Fabrication of Amphiphilic Graphene Oxide—Polyoxometalate with Temperature Response for Epoxidation of Olefin in Water
Abstract
1. Introduction
2. Materials and Methods
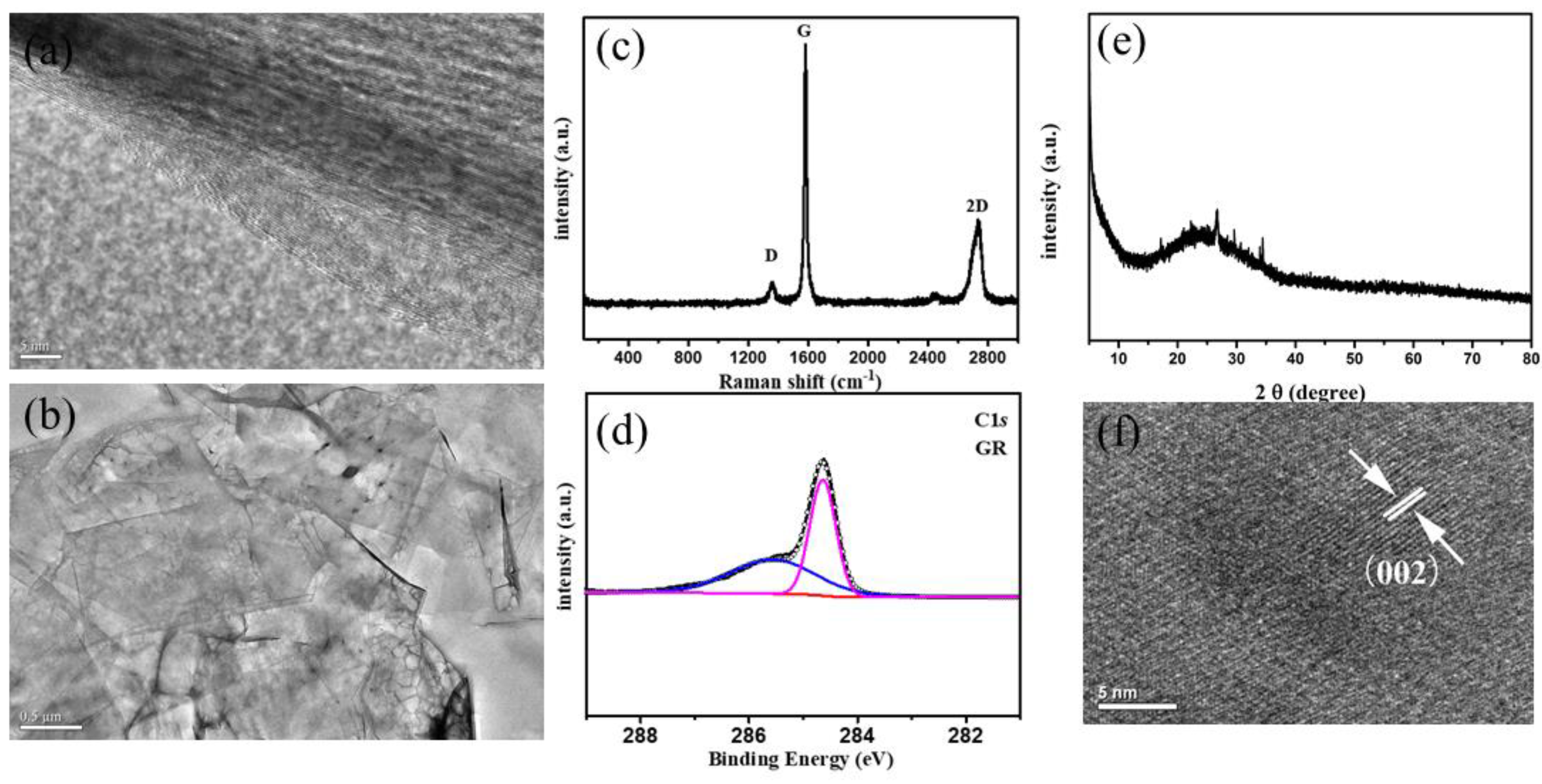
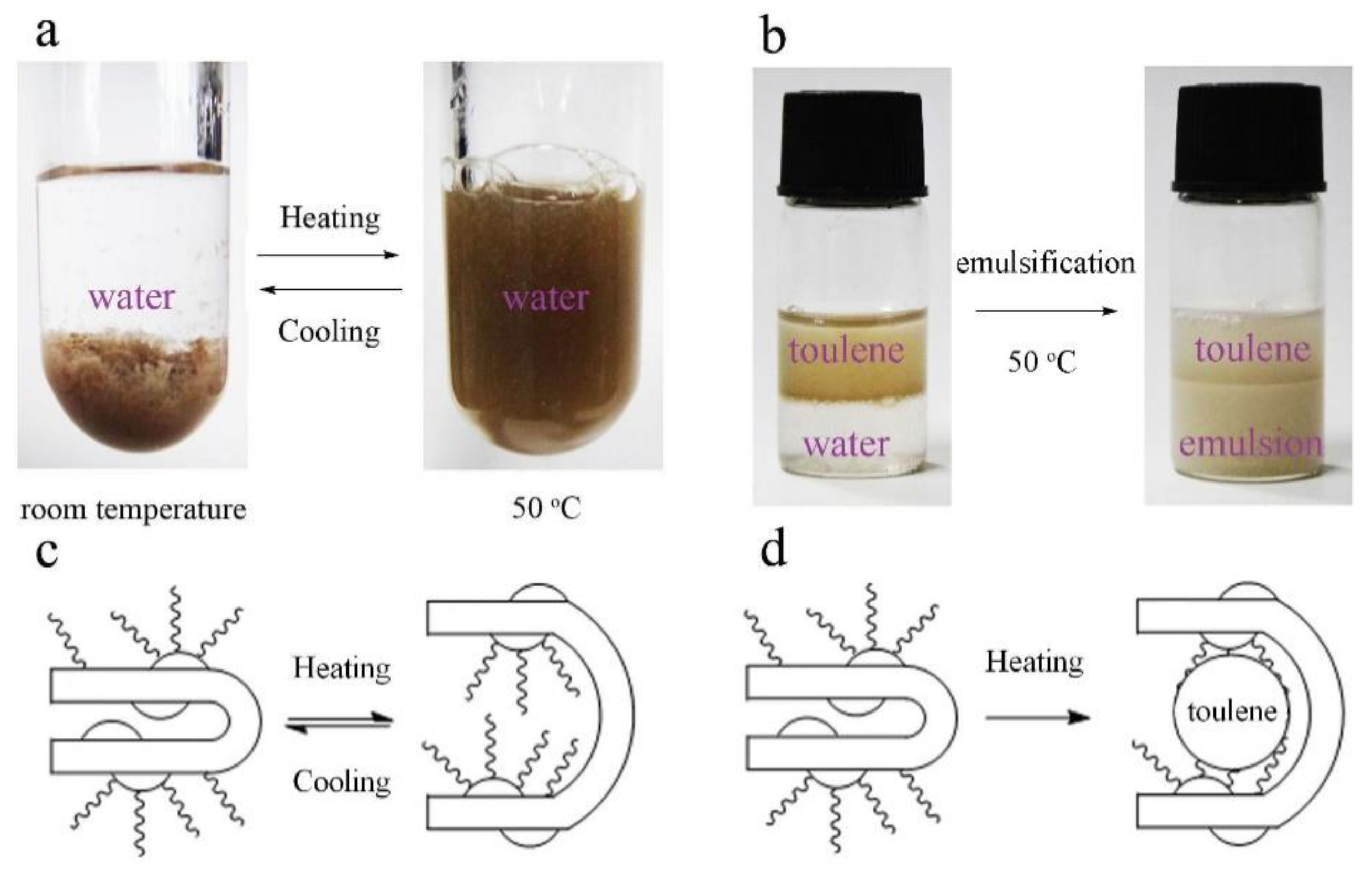
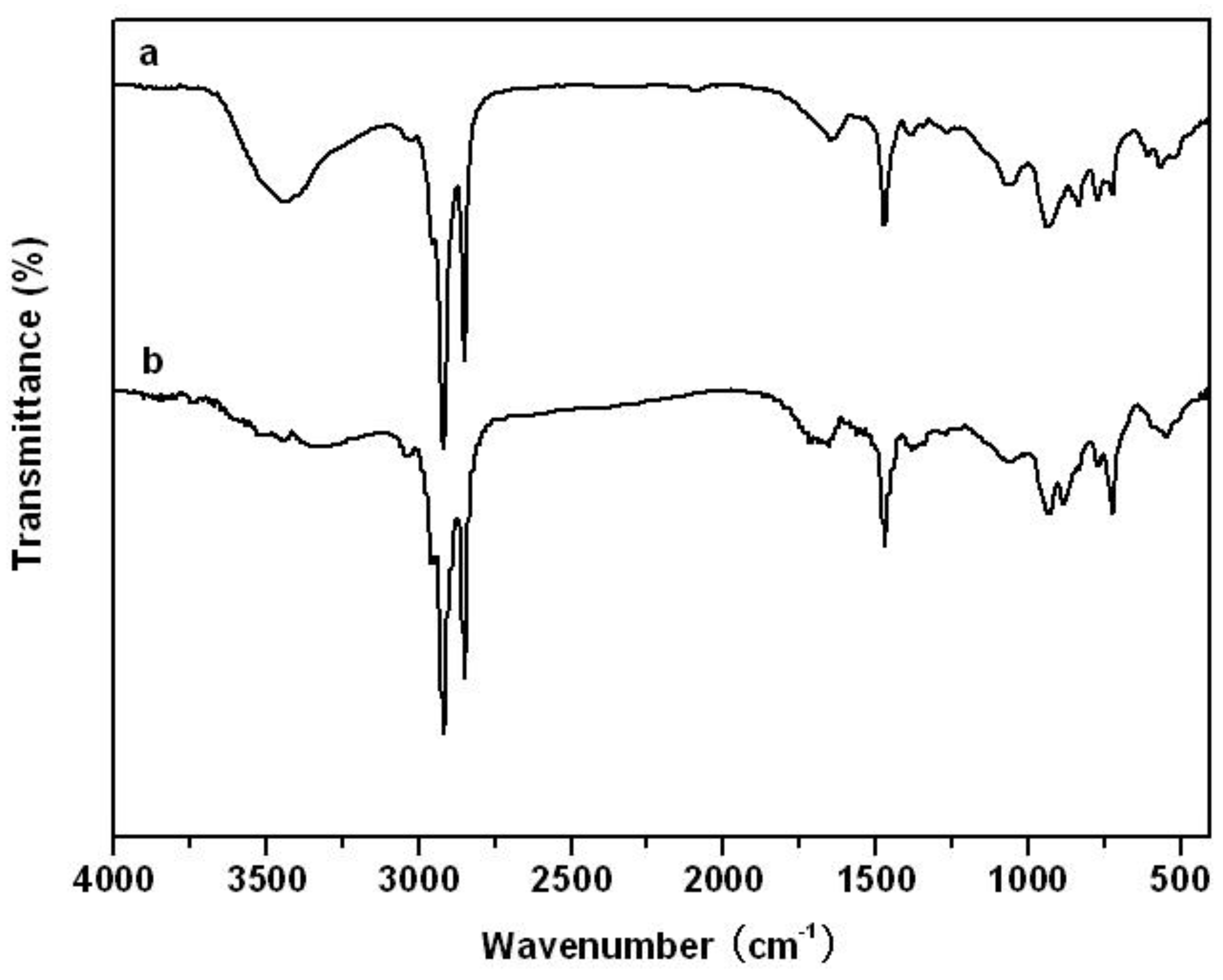
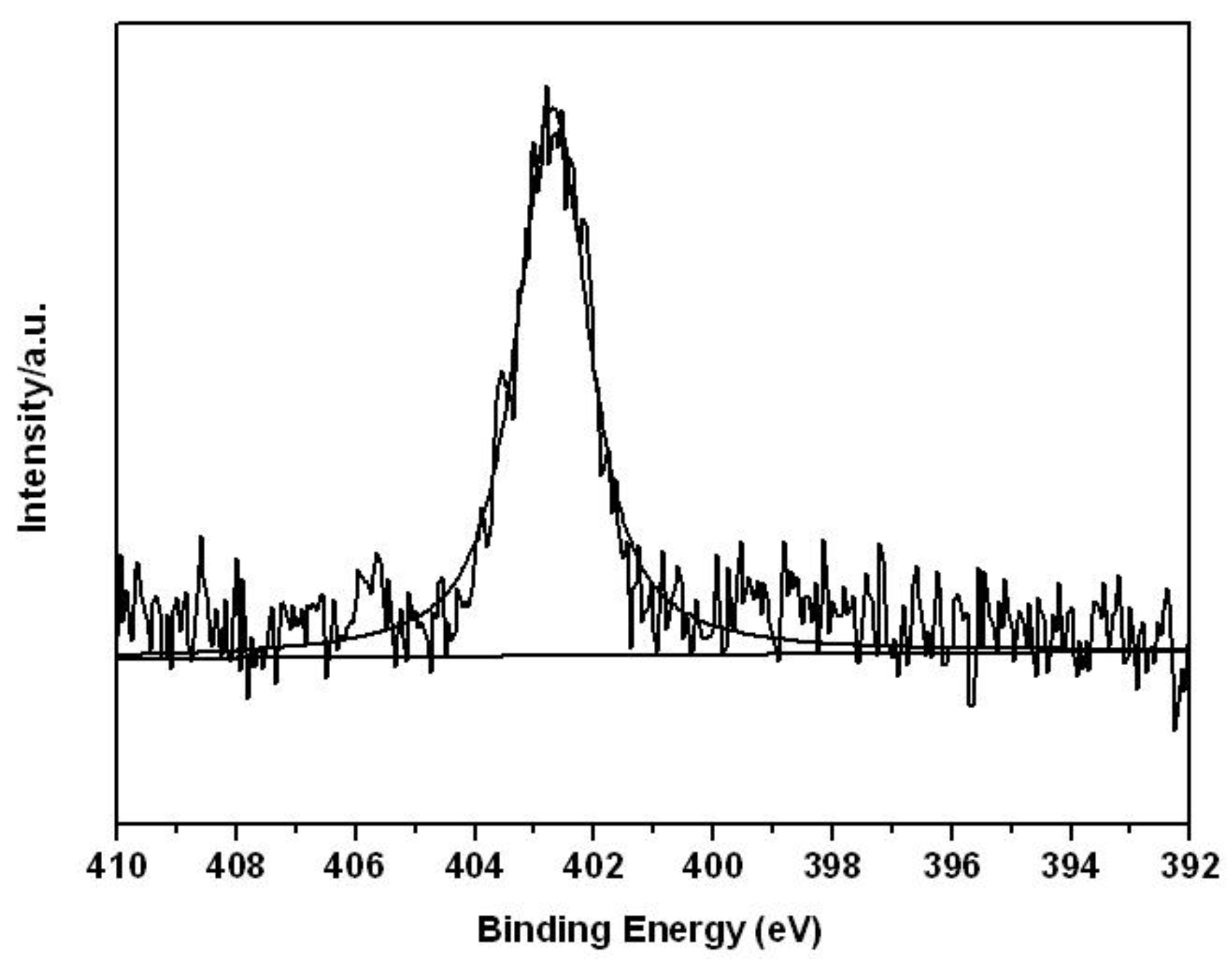
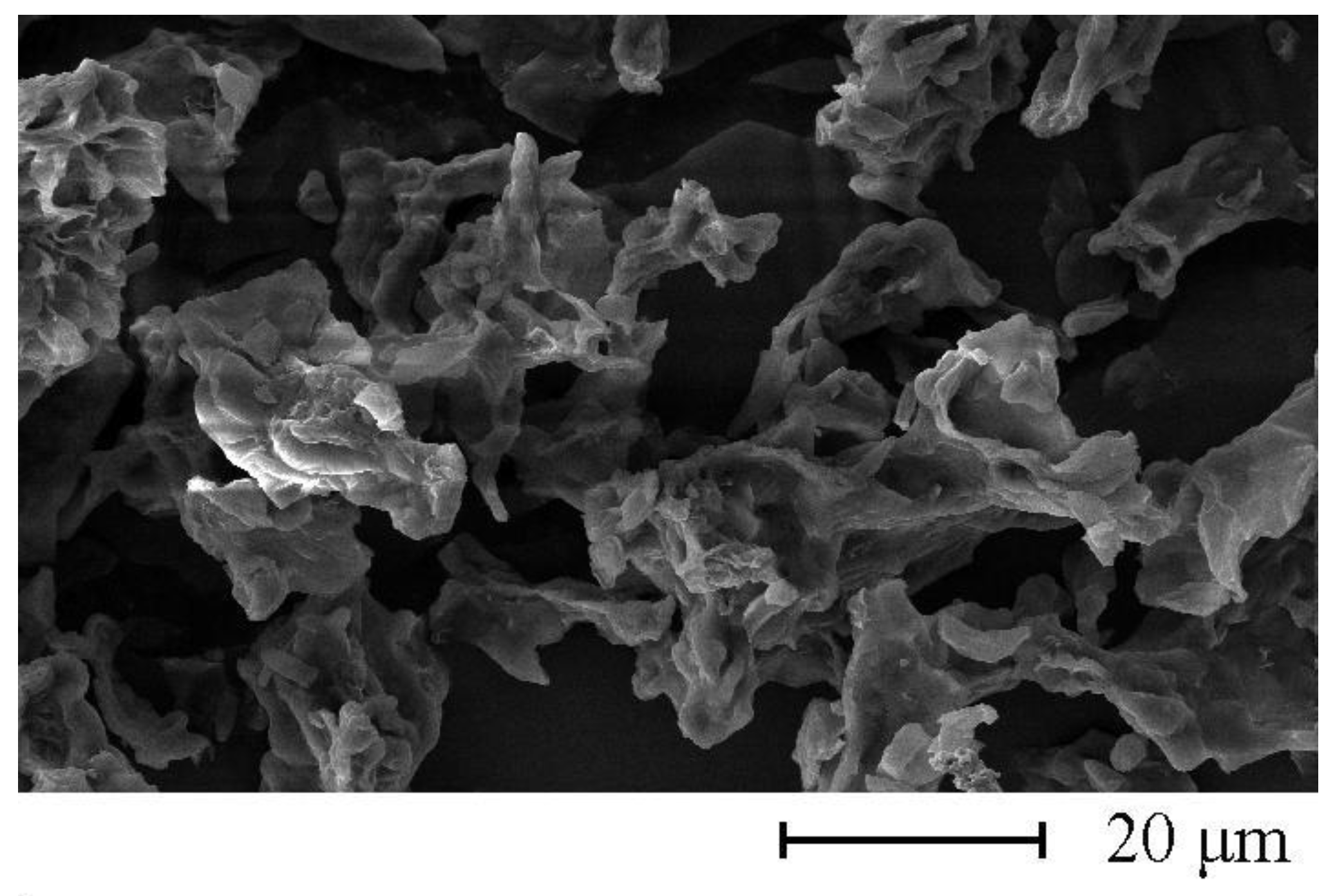
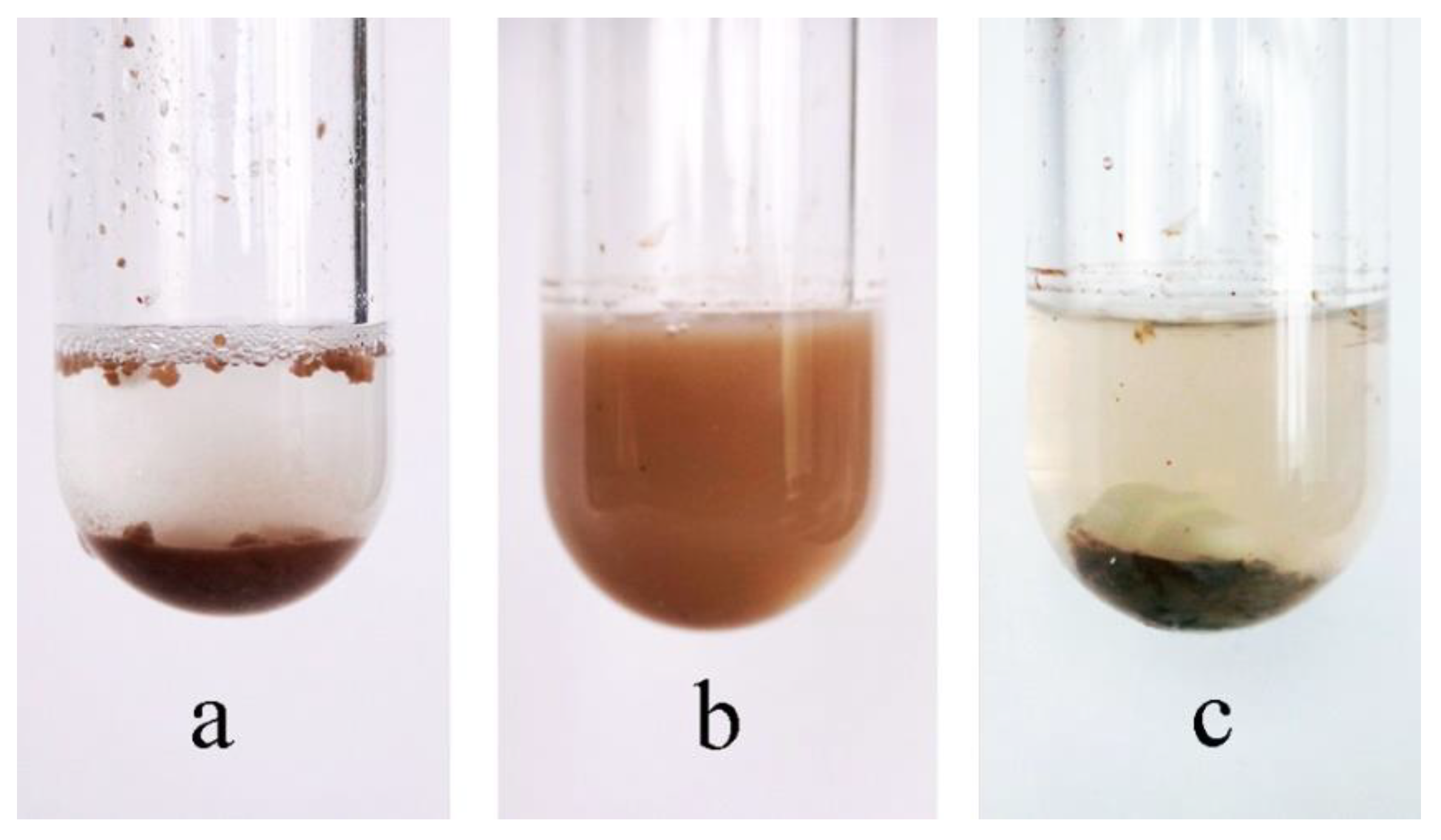
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Weinstock, I.A. Polyoxometalate-decorated nanoparticles. Chem. Soc. Rev. 2012, 41, 7479–7496. [Google Scholar] [CrossRef] [PubMed]
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and post-functionalization: A step towards polyoxometalate-based materials. Chem. Soc. Rev. 2012, 41, 7605–7622. [Google Scholar] [CrossRef] [PubMed]
- Fraqueza, G.; Ohlin, C.A.; Casey, W.H.; Aureliano, M. Sarcoplasmic reticulum calcium ATPase interactions with decaniobate, decavanadate, vanadate, tungstate and molybdate. J. Inorg. Biochem. 2012, 107, 82–89. [Google Scholar] [CrossRef]
- Putaj, P.; Lefebvre, F. Polyoxometalates containing late transition and noble metal atoms. Coord. Chem. Rev. 2011, 255, 1642–1685. [Google Scholar] [CrossRef]
- Song, Y.-F.; Tsunashima, R. Recent advances on polyoxometalate-based molecular and composite materials. Chem. Soc. Rev. 2012, 41, 7384–7402. [Google Scholar] [CrossRef]
- Du, D.-Y.; Yan, L.-K.; Su, Z.-M.; Li, S.-L.; Lan, Y.-Q.; Wang, E.-B. Chiral polyoxometalate-based materials: From design syntheses to functional applications. Coord. Chem. Rev. 2013, 257, 702–717. [Google Scholar] [CrossRef]
- Doherty, S.; Knight, J.G.; Ellison, J.R.; Weekes, D.; Harrington, R.W.; Hardacre, C.; Manyar, H. An efficient recyclable peroxometalate-based polymer-immobilised ionic liquid phase (PIILP) catalyst for hydrogen peroxide-mediated oxidation. Green Chem. 2012, 14, 925–929. [Google Scholar] [CrossRef]
- Neumann, R.; Khenkin, A.M. Peroxometalate Catalyzed Oxidations with Hydrogen Peroxide in Biphasic Reaction Media: Reactions in Inverse Emulsions. J. Org. Chem. 1994, 59, 7577–7579. [Google Scholar] [CrossRef]
- Lambert, A.; Plucinski, P.; Kozhevnikov, I.V. Polyoxometalate-catalysed epoxidation of 1-octene with hydrogen peroxide in microemulsions coupled with ultrafiltration. Chem. Commun. 2003, 714–715. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, G.; Gao, S.; Xi, Z.; Xu, J. Secondary alcohols oxidation with hydrogen peroxide catalyzed by [n-C16H33N(CH3)3]3PW12O40: Transform-and-retransform process between catalytic precursor and catalytic activity species. J. Mol. Catal. A Chem. 2008, 289, 22–27. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Y.; Jia, G.; Jiang, Z.; Wang, S.; Lu, H.; Song, B.; Li, C. A direct imaging of amphiphilic catalysts assembled at the interface of emulsion droplets using fluorescence microscopy. Chem. Commun. 2008, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, X.; Wang, L.; Mi, Z. Epoxidation of hydroxyl-terminated polybutadiene with hydrogen peroxide under phase-transfer catalysis. J. Mol. Catal. A Chem. 2009, 309, 89–94. [Google Scholar] [CrossRef]
- Xue, J.; Wang, A.; Yin, H.; Wang, J.; Zhang, D.; Chen, W.; Yu, L.; Jiang, T. Oxidation of cyclopentene catalyzed by phosphotungstic quaternary ammonium salt catalysts. J. Ind. Eng. Chem. 2010, 16, 288–292. [Google Scholar] [CrossRef]
- Duncan, D.C.; Chambers, R.C.; Hecht, E.; Hill, C.L. Mechanism and Dynamics in the H3[PW12O40]-Catalyzed Selective Epoxidation of Terminal Olefins by H2O2. Formation, Reactivity, and Stability of {PO4[WO(O2)2]4}3. J. Am. Chem. Soc. 1995, 117, 681–691. [Google Scholar] [CrossRef]
- Casuscelli, S.G.; Crivello, M.E.; Perez, C.F.; Ghione, G.; Herrero, E.R.; Pizzio, L.R.; Vázquez, P.G.; Cáceres, C.V.; Blanco, M.N. Effect of reaction conditions on limonene epoxidation with H2O2 catalyzed by supported Keggin heteropolycompounds. Appl. Catal. A 2004, 274, 115–122. [Google Scholar] [CrossRef]
- Ding, Y.; Gao, Q.; Li, G.; Zhang, H.; Wang, J.; Yan, L.; Suo, J. Selective epoxidation of cyclohexene to cyclohexene oxide catalyzed by Keggin-type heteropoly compounds using anhydrous urea–hydrogen peroxide as oxidizing reagent and acetonitrile as the solvent. J. Mol. Catal. A Chem. 2004, 218, 161–170. [Google Scholar] [CrossRef]
- Tayebee, R. Epoxidation of some olefins with hydrogen peroxide catalyzed by heteropolyoxometalates. Asian J. Chem. 2008, 20, 8–14. [Google Scholar]
- Liu, L.; Chen, C.; Hu, X.; Mohamood, T.; Ma, W.; Lin, J.; Zhao, J. A role of ionic liquid as an activator for efficient olefin epoxidation catalyzed by polyoxometalate. New J. Chem. 2008, 32, 283–289. [Google Scholar] [CrossRef]
- Leclercq, L.; Mouret, A.; Proust, A.; Schmitt, V.; Bauduin, P.; Aubry, J.-M.; Nardello-Rataj, V. Pickering Emulsion Stabilized by Catalytic Polyoxometalate Nanoparticles: A New Effective Medium for Oxidation Reactions. Chem. Eur. J. 2012, 18, 14352–14358. [Google Scholar] [CrossRef]
- Vasylyev, M.V.; Neumann, R. New Heterogeneous Polyoxometalate Based Mesoporous Catalysts for Hydrogen Peroxide Mediated Oxidation Reactions. J. Am. Chem. Soc. 2003, 126, 884–890. [Google Scholar] [CrossRef]
- Jahier, C.; Plault, L.; Nlate, S. Encapsulation of Polyoxotungstate into Dendrimers by Ionic Bonding and Their Use As Oxidation Catalyst. Isr. J. Chem. 2009, 49, 109–118. [Google Scholar] [CrossRef]
- Haimov, A.; Cohen, H.; Neumann, R. Alkylated Polyethyleneimine/Polyoxometalate Synzymes as Catalysts for the Oxidation of Hydrophobic Substrates in Water with Hydrogen Peroxide. J. Am. Chem. Soc. 2004, 126, 11762–11763. [Google Scholar] [CrossRef] [PubMed]
- Rezaeifard, A.; Jafarpour, M.; Naeimi, A.; Haddad, R. Aqueous heterogeneous oxygenation of hydrocarbons and sulfides catalyzed by recoverable magnetite nanoparticles coated with copper(ii) phthalocyanine. Green Chem. 2012, 14, 3386–3394. [Google Scholar] [CrossRef]
- Hsiao, M.-C.; Liu, S.-T. Polymer Supported Vanadium Complexes as Catalysts for the Oxidation of Alkenes in Water. Catal. Lett. 2010, 139, 61–66. [Google Scholar] [CrossRef]
- Sakamoto, T.; Pac, C.J. Selective epoxidation of olefins by hydrogen peroxide in water using a polyoxometalate catalyst supported on chemically modified hydrophobic mesoporous silica gel. Tetrahedron Lett. 2000, 41, 10009–10012. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, H.; Hua, L.; Orzechowski, L.; Yan, K.; Feng, B.; Pan, Z.; Theyssen, N.; Leitner, W.; Hou, Z. Peroxometalates Immobilized on Magnetically Recoverable Catalysts for Epoxidation. ChemPlusChem 2012, 77, 1128–1138. [Google Scholar] [CrossRef]
- Shi, Z.; Mei, C.; Niu, G.; Han, Q. Two inorganic–organic hybrids based on a polyoxometalate: Structures, characterizations, and epoxidation of olefins. J. Coord. Chem. 2018, 71, 1460–1468. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Rani, J.R.; Thangavel, R.; Kim, M.; Lee, Y.S.; Jang, J.-H. Ultra-High Energy Density Hybrid Supercapacitors Using MnO2/Reduced Graphene Oxide Hybrid Nanoscrolls. Nanomaterials 2020, 10, 2049. [Google Scholar] [CrossRef]
- Thangavel, R.; Kannan, A.G.; Ponraj, R.; Yoon, G.; Aravindan, V.; Kim, D.; Kang, K.; Yoon, W.; Lee, Y. Surface enriched graphene hollow spheres towards building ultra-high power sodium-ion capacitor with long durability. Energy Storage Mater. 2020, 25, 702–713. [Google Scholar] [CrossRef]
- Rani, J.R.; Thangavel, R.; Oh, S.-I.; Lee, Y.S.; Jang, J.-H. An Ultra-High-Energy Density Supercapacitor; Fabrication Based on Thiol-functionalized Graphene Oxide Scrolls. Nanomaterials 2019, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Jia, H.-P.; Bielawski, C.W. Graphene Oxide: A Convenient Carbocatalyst for Facilitating Oxidation and Hydration Reactions. Angew. Chem. Int. Ed. 2010, 49, 6813–6816. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Concepcion, P.; Fornes, V.; Garcia, H. Graphene oxide as an acid catalyst for the room temperature ring opening of epoxides. Chem. Commun. 2012, 48, 5443–5445. [Google Scholar] [CrossRef] [PubMed]
- Mirza-Aghayan, M.; Kashef-Azar, E.; Boukherroub, R. Graphite oxide: An efficient reagent for oxidation of alcohols under sonication. Tetrahedron Lett. 2012, 53, 4962–4965. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, C.; Wang, X.; Gao, J.; Wang, W.; Liu, Y. Oxidation of SO2 to SO3 catalyzed by graphene oxide foams. J. Mater. Chem. 2011, 21, 13934–13941. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Jia, H.-P.; Todd, A.D.; Geng, J.; Bielawski, C.W. Graphite oxide: A selective and highly efficient oxidant of thiols and sulfides. Org. Biomol. Chem. 2011, 9, 7292–7295. [Google Scholar] [CrossRef]
- Chen, J.; Liu, S.; Feng, W.; Zhang, G.; Yang, F. Fabrication phosphomolybdic acid-reduced graphene oxide nanocomposite by UV photo-reduction and its electrochemical properties. PCCP 2013, 15, 5664–5669. [Google Scholar] [CrossRef]
- Li, S.; Liu, R.; Ngo Biboum, R.; Lepoittevin, B.; Zhang, G.; Dolbecq, A.; Mialane, P.; Keita, B. First Examples of Hybrids Based on Graphene and a Ring-Shaped Macrocyclic Polyoxometalate: Synthesis, Characterization, and Properties. Eur. J. Inorg. Chem. 2013, 2013, 1882–1889. [Google Scholar] [CrossRef]
- Rodriguez-Albelo, L.M.; Rousseau, G.; Mialane, P.; Marrot, J.; Mellot-Draznieks, C.; Ruiz-Salvador, A.R.; Li, S.; Liu, R.; Zhang, G.; Keita, B.; et al. Keggin-based coordination networks: Synthesis, structure and application toward green synthesis of polyoxometalate@graphene hybrids. Dalton Trans. 2012, 41, 9989–9999. [Google Scholar] [CrossRef]
- Kim, Y.; Shanmugam, S. Polyoxometalate–Reduced Graphene Oxide Hybrid Catalyst: Synthesis, Structure, and Electrochemical Properties. ACS Appl. Mat. Interfaces 2013, 5, 12197–12204. [Google Scholar] [CrossRef]
- Szabó, T.; Tombácz, E.; Illés, E.; Dékány, I. Enhanced acidity and pH-dependent surface charge characterization of successively oxidized graphite oxides. Carbon 2006, 44, 537–545. [Google Scholar] [CrossRef]
- Venturello, C.; D’Aloisio, R. Quaternary ammonium tetrakis(diperoxotungsto)phosphates(3-) as a new class of catalysts for efficient alkene epoxidation with hydrogen peroxide. J. Org. Chem. 1988, 53, 1553–1557. [Google Scholar] [CrossRef]
- Gao, J.; Chen, Y.; Han, B.; Feng, Z.; Li, C.; Zhou, N.; Gao, S.; Xi, Z. A spectroscopic study on the reaction-controlled phase transfer catalyst in the epoxidation of cyclohexene. J. Mol. Catal. A Chem. 2004, 210, 197–204. [Google Scholar] [CrossRef]
- Yang, X.-L.; Dai, W.-L.; Gao, R.; Fan, K. Characterization and catalytic behavior of highly active tungsten-doped SBA-15 catalyst in the synthesis of glutaraldehyde using an anhydrous approach. J. Catal. 2007, 249, 278–288. [Google Scholar] [CrossRef]
- Lindberg, B.J.; Hedman, J. Molecular Spectroscopy by Means of Esca 6. Group Shifts for N, P and as Compounds. Chem. Scr. 1975, 7, 155–166. [Google Scholar]
- Barr, T.L. An ESCA study of the termination of the passivation of elemental metals. J. Phys. Chem. 1978, 82, 1801–1810. [Google Scholar] [CrossRef]
- Radkov, E.; Beer, R.H. High yield synthesis of mixed-metal keggin polyoxoanions in non-aqueous solvents: Preparation of (n-Bu4N)4[PMW11O40] (M = V, Nb, Ta). Polyhedron 1995, 14, 2139–2143. [Google Scholar] [CrossRef]
- Abdalla, Z.E.A.; Li, B.; Tufail, A. Direct synthesis of mesoporous (C19H42N)4H3(PW11O39)/SiO2 and its catalytic performance in oxidative desulfurization. Colloids Surf. A 2009, 341, 86–92. [Google Scholar] [CrossRef]
- Salles, L.; Aubry, C.; Thouvenot, R.; Robert, F.; Doremieux-Morin, C.; Chottard, G.; Ledon, H.; Jeannin, Y.; Bregeault, J.M. 31P and 183W NMR Spectroscopic Evidence for Novel Peroxo Species in the “H3[PW12O40].cntdot.yH2O/H2O2” System. Synthesis and X-ray Structure of Tetrabutylammonium (.mu.-Hydrogen phosphato)bis(.mu.-peroxo)bis(oxoperoxotungstate) (2-): A Catalyst of Olefin Epoxidation in a Biphase Medium. Inorg. Chem. 1994, 33, 871–878. [Google Scholar] [CrossRef]
- Salles, L.; Piquemal, J.-Y.; Thouvenot, R.; Minot, C.; Brégeault, J.-M. Catalytic epoxidation by heteropolyoxoperoxo complexes: From novel precursors or catalysts to a mechanistic approach. J. Mol. Catal. A Chem. 1997, 117, 375–387. [Google Scholar] [CrossRef]
- Gresley, N.M.; Griffith, W.P.; Laemmel, A.C.; Nogueira, H.I.S.; Parkin, B.C. Studies on polyoxo and polyperoxo-metalates part 5: Peroxide-catalysed oxidations with heteropolyperoxo-tungstates and -molybdates. J. Mol. Catal. A Chem. 1997, 117, 185–198. [Google Scholar] [CrossRef]





| Entry | Catalyst | Solution | Conversion | Yield |
|---|---|---|---|---|
| 1 | CuPcS @ASMNP [23] | water | 92% | 92% |
| 2 | c-PMAn-V [24] | water | 100% | 92% |
| 3 | MNP-[HDMIM]2[W2O11] [26] | water | 59.5% | 58.9% |
| 4 | [p-C5H5N+(CH2)15CH3]3(PW12O40) [25] | water | 100% | 98% |
| 5 | [(C4H9)4 N]4[SiW12O40] [27] | water | 87.1% | 88% |
| Peak | Position (eV) | Area | FWHM (eV) | % GL |
|---|---|---|---|---|
| 0 | 37.374 | 837.542 | 1.024 | 80 |
| 1 | 35.224 | 1116.722 | 1.037 | 80 |
| 2 | 36.65 | 1058.789 | 0.855 | 80 |
| 3 | 34.5 | 1411.719 | 0.824 | 80 |
| Entry | Catalyst | Solvent | Oxidant | Time (h) | Conversion (%) | Yield (%) | Selectivity (%) |
|---|---|---|---|---|---|---|---|
| 1 | CAT I | H2O | H2O2 | 20 | 92 | 84 | 92 |
| 2 | CAT I | H2O | Oxone® | 20 | 98 | 6 | 6 |
| 3 | CAT I | H2O | TBHP | 20 | 3 | 0.3 | 10 |
| 4 a | CAT I | [Bmim]BF4 | H2O2 | 20 | 76 | 45 | 59 |
| 5 | CAT I | Ethyl acetate | H2O2 | 20 | 11 | 9 | 80 |
| 6 | CAT I | acetonitrile | H2O2 | 20 | 12 | 3 | 25 |
| 7 b | CAT I | H2O | H2O2 | 12 | 100 | 94 | 94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Zheng, H.; Wang, C.; Gao, Y.; Wang, X.; Wang, X.; Huo, M. Fabrication of Amphiphilic Graphene Oxide—Polyoxometalate with Temperature Response for Epoxidation of Olefin in Water. Crystals 2020, 10, 1077. https://doi.org/10.3390/cryst10121077
Wu J, Zheng H, Wang C, Gao Y, Wang X, Wang X, Huo M. Fabrication of Amphiphilic Graphene Oxide—Polyoxometalate with Temperature Response for Epoxidation of Olefin in Water. Crystals. 2020; 10(12):1077. https://doi.org/10.3390/cryst10121077
Chicago/Turabian StyleWu, Jinghui, Hongwei Zheng, Chi Wang, Ya Gao, Xianze Wang, Xiaohong Wang, and Mingxin Huo. 2020. "Fabrication of Amphiphilic Graphene Oxide—Polyoxometalate with Temperature Response for Epoxidation of Olefin in Water" Crystals 10, no. 12: 1077. https://doi.org/10.3390/cryst10121077
APA StyleWu, J., Zheng, H., Wang, C., Gao, Y., Wang, X., Wang, X., & Huo, M. (2020). Fabrication of Amphiphilic Graphene Oxide—Polyoxometalate with Temperature Response for Epoxidation of Olefin in Water. Crystals, 10(12), 1077. https://doi.org/10.3390/cryst10121077





