Development of Co(OH)xF2−x Nanosheets for Acetone Gas Sensor Applications: Material Characterization and Sensor Performance Evaluation
Abstract
1. Introduction
2. Materials and Methods
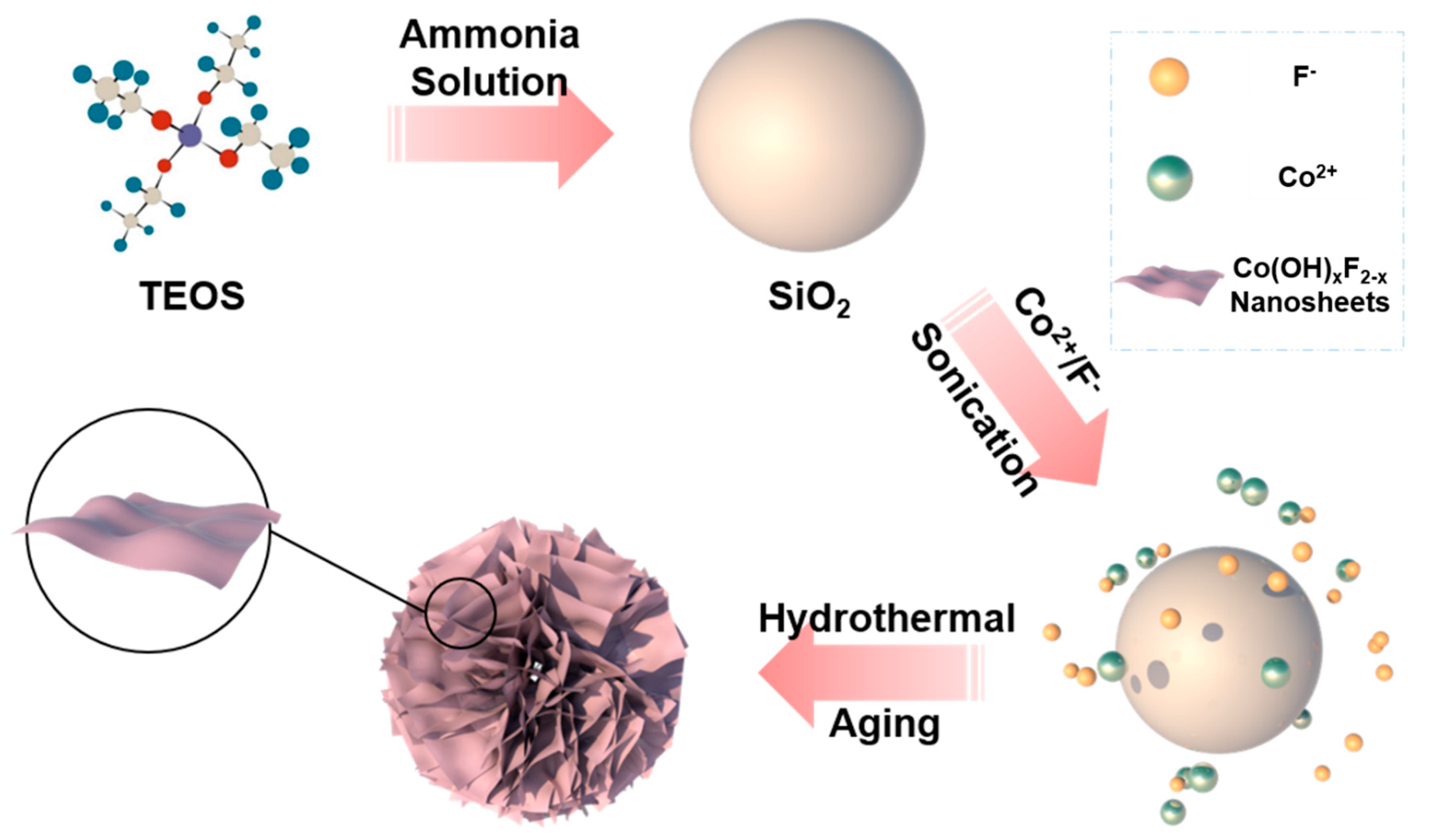
2.1. Fabrication of Co(OH)xF2−x Nanosheets
2.2. Characterization
2.3. Fabrication of Gas Sensor and Gas-Sensing Measurements
3. Results
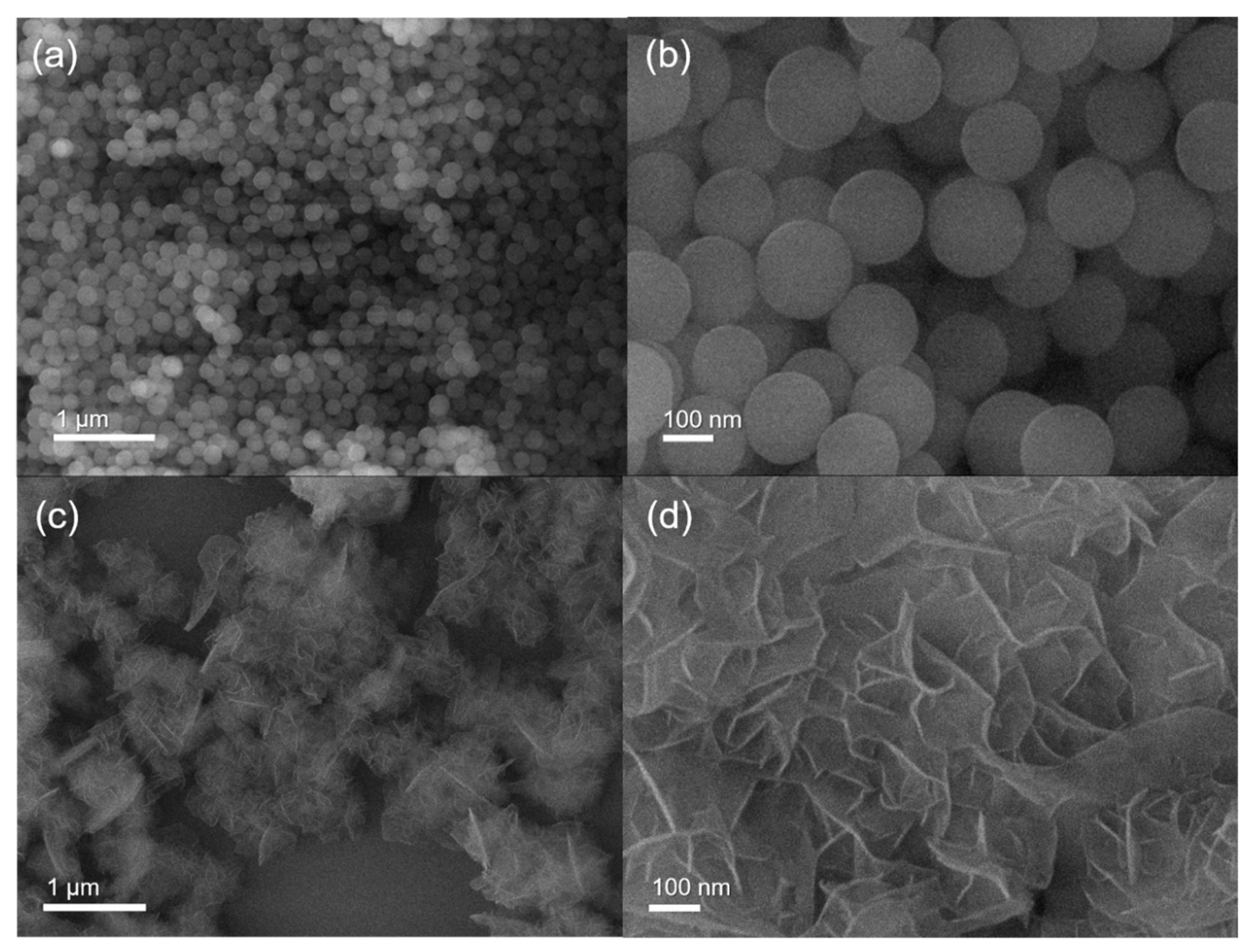
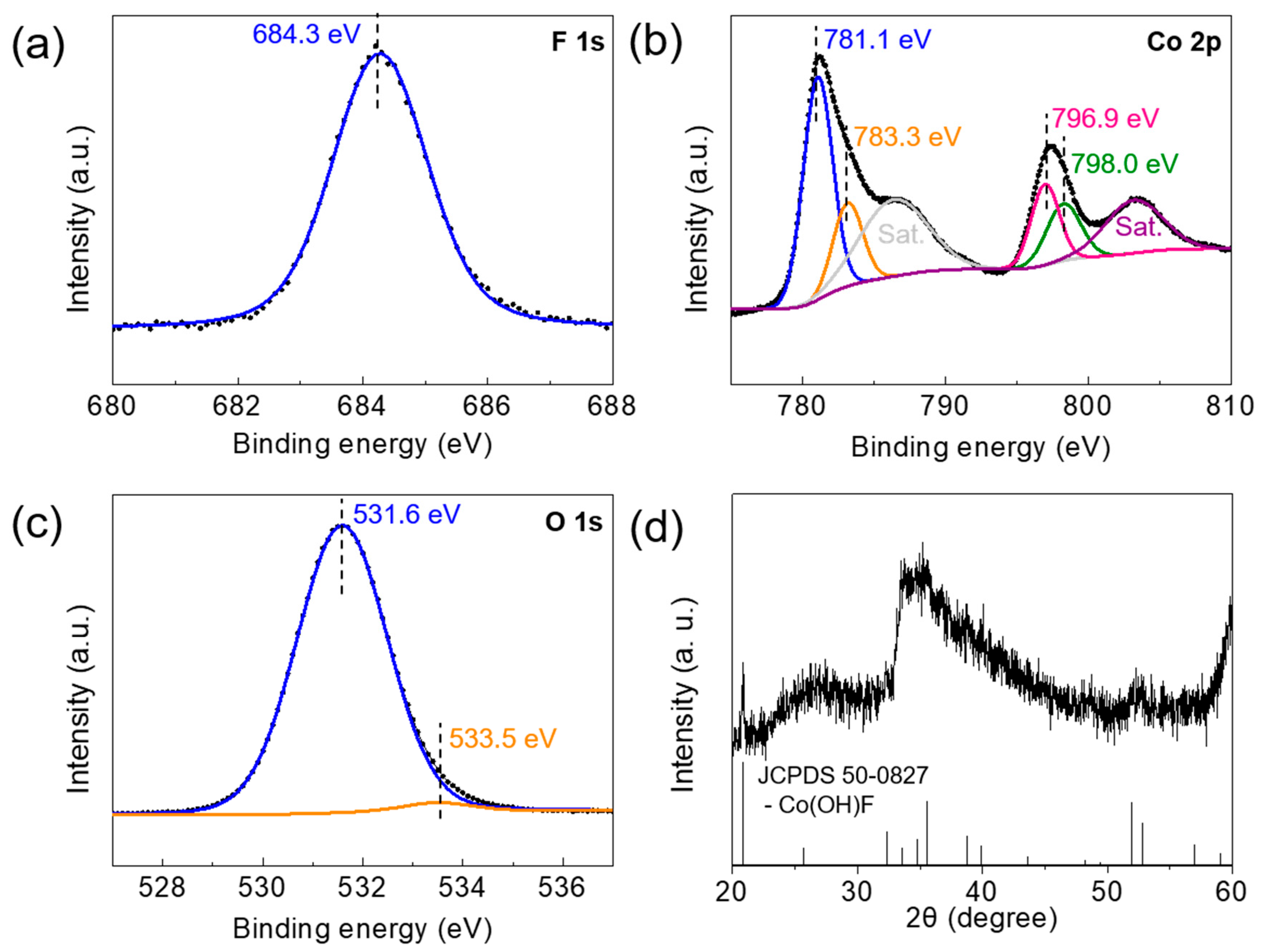
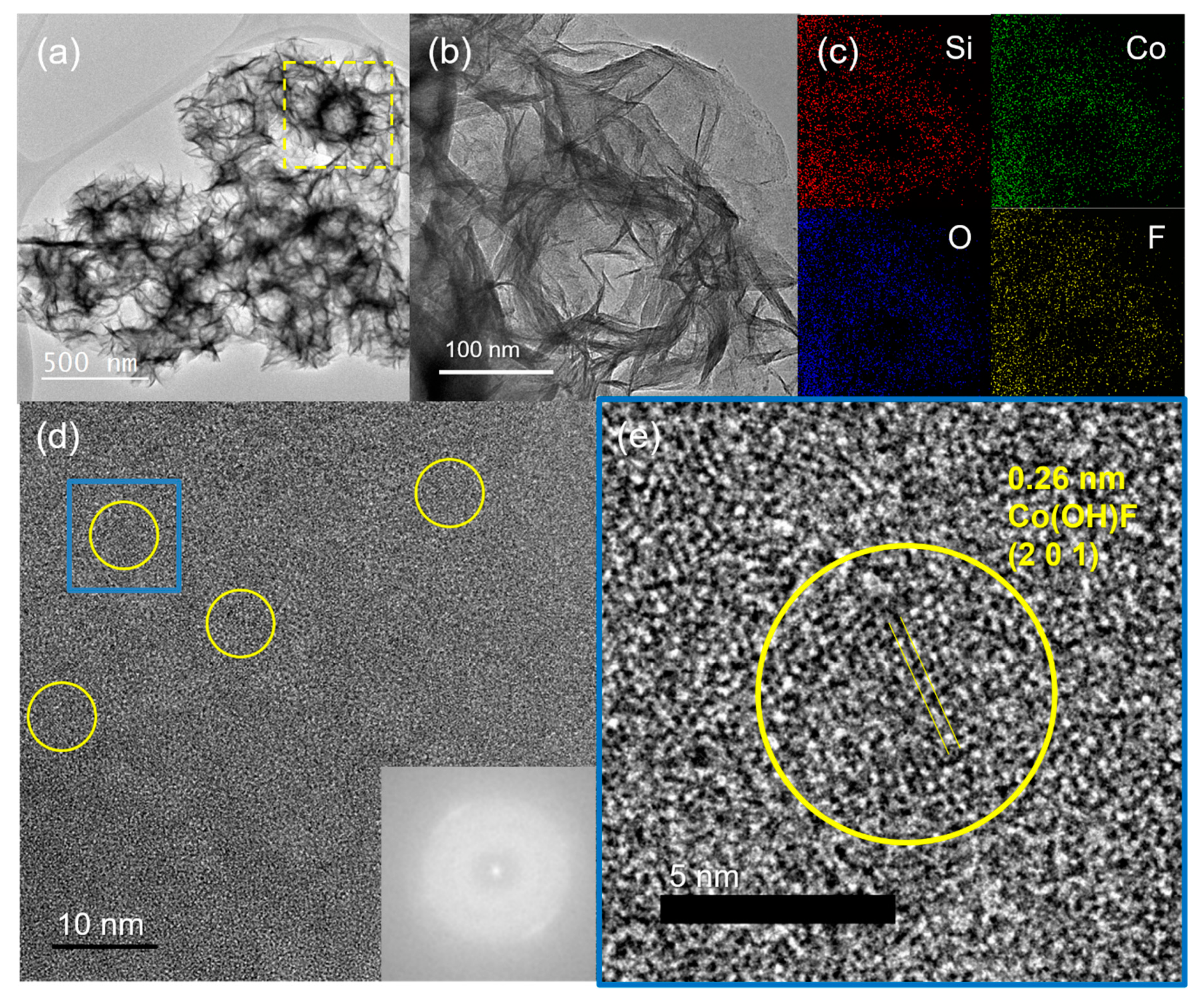
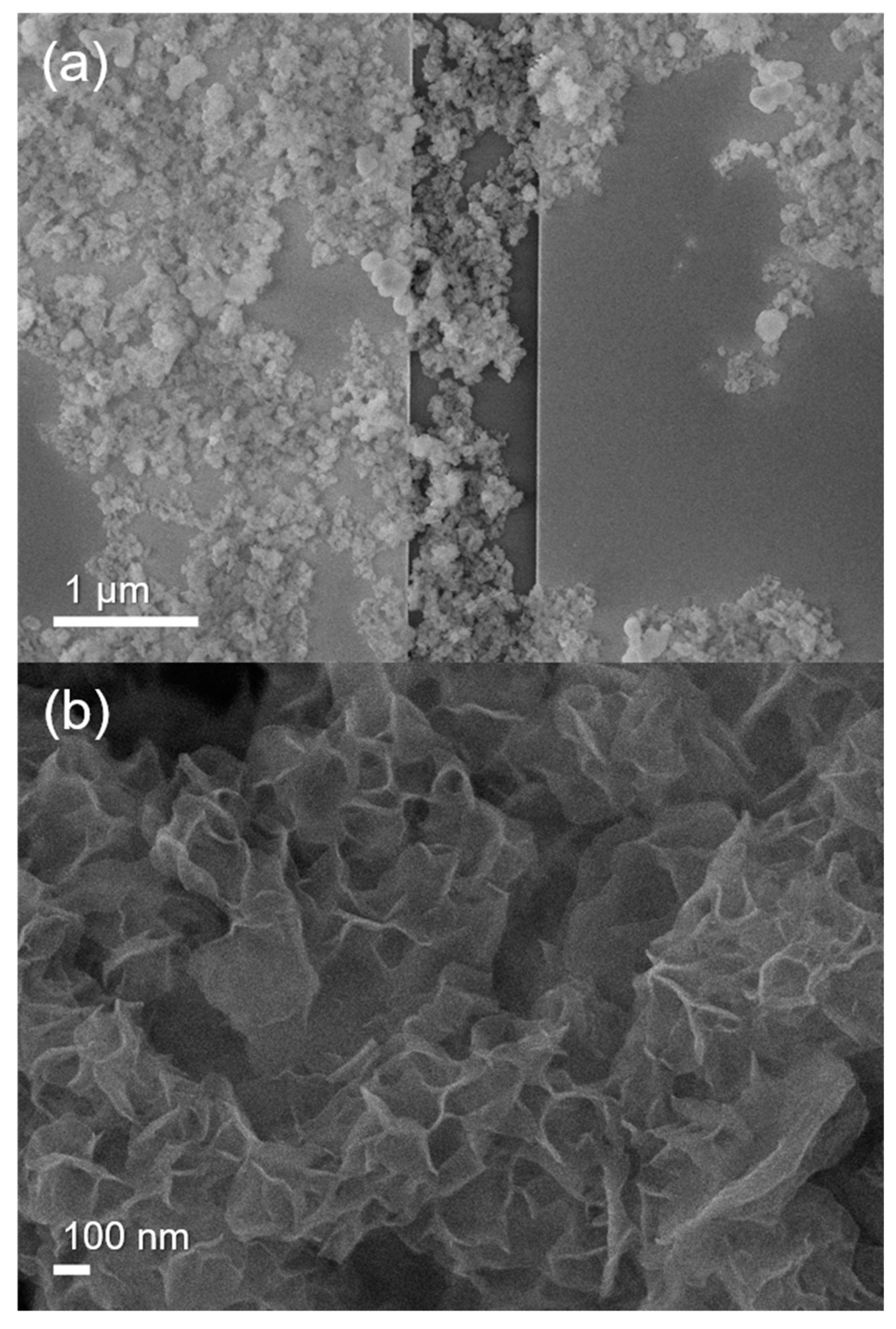
3.1. Synthesis and Characterization of Co(OH)xF2−x Nanosheets
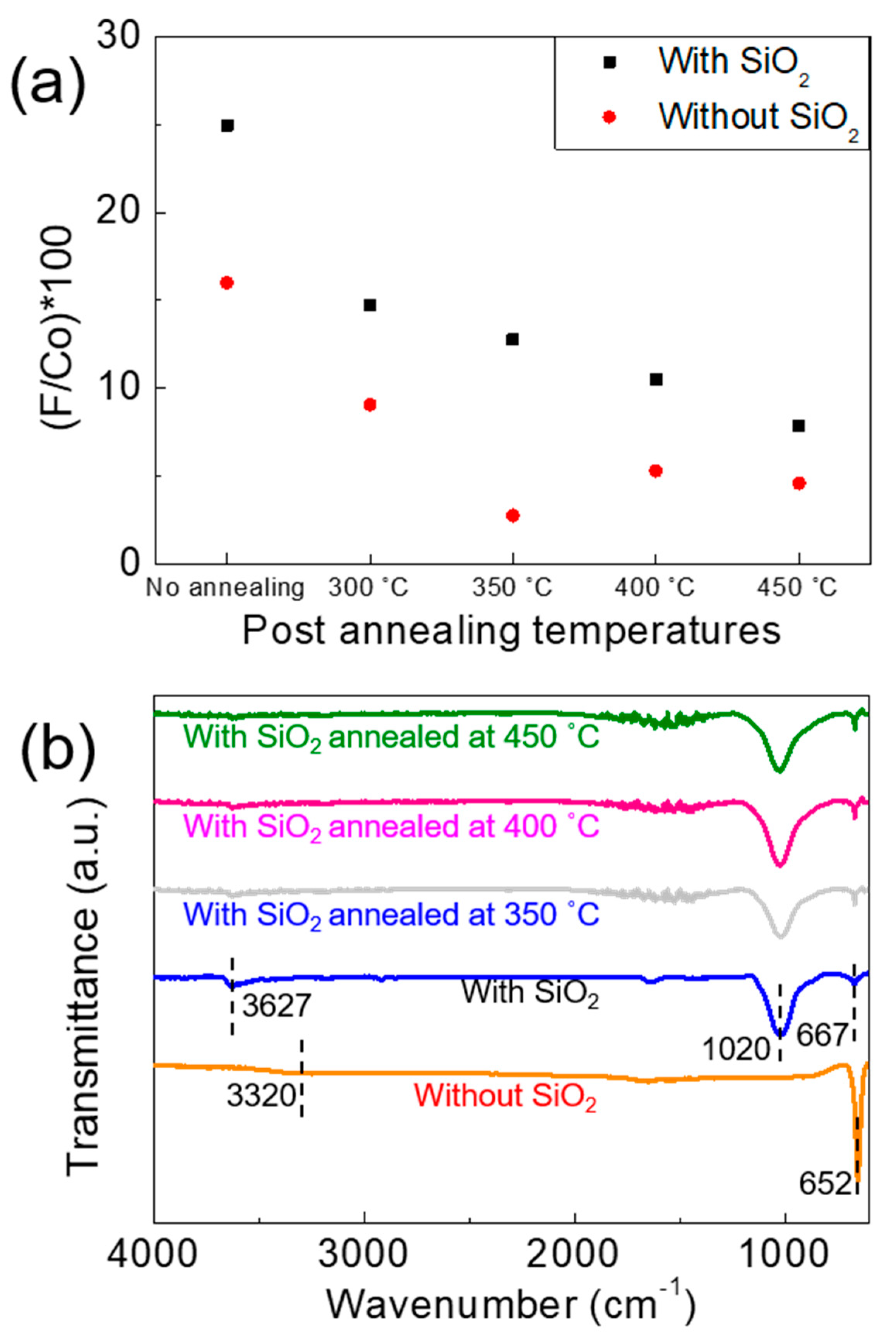
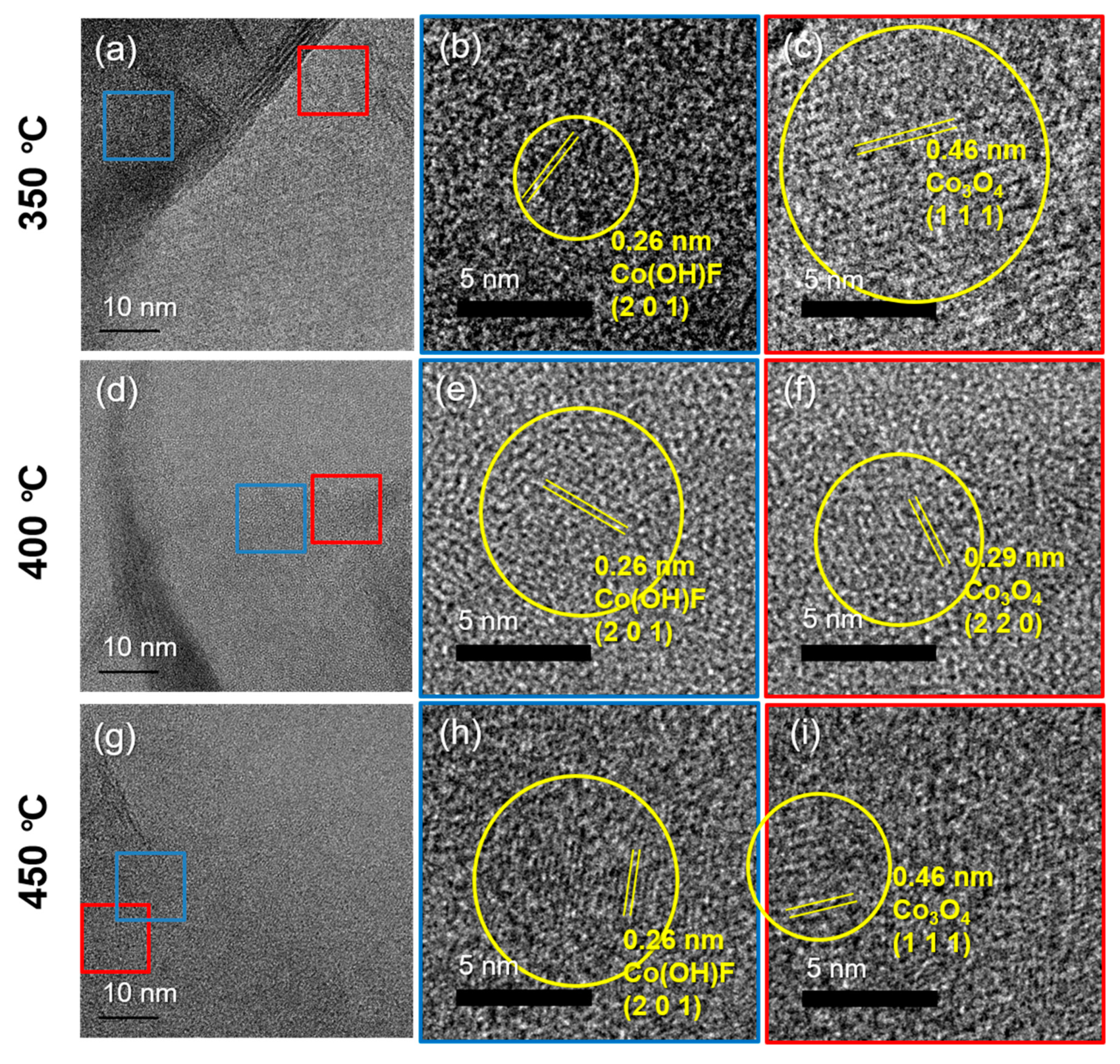
3.2. Post-Annealing Effects on Co(OH)xF2−x Nanosheets
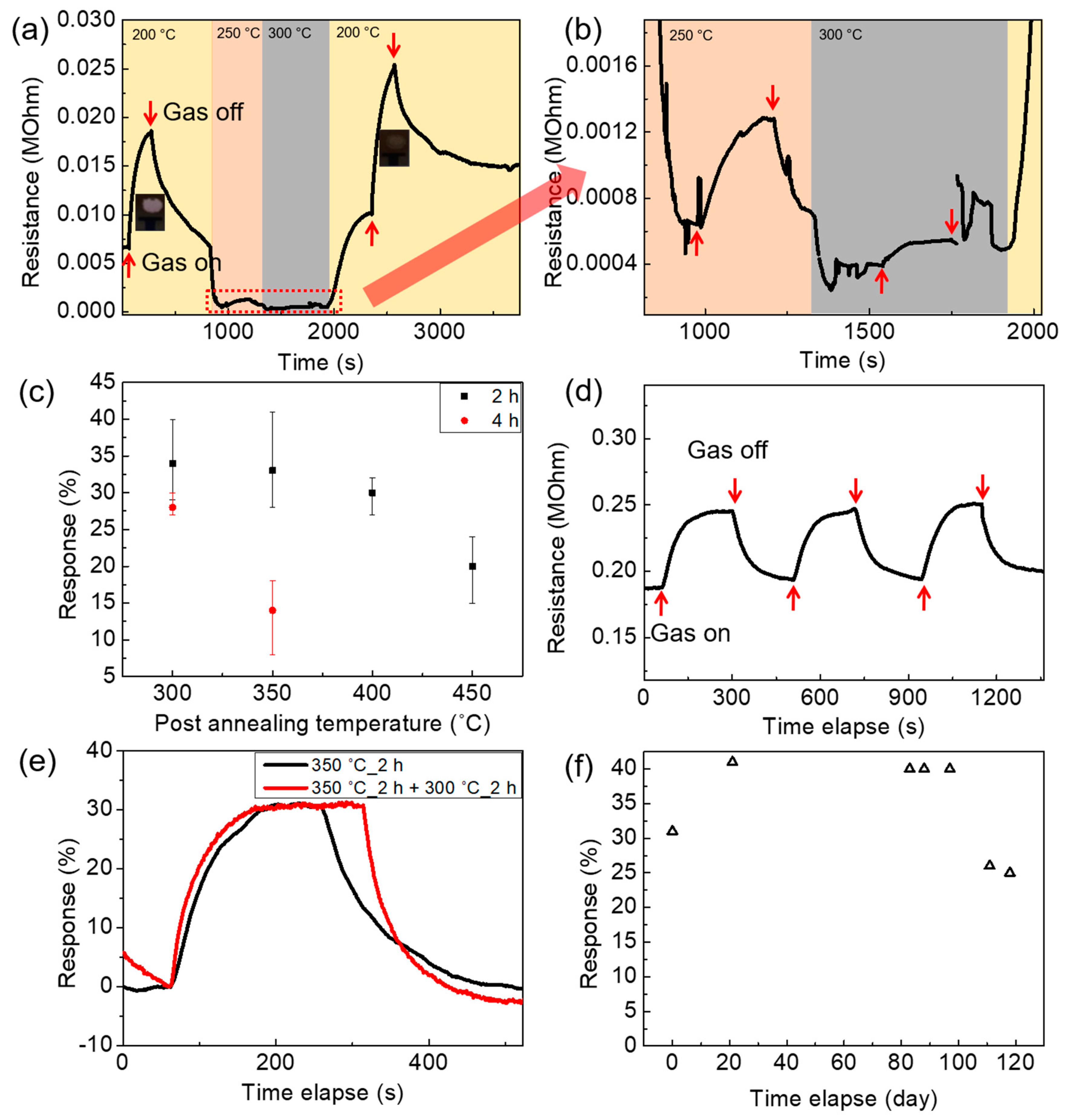
3.3. Gas-Sensing Characteristics of Co(OH)xF2−x Nanosheet Sensors
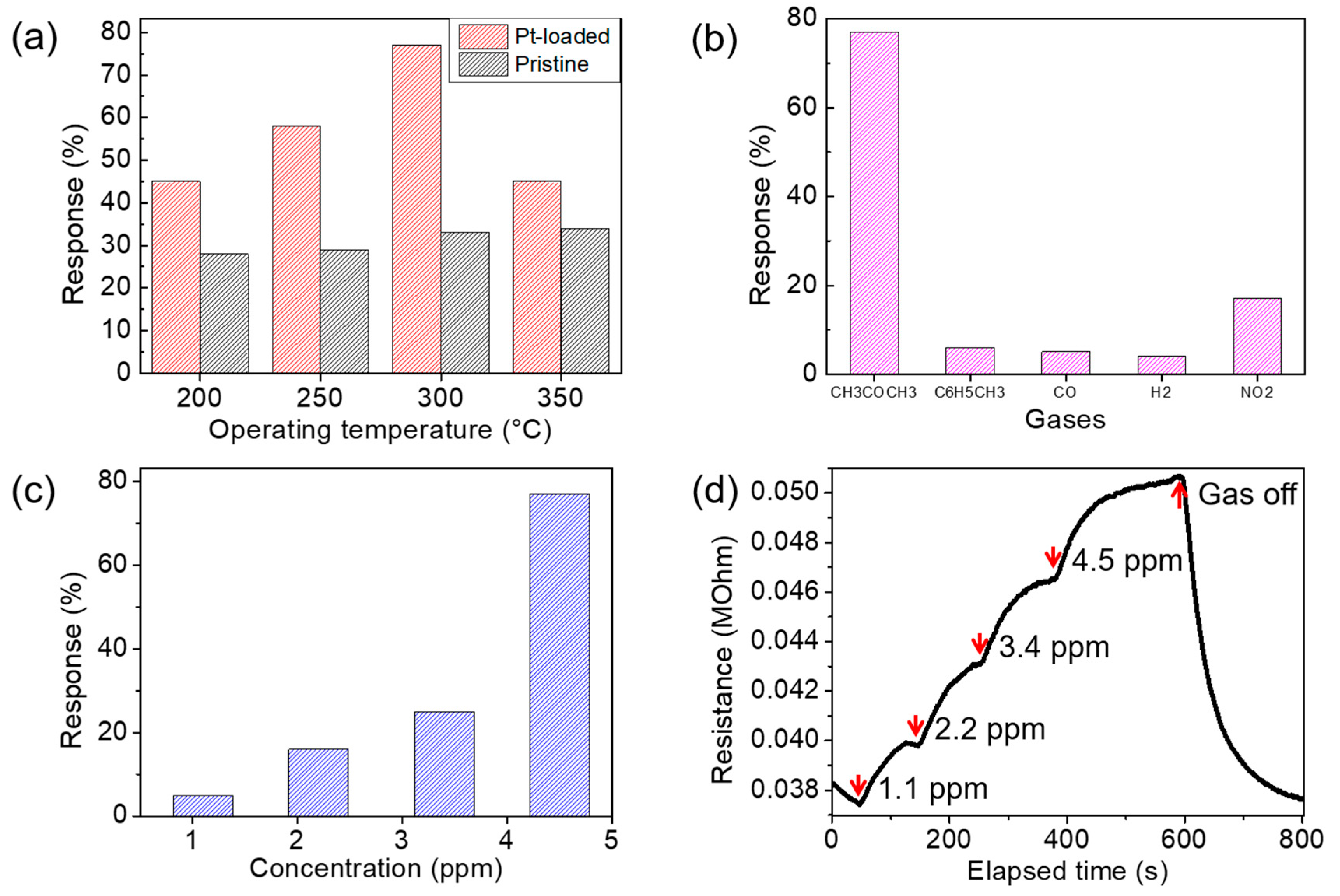
3.4. Gas-Sensing Characteristics of Annealed Co(OH)xF2−x Nanosheet Sensors and Pt-Doping Effect
3.5. Gas-Sensing Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.; Zhu, L.; Wen, Z.; Ye, Z. Controllable synthesis of Co3O4 crossed nanosheet arrays toward an acetone gas sensor. Sens. Actuators B Chem. 2017, 238, 1052–1059. [Google Scholar] [CrossRef]
- Kao, K.-W.; Hsu, M.-C.; Chang, Y.-H.; Gwo, S.; Yeh, J.A. A sub-ppm acetone gas sensor for diabetes detection using 10 nm thick ultrathin InN FETs. Sensors 2012, 12, 7157–7168. [Google Scholar] [CrossRef]
- Xie, Y.; Xing, R.; Li, Q.; Xu, L.; Song, H. Three-dimensional ordered ZnO–CuO inverse opals toward low concentration acetone detection for exhaled breath sensing. Sens. Actuators B Chem. 2015, 211, 255–262. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, T.; Yuan, M.; Kang, M.; Lu, G.; Wang, R.; Fan, H.; He, Y.; Yang, H. Growth and selective acetone detection based on ZnO nanorod arrays. Sens. Actuators B Chem. 2009, 143, 93–98. [Google Scholar] [CrossRef]
- Kou, X.; Xie, N.; Chen, F.; Wang, T.; Guo, L.; Wang, C.; Wang, Q.; Ma, J.; Sun, Y.; Zhang, H. Superior acetone gas sensor based on electrospun SnO2 nanofibers by Rh doping. Sens. Actuators B Chem. 2018, 256, 861–869. [Google Scholar] [CrossRef]
- Koo, W.-T.; Yu, S.; Choi, S.-J.; Jang, J.-S.; Cheong, J.Y.; Kim, I.-D. Nanoscale PdO catalyst functionalized Co3O4 hollow nanocages using MOF templates for selective detection of acetone molecules in exhaled breath. ACS Appl. Mater. Interfaces 2017, 9, 8201–8210. [Google Scholar] [CrossRef]
- Li, W.-Y.; Xu, L.-N.; Chen, J. Co3O4 nanomaterials in lithium-ion batteries and gas sensors. Adv. Funct. Mater. 2005, 15, 851–857. [Google Scholar] [CrossRef]
- Wan, S.; Qi, J.; Zhang, W.; Wang, W.; Zhang, S.; Liu, K.; Zheng, H.; Sun, J.; Wang, S.; Cao, R. Hierarchical Co(OH)F Superstructure Built by Low-Dimensional Substructures for Electrocatalytic Water Oxidation. Adv. Mater. 2017, 29, 1700286. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T.; Deng, J.; Zhang, R.; Lou, Z.; Wang, L. P-type Co3O4 nanomaterials-based gas sensor: Preparation and acetone sensing performance. Sens. Actuators B Chem. 2017, 242, 369–377. [Google Scholar] [CrossRef]
- Kang, J.-S.; Park, J.-S.; An, B.-S.; Yang, C.-W.; Lee, H.-J. Nickel Doping on Cobalt Oxide Thin Film Using by Sputtering Process-a Route for Surface Modification for p-type Metal Oxide Gas Sensors. J. Korean Phys. Soc. 2018, 73, 1867–1872. [Google Scholar] [CrossRef]
- Vladimirova, S.; Krivetskiy, V.; Rumyantseva, M.; Gaskov, A.; Mordvinova, N.; Lebedev, O.; Martyshov, M.; Forsh, P. Co3O4 as p-type material for CO sensing in humid air. Sensors 2017, 17, 2216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, L.; Hou, L.; Chen, W. Fabrication of Co3O4 nanowires assembled on the surface of hollow carbon spheres for acetone gas sensing. Sens. Actuators B Chem. 2019, 291, 130–140. [Google Scholar] [CrossRef]
- Xu, K.; Zhao, W.; Yu, X.; Duan, S.; Zeng, W. MOF-derived Co3O4/Fe2O3 pn hollow cubes for improved acetone sensing characteristics. Phys. E Low Dimens. Syst. Nanostruct. 2020, 118, 113869. [Google Scholar] [CrossRef]
- Barreca, D.; Bekermann, D.; Comini, E.; Devi, A.; Fischer, R.A.; Gasparotto, A.; Gavagnin, M.; Maccato, C.; Sada, C.; Sberveglieri, G. Plasma enhanced-CVD of undoped and fluorine-doped Co3O4 nanosystems for novel gas sensors. Sens. Actuators B Chem. 2011, 160, 79–86. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, X.; Wang, X.; He, J.; Yu, Y.; He, H. Effects of F doping on TiO2 acidic sites and their application in QCM based gas sensors. Sens. Actuators B Chem. 2010, 151, 205–211. [Google Scholar] [CrossRef]
- Song, M.; Zhang, Z.; Li, Q.; Jin, W.; Wu, Z.; Fu, G.; Liu, X. Ni-foam supported Co(OH)F and Co–P nanoarrays for energy-efficient hydrogen production via urea electrolysis. J. Mater. Chem. A 2019, 7, 3697–3703. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Du, G.; Asiri, A.M.; Wang, L.; Li, X.; Wang, H.; Sun, X.; Chen, L.; Zhang, Q. Ultrafine PtO2 nanoparticles coupled with a Co(OH)F nanowire array for enhanced hydrogen evolution. Chem. Commun. 2018, 54, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Yang, X.; Zang, H.-Y.; Wang, Y.-H.; Li, Y.-G. Ultralong needle-like N-doped Co(OH)F on carbon fiber paper with abundant oxygen vacancies as an efficient oxygen evolution reaction catalyst. Mater. Chem. Front. 2018, 2, 2045–2053. [Google Scholar] [CrossRef]
- Wen, Z.; Zhu, L.; Mei, W.; Hu, L.; Li, Y.; Sun, L.; Cai, H.; Ye, Z. Rhombus-shaped Co3O4 nanorod arrays for high-performance gas sensor. Sens. Actuators B Chem. 2013, 186, 172–179. [Google Scholar] [CrossRef]
- Wang, W.; Gu, B.; Liang, L.; Hamilton, W.A. Fabrication of near-infrared photonic crystals using highly-monodispersed submicrometer SiO2 spheres. J. Phys. Chem. B 2003, 107, 12113–12117. [Google Scholar] [CrossRef]
- Simmonov, Y.A.; Kritskii, A.A.; Rychkov, V.N.; Tomashov, V.A. Investigation of the treatment process of associated dioxides of zirconium and silicon by an aqueous solution of ammonium fluoride. Rus. J. Non Ferr. Met. 2010, 51, 320–323. [Google Scholar] [CrossRef]
- Nundy, S.; Eom, T.-Y.; Song, K.-Y.; Park, J.-S.; Lee, H.-J. Hydrothermal synthesis of mesoporous ZnO microspheres as NOX gas sensor materials—Calcination effects on microstructure and sensing performance. Ceram. Int. 2020, 46, 19354–19364. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Martens, W.N.; Frost, R.L. Synthesis and characterization of cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J. Phys. Chem. C 2010, 114, 111–119. [Google Scholar] [CrossRef]
- Kong, L.-B.; Liu, M.-C.; Lang, J.-W.; Liu, M.; Luo, Y.-C.; Kang, L. Porous cobalt hydroxide film electrodeposited on nickel foam with excellent electrochemical capacitive behavior. J. Solid State Electrochem. 2011, 15, 571–577. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Zhang, J.; Xue, T.; Zhao, D.-D.; Li, H.-L. Electrodeposition of ordered mesoporous cobalt hydroxide film from lyotropic liquid crystal media for electrochemical capacitors. J. Mater. Chem. 2008, 18, 905–910. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, R.; Jiao, W.; Ding, G.; Hao, L.; Yang, F.; He, X. MoS2 graphene fiber based gas sensing devices. Carbon 2015, 95, 34–41. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, A.; Chang, H.; Xia, B. Room-temperature high-performance acetone gas sensor based on hydrothermal synthesized SnO2-reduced graphene oxide hybrid composite. RSC Adv. 2015, 5, 3016–3022. [Google Scholar] [CrossRef]
- Yang, C.; Xiao, F.; Wang, J.; Su, X. 3D flower-and 2D sheet-like CuO nanostructures: Microwave-assisted synthesis and application in gas sensors. Sens. Actuators B Chem. 2015, 207, 177–185. [Google Scholar] [CrossRef]
- Liptak, R.; Yang, J.; Kramer, N.; Kortshagen, U.; Campbell, S. Environmental photostability of SF6-etched silicon nanocrystals. Nanotechnology 2012, 23, 395205. [Google Scholar] [CrossRef]
- Adam, F.; Thiam-Seng, C. The Production of Hydrogen by Catalytic Oxidation of Hydrocarbons using a Rice Husk Based Cobalt Phyllosilicate Catalyst. J. Phys. Sci. 2013, 24, 61. [Google Scholar]
- Xu, J.; Zhang, J.; Wang, B.; Liu, F. Shape-regulated synthesis of cobalt oxide and its gas-sensing property. J. Alloys Compd. 2015, 619, 361–367. [Google Scholar] [CrossRef]
- Nguyen, H.; El-Safty, S.A. Meso-and macroporous Co3O4 nanorods for effective VOC gas sensors. J. Phys. Chem. C 2011, 115, 8466–8474. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, T.; Wang, L.; Zhang, T. Metal–organic frameworks-derived hierarchical Co3O4 structures as efficient sensing materials for acetone detection. ACS Appl. Mater. Interfaces 2018, 10, 9765–9773. [Google Scholar] [CrossRef]
- Wang, S.; Cao, J.; Cui, W.; Fan, L.; Li, X.; Li, D. Facile synthesis of bamboo raft-like Co3O4 with enhanced acetone gas sensing performances. J. Alloys Compd. 2018, 758, 45–53. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, Z.; Ye, Z.; Zhu, L. Gas sensors based on ultrathin porous Co3O4 nanosheets to detect acetone at low temperature. RSC Adv. 2015, 5, 59976–59982. [Google Scholar] [CrossRef]
- Lin, Y.; Ji, H.; Shen, Z.; Jia, Q.; Wang, D. Enhanced acetone sensing properties of Co3O4 nanosheets with highly exposed (111) planes. J. Mater. Sci. Mater. Electron. 2016, 27, 2086–2095. [Google Scholar] [CrossRef]
- Khandekar, M.; Tarwal, N.; Mulla, I.; Suryavanshi, S. Nanocrystalline Ce doped CoFe2O4 as an acetone gas sensor. Ceram. Int. 2014, 40, 447–452. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Wu, Z.; Dong, G. Metal-organic frameworks-derived hollow zinc oxide/cobalt oxide nanoheterostructure for highly sensitive acetone sensing. Sens. Actuators B Chem. 2019, 283, 42–51. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, Z.; Ding, D.; Lu, W.; Xue, Q. Multi-shelled ZnCo2O4 yolk-shell spheres for high-performance acetone gas sensor. Appl. Surf. Sci. 2018, 443, 114–121. [Google Scholar] [CrossRef]
- Alali, K.T.; Liu, J.; Liu, Q.; Li, R.; Zhang, H.; Aljebawi, K.; Liu, P.; Wang, J. Enhanced acetone gas sensing response of ZnO/ZnCo2O4 nanotubes synthesized by single capillary electrospinning technology. Sens. Actuators B Chem. 2017, 252, 511–522. [Google Scholar] [CrossRef]
- Qu, F.; Thomas, T.; Zhang, B.; Zhou, X.; Zhang, S.; Ruan, S.; Yang, M. Self-sacrificing templated formation of Co3O4/ZnCo2O4 composite hollow nanostructures for highly sensitive detecting acetone vapor. Sens. Actuators B Chem. 2018, 273, 1202–1210. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Wu, X.-H.; Su, Q.; Li, Y.-F.; Zhou, Z.-L. Ammonia-sensing characteristics of Pt and SiO2 doped SnO2 materials. Solid State Electron. 2001, 45, 347–350. [Google Scholar] [CrossRef]
- Lim, S.K.; Hwang, S.-H.; Kim, S.; Park, H. Preparation of ZnO nanorods by microemulsion synthesis and their application as a CO gas sensor. Sens. Actuators B Chem. 2011, 160, 94–98. [Google Scholar] [CrossRef]
- Ge, Y.; Kan, K.; Yang, Y.; Zhou, L.; Jing, L.; Shen, P.; Li, L.; Shi, K. Highly mesoporous hierarchical nickel and cobalt double hydroxide composite: Fabrication, characterization and ultrafast NOx gas sensors at room temperature. J. Mater. Chem. A 2014, 2, 4961–4969. [Google Scholar] [CrossRef]
- Gao, T.; Wang, T. Synthesis and properties of multipod-shaped ZnO nanorods for gas-sensor applications. Appl. Phys. A 2005, 80, 1451–1454. [Google Scholar] [CrossRef]
- Bie, L.-J.; Yan, X.-N.; Yin, J.; Duan, Y.-Q.; Yuan, Z.-H. Nanopillar ZnO gas sensor for hydrogen and ethanol. Sens. Actuators B Chem. 2007, 126, 604–608. [Google Scholar] [CrossRef]

| Material | Operating Temp. (°C) | Concentration (ppm) | Response (Rg/Ra) | Reference |
|---|---|---|---|---|
| Hierarchical and hexagonal Co3O4 | 240 | 2 | 1.32 | [32] |
| Co3O4 crossed nanosheet arrays | 111 | 20 | 6.8 | [1] |
| Meso- and macroporous Co3O4 nanorods | 300 | 74,570 | 18.5 | [33] |
| Rhombus-shaped Co3O4 nanorod arrays | 160 | 500 | 20.1 | [20] |
| Co3O4 core-shell | 190 | 200 | 13 | [34] |
| Bamboo raft-like Co3O4 | 180 | 200 | 10.5 | [35] |
| Ultrathin porous Co3O4 nanosheets | 150 | 100 | 11.4 | [36] |
| Co3O4 nanosheets | 160 | 100 | 6.1 | [37] |
| Ce-doped CoFe2O4 | 225 | 5000 | 1.77 | [38] |
| Hollow ZnO/Co3O4 | 300 | 1 | 1.5 | [39] |
| Multi-shelled ZnCo2O4 | 200 | 0.5 | 1.36 | [40] |
| PdO Catalyst functionalized Co3O4 hollow nanocages | 350 | 5 | 2.51 | [7] |
| ZnO/ZnCo2O4 nanotubes | 175 | 50 | 15 | [41] |
| Co3O4/ZnCo2O4 | 255 | 100 | 16.3 | [42] |
| Co(OH)xF2−x nanosheets | 200 | 4.5 | 3.69 (269%) | Our study |
| 1.1 | 2.17 (117%) | |||
| 0.04 | 1.17 (17%) | |||
| Pt-doped Co(OH)xF2−x nanosheets-annealed at 300 °C | 300 | 4.5 | 1.77 (77%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Eom, T.-y.; Xu, S.; Yoo, P.J.; Yan, C.; Park, J.-S.; Lee, H.-J. Development of Co(OH)xF2−x Nanosheets for Acetone Gas Sensor Applications: Material Characterization and Sensor Performance Evaluation. Crystals 2020, 10, 968. https://doi.org/10.3390/cryst10110968
Yan Y, Eom T-y, Xu S, Yoo PJ, Yan C, Park J-S, Lee H-J. Development of Co(OH)xF2−x Nanosheets for Acetone Gas Sensor Applications: Material Characterization and Sensor Performance Evaluation. Crystals. 2020; 10(11):968. https://doi.org/10.3390/cryst10110968
Chicago/Turabian StyleYan, Yaping, Tae-yil Eom, Shiyu Xu, Pil J. Yoo, Changzeng Yan, Joon-Shik Park, and Hoo-Jeong Lee. 2020. "Development of Co(OH)xF2−x Nanosheets for Acetone Gas Sensor Applications: Material Characterization and Sensor Performance Evaluation" Crystals 10, no. 11: 968. https://doi.org/10.3390/cryst10110968
APA StyleYan, Y., Eom, T.-y., Xu, S., Yoo, P. J., Yan, C., Park, J.-S., & Lee, H.-J. (2020). Development of Co(OH)xF2−x Nanosheets for Acetone Gas Sensor Applications: Material Characterization and Sensor Performance Evaluation. Crystals, 10(11), 968. https://doi.org/10.3390/cryst10110968








