Growth of Nacre Biocrystals by Self-Assembly of Aragonite Nanoparticles with Novel Subhedral Morphology
Abstract
1. Introduction
2. Materials and Methods
3. Results
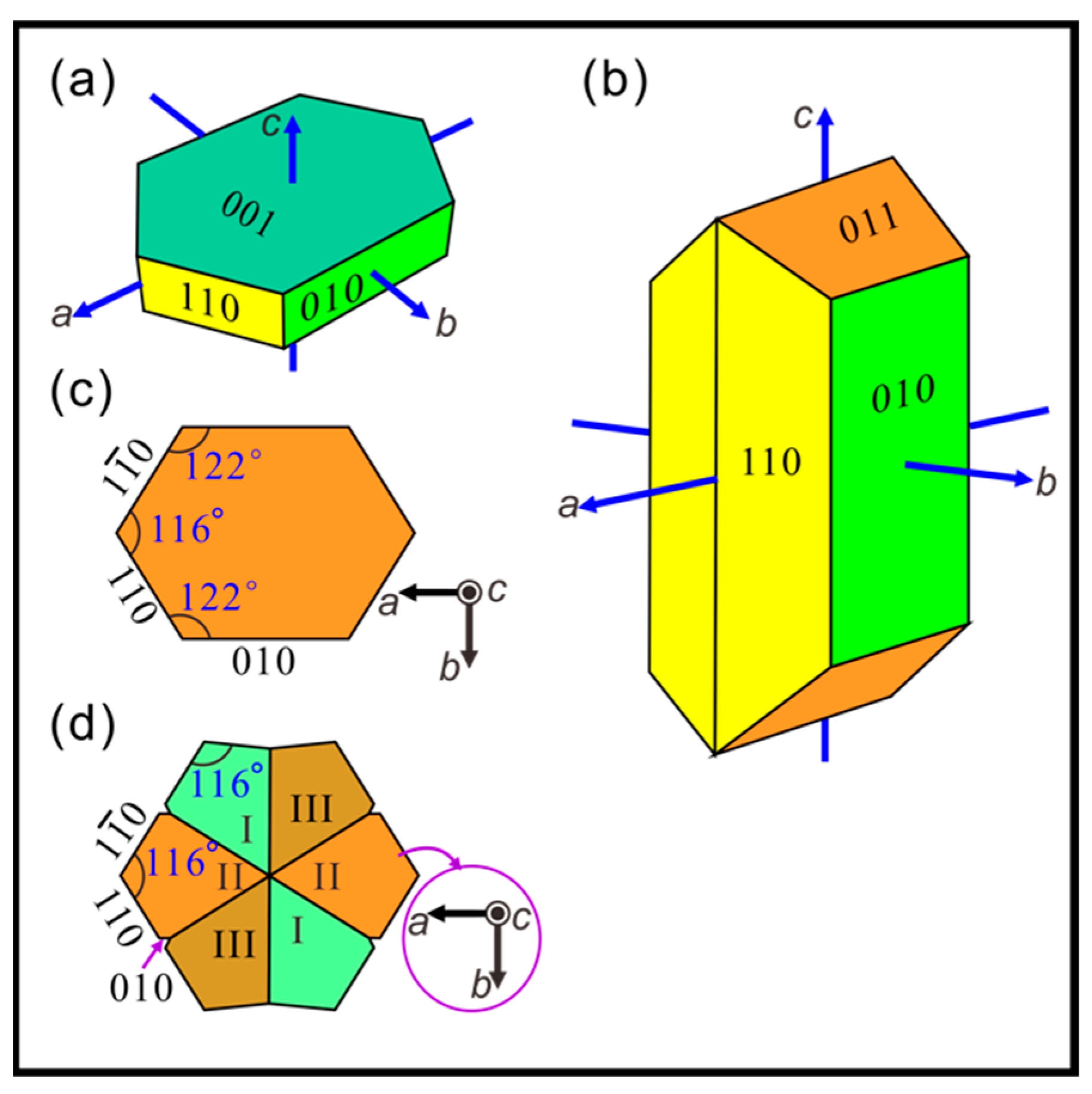
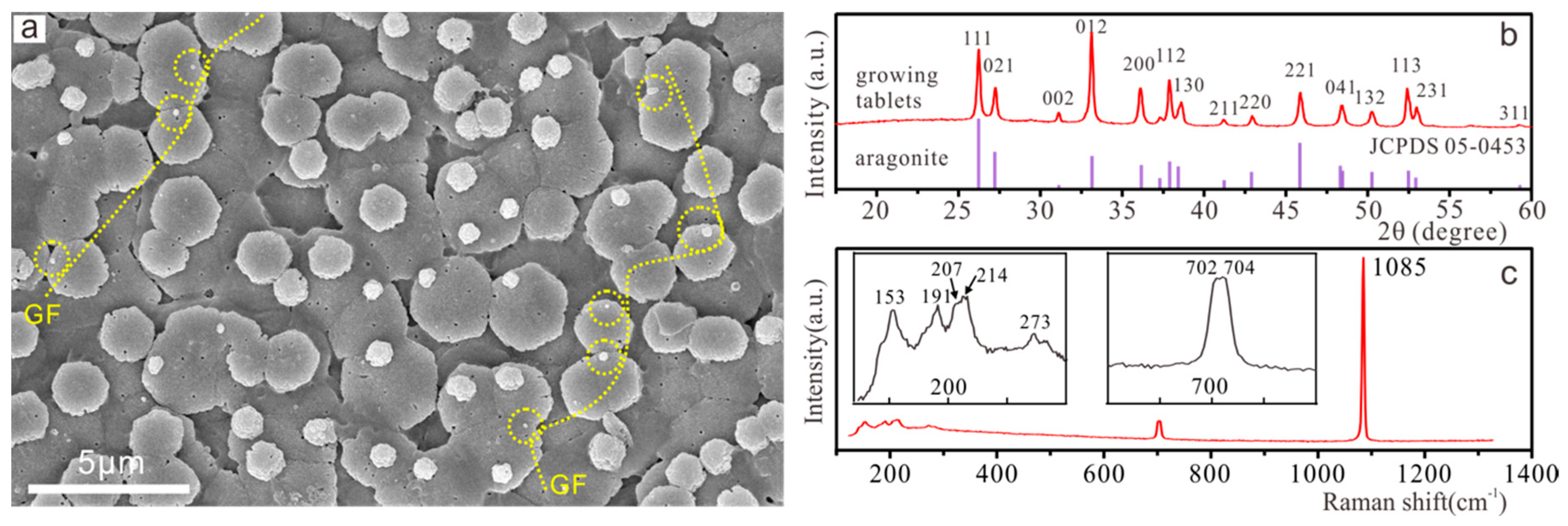
3.1. Nacre Growth Pattern and Composition
3.2. SEM Observation of Growing Tablets
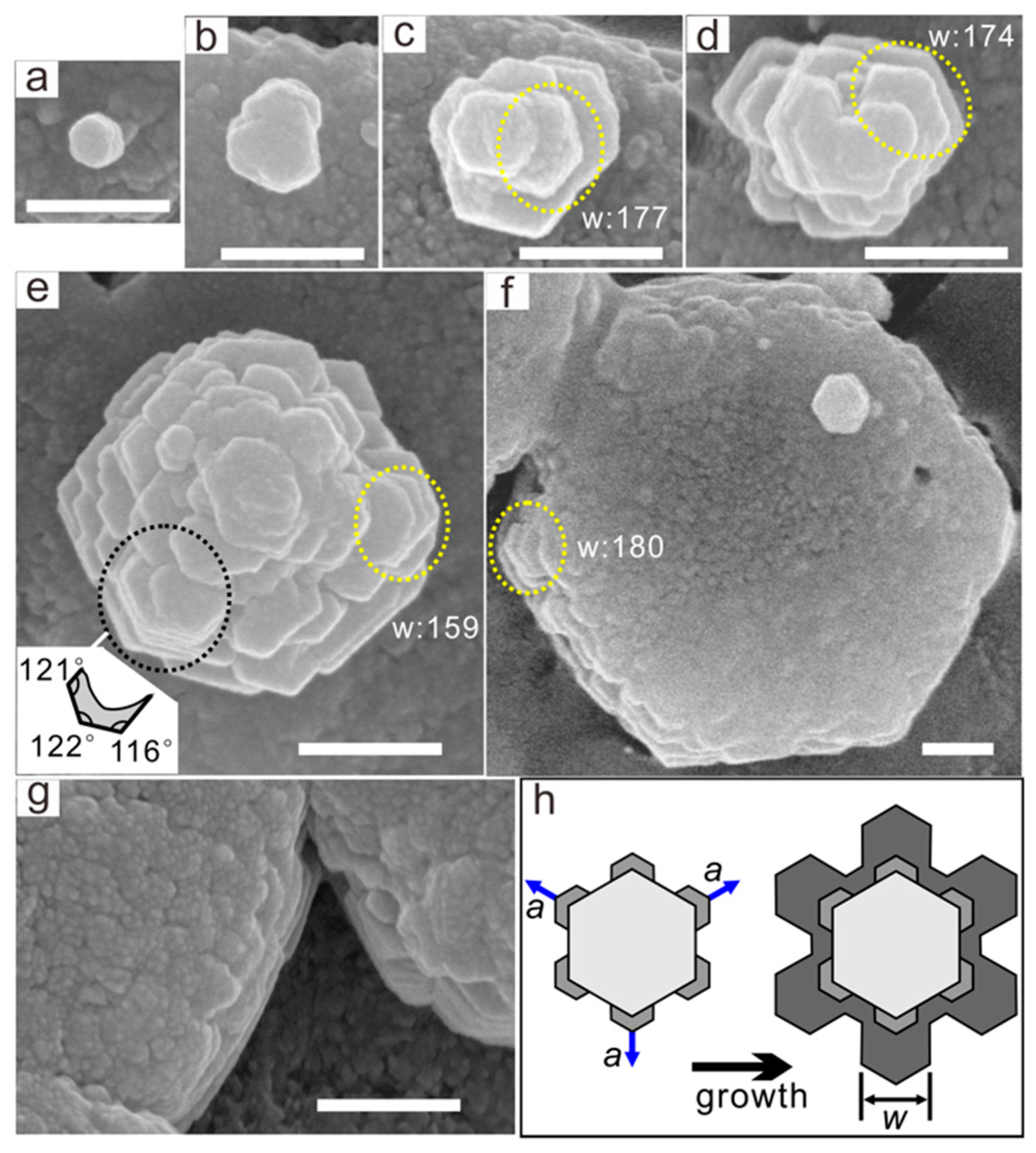
3.2.1. Nanoparticle Shape and Size
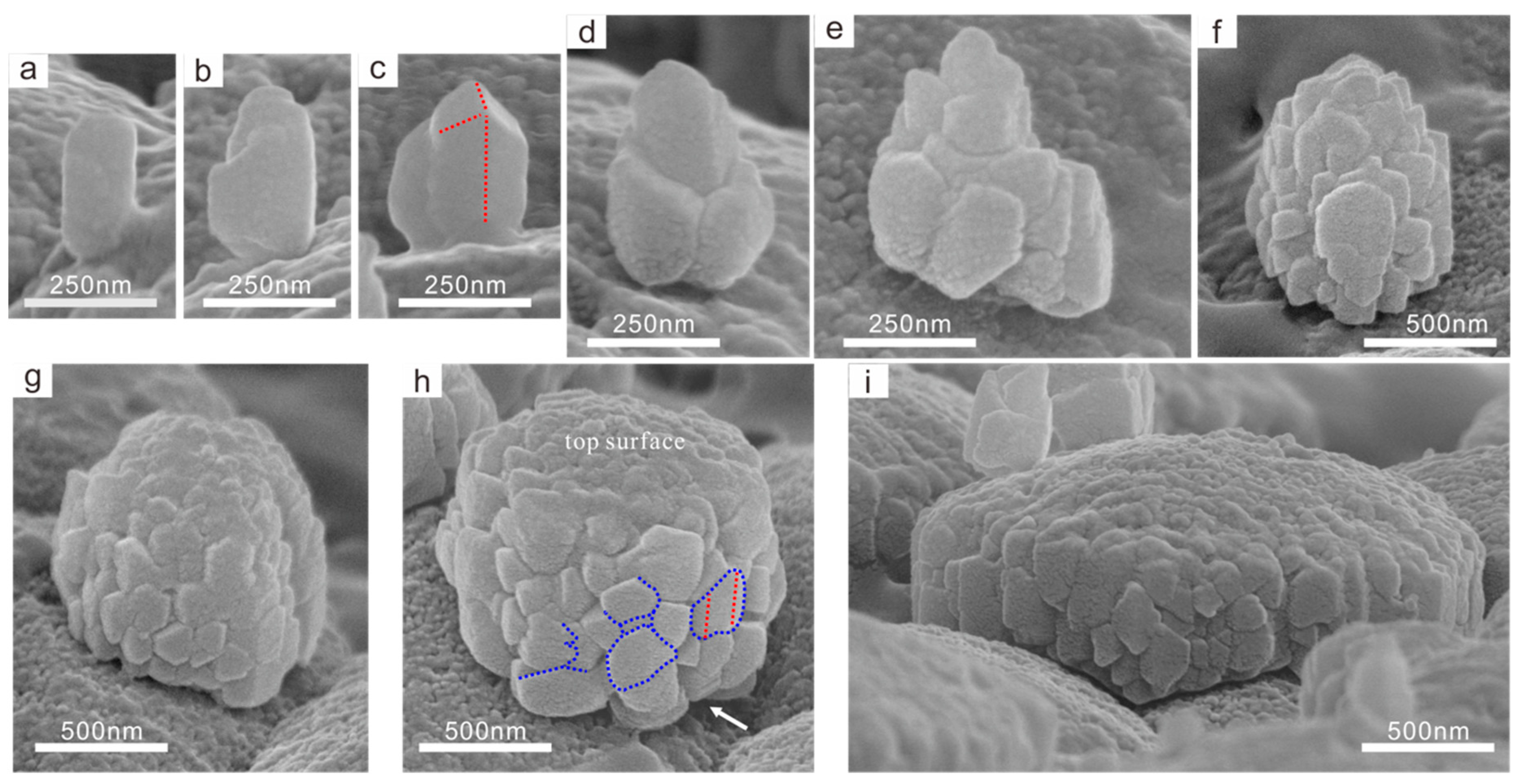
3.2.2. Nanoparticle Self-Assembly Process
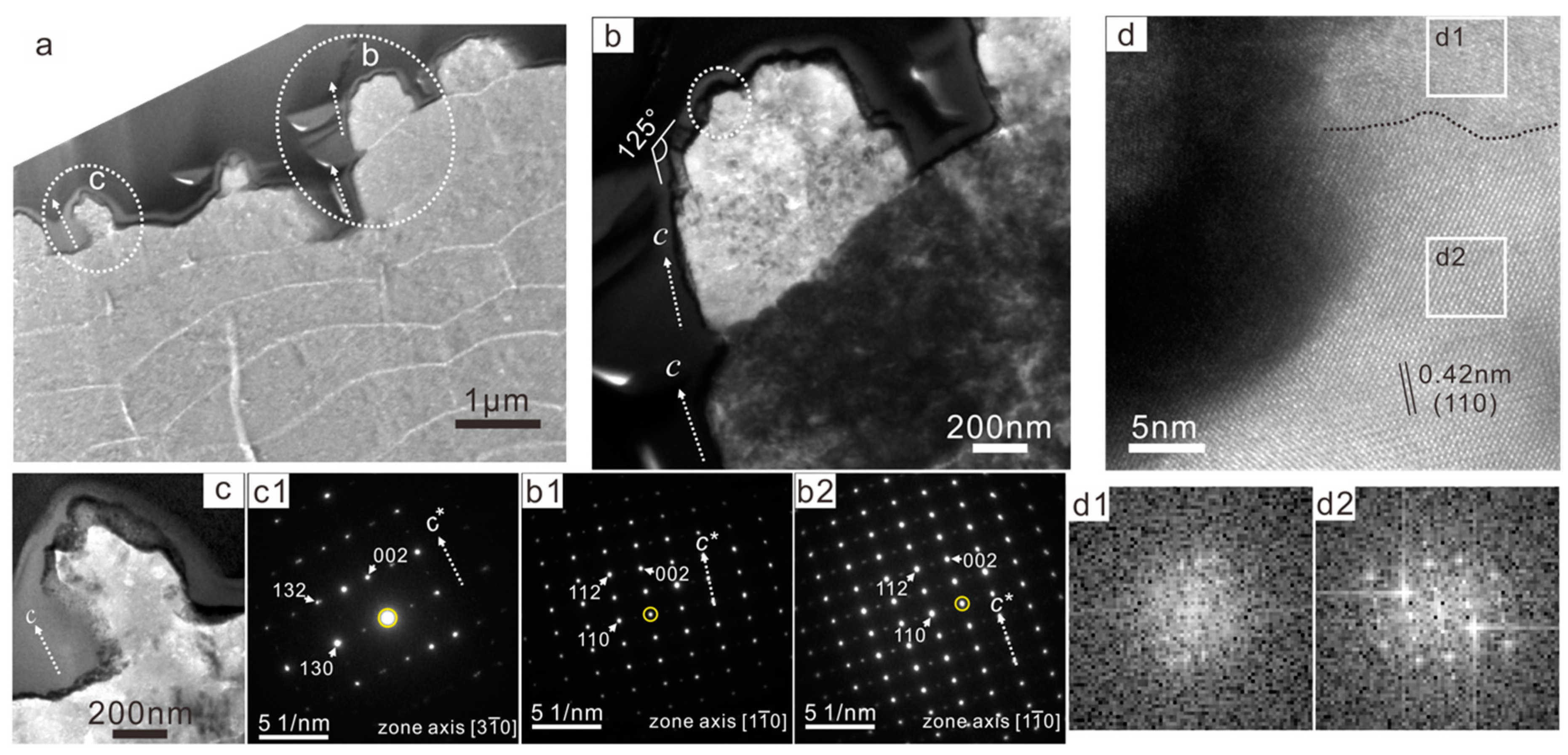
3.3. TEM Observation of the Growing Tablets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meyers, M.A.; Chen, P.Y.; Lin, A.Y.M.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.B.; Gao, H.L.; Yao, H.B.; Liu, L.; Colfen, H.; Liu, G.; Chen, S.M.; Li, S.K.; Yan, Y.X.; Liu, Y.Y.; et al. Synthetic nacre by predesigned matrix-directed mineralization. Science 2016, 354, 107–110. [Google Scholar] [CrossRef]
- Pan, X.F.; Gao, H.L.; Lu, Y.; Wu, C.Y.; Wu, Y.D.; Wang, X.Y.; Pan, Z.Q.; Dong, L.; Song, Y.H.; Cong, H.P.; et al. Transforming ground mica into high-performance biomimetic polymeric mica film. Nat. Commun. 2018, 9, 2974. [Google Scholar] [CrossRef]
- Cao, W.T.; Chen, F.F.; Zhu, Y.J.; Zhang, Y.G.; Jiang, Y.Y.; Ma, M.G.; Chen, F. Binary strengthening and toughening of MXene/Cellulose nanofiber composite paper with nacre-inspired structure and superior electromagnetic interference shielding properties. ACS Nano 2018, 12, 4583–4593. [Google Scholar] [CrossRef]
- Yaraghi, N.A.; Kisailus, D. Biomimetic structural materials: Inspiration from design and assembly. Annu. Rev. Phys. Chem. 2018, 69, 23–57. [Google Scholar] [CrossRef]
- Taylor, J.D. The shell structure and mineralogy of the Bivalvia. Introduction. Nuculacea-Trigonacea. Bull. Br. Mus. (Nat. Hist.) 1969, 3, 1–125. [Google Scholar]
- Wada, K. Nucleation and growth of aragonite crystals in the nacre of some bivalve molluscs. Biomineralisation 1972, 6, 141–159. [Google Scholar]
- Rousseau, M.; Rollion-Bard, C. Influence of the depth on the shape and thickness of nacre tablets of pinctada margaritifera pearl oyster, and on oxygen isotopic composition. Minerals 2012, 2, 55–64. [Google Scholar] [CrossRef]
- Yao, N.; Epstein, A.K.; Liu, W.W.; Sauer, F.; Yang, N. Organic–inorganic interfaces and spiral growth in nacre. J. R. Soc. Interface 2009, 6, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Joan, F.B.; Louis, F.; Gainey, J.; Michael, J.G. Shell ultrastructure in two subspecies of the ribbed mussel. Biol. Bull. 1977, 152, 1–11. [Google Scholar]
- Yoshimi, K.; Shoji, M.; Ogawa, T.; Yamauchi, A.; Naganuma, T.; Muramoto, K.; Hanada, S. Microstructure and orientation distribution of aragonite crystals in nacreous layer of pearl shells. Mater. Trans. 2004, 45, 999–1004. [Google Scholar] [CrossRef]
- Mukai, H.; Saruwatari, K.; Nagasawa, H.; Kogure, T. Aragonite twinning in gastropod nacre. J. Cryst. Growth 2010, 312, 3014–3019. [Google Scholar] [CrossRef]
- Gower, L.B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef]
- Feng, Q.L.; Cui, F.Z.; Pu, G.; Wang, R.Z.; Li, H.D. Crystal orientation, toughening mechanisms and a mimic of nacre. Mater. Sci. Eng. C 2000, 11, 19–25. [Google Scholar] [CrossRef]
- DiMasi, E.; Sarikaya, M. Synchrotron x-ray microbeam diffraction from abalone shell. J. Mater. Res. 2004, 19, 1471–1476. [Google Scholar] [CrossRef]
- De Leeuw, N.H.; Parker, S.C. Surface structure and morphology of calcium carbonate polymorphs calcite, aragonite, and vaterite: An atomistic approach. J. Phys. Chem. B 1998, 102, 2914–2922. [Google Scholar] [CrossRef]
- Sekkal, W.; Zaoui, A. Nanoscale analysis of the morphology and surface stability of calcium carbonate polymorphs. Sci. Rep. 2013, 3, 1587. [Google Scholar] [CrossRef]
- Sand, K.K.; Rodriguez-Blanco, J.D.; Makovicky, E.; Benning, L.G.; Stipp, S.L.S. Crystallization of CaCO3 in water–alcohol mixtures: Spherulitic growth, polymorph stabilization, and morphology change. Cryst. Growth Des. 2012, 12, 842–853. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef] [PubMed]
- Oaki, Y.; Imai, H. The hierarchical architecture of nacre and its mimetic material. Angew. Chem. Int. Ed. 2005, 44, 6571–6575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G. Biomineralization on the wavy substrate: Shape transition of nacreous tablets from pyramids of amorphous nanoparticles to dome-capped prisms of single crystals. Acta Biomater. 2016, 36, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Power, A.J.; Walker, R.; Payne, K.; Hurley, D. First occurrence of the nonindigenous green mussel, Perna viridis (Linnaeus, 1758) in coastal Georgia, United States. J. Shellfish Res. 2004, 23, 741–744. [Google Scholar]
- Wise Sherwood, W., Jr. Microarchitecture and mode of formation of nacre (mother-of-pearl) in pelecypods, gastropods, and cephalopods. Eclogae Geol. Helv. 1970, 63, 775–797. [Google Scholar]
- De La Pierre, M.; Carteret, C.; Maschio, L.; André, E.; Orlando, R.; Dovesi, R. The Raman spectrum of CaCO3 polymorphs calcite and aragonite: A combined experimental and computational study. J. Chem. Phys. 2014, 140, 164509. [Google Scholar] [CrossRef]
- Libbrecht, K. The physics of snow crystals. Rep. Prog. Phys. 2005, 68, 855–895. [Google Scholar] [CrossRef]
- Weiner, S.; Traub, W.; Parker, S.B. Macromolecules in Mollusc Shells and Their Functions in Biomineralization [and Discussion]. Philos. Trans. R. Soc. B Biol. Sci. 1984, 304, 425–434. [Google Scholar] [CrossRef]
- Watabe, N. Crystal growth of calcium carbonate in the invertebrates. Prog. Cryst. Growth Charact. 1981, 4, 99–147. [Google Scholar] [CrossRef]
- Rousseau, M.; Meibom, A.; Gèze, M.; Bourrat, X.; Angellier, M.; Lopez, E. Dynamics of sheet nacre formation in bivalves. J. Struct. Biol. 2009, 165, 190–195. [Google Scholar] [CrossRef]
- Nassif, N.; Pinna, N.; Gehrke, N.; Antonietti, M.; Jager, C.; Colfen, H. Amorphous layer around aragonite platelets in nacre. Proc. Natl. Acad. Sci. USA 2005, 102, 12653–12655. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.E.; Böhm, C.F.; Harris, J.; Demmert, B.; Jacob, D.E.; Mondeshki, M.; Ruiz-Agudo, E.; Rodríguez-Navarro, C. Nonclassical crystallization in vivo et in vitro (I): Process-structure-property relationships of nanogranular biominerals. J. Struct. Biol. 2016, 196, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.; Weiner, S.; Addadi, L. A perspective on underlying crystal growth mechanisms in biomineralization: Solution mediated growth versus nanosphere particle accretion. CrystEngComm 2015, 17, 2606–2615. [Google Scholar] [CrossRef]
- Van Der Merwe, J.H. Theoretical considerations in growing uniform epilayers. Interface Sci. 1993, 1, 77–86. [Google Scholar] [CrossRef]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk shell formation: A source of new concepts for understanding biomineralization processes. Chem. A Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Macías-Sánchez, E.; Willinger, M.G.; Pina, C.M.; Checa, A.G. Transformation of ACC into aragonite and the origin of the nanogranular structure of nacre. Sci. Rep. 2017, 7, 12728. [Google Scholar] [CrossRef]
- Schäffer, T.E.; Ionescu-Zanetti, C.; Proksch, R.; Fritz, M.; Walters, D.A.; Almqvist, N.; Zaremba, C.M.; Belcher, A.M.; Smith, B.L.; Stucky, G.D.; et al. Does abalone nacre form by heteroepitaxial nucleation or by growth through mineral bridges? Chem. Mater. 1997, 9, 1731–1740. [Google Scholar] [CrossRef]
- Checa, A.G. Physical and biological determinants of the fabrication of molluscan shell microstructures. Front. Mar. Sci. 2018, 5, 1–21. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, J. From colloidal nanoparticles to a single crystal: New insights into the formation of nacre’s aragonite tablets. J. Struct. Biol. 2013, 182, 36–43. [Google Scholar] [CrossRef]
- DeVol, R.T.; Sun, C.-Y.; Marcus, M.A.; Coppersmith, S.N.; Myneni, S.C.B.; Gilbert, P.U.P.A. Nanoscale transforming mineral phases in fresh nacre. J. Am. Chem. Soc. 2015, 137, 13325–13333. [Google Scholar] [CrossRef]
- Gilbert, P.U.P.A.; Porter, S.M.; Sun, C.Y.; Xiao, S.; Gibson, B.M.; Shenkar, N.; Knoll, A.H. Biomineralization by particle attachment in early animals. Proc. Natl. Acad. Sci. USA 2019, 116, 17659–17665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.T.; Yao, Q.Z.; Ni, J.; Jin, G. Formation of aragonite mesocrystals and implication for biomineralization. Am. Mineral. 2009, 94, 293–302. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Wang, R.; Feng, X.; Zhang, G. Growth of Nacre Biocrystals by Self-Assembly of Aragonite Nanoparticles with Novel Subhedral Morphology. Crystals 2020, 10, 3. https://doi.org/10.3390/cryst10010003
Gao R, Wang R, Feng X, Zhang G. Growth of Nacre Biocrystals by Self-Assembly of Aragonite Nanoparticles with Novel Subhedral Morphology. Crystals. 2020; 10(1):3. https://doi.org/10.3390/cryst10010003
Chicago/Turabian StyleGao, Ruohe, Rize Wang, Xin Feng, and Gangsheng Zhang. 2020. "Growth of Nacre Biocrystals by Self-Assembly of Aragonite Nanoparticles with Novel Subhedral Morphology" Crystals 10, no. 1: 3. https://doi.org/10.3390/cryst10010003
APA StyleGao, R., Wang, R., Feng, X., & Zhang, G. (2020). Growth of Nacre Biocrystals by Self-Assembly of Aragonite Nanoparticles with Novel Subhedral Morphology. Crystals, 10(1), 3. https://doi.org/10.3390/cryst10010003



