Study on Co-Crystallization of LCZ696 Using In Situ ATR-FTIR and Imaging
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Apparatus and Analysis Methods
2.3. Synthesis and Characterization of LCZ696 Crystals
2.4. Monitoring the Co-Crystallization of LCZ696 Using ATR-FTIR and Imaging
3. Result and Discussion
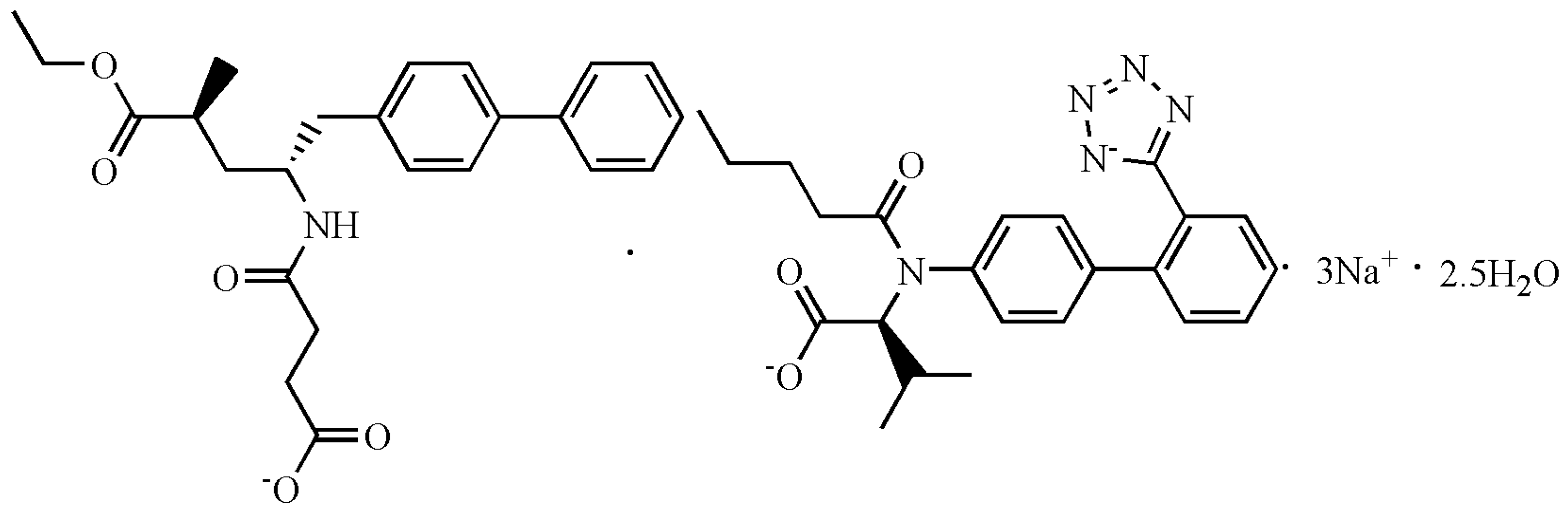
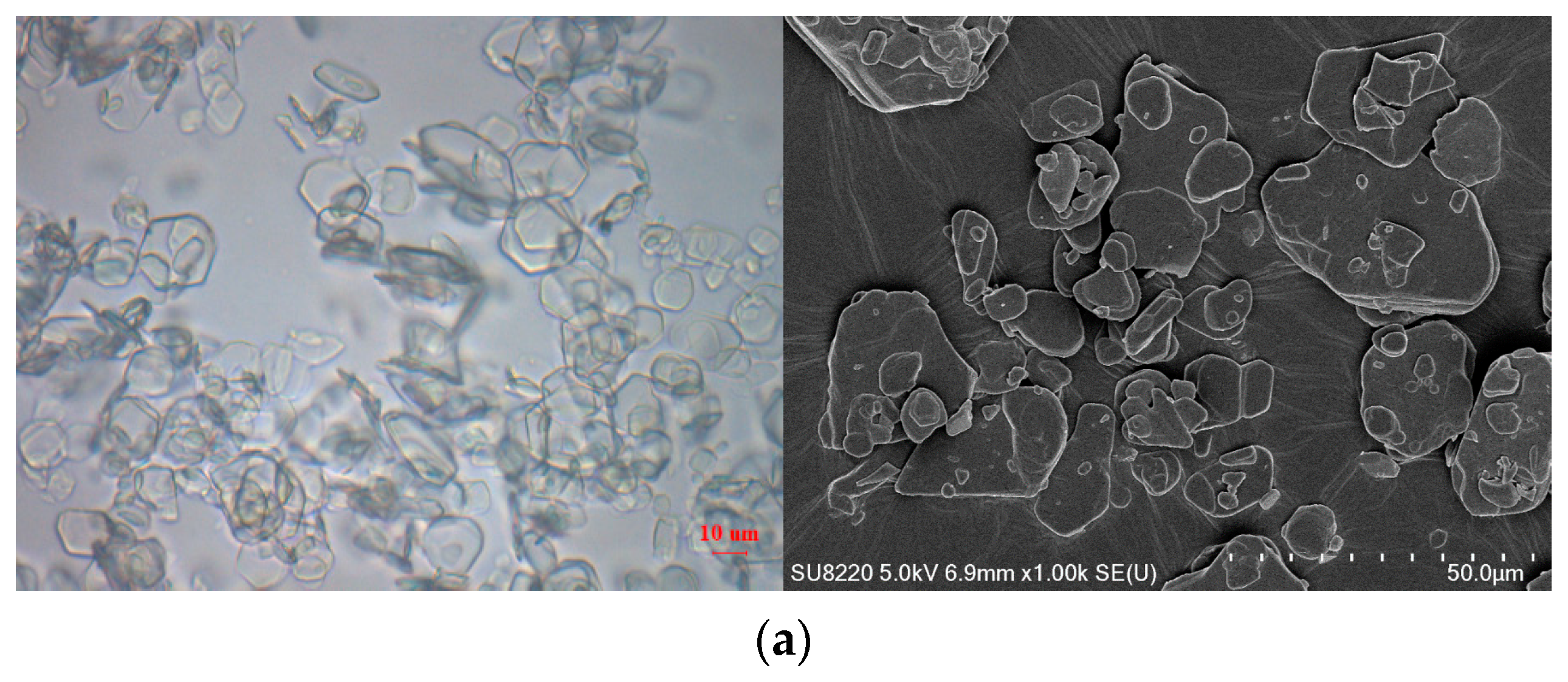
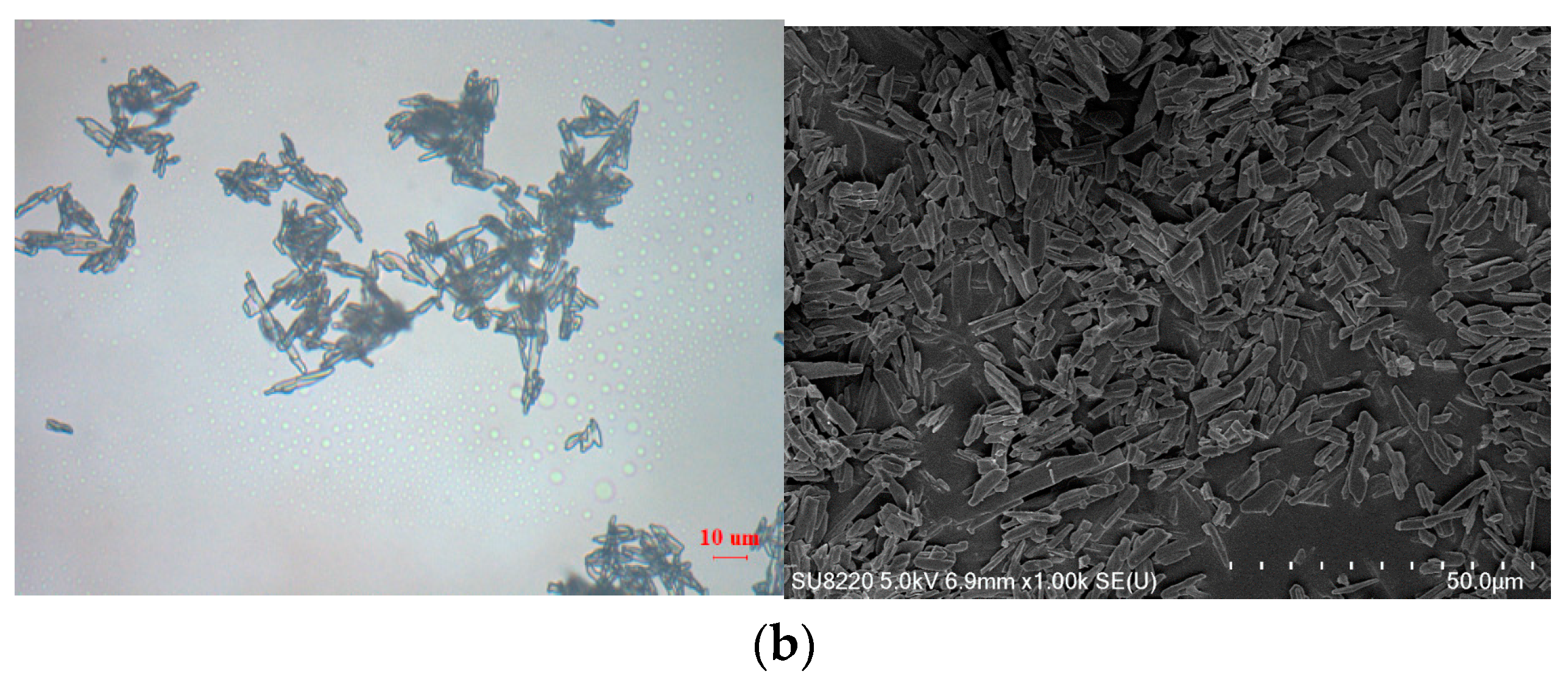
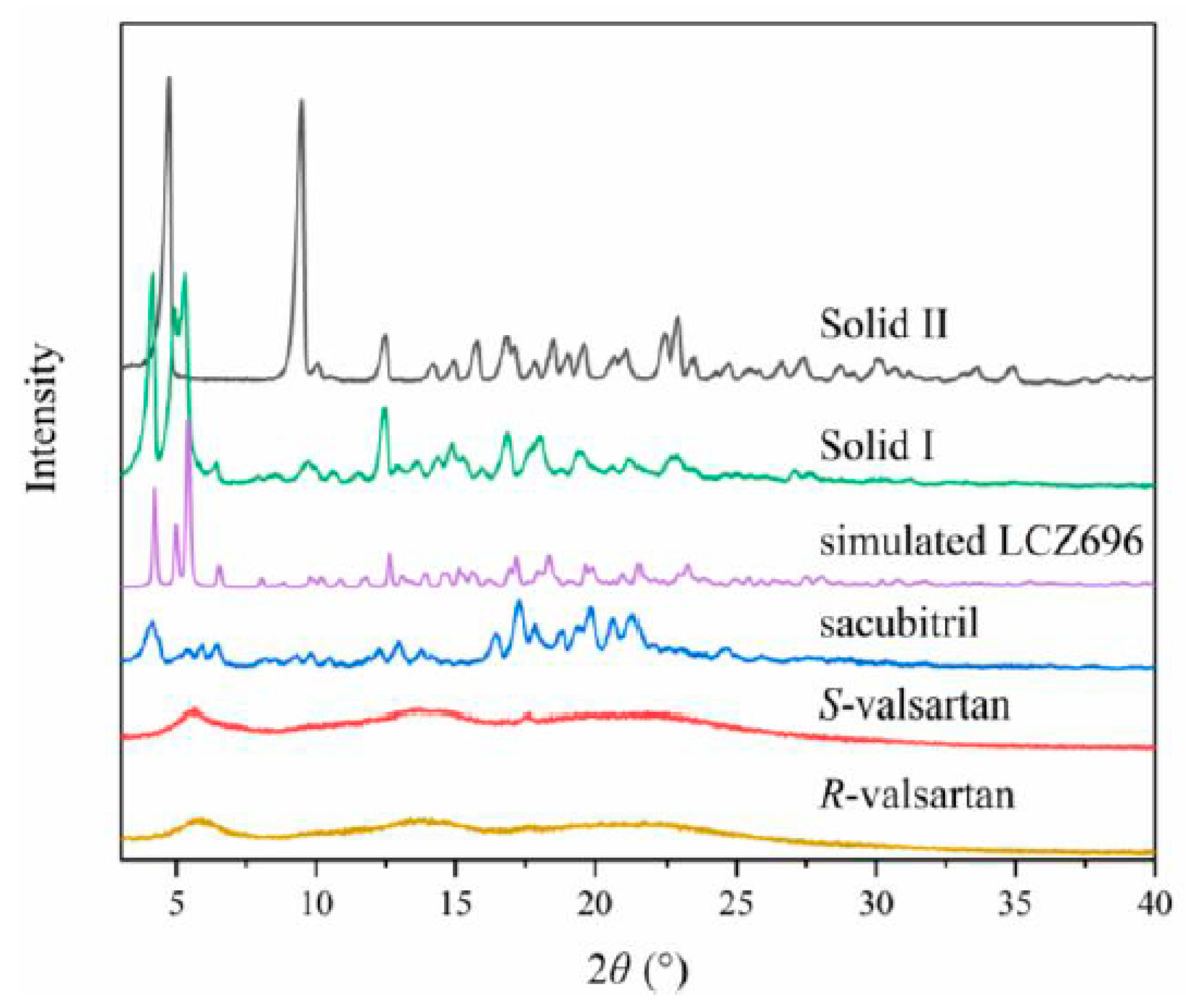
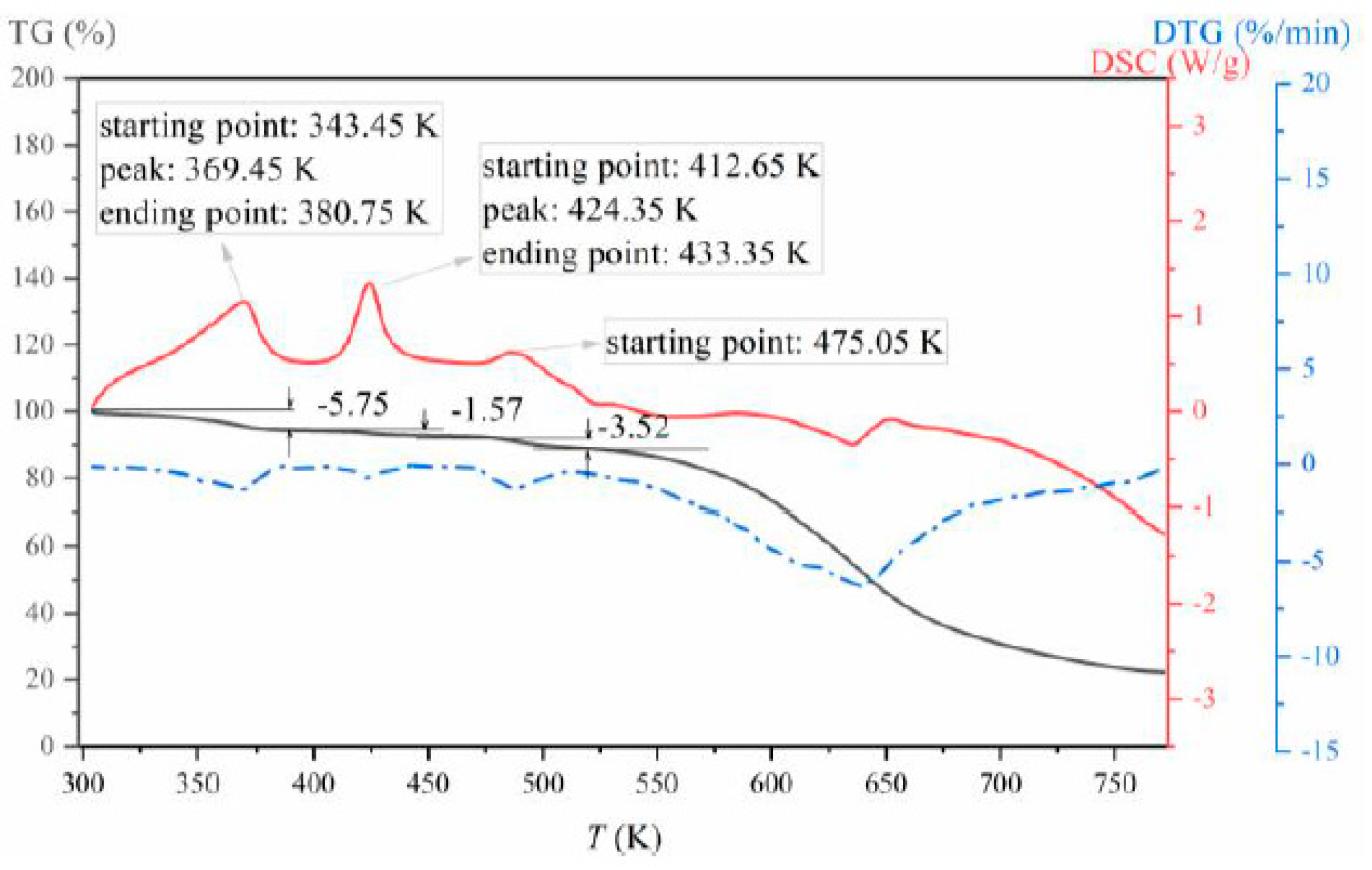
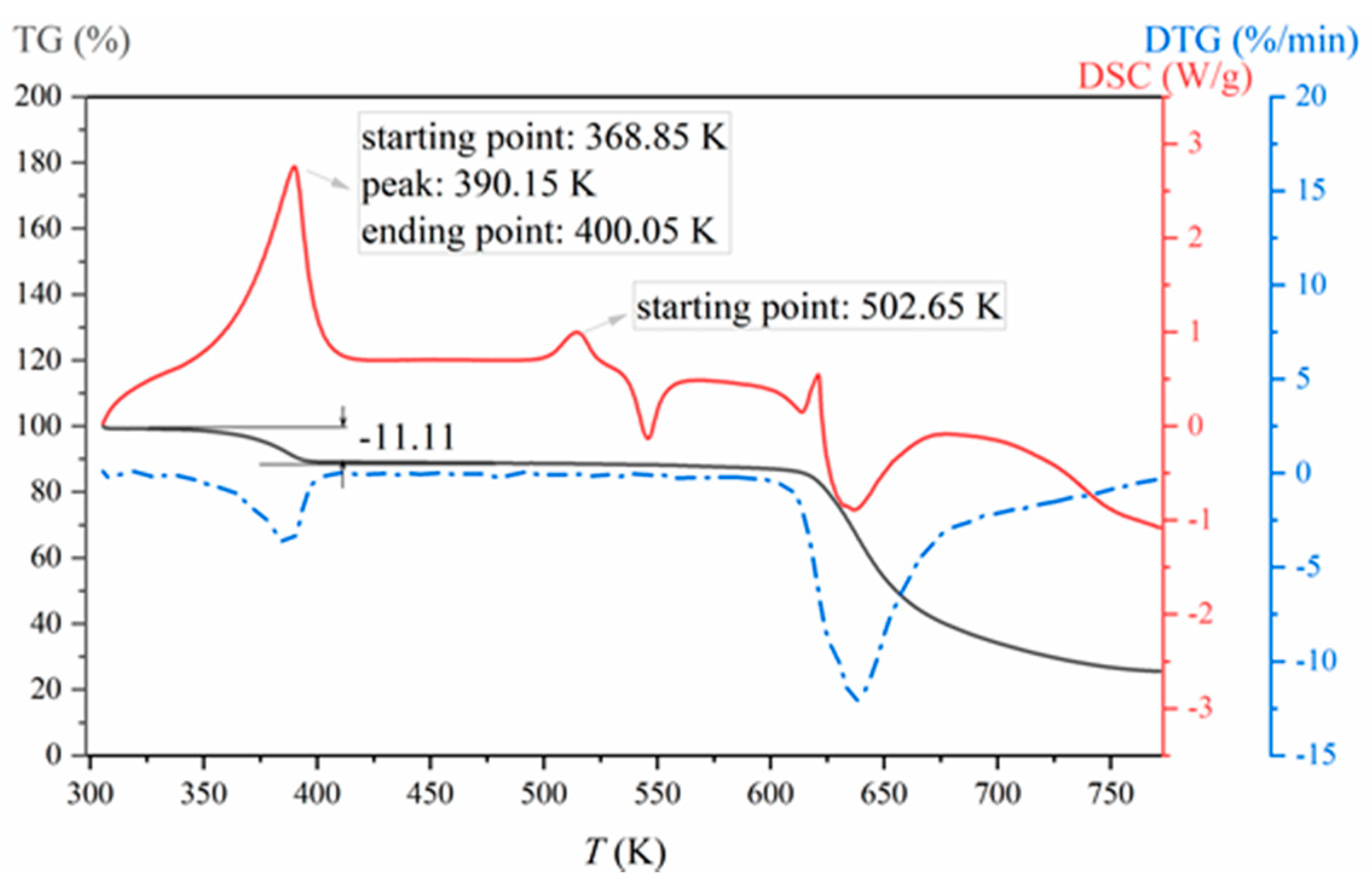
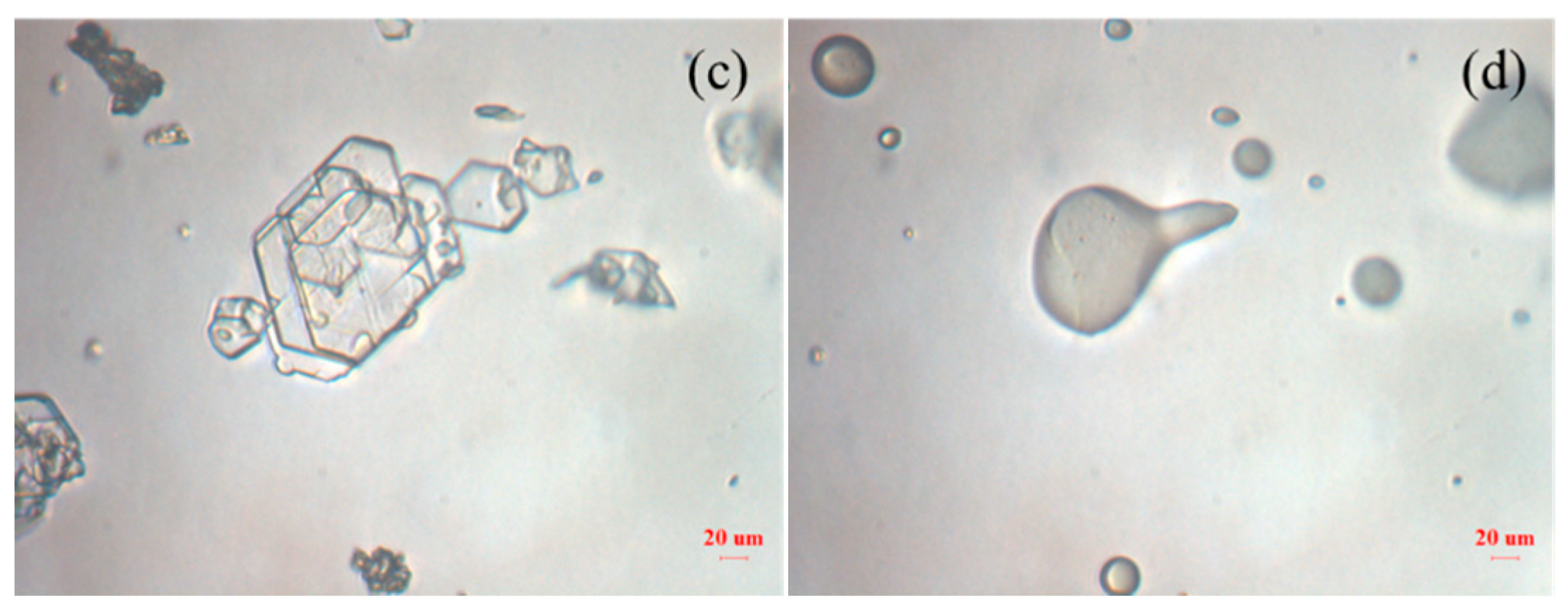
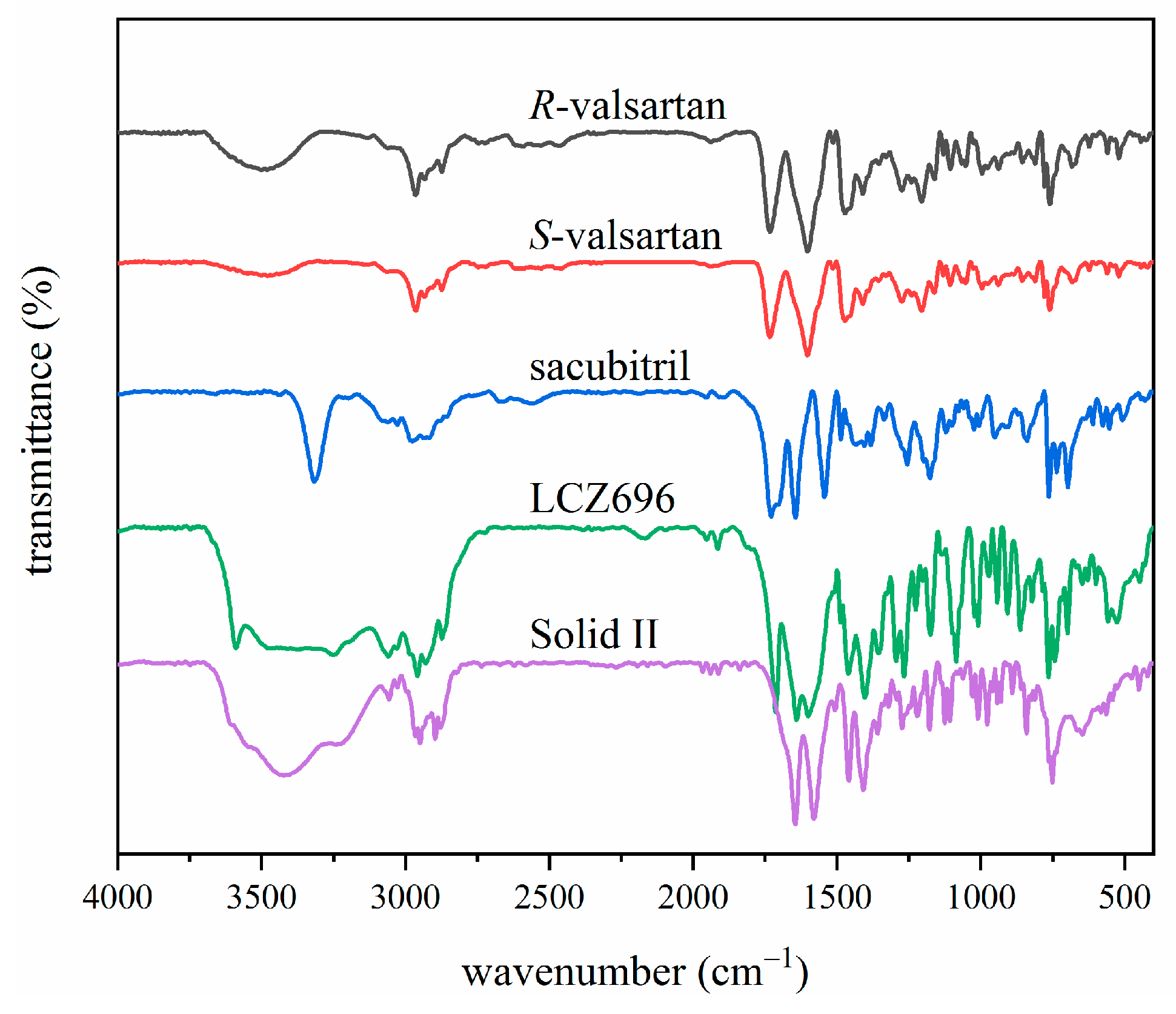
3.1. Formation and Characterization of LCZ696
3.2. ATR-FTIR Model Building
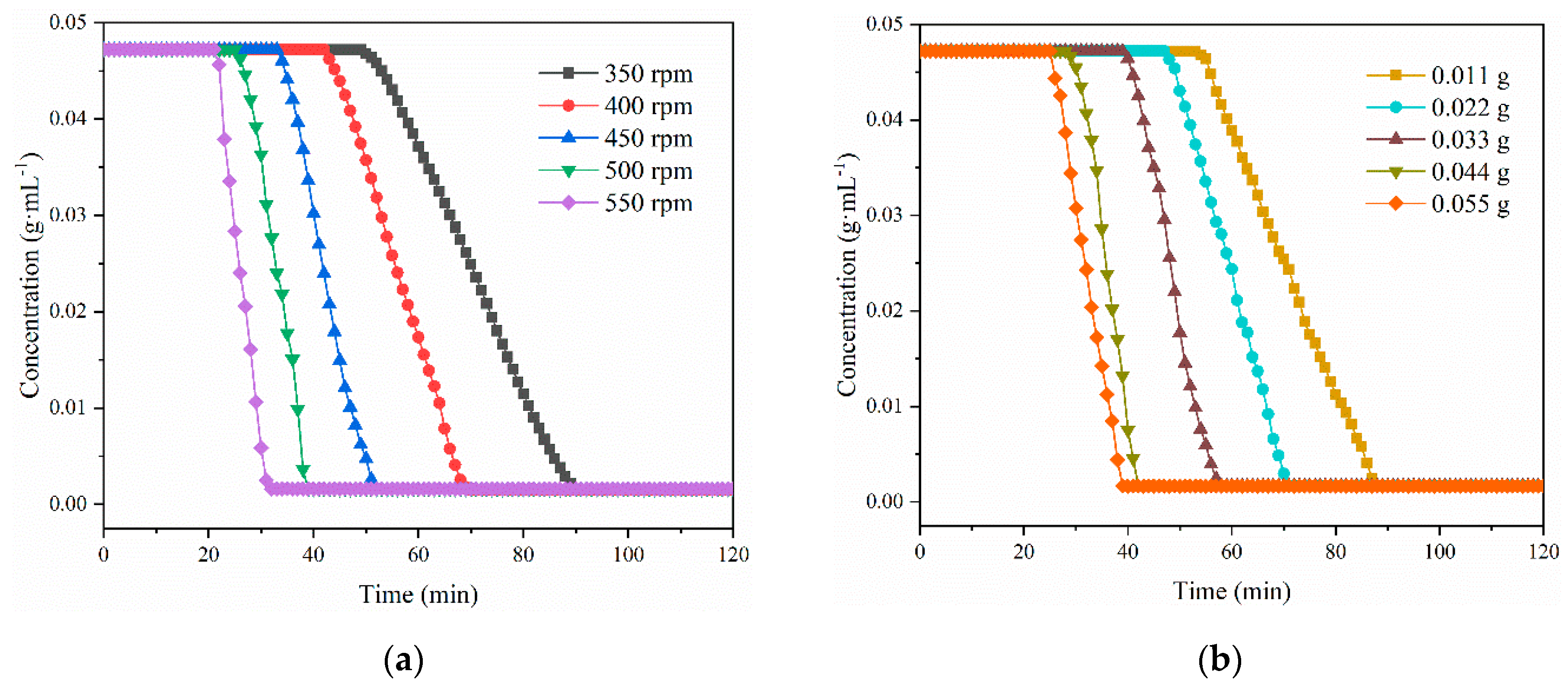
3.3. In Situ Pat Monitoring
3.4. Kinetics of the Co-Crystallization of LCZ696
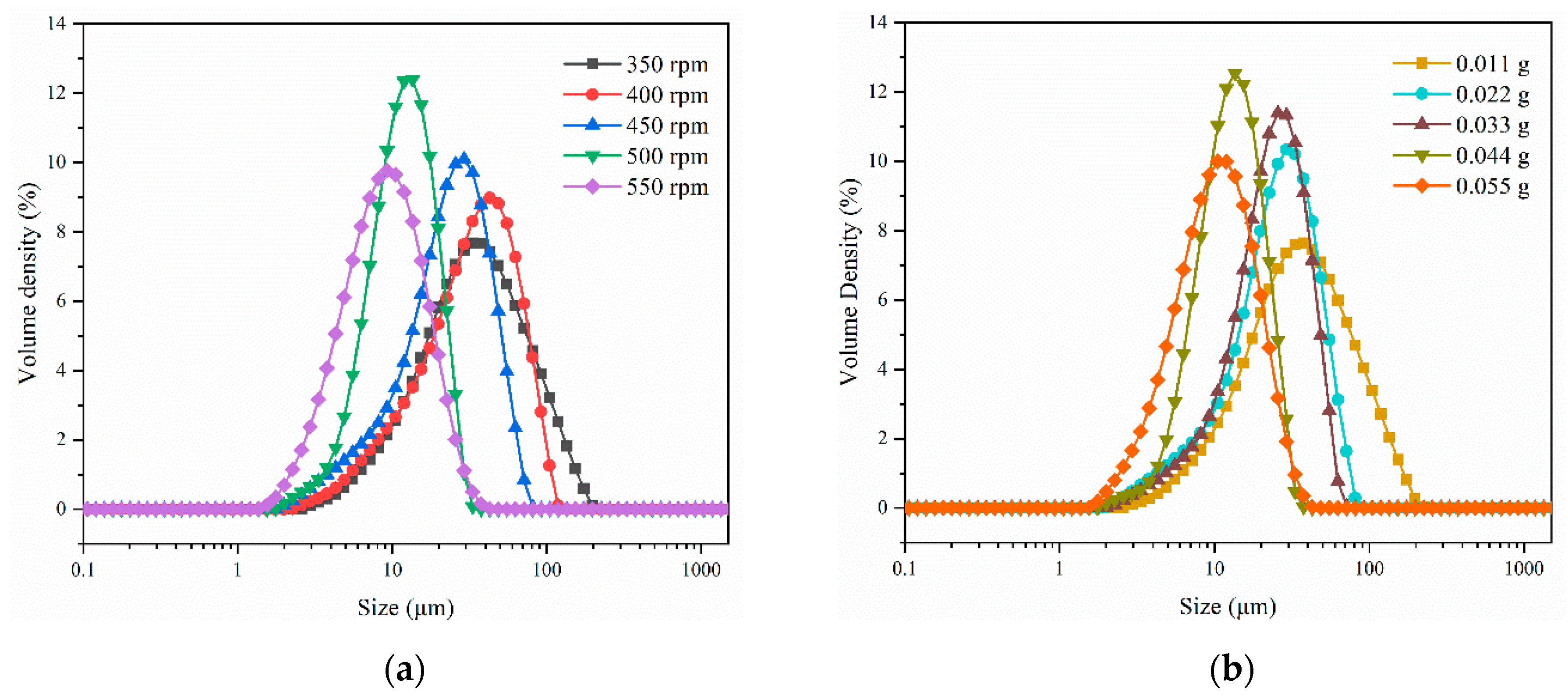
3.5. Effects on Crystal Size Distribution of LCZ696
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.-R.; Wang, X.; Lu, L.; Mei, X. Highly Crystalline Forms of Valsartan with Superior Physicochemical Stability. Cryst. Growth Des. 2013, 13, 3261–3269. [Google Scholar] [CrossRef]
- Flesch, G.; Muller, P.; Lloyd, P. Absolute bioavailability and pharmacokinetics of valsartan, an angiotensin II receptor antagonist, in man. Eur. J. Clin. Pharmacol. 1997, 52, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.R.; Nguyen, N.T.; Lee, Y.J.; Choi, S.; Kang, J.S.; Mar, W.; Kim, K.H. Determination of the R-enantiomer of valsartan in pharmaceutical formulation by capillary electrophoresis. Arch. Pharmacal Res. 2015, 38, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T.; Malachi, O.; Sasson, N.; Gold, A.; Tamas, K.; Omer, M.; Nisim, S.; Amir, G. Preparation of Amorphous Valsartan Useful to Treat Diabetes Related Hypertension and Cancer, Comprising Preparing Solution of Valsartan in Solvent (e.g., Acetonitrile, Toluene and Water) and Removing Solvent. U.S. Patent 2006270723-A1, 30 November 2006. [Google Scholar]
- Yu, S.; Cheng, Y.; Xing, W.; Xue, F. Solubility determination and thermodynamic modelling of gliclazide in five binary solvent mixtures. J. Mol. Liq. 2020, 311, 113258. [Google Scholar] [CrossRef]
- De Matos Jensen, C.E.; Souza dos Santos, R.A.; Leite Denadai, A.M.; Ferreira Santos, C.F.; Goncalves Braga, A.N.; Sinisterra, R.D. Pharmaceutical Composition of Valsartan: β-Cyclodextrin: Physico-Chemical and Characterization Anti-Hypertensive Evaluation. Molecules 2010, 15, 4067–4084. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Xing, W.; Xue, F.; Cheng, Y.; Li, B. Solubility and thermodynamic properties of nimodipine in pure and binary solvents at a series of temperatures. J. Chem. Thermodyn. 2020, 106259. [Google Scholar] [CrossRef]
- Reguri, B.R.; Sunkari, S. New Crystalline Polymorphic Forms (I) and (II) of (S)-N-(1-carboxy-2-methyl-prop-1-yl)-N-pentanoyl-N-(2′-(1H-tetrazol-5-yl)-bi-phenyl-4-yl methyl)amine Useful in the Treatment of e.g., Hypertension, Heart Failure. U.S. Patent 2004072886-A1, 15 April 2004. [Google Scholar]
- Rukhman, I.; Flyaks, E.; Koltai, T.; Aronhime, J. New Amorphous and Various Crystalline Forms of Valsartan Useful in the Treatment of Hypertension. Patent WO2004083192-A1, 30 September 2004. [Google Scholar]
- Burgbacher, J.; Hahn, B.T.; Rampf, F.A.; Schneeberger, R. New Highly Crystalline form of Valsartan, Useful for Treating Hypertension or Elevated Blood Pressure. Patent WO2012016969-A1, 9 February 2012. [Google Scholar]
- Yan, Y.-D.; Sung, J.H.; Kim, K.K.; Kim, D.W.; Kim, J.O.; Lee, B.-J.; Yong, C.S.; Choi, H.-G. Novel valsartan-loaded solid dispersion with enhanced bioavailability and no crystalline changes. Int. J. Pharm. 2012, 422, 202–210. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Xing, W.; Xue, F.; Cheng, Y. Solubility, thermodynamic parameters, and dissolution properties of gliclazide in seventeen pure solvents at temperatures from 278.15 to 318.15 K. J. Mol. Liq. 2020, 312, 113425. [Google Scholar] [CrossRef]
- Yu, S.; Xing, W.; Xue, F.; Cheng, Y.; Liu, Y.; Chen, H.; Hao, C.; Sun, Y. Measurement and Correlation of Solubility and Thermodynamic Properties of Fluoxetine Hydrochloride in 15 Pure Solvents and a Methanol + Water Binary Solvent System. J. Chem. Eng. Data 2020, 65, 4656–4668. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Das, S.; Lal, G.; Soni, S.R.; Ghosh, A.; Reddy, C.M.; Ghosh, A. Screening, crystal structures and solubility studies of a series of multidrug salt hydrates and cocrystals of fenamic acids with trimethoprim and sulfamethazine. J. Mol. Struct. 2020, 1199, 127028. [Google Scholar] [CrossRef]
- Mazur, L.; Materek, I.; Bond, A.D.; Jones, W. Multicomponent Crystal Forms of a Biologically Active Hydrazone with Some Dicarboxylic Acids: Salts or Cocrystals? Cryst. Growth Des. 2019, 19, 2663–2678. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Z.-H.; Guo, W.; Fu, X.-J. Multi-Component Hydrogen-Bonding Assembly of a Pharmaceutical Agent Pamoic Acid with Piperazine or 4,4′-Bipyridyl: A Channel Hydrated Salt with Multiple-Helical Motifs vs a Bimolecular Cocrystal. Cryst. Growth Des. 2009, 9, 1655–1657. [Google Scholar] [CrossRef]
- Atwood, J.L.; Lehn, J.M. Comprehensive supramolecular chemistry. Solid State Supramol. Chem. Cryst. Eng. 1996, 6, 199–206. [Google Scholar]
- Lehn, J. Supramolecular Chemistry: Concepts and Perspectives; Wiley: Hoboken, NJ, USA, 1995; Volume 34. [Google Scholar]
- Aakeröy, C.B.; Salmon, D.J. Building co-crystals with molecular sense and supramolecular sensibility. CrystEngComm 2005, 7, 439–448. [Google Scholar] [CrossRef]
- Feng, L.; Karpiński, P.H.; Sutton, P.; Liu, Y.; Hook, D.F.; Hu, B.; Blacklock, T.J.; Fanwick, P.E.; Prashad, M.; Godtfredsen, S.; et al. LCZ696: A dual-acting sodium supramolecular complex. Tetrahedron Lett. 2012, 53, 275–276. [Google Scholar] [CrossRef]
- Ayalasomayajula, S.; Langenickel, T.; Pal, P.; Boggarapu, S.; Sunkara, G. Clinical Pharmacokinetics of Sacubitril/Valsartan (LCZ696): A Novel Angiotensin Receptor-Neprilysin Inhibitor. Clin. Pharmacokinet. 2017, 56, 1461–1478. [Google Scholar] [CrossRef]
- Gu, J.; Noe, A.; Chandra, R.; Al-Fayoumi, S.; Ligueros, M.; Sarangapani, R.; Maahs, S.; Ksander, G.; Rigel, D.; Jeng, A.; et al. Pharmacokinetic and pharmacodynamic properties of LCZ696, a novel dual-acting angiotensin receptor neprilysin inhibitor (ARNI). J. Hypertens. 2009, 27, S9. [Google Scholar]
- Feng, L.L.; Godtfredsen, S.E.; Karpinski, P. Pharmeceutical Combinations of An Angiotensin Receptor Antagonist and An NEP Inhibitor. Patent WO2007056546, 18 May 2007. [Google Scholar]
- Kulmatycki, K.M.; Langenickel, T.; Ng, D.; Pal, P.; Zhou, W.; Lin, T.-H.; Rajman, I.; Chandra, P.; Sunkara, G. Pharmacokinetics and safety of sacubitril/valsartan (LCZ696) in patients with mild and moderate hepatic impairment. Int. J. Clin. Pharmacol. Ther. 2017, 55, 728–739. [Google Scholar] [CrossRef]
- Kobalava, Z.K.; Kotovskaya, Y.; Averkov, O.; Pavlikova, E.; Moiseev, V.; Albrecht, D.; Chandra, P.; Ayalasomayajula, S.; Prescott, M.F.; Pal, P.; et al. Pharmacodynamic and Pharmacokinetic Profiles of Sacubitril/Valsartan (LCZ696) in Patients with Heart Failure and Reduced Ejection Fraction. Cardiovasc. Ther. 2016, 34, 191–198. [Google Scholar] [CrossRef]
- Li, S.; Xu, W.; Zhai, H. Study on crystallization solvents of sacbitril/valsartan. Guangdong Chem. Ind. 2018, 45, 85–88. [Google Scholar]
- Chang, P.-C.; Lin, S.-F.; Chu, Y.; Wo, H.-T.; Lee, H.-L.; Huang, Y.-C.; Wen, M.-S.; Chou, C.-C. LCZ696 Therapy Reduces Ventricular Tachyarrhythmia Inducibility in a Myocardial Infarction-Induced Heart Failure Rat Model. Cardiovasc. Ther. 2019, 2019, 6032631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, A.S.; McMurray, J.J.; Packer, M.; Swedberg, K.; Rouleau, J.L.; Chen, F.; Gong, J.; Rizkala, A.R.; Brahimi, A.; Claggett, B.; et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur. Heart J. 2015, 36, 1990–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandala, V.S.; Loewus, S.J.; Mehta, M.A. Monitoring Cocrystal Formation via In Situ Solid-State NMR. J. Phys. Chem. Lett. 2014, 5, 3340–3344. [Google Scholar] [CrossRef] [PubMed]
- Sarraguça, M.; Paisana, M.; Pinto, J.F.; Lopes, J.A. Real-time monitoring of cocrystallization processes by solvent evaporation: A near infrared study. Eur. J. Pharm. Sci. 2016, 90, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Hattori, Y.; Ashizawa, K.; Otsuka, M. Kinetics Study of Cocrystal Formation between Indomethacin and Saccharin Using High-Shear Granulation with In Situ Raman Spectroscopy. J. Pharm. Sci. 2019, 108, 3201–3208. [Google Scholar] [CrossRef] [Green Version]
- Kelly, A.; Gough, T.; Dhumal, R.; Halsey, S.; Paradkar, A. Monitoring ibuprofen–nicotinamide cocrystal formation during solvent free continuous cocrystallization (SFCC) using near infrared spectroscopy as a PAT tool. Int. J. Pharm. 2012, 426, 15–20. [Google Scholar] [CrossRef]
- Qiao, N.; Wang, K.; Schlindwein, W.; Davies, A.; Li, M. In situ monitoring of carbamazepine-nicotinamide cocrystal intrinsic dissolution behaviour. Eur. J. Pharm. Biopharm. 2013, 83, 415–426. [Google Scholar] [CrossRef]
- Watari, M. A Review of Online Real-Time Process Analyses of Melt-State Polymer Using the Near-Infrared Spectroscopy and Chemometrics. Appl. Spectrosc. Rev. 2013, 49, 462–491. [Google Scholar] [CrossRef]
- Griffin, D.J.; Grover, M.A.; Kawajiri, Y.; Rousseau, R.W. Combining ATR-FTIR and FBRM for feedback on crystal size. In Proceedings of the 2015 American Control Conference (ACC), Chicago, IL, USA, 1–3 July 2015; pp. 4308–4313. [Google Scholar]
- Yu, S.; Zhang, Y.; Wang, X.Z. Improved Understanding of Cefixime Trihydrate Reactive Crystallization and Process Scale-up with the Aid of PAT. Org. Process. Res. Dev. 2019, 23, 177–188. [Google Scholar] [CrossRef]
- Feng, Y.; Dang, L.; Wei, H. Analyzing Solution Complexation of Cocrystals by Mathematic Models and In-Situ ATR-FTIR Spectroscopy. Cryst. Growth Des. 2012, 12, 2068–2078. [Google Scholar] [CrossRef]
- Gagniere, E.; Mangin, D.; Puel, F.; Bebon, C.; Klein, J.-P.; Monnier, O.; Garcia, E. Cocrystal Formation in Solution: In Situ Solute Concentration Monitoring of the Two Components and Kinetic Pathways. Cryst. Growth Des. 2009, 9, 3376–3383. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, Z.; Yang, E.; Pan, B.; Dang, L.; Wei, H. Insights into Cocrystal Polymorphic Transformation Mechanism of Ethenzamide-Saccharin: A Combined Experimental and Simulative Study. Cryst. Growth Des. 2016, 16, 5118–5126. [Google Scholar] [CrossRef]
- Soares, F.L.F.; Carneiro, R.L. In-line monitoring of cocrystallization process and quantification of carbamazepine-nicotinamide cocrystal using Raman spectroscopy and chemometric tools. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2017, 180, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, C.; Balss, K.; Hartnett, C.O.; Madden, F.; McCarthy, B.; Schaefer, E.; Barnthouse, K.; Rea, R.; O’Mahony, J.; Shivappa, R. Monitoring and Controlling Critical Components in Cell Culture Process Using PAT Tool, Raman Spectroscopy. In Abstracts of Papers of the American Chemical Society, Proceedings of the 257th National Meeting of the American Chemical Society (ACS), Orlando, FL, USA, 31 March–4 April 2019; Abstract 426; American Chemical Society: Washington, DC, USA, 2019; Volume 257. [Google Scholar]
- Yu, Z.-Q.; Chow, P.S.; Tan, R.B.H.; Ang, W.H. Supersaturation Control in Cooling Polymorphic Co-Crystallization of Caffeine and Glutaric Acid. Cryst. Growth Des. 2011, 11, 4525–4532. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, J.-S.; Ulrich, J.; Wang, J.-K. Online Measurement of Hydrocortisone Particles and Improvement of the Crystallization Process. Chem. Eng. Technol. 2009, 32, 1073–1077. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Ryšavá, N.; Spasov, T.; Tichý, L. Isothermal DSC method for evaluation of the kinetics of crystallization in the Ge-Sb-S glassy system. J. Therm. Anal. Calorim. 1987, 32, 1015–1021. [Google Scholar] [CrossRef]
- Azéma, E.; Linero, S.; Estrada, N.; Lizcano, A. Shear strength and microstructure of polydisperse packings: The effect of size span and shape of particle size distribution. Phys. Rev. E 2017, 96, 022902. [Google Scholar] [CrossRef]




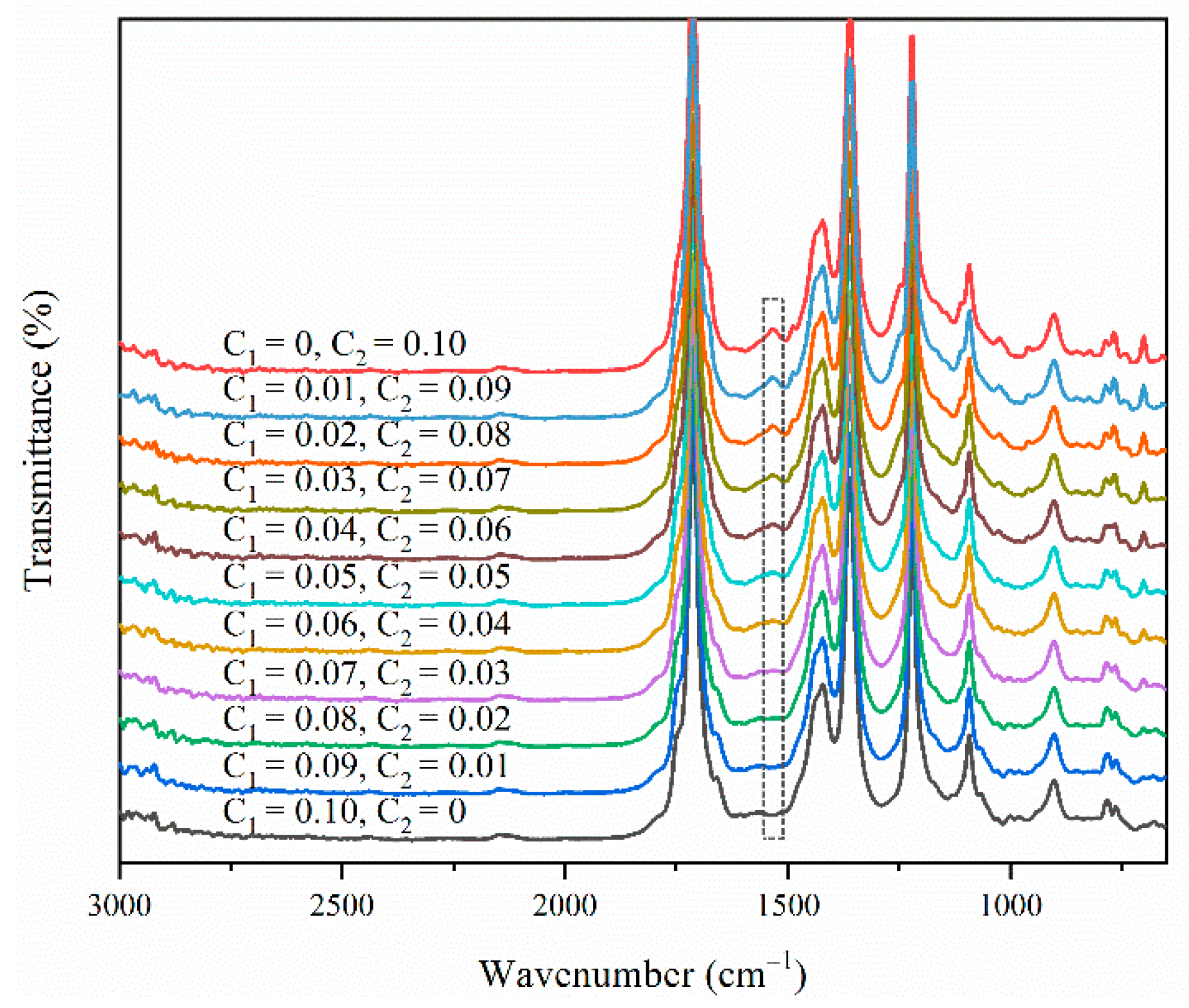

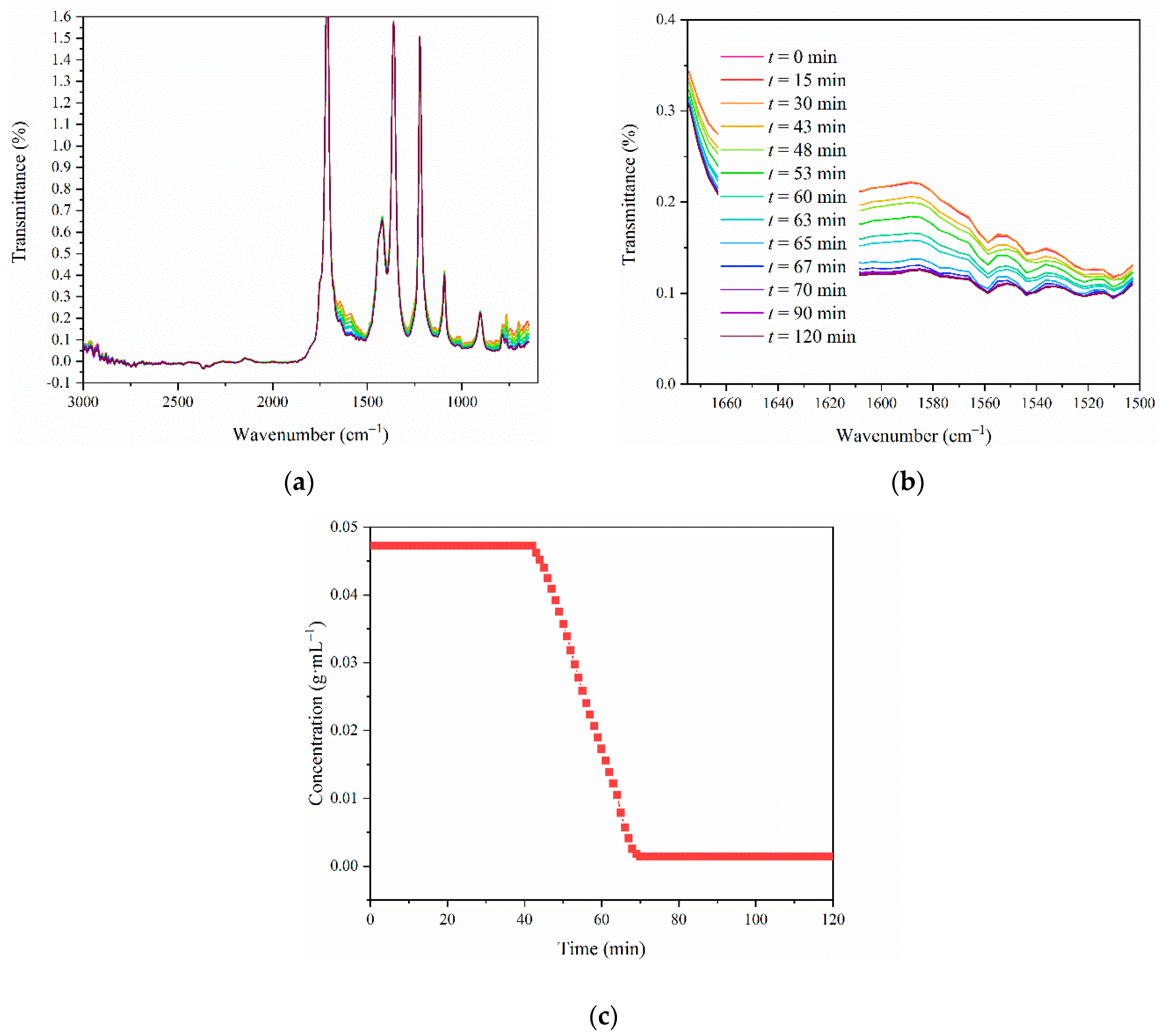
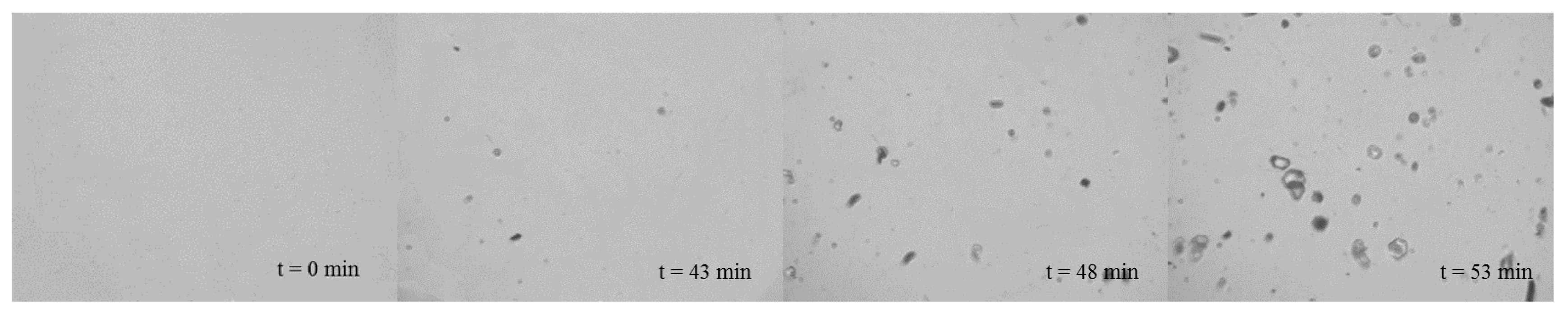
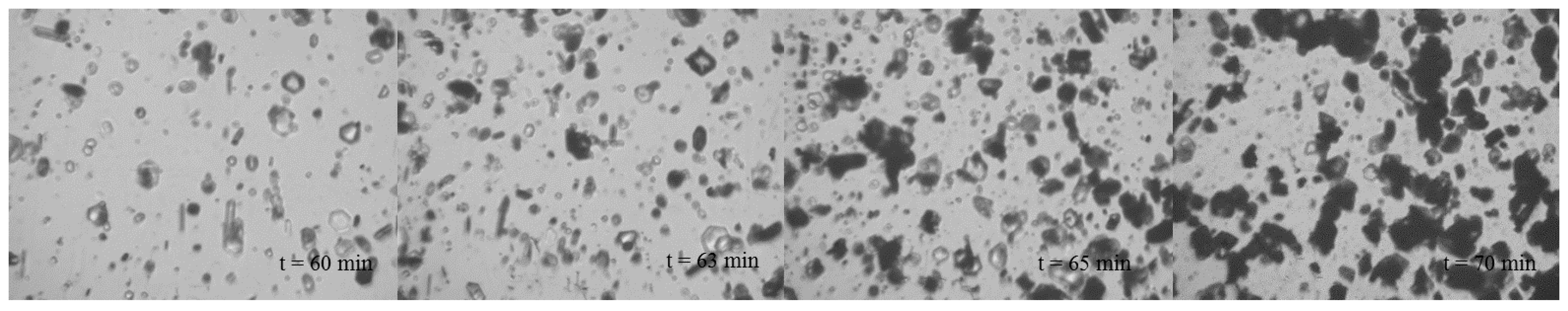
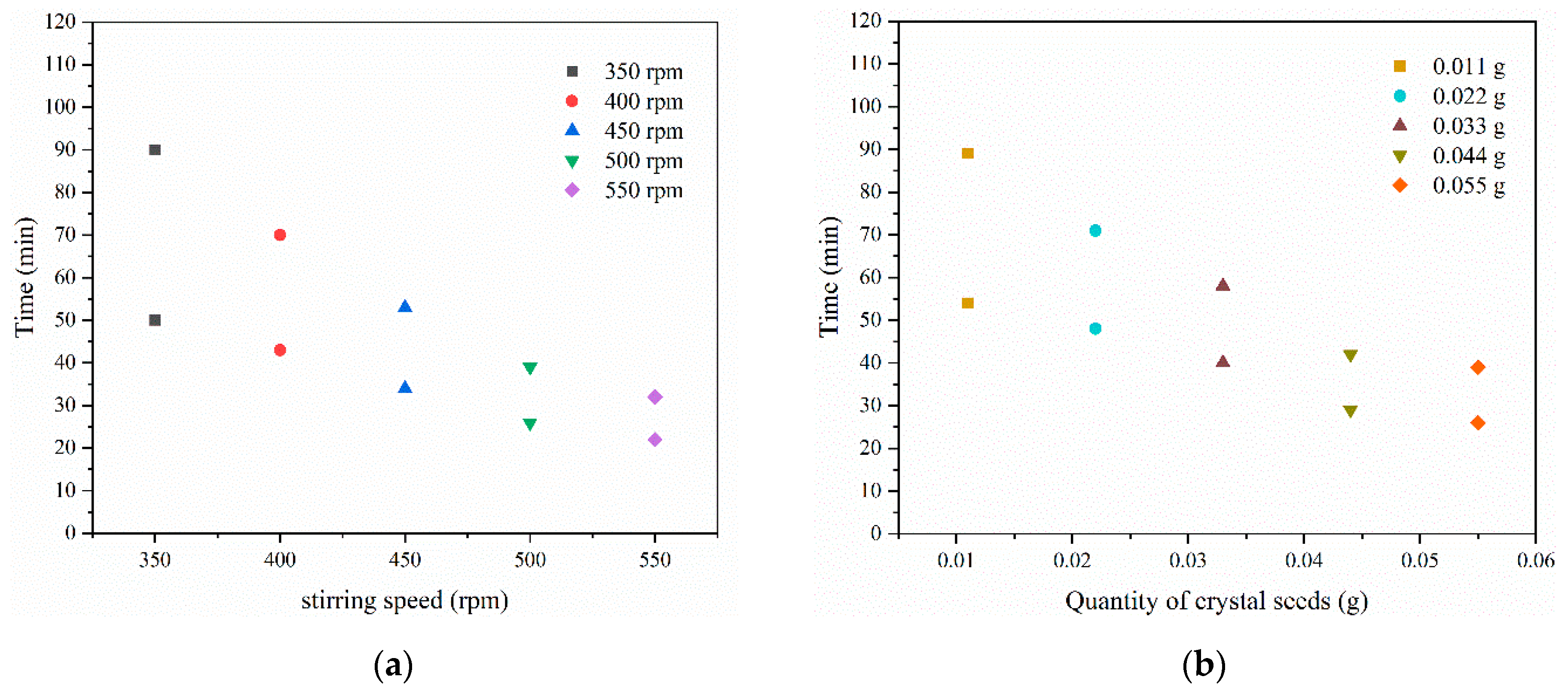

| Experiment | Stirrer Speed/rpm | Quality/g | tind/min | ttrans/min | K/min−3 | n | R2 |
|---|---|---|---|---|---|---|---|
| 1 | 350 | 0 | 50 | 90 | 0.0007 | 1.8219 | 0.9958 |
| 2 | 400 | 0 | 43 | 70 | 0.0010 | 1.3897 | 0.9954 |
| 3 | 450 | 0 | 34 | 53 | 0.0014 | 2.1293 | 0.9973 |
| 4 | 500 | 0 | 26 | 39 | 0.0023 | 1.5927 | 0.9959 |
| 5 | 550 | 0 | 22 | 32 | 0.0034 | 1.4365 | 0.9955 |
| 6 | 400 | 0.011 | 54 | 89 | 0.0008 | 1.6588 | 0.9958 |
| 7 | 400 | 0.022 | 48 | 71 | 0.0012 | 1.8750 | 0.9955 |
| 8 | 400 | 0.033 | 40 | 58 | 0.0016 | 1.7623 | 0.9915 |
| 9 | 400 | 0.044 | 29 | 42 | 0.0024 | 1.7897 | 0.9979 |
| 10 | 400 | 0.055 | 26 | 39 | 0.0027 | 2.7788 | 0.9939 |
| Experiment | Stirrer Speed/rpm | Quality/g | D10 | D50 | RSD (D50) | D90 | Span |
|---|---|---|---|---|---|---|---|
| 1 | 350 | 0 | 11.4 | 34 | 0.42 | 89.9 | 2.308 |
| 2 | 400 | 0 | 3.84 | 8.83 | 0.53 | 18.4 | 1.648 |
| 3 | 450 | 0 | 9.3 | 34.6 | 0.38 | 68.3 | 1.705 |
| 4 | 500 | 0 | 5.93 | 11.8 | 0.81 | 20.5 | 1.234 |
| 5 | 550 | 0 | 7.86 | 23.7 | 0.29 | 46.4 | 1.626 |
| 6 | 400 | 0.011 | 11.7 | 35.1 | 0.35 | 93.8 | 2.339 |
| 7 | 400 | 0.022 | 10.3 | 33.6 | 0.37 | 70.4 | 1.788 |
| 8 | 400 | 0.033 | 4.42 | 10.3 | 0.43 | 20.7 | 1.580 |
| 9 | 400 | 0.044 | 6.54 | 12.8 | 0.70 | 22.3 | 1.231 |
| 10 | 400 | 0.055 | 9.44 | 23.6 | 0.18 | 42.8 | 1.413 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.J.; Zhang, Y.; Wang, X.Z. Study on Co-Crystallization of LCZ696 Using In Situ ATR-FTIR and Imaging. Crystals 2020, 10, 922. https://doi.org/10.3390/cryst10100922
Liu XJ, Zhang Y, Wang XZ. Study on Co-Crystallization of LCZ696 Using In Situ ATR-FTIR and Imaging. Crystals. 2020; 10(10):922. https://doi.org/10.3390/cryst10100922
Chicago/Turabian StyleLiu, Xiao Juan, Yang Zhang, and Xue Zhong Wang. 2020. "Study on Co-Crystallization of LCZ696 Using In Situ ATR-FTIR and Imaging" Crystals 10, no. 10: 922. https://doi.org/10.3390/cryst10100922
APA StyleLiu, X. J., Zhang, Y., & Wang, X. Z. (2020). Study on Co-Crystallization of LCZ696 Using In Situ ATR-FTIR and Imaging. Crystals, 10(10), 922. https://doi.org/10.3390/cryst10100922





