries. ACS Appl. Mater. Interfaces 2017, 9, 4587–4596. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Ran, J.; Qiao, S.-Z. Scalable Self-Supported Graphene Foam for High-Performance Electrocatalytic Oxygen Evolution. ACS Appl. Mater. Interfaces 2017, 9, 41980–41987. [Google Scholar] [CrossRef]
- Lin, C.; Li, X.; Shinde, S.S.; Kim, D.-H.; Song, X.; Zhang, H.; Lee, J.-H. Long-Life Rechargeable Zn Air Battery Based on Binary Metal Carbide Armored by Nitrogen-Doped Carbon. ACS Appl. Energy Mater. 2019, 2, 1747–1755. [Google Scholar] [CrossRef]
- Yang, H.B.; Miao, J.; Hung, S.-F.; Chen, J.; Tao, H.B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen, H.M.; et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, e1501122. [Google Scholar] [CrossRef]
- Wang, Z.-D.; Bai, C.-K.; Chen, X.-Y.; Wang, B.-D.; Lu, G.-L.; Sun, H.; Liu, Z.-N.; Huang, H.; Liang, S.; Zang, H.-Y. Co/Co9S8 nanoparticles coupled with N,S-doped graphene-based mixed-dimensional heterostructures as bifunctional electrocatalysts for the overall oxygen electrode. Inorg. Chem. Front. 2019, 6, 2558–2565. [Google Scholar] [CrossRef]
- Qin, X.; Huang, Y.; Wang, K.; Xu, T.; Wang, Y.; Liu, P.; Kang, Y.; Zhang, Y. Novel hierarchically porous Ti-MOFs/nitrogen-doped graphene nanocomposite served as high efficient oxygen reduction reaction catalyst for fuel cells application. Electrochim. Acta 2019, 297, 805–813. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, Y.; Lei, Y.; Wang, Y.; Wang, Y.; Li, Y.; Wang, S. Pyridinic-N-Dominated Doped Defective Graphene as a Superior Oxygen Electrocatalyst for Ultrahigh-Energy-Density Zn–Air Batteries. ACS Energy Lett. 2018, 3, 1183–1191. [Google Scholar] [CrossRef]
- Wen, Z.; Shen, C.; Lu, Y. Air electrode for the lithium–air batteries: Materials and structure designs. ChemPlusChem 2015, 80, 270–287. [Google Scholar] [CrossRef]
- Kunanusont, N.; Shimoyama, Y. Porous carbon electrode for Li-air battery fabricated from solvent expansion during supercritical drying. J. Supercrit. Fluids 2018, 133, 77–85. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.; Liu, H.; Xia, B.Y. Advanced Architectures and Relatives of Air Electrodes in Zn–Air Batteries. Adv. Sci. 2018, 5, 1700691. [Google Scholar] [CrossRef] [PubMed]





| N-Doped Electrodes | Current (mA g−1) | Reversible Capacity (mA h g−1) | Voltage Window (V) | Initial Coulombic eff. (%) | N-Content | Surface Area (m2 g−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Gp nanosheets | 42 | 900 | 0.01–3.0 | - | 2% | - | [129] |
| CNFs | 100 | 1280 | 0.01–3.0 | 48.4 | 10.25 wt% | 2381 | [130] |
| Mesoporous carbon | 100 | 1780 | 0.01–3.0 | 55 | 10.1 wt% | 805.7 | [131] |
| Porous carbon | 100 | 1181 | 0.01–3.0 | 55 | 5.5% | 1300 | [132] |
| Porous Gp | 100 | 742 | 0.01–3.0 | 62% | 5.8 at% | 1170 | [82] |
| Carbon nanosheets | 100 | 1913 | 0.01–3.0 | 49.2% | 4.7% | 2494 | [133] |
| Gp nanoribbons | 100 | 740 | 0.01–3.0 | 63% | 3.7 at% | - | [134] |
| Gp nanosheets | 100 | 832.4 | 0.01–3.0 | 44.8% | 19.46 at% | 504 | [135] |
| CFs | 30 | 650 | 0.005–3.0 | 82.8% | 12.6 wt% | 381 | [136] |
| Carbon sponge | 500 | 553 | 0.001–3.0 | 34.7% | 2.38 at% | 613 | [137] |
| Carbon spheres | 500 | 666 | 0.01–3.0 | 60% | 5% | 67.4 | [138] |
| Carbon capsules | 50 | 1046 | 0.01–3.0 | 90% | 13.3% | 595 | [139] |
| Gp frameworks | 200 | 700 | 0.005–3.0 | 52.3% | 2.6 wt% | 610 | [140] |
| Gp microspheres | 100 | 1643 | 0.005–3.0 | 66.9% | 9.63% | 948 | [141] |
| N-doped Gp | 50 | 1177 | 0.01–3.0 | - | 2.1 at% | - | [142] |
| Carbon/rGO | 100 | 1100 | 0.003–3.0 | 58.3% | 15.4 wt% | 327 | [85] |
| Gp/N-doped carbon nanosheets | 500 | 554 | - | - | - | - | [85] |
| Gp/CNTs nano-lamellar design | 1000 | ≈550 | 0.01–3.0 | 60.1% | 11.2 at % | 293 | [88] |
| 3D-Gp | 1500 | ≈670 | 1.7–2.7 | ≈100% | 10.1 at% | 398 | [107] |
| CNTs/Gp sandwich design | 1C | ≈1150 | 1.6–3 | ≈98% | 0.86 | 217 | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikram, R.; Mohamed Jan, B.; Atif Pervez, S.; Papadakis, V.M.; Ahmad, W.; Bushra, R.; Kenanakis, G.; Rana, M. Recent Advancements of N-Doped Graphene for Rechargeable Batteries: A Review. Crystals 2020, 10, 1080. https://doi.org/10.3390/cryst10121080
Ikram R, Mohamed Jan B, Atif Pervez S, Papadakis VM, Ahmad W, Bushra R, Kenanakis G, Rana M. Recent Advancements of N-Doped Graphene for Rechargeable Batteries: A Review. Crystals. 2020; 10(12):1080. https://doi.org/10.3390/cryst10121080
Chicago/Turabian StyleIkram, Rabia, Badrul Mohamed Jan, Syed Atif Pervez, Vassilis M. Papadakis, Waqas Ahmad, Rani Bushra, George Kenanakis, and Masud Rana. 2020. "Recent Advancements of N-Doped Graphene for Rechargeable Batteries: A Review" Crystals 10, no. 12: 1080. https://doi.org/10.3390/cryst10121080
APA StyleIkram, R., Mohamed Jan, B., Atif Pervez, S., Papadakis, V. M., Ahmad, W., Bushra, R., Kenanakis, G., & Rana, M. (2020). Recent Advancements of N-Doped Graphene for Rechargeable Batteries: A Review. Crystals, 10(12), 1080. https://doi.org/10.3390/cryst10121080








