3.1. Thermodynamic Analysis
As shown in
Figure 1 and
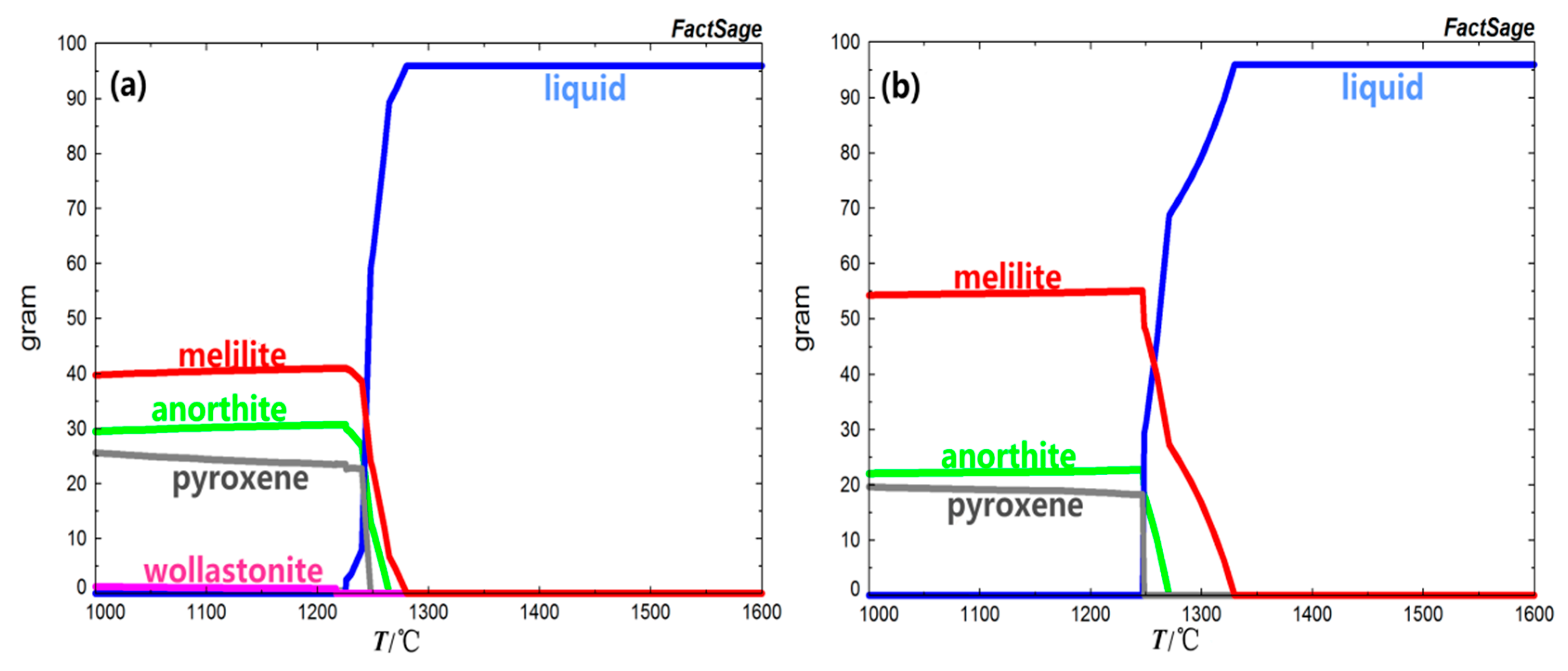
Figure 2, when the basicity was 0.7, the liquid phase of BFS decreased gradually with the decrease of temperature. Finally, the liquid phase completely disappeared and the melilite (Ca
2(MgAl)(AlSi)SiO
7), anorthite (CaAl
2Si
2O
8), pyroxene (Ca(Mg,Al)(Si,Al)
2O
6), and wollastonite (CaSiO
3) were precipitated at the same time. The melilite phase was precipitated at 1280.40 °C firstly, and then the anorthite, pyroxene, and wollastonite were precipitated at 1264.70 °C 1248.00 °C, and 1216.60 °C respectively. At 1205.40 °C the crystallization phases were completely precipitated, and the precipitation amount of melilite, anorthite, pyroxene, and wollastonite was 39.69%, 29.42%, 25.53%, and 1.24%, respectively.
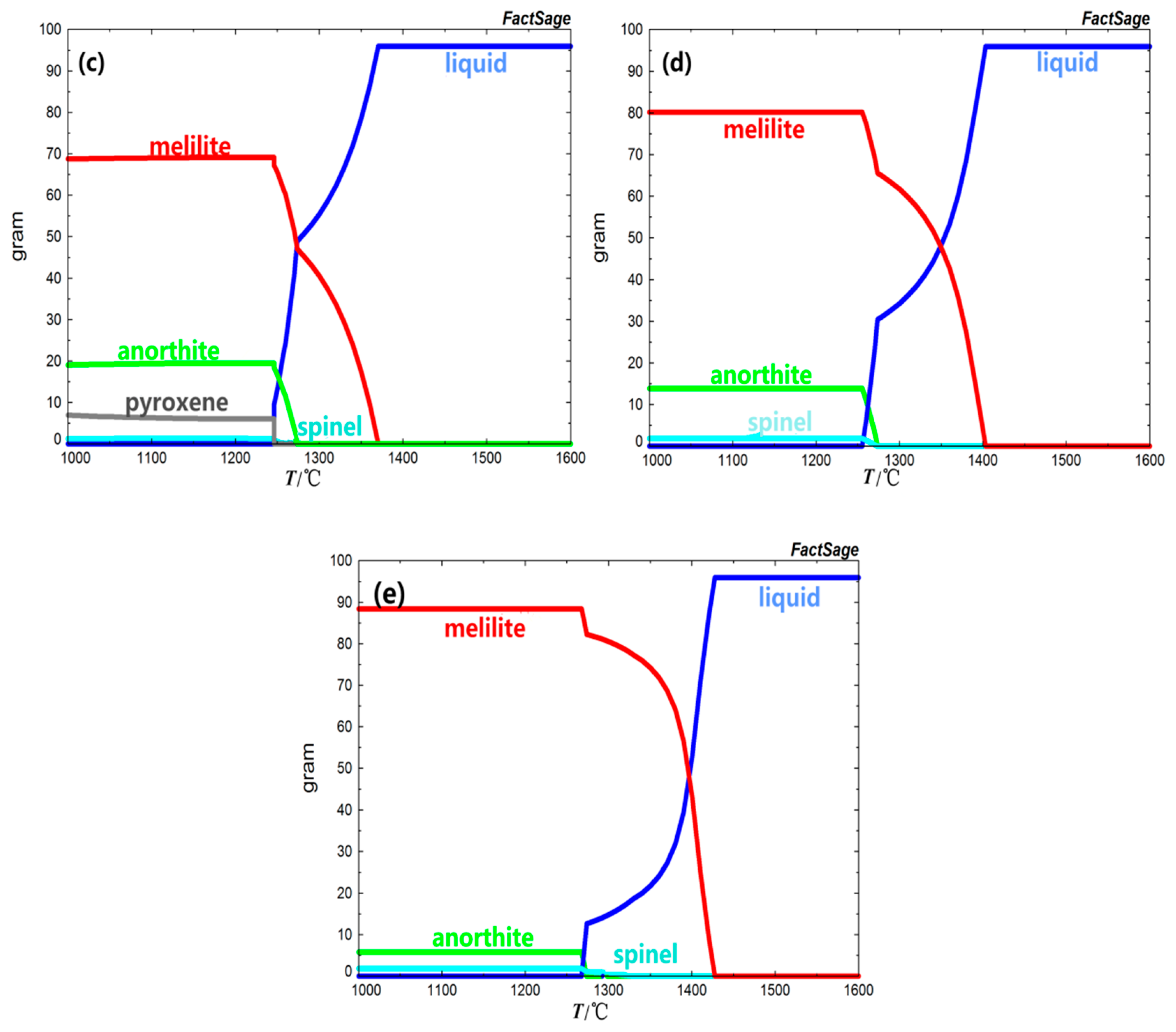
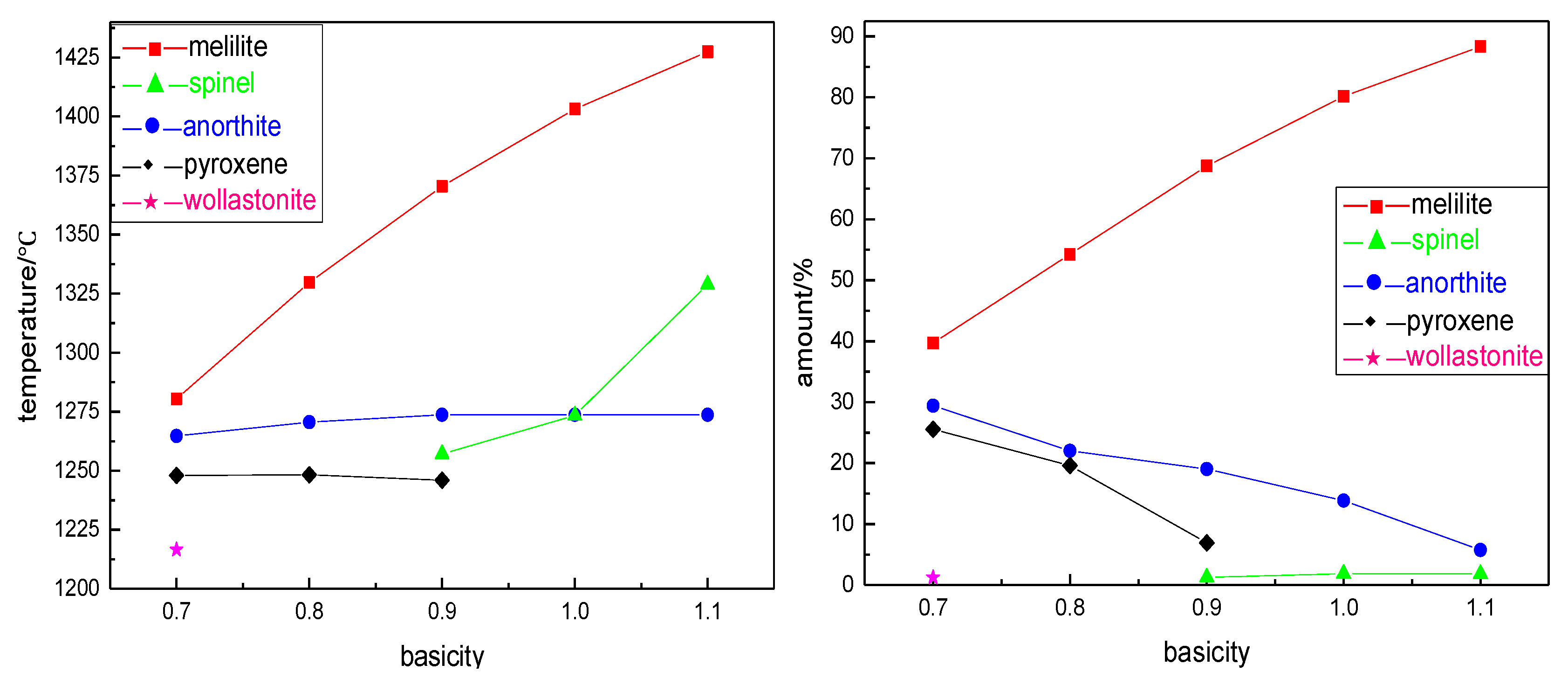
With the increase of basicity, due to the relative decrease of Mg
2+ and Si
2+ in the slag, the precipitation amount of pyroxene and wollastonite in BFS decreased gradually, and did not precipitate when the basicity was 0.9. The spinel began to crystallize when the basicity increased to 0.9. At the same time, the precipitation amount of melilite and spinel increased from 39.69% and 1.24% to 88.32% and 1.83% with the increase of basicity, respectively. The precipitation amount of anorthite decreased from 29.42% to 5.74%. The initial crystallization phases were all melilite and the crystallization temperature of BFS were 1280.40 °C, 1329.70 °C, 1370.4 °C, 1403.20 °C, and 1427.40 °C with the increase of basicity. The initial crystallization temperature increased with the increase of basicity. That was to say, the higher the basicity was, the easier it was to reach the crystallization temperature, and the faster the crystal precipitated in the cooling process. The crystallization precipitation in slag was closely related to the degree of polymerization of negative ion groups [
15]. From
Table 3, the slag viscosity had a significant decrease trend with the increase of basicity. Therefore, the increase of basicity was more likely to cause the relative movement between slag molecules, thus making the molecules structural unit rearrange. At the same time, the amount of the compound anionic group in the slag was relatively decreased, so that the degree of polymerization of the slag was obviously increased and promoted the precipitation of the crystals.
According to the Bowen’s reaction series [
16], the precipitation of the crystallization phase in minerals has a certain order because of the gradual change of composition during gradual condensation of mineral temperature from high to low, thus the precipitation sequence of crystallization phase in slag was obtained by analyzing the components of slag. When the basicity was lower than 0.7, the melilite phase was precipitated firstly in the BFS, the slag consumed more Ca
2+ and still had more Si
2+, which promoted the precipitation of the anorthite phase, and then the pyroxene phase precipitated, and the final phase was wollastonite. When the basicity increased to 0.8, the initial crystal phase was still melilite, and then anorthite and pyroxene were respectively precipitated. Due to the increase of the basicity, the Si
2+ content in the slag was relatively decreased, and the wollatonite was no longer precipitated in the slag. When the basicity of slag increased to 0.9, spinel appeared in the crystal phase of slag and the precipitation order was before pyroxene. The reason might be that the decrease of Si
2+ content made it insufficient to provide enough Si
2+ in slag to form pyroxene, and the Mg
2+ and Al
3+ contents in slag occupied the majority, which promoted the precipitation of spinel first. When basicity increased to 1.0, the slag first precipitated melilite, and then precipitated anorthite. With the further decrease of Si
2+ content, there was no pyroxene precipitation in slag. When basicity increased to 1.1, the Ca
2+ content in the slag increased relatively, which promoted the precipitation of melilite, but with the decrease of Si
2+ content, it was not enough to precipitate anorthite in the slag. After the precipitation of melilite, the Mg
2+ and Al
3+ contents in the slag accounted for the majority, so the spinel precipitated first and the anorthite phase was precipitated last.
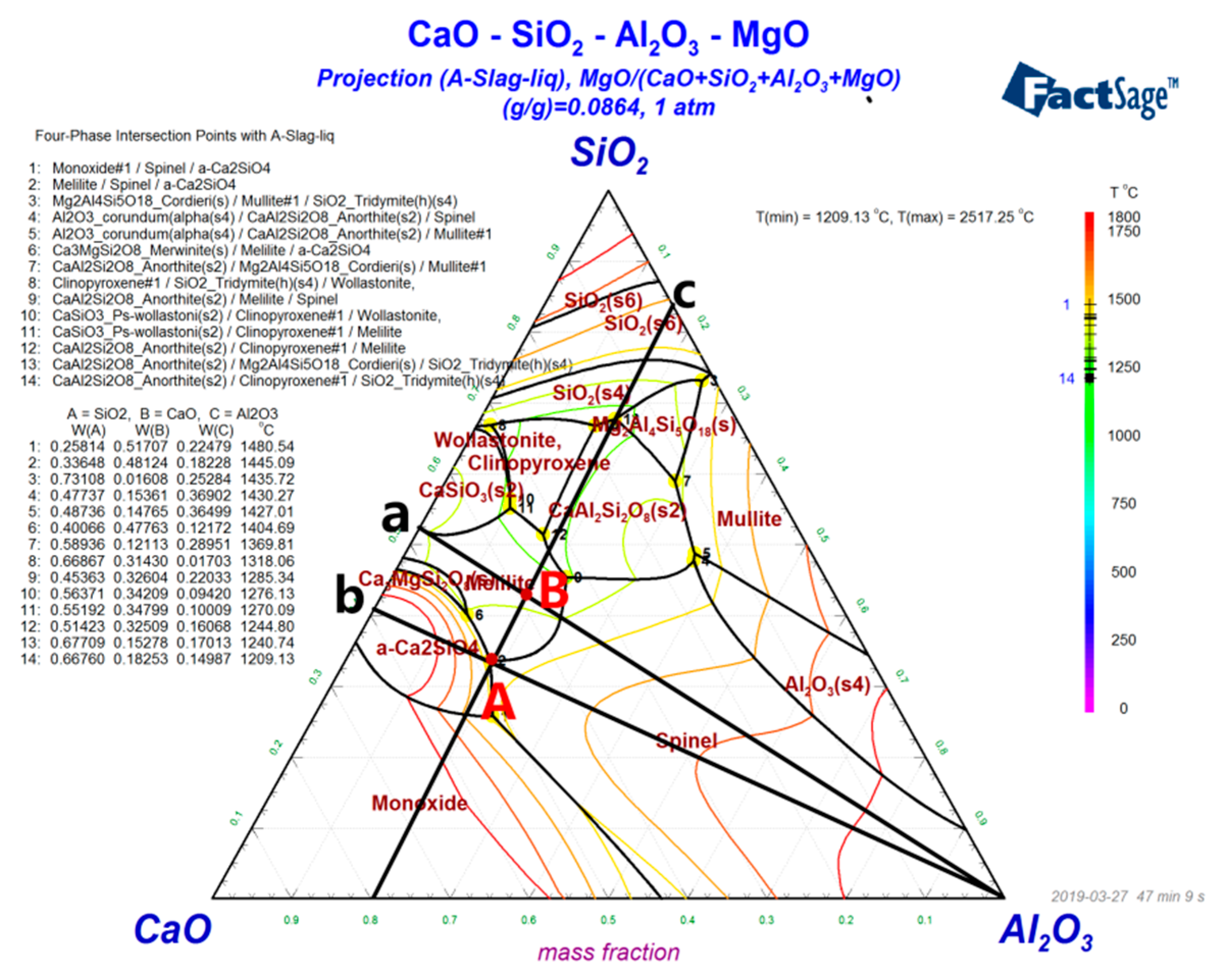
The phase diagram of BFS with different components was drawn using Factsage in order to better understand the influence of different basicities on crystallization. From
Figure 3, with the basicity ranging 0.7–1.1, the BFS components region moved gradually from the B components’ point melilite low melting point region to the A components’ point dicalcium silicate high melting point region. From the temperature strip on the right, we could see that the isotherm of point B was lower than that of point A. Therefore, the precipitation temperature of the initial crystallization phase increased as the basicity increased from 0.7 to 1.1. In conclusion, the smaller basicity would be more beneficial to the preparation of amorphous slag beads.
3.2. Determination of the Mineral Phase
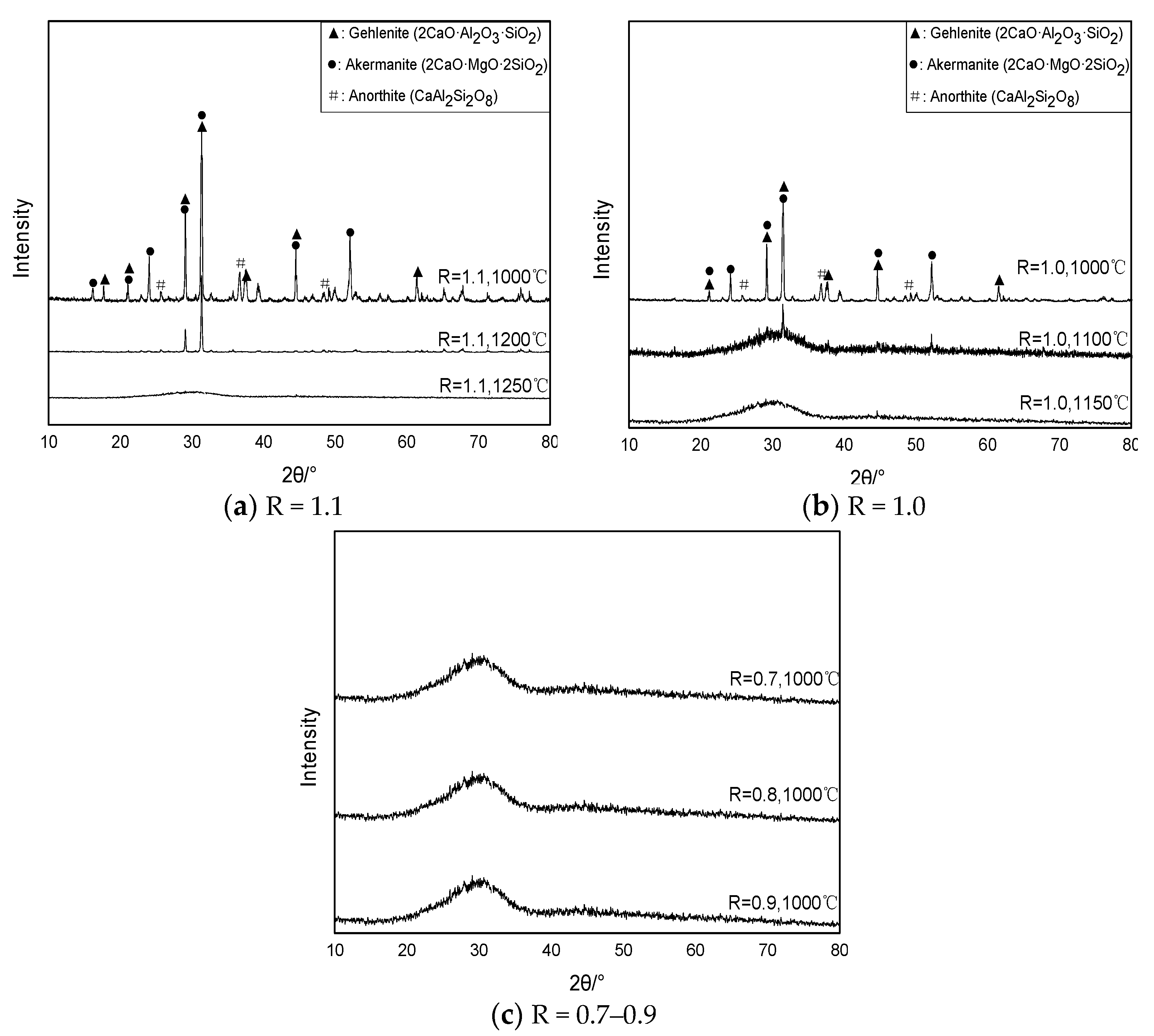
In order to verify the accuracy of thermodynamic simulation results, the BFS samples with different basicities were cooled at specified temperatures, and the mineral phase analysis was carried out. As can be seen from
Figure 4a, when the sample with 1.1 basicity was cooled to 1250 °C, there was still no mineral phase precipitation, and the mineral phase was basically amorphous phase. When the temperature continued to cool down to 1200 °C, a small amount of gehlenite and akermanite phase began to precipitate. When the temperature continued to cool to 1000 °C, a large amount of crystallization phase began to precipitate, and the main crystallization phase was the melilite and the anothite. The intensity of the diffraction peak of the melilite was large and the quantity was high, and the precipitation amount occupied the majority. When the basicity decreased to 1.0 in
Figure 4b, the precipitation phase was almost amorphous phase when the samples cooled to 1150 °C, and when the temperature continued to decrease to 1100 °C, the melilite phase began to precipitate; when the temperature decreased to 1000 °C, a large amount of crystallization phase was precipitated in the BFS, and the main crystallization phase was the melilite and the anothite. There was no spinel phase precipitation in the slag. It might be that at the specified temperature, spinel phase was not precipitated in the cooling process of basicity 1.0 and 1.1, or the diffraction peak was not formed for the lesser precipitation amount. When the basicity continued to decrease to 0.9–0.7, there was no crystallization precipitation of BFS in
Figure 4c, which indicated that the precipitation temperature of the initial crystallization phase was below 1000 °C.
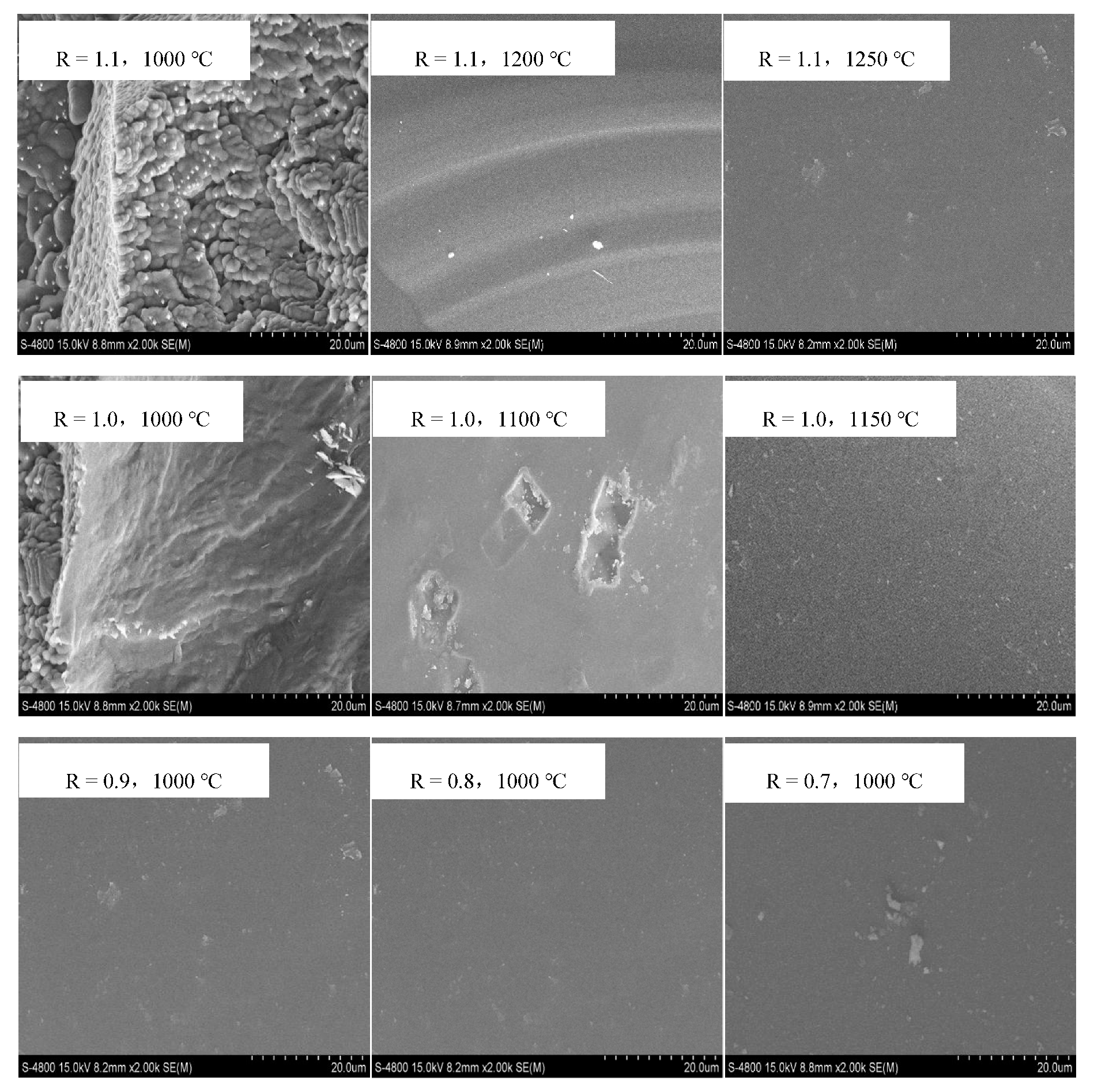
Figure 5 shows the microstructure of BFS with different basicities cooling to the specified temperatures. When the basicity was 1.1, there was basically no crystallization precipitation when the slag was cooled to 1250 °C, and the surface of the slag was smooth glass phase. When the temperature cooled to 1200 °C, the strip crystallization phase began to precipitate, but there were still more amorphous phases and the surface was still smooth. When the temperature decreased to 1000 °C, a large number of bulk crystallization phases were precipitated from the BFS, which indicated that the initial crystallization temperature of BFS should be 1200 °C. When the basicity decreased to 1.0, some of the crystallization phases began to precipitate when the temperature decreased to 1100 °C, which showed the initial crystallization temperature, and the temperature was lower than that of the BFS with basicity 1.1. When the basicity continued to decrease to 0.9–0.7, there was no crystallization phase when the temperature decreased to 1000 °C, and the microstructure was basically a smooth surface, which showed that the initial crystallization temperature of BFS was still below this temperature. The above results further confirmed that the initial crystallization temperature of BFS decreased gradually with the decrease of basicity, which matched with the thermodynamic simulation results. However, the temperature of the initial crystallization phase was slightly lower than that of the thermodynamic simulation temperature, mainly because the thermodynamic simulation was carried out under the condition of complete equilibrium, and the reaction was more equal. The mineral analysis experiment cooled the slag in the furnace at a specified temperature, the cooling process was faster, and the crystallization temperature was slightly lower.
3.3. DSC Analysis
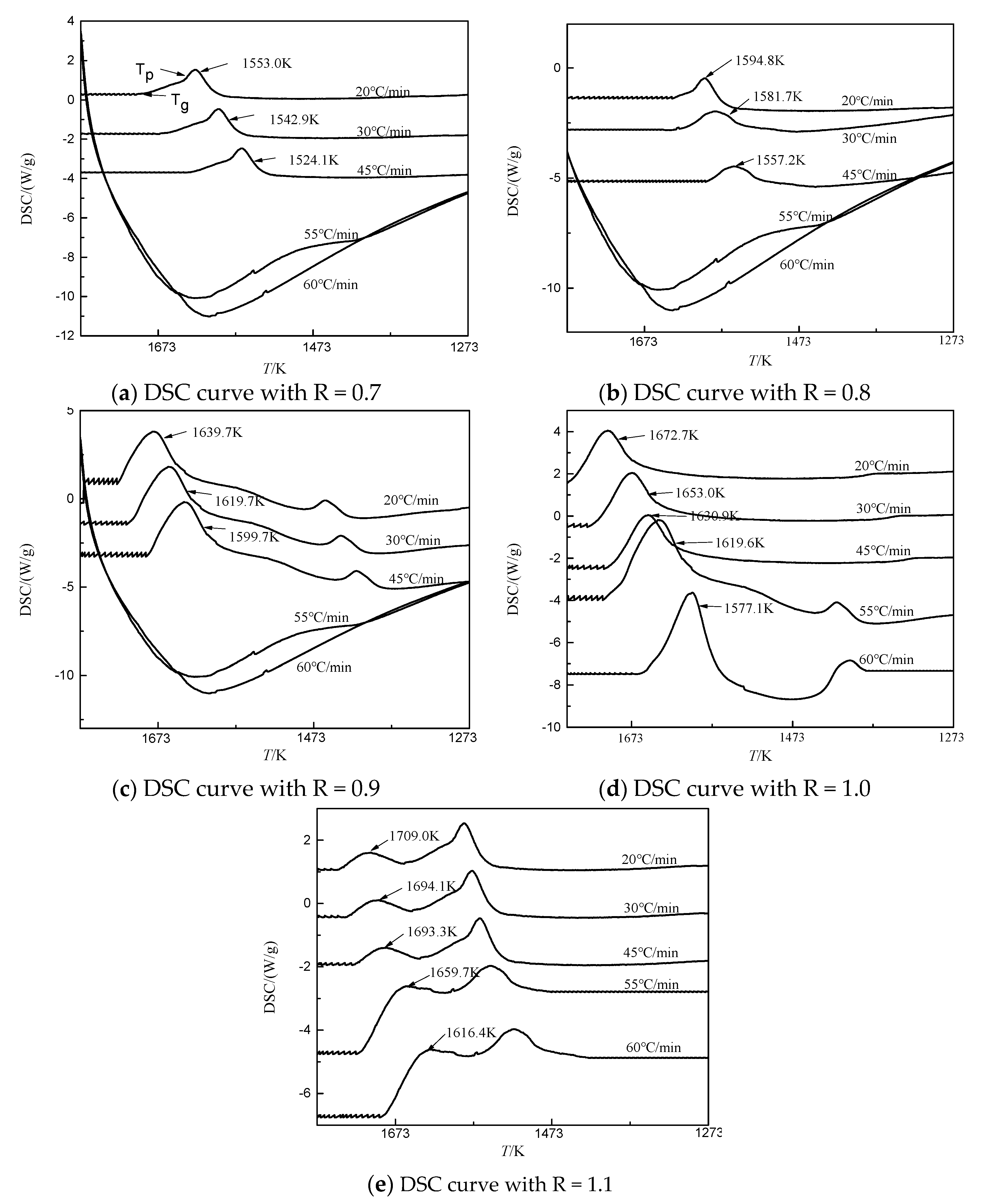
The crystallization ability of BFS with different basicities was further investigated using kinetic differential scanning calorimeter (DSC) methods, as shown in
Figure 6. The DSC curve was characterized by the endothermic peak represented by Tg (glass transition temperature), and the exothermic peak represented by Tp (crystallization peak temperature). In order to restrain the precipitation of crystallization phase in BFS as much as possible and obtain more amorphous phase to increase the activity of slag beads, it was only necessary to study the precipitation temperature of the initial crystallization phase of BFS, thus determining the gas quenching temperature. So only the values of the first Tp for each curve were taken, analyzed using Proteus analysis software, and the values were listed in
Table 4.
As shown in
Figure 6a–e, there were obvious exothermic peaks at the cooling rates from 20 °C/min to 45 °C/min, whereas basically no peaks were observed when the cooling rate was higher than 55 °C/min with basicities lower than 0.9. The initial crystallization temperature, which was the first exothermic peak temperature, decreased with the basicity decreased at the same cooling rate, which indicated that the greater the basicity, the stronger the slag crystallization capability. It was mainly because the Ca
2+ content increased with increase of the basicity, so that the main viscous flow unit Si
xO
y2− disintegrated in the slag. The viscosity of BFS decreased, so that the atoms in the slag only needed a small amount of energy to overcome the crystallization barrier from one equilibrium position to another [
17,
18]. At the same basicity, the crystallization temperature of BFS decreased gradually with the increase of cooling rate. When the basicity was less than 0.9 and the cooling rate was greater than 55 °C/min, the crystallization of BFS could be restrained. Overall, the decrease of basicity could restrain crystallization precipitation, and the slag with higher basicity could obtain higher amorphous content by increasing the cooling rate.
The crystallization ability of melt in the DSC kinetics analysis is usually characterized by the crystallization activation energy (Ec). When a melt changes from the glass state to the crystalline state, it is necessary to overcome rearrangements of the structural units, and the energy required for this is usually characterized by the crystallization activation energy. The more energy required, the more difficult the crystallization. The Johnson–Mehl–Avrami (JMA) equation which is the classical theoretical equation usually used to analyze Ec, which is mainly used to study the crystallization kinetics of the melt.
The Kissinger method [
19,
20], in which crystallization is treated as a first-order reaction, is the method most commonly used to analyze crystallization kinetics based on the JMA equation, which is shown as follows:
where β is defined as the cooling rate in DSC curves, Tp is the crystallization peak temperature of DSC curves, and R is the gas constant.
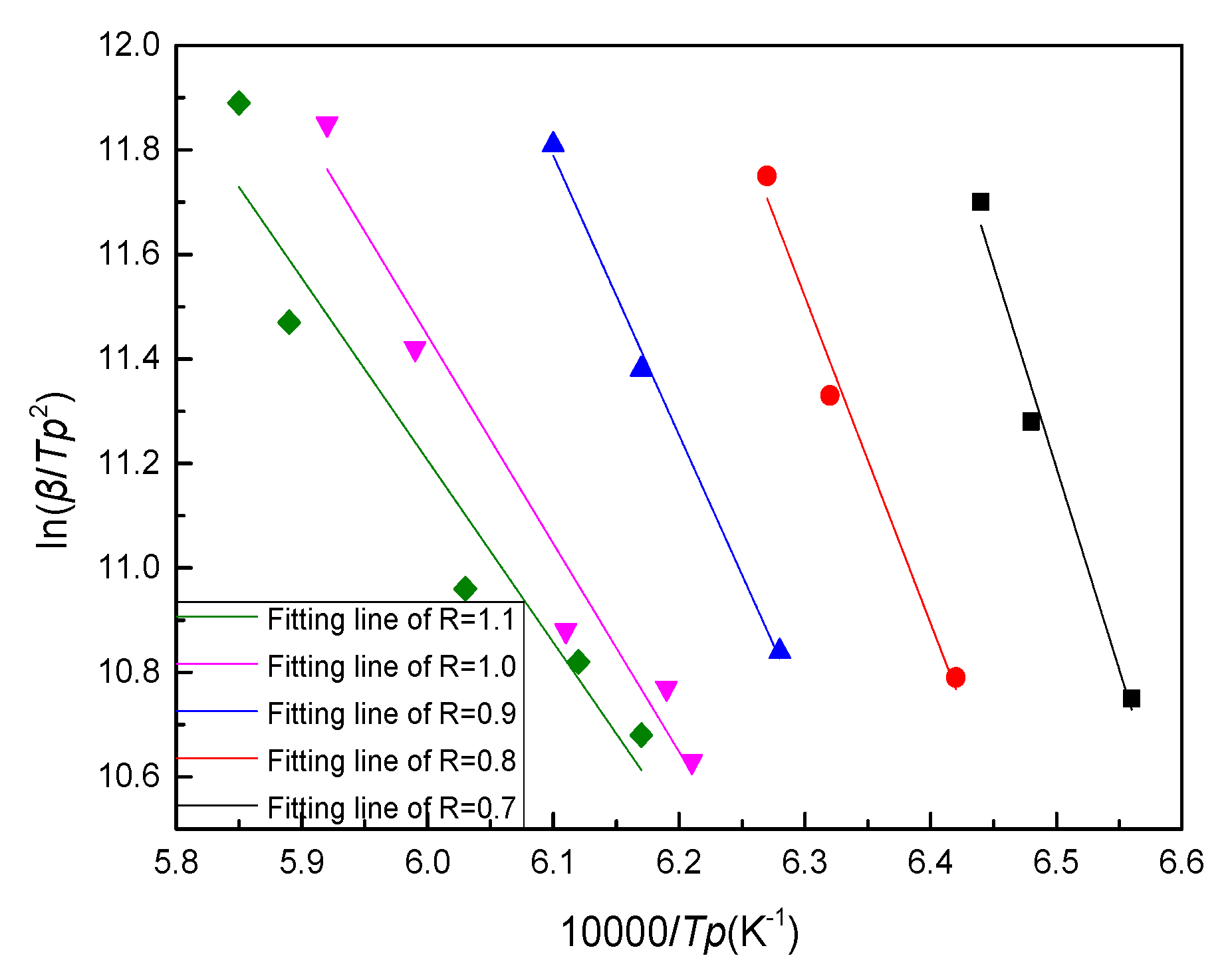
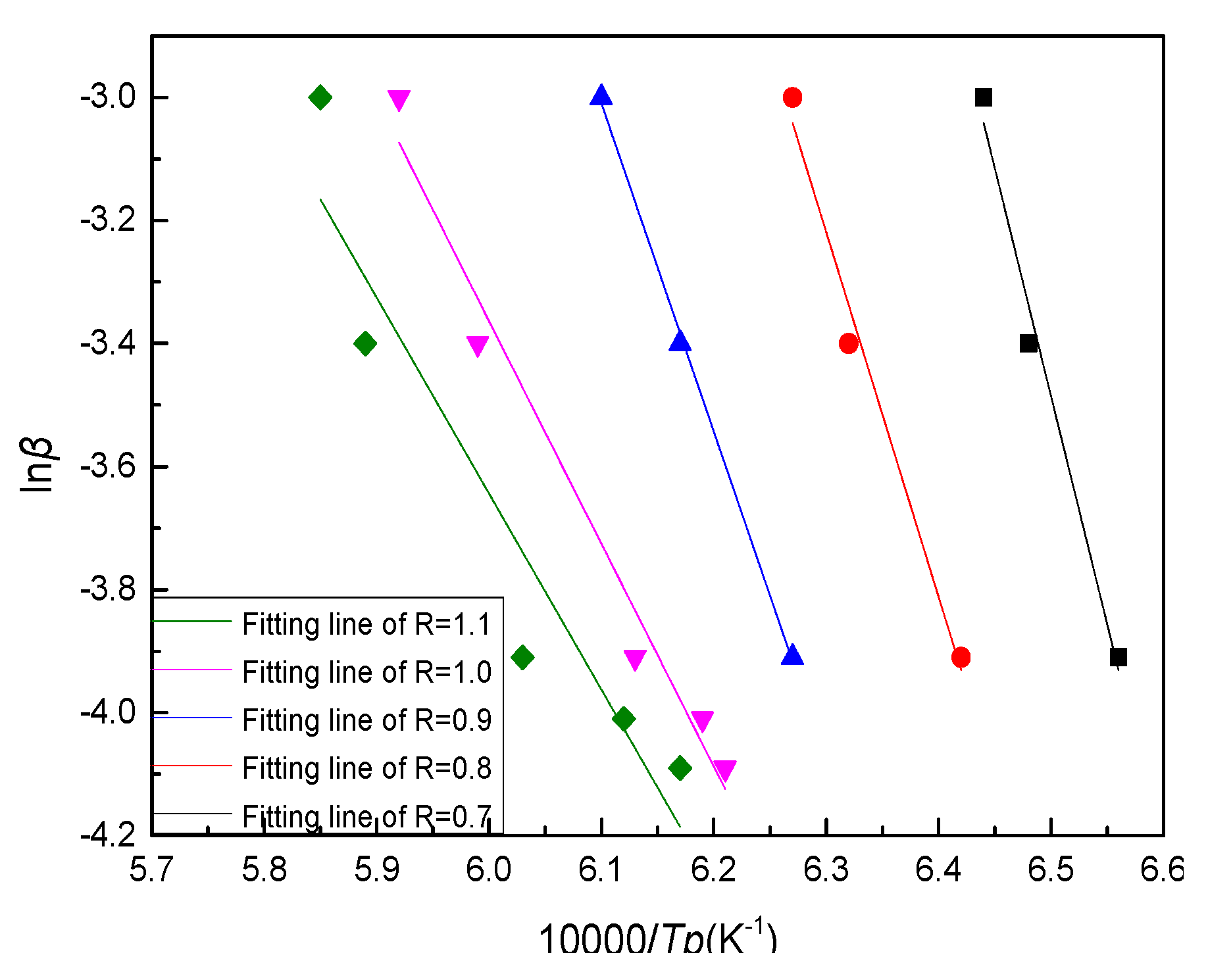
The Tp corresponding to different cooling rate β was obtained using Proteus analysis software in the DSC diagram, and then the ln(β/Tp
2) values could be calculated separately. The corresponding ln(β/Tp
2) values under different 10,000/Tp were plotted by Origin drawing software, and the fitting curves were obtained by fitting each numerical point. By plotting ln(β/Tp
2) versus 10,000/Tp curves and fitting them to the straight lines shown in
Figure 7, the fitting equations and R
2 could be obtained as shown in
Table 5. Ec could be calculated from the slopes of the fitting lines listed in
Table 4.
The Ozawa [
19] method is another method widely used to calculate
Ec, the equation is as follows:
By plotting ln(β/Tp
2) versus 10,000/Tp curves and fitting them to the straight lines shown in
Figure 8, the fitting equations and R
2 could be obtained as shown in
Table 6. Ec could be calculated from the slopes of the fitting lines listed in
Table 4.
As shown in
Table 4, all the results showed that the Ec increased when basicity decreased. This indicated that when the basicity was small, the atom needed more energy to overcome increasingly large rearrangement barriers of structural units, so the state was not easy to change, therefore the crystallization of BFS could be restrained by decreasing basicity. All of these showed that decreasing basicity of BFS could effectively inhibit crystallization precipitation in BFS and prepare slag beads with high amorphous phase content.