Enhanced Selective Production of Carbonyl Products for Aerobic Oxidation of Benzylic Alcohols over Mesoporous Fe2O3 Supported Gold Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalysts
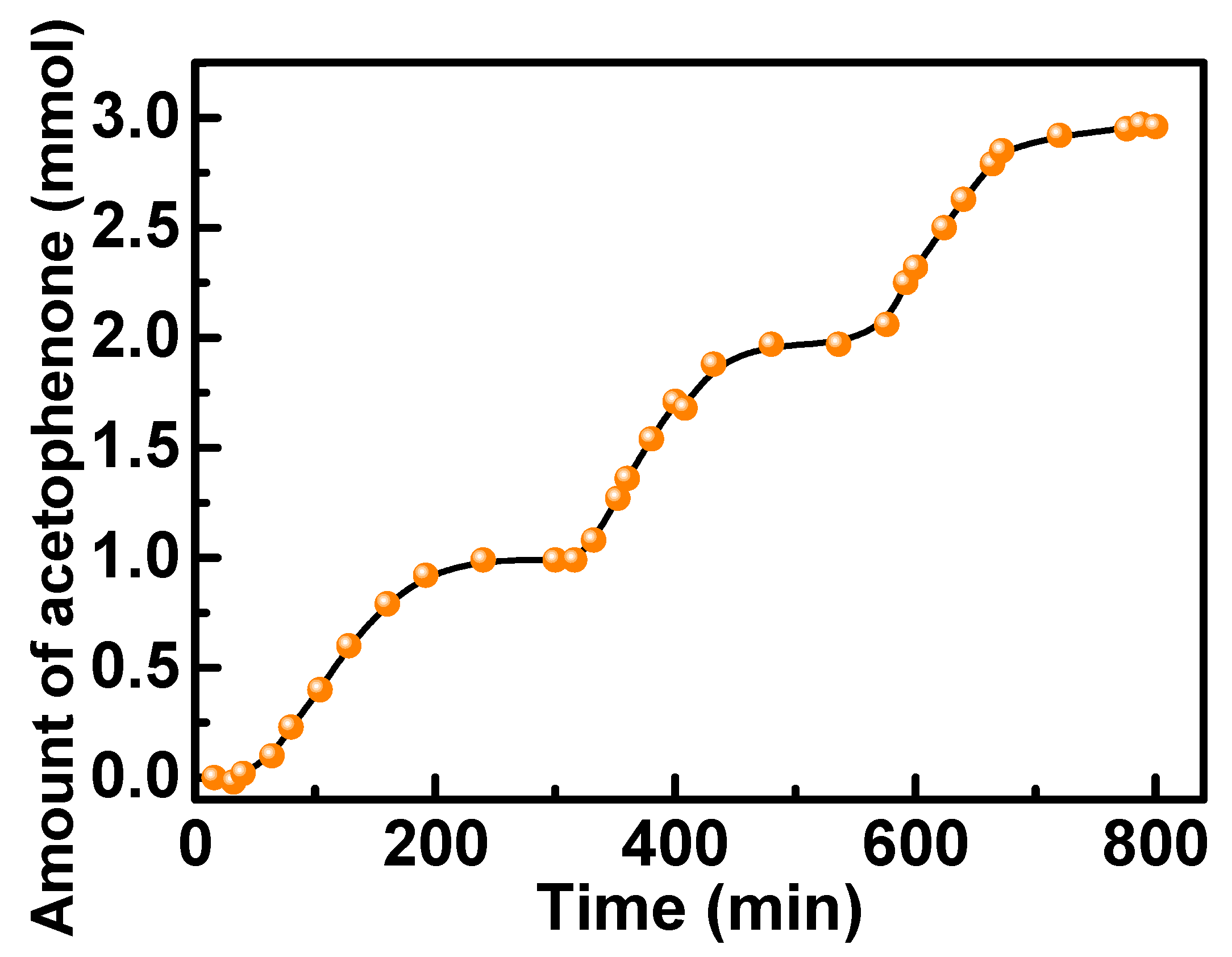
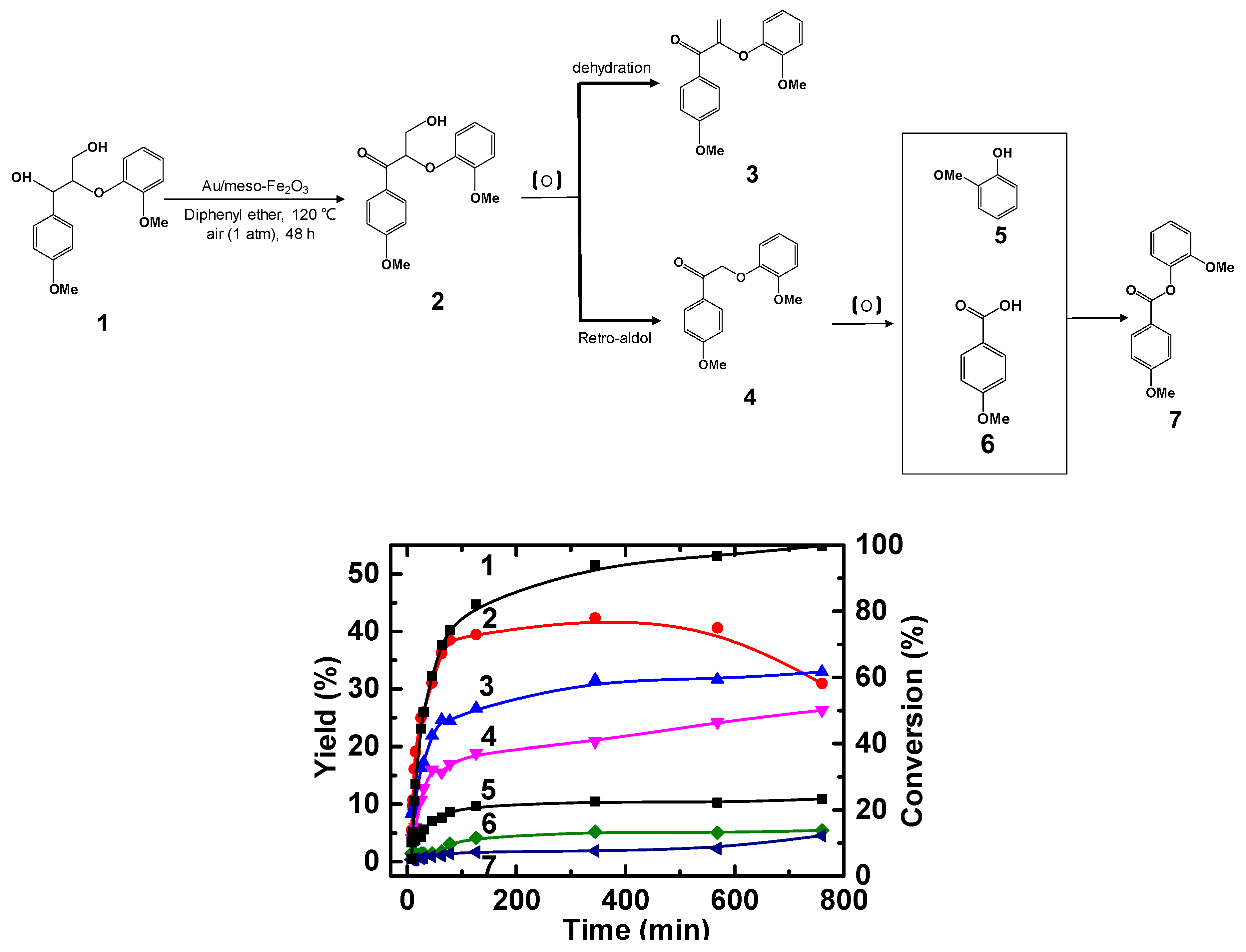
2.2. Oxidation of Benzylic Alcohols
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Oxidation of Benzylic Alcohols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, P.; Hu, C. Controlling the cleavage of the inter- and intra-molecular linkages in lignocellulosic biomass for further biorefining: A review. Bioresour. Technol. 2018, 256, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed]
- Teong, S.P.; Yi, G.; Zhang, Y. Hydroxymethylfurfural production from bioresources: Past, present and future. Green Chem. 2014, 16, 2015–2026. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Zhang, Q.; Xu, Y.; Long, J.; Chen, L.; Wang, C.; Ma, L. Production of alkanes from lignin-derived phenolic compounds over in situ formed Ni catalyst with solid acid. Chem. Lett. 2015, 44, 648–650. [Google Scholar] [CrossRef]
- Behling, R.; Valange, S.; Chatel, G. Heterogeneous catalytic oxidation for lignin valorization into valuable chemicals: What results? What limitations? What trends? Green Chem. 2016, 18, 1839–1854. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef]
- Das, L.; Kolar, P.; Sharma-Shivappa, R. Heterogeneous catalytic oxidation of lignin into value-added chemicals. Biofuels 2012, 3, 155–166. [Google Scholar] [CrossRef]
- Deng, W.P.; Zhang, H.X.; Wu, X.J.; Li, R.S.; Zhang, Q.H.; Wang, Y. Oxidative conversion of lignin and lignin model compounds catalyzed by CeO2-supported Pd nanoparticles. Green Chem. 2015, 17, 5009–5018. [Google Scholar] [CrossRef]
- Mottweiler, J.; Puche, M.; Raeuber, C.; Schmidt, T.; Concepcion, P.; Corma, A.; Bolm, C. Copper- and vanadium-catalyzed oxidative cleavage of lignin using dioxygen. ChemSusChem 2015, 8, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, M.R.; O’Brien, M.H.; Ciesielski, P.N.; Katahira, R.; Kruger, J.S.; Chmely, S.C.; Hamlin, J.; Lawrence, K.; Hunsinger, G.B.; Foust, T.D. Lignin depolymerisation by nickel supported layered-double hydroxide catalysts. Green Chem. 2014, 16, 824–835. [Google Scholar] [CrossRef]
- Kruger, J.S.; Cleveland, N.S.; Zhang, S.; Katahira, R.; Black, B.A.; Chupka, G.M.; Lammens, T.; Hamilton, P.G.; Biddy, M.J.; Beckham, G.T. Lignin depolymerization with nitrate-intercalated hydrotalcite catalysts. ACS Cata. 2016, 6, 1316–1328. [Google Scholar] [CrossRef]
- Wu, X.; Guo, S.; Zhang, J. Selective oxidation of veratryl alcohol with composites of Au nanoparticles and graphene quantum dots as catalysts. Chem. Commun. 2015, 51, 6318–6321. [Google Scholar] [CrossRef] [PubMed]
- Luc, W.; Jiao, F. Synthesis of nanoporous metals, oxides, carbides, and sulfides: Beyond nanocasting. Acc. Chem. Res. 2016, 49, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 17, 2136–2137. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Zheng, B.; Wang, X.; Zhang, W.; Wu, S.; Jia, M.; Yan, W.; Liu, G. Fe3O4 nanoparticles anchored on carbon serve the dual role of catalyst and magnetically recoverable entity in the aerobic oxidation of alcohols. ChemCatChem 2016, 8, 805–811. [Google Scholar] [CrossRef]
- Yang, X.; Huang, C.; Fu, Z.; Song, H.; Liao, S.; Su, Y.; Du, L.; Li, X. An effective pd-promoted gold catalyst supported on mesoporous silica particles for the oxidation of benzyl alcohol. Appl. Catal. B Environ. 2013, 140, 419–425. [Google Scholar] [CrossRef]
- Bingwa, N.; Patala, R.; Noh, J.-H.; Ndolomingo, M.J.; Tetyana, S.; Bewana, S.; Meijboom, R. Synergistic effects of gold-palladium nanoalloys and reducible supports on the catalytic reduction of 4-nitrophenol. Langmuir 2017, 33, 7086–7095. [Google Scholar] [CrossRef]
- Mobley, J.K.; Crocker, M. Catalytic oxidation of alcohols to carbonyl compounds over hydrotalcite and hydrotalcite-supported catalysts. RSC Adv. 2015, 5, 65780–65797. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, S.; Gomez-Aviles, A.; Martynyuk, O.; Pestryakov, A.; Bogdanchikova, N.; Cortes Corberan, V. Selective oxidation of 1-octanol over gold supported on mesoporous metal-modified hms: The effect of the support. Catal. Today 2014, 227, 65–70. [Google Scholar] [CrossRef]
- Song, Y.; Mobley, J.K.; Motagamwala, A.H.; Isaacs, M.; Dumesic, J.A.; Ralph, J.; Lee, A.F.; Wilson, K.; Crocker, M. Gold-catalyzed conversion of lignin to low molecular weight aromatics. Chem. Sci. 2018, 9, 8127–8133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, S.; Li, J.; Zhang, H. Hierarchical hollow nanostructured core@shell recyclable catalysts gamma-Fe2O3@LDH@Au25-x for highly efficient alcohol oxidation. Green Chem. 2016, 18, 5900–5914. [Google Scholar] [CrossRef]
- Rakap, M.; Ozkar, S. Hydroxyapatite-supported palladium(0) nanoclusters as effective and reusable catalyst for hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hydrog. Energ. 2011, 36, 7019–7027. [Google Scholar] [CrossRef]
- Liu, Y.; Tsunoyama, H.; Akita, T.; Xie, S.; Tsukuda, T. Aerobic oxidation of cyclohexane catalyzed by size-controlled au clusters on hydroxyapatite: Size effect in the sub-2 nm regime. ACS Cata. 2011, 1, 2–6. [Google Scholar] [CrossRef]
- Baker, T.A.; Liu, X.; Friend, C.M. The mystery of gold’s chemical activity: Local bonding, morphology and reactivity of atomic oxygen. Phy. Chem. Chem. Phy. 2011, 13, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Hu, Y.; Biasini, M.; Beyermann, W.P.; Yin, Y. Superparamagnetic magnetite colloidal nanocrystal clusters. Angew. Chem. Int. Edit. 2007, 46, 4342–4345. [Google Scholar] [CrossRef]
- Megias-Sayago, C.; Chakarova, K.; Penkova, A.; Lolli, A.; Ivanova, S.; Albonetti, S.; Cavani, F.; Antonio Odriozola, J. Understanding the role of the acid sites in 5-hydroxymethylfurfural oxidation to 2,5-furandicarboxylic acid reaction over gold catalysts: Surface investigation on CexZr1-xO2 compounds. ACS Cata. 2018, 8, 11154–11164. [Google Scholar] [CrossRef]
- Ma, R.; Xu, Y.; Zhang, X. Catalytic oxidation of biorefinery lignin to value-added chemicals to support sustainable biofuel production. ChemSusChem 2015, 8, 24–51. [Google Scholar] [CrossRef]
- Wang, S.; Yin, S.; Chen, G.; Li, L.; Zhang, H. Nearly atomic precise gold nanoclusters on nickel-based layered double hydroxides for extraordinarily efficient aerobic oxidation of alcohols. Catal. Sci. Technol. 2016, 6, 4090–4104. [Google Scholar] [CrossRef]
- Masunga, N.; Tito, G.S.; Meijboom, R. Catalytic evaluation of mesoporous metal oxides for liquid phase oxidation of styrene. Appl. Catal. A Gen. 2018, 552, 154–167. [Google Scholar] [CrossRef]
- Nishimura, S.; Yakita, Y.; Katayama, M.; Higashimine, K.; Ebitani, K. The role of negatively charged au states in aerobic oxidation of alcohols over hydrotalcite supported aupd nanoclusters. Catal. Sci. Technol. 2013, 3, 351–359. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Wu, H.; Li, X.; He, M.; Wu, P. Ets-10 supported Au nanoparticles for solvent-free oxidation of 1-phenylethanol with oxygen. Catal. Lett. 2011, 141, 860–865. [Google Scholar] [CrossRef]
- Haider, P.; Grunwaldt, J.-D.; Baiker, A. Gold supported on mg, al and cu containing mixed oxides: Relation between surface properties and behavior in catalytic aerobic oxidation of 1-phenylethanol. Catal. Today 2009, 141, 349–354. [Google Scholar] [CrossRef]
- Wang, L.; Meng, X.; Xiao, F. Au nanoparticles supported on a layered double hydroxide with excellent catalytic properties for the aerobic oxidation of alcohols. Chin. J. Catal. 2010, 31, 943–947. [Google Scholar] [CrossRef]
- Li, L.; Dou, L.; Zhang, H. Layered double hydroxide supported gold nanoclusters by glutathione-capped Au nanoclusters precursor method for highly efficient aerobic oxidation of alcohols. Nanoscale 2014, 6, 3753–3763. [Google Scholar] [CrossRef]
- Ballarin, B.; Barreca, D.; Boanini, E.; Cassani, M.C.; Dambruoso, P.; Massi, A.; Mignani, A.; Nanni, D.; Parise, C.; Zaghi, A. Supported gold nanoparticles for alcohols oxidation in continuous flow heterogeneous systems. ACS Sustain. Chem. Eng. 2017, 5, 4746–4756. [Google Scholar] [CrossRef]
- Buonerba, A.; Cuomo, C.; Sanchez, S.O.; Canton, P.; Grassi, A. Gold nanoparticles incarcerated in nanoporous syndiotactic polystyrene matrices as new and efficient catalysts for alcohol oxidations. Chem. Eur. J. 2012, 18, 709–715. [Google Scholar]
- Asao, N.; Hatakeyama, N.; Menggenbateer; Minato, T.; Ito, E.; Hara, M.; Kim, Y.; Yamamoto, Y.; Chen, M.; Zhang, W.; et al. Aerobic oxidation of alcohols in the liquid phase with nanoporous gold catalysts. Chem. Commun. 2012, 48, 4540–4542. [Google Scholar] [CrossRef]
- Rinesch, T.; Mottweiler, J.; Puche, M.; Concepcion, P.; Corma, A.; Bolm, C. Mechanistic investigation of the catalyzed cleavage for the lignin β-O-4 linkage: Implications for vanillin and vanillic acid formation. ACS Sustain. Chem. Eng. 2017, 5, 9818–9825. [Google Scholar] [CrossRef]
- Tsang, A.S.K.; Kapat, A.; Schoenebeck, F. Factors that control c-c cleavage versus c-h bond hydroxylation in copper-catalyzed oxidations of ketones with O2. J. Am. Chem. Soc. 2016, 138, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Cai, J.; Zhang, J.; Yu, W.; Wang, F.; Xu, J. Hydrogenation and cleavage of the C-O bonds in the lignin model compound phenethyl phenyl ether over a nickel-based catalyst. Chin. J. Catal. 2013, 34, 651–658. [Google Scholar] [CrossRef]
- Sun, X.; Shi, Y.; Zhang, P.; Zheng, C.; Zheng, X.; Zhang, F.; Zhang, Y.; Guan, N.; Zhao, D.; Stucky, G.D. Container effect in nanocasting synthesis of mesoporous metal oxides. J. Am. Chem. Soc. 2011, 133, 14542–14545. [Google Scholar] [CrossRef] [PubMed]
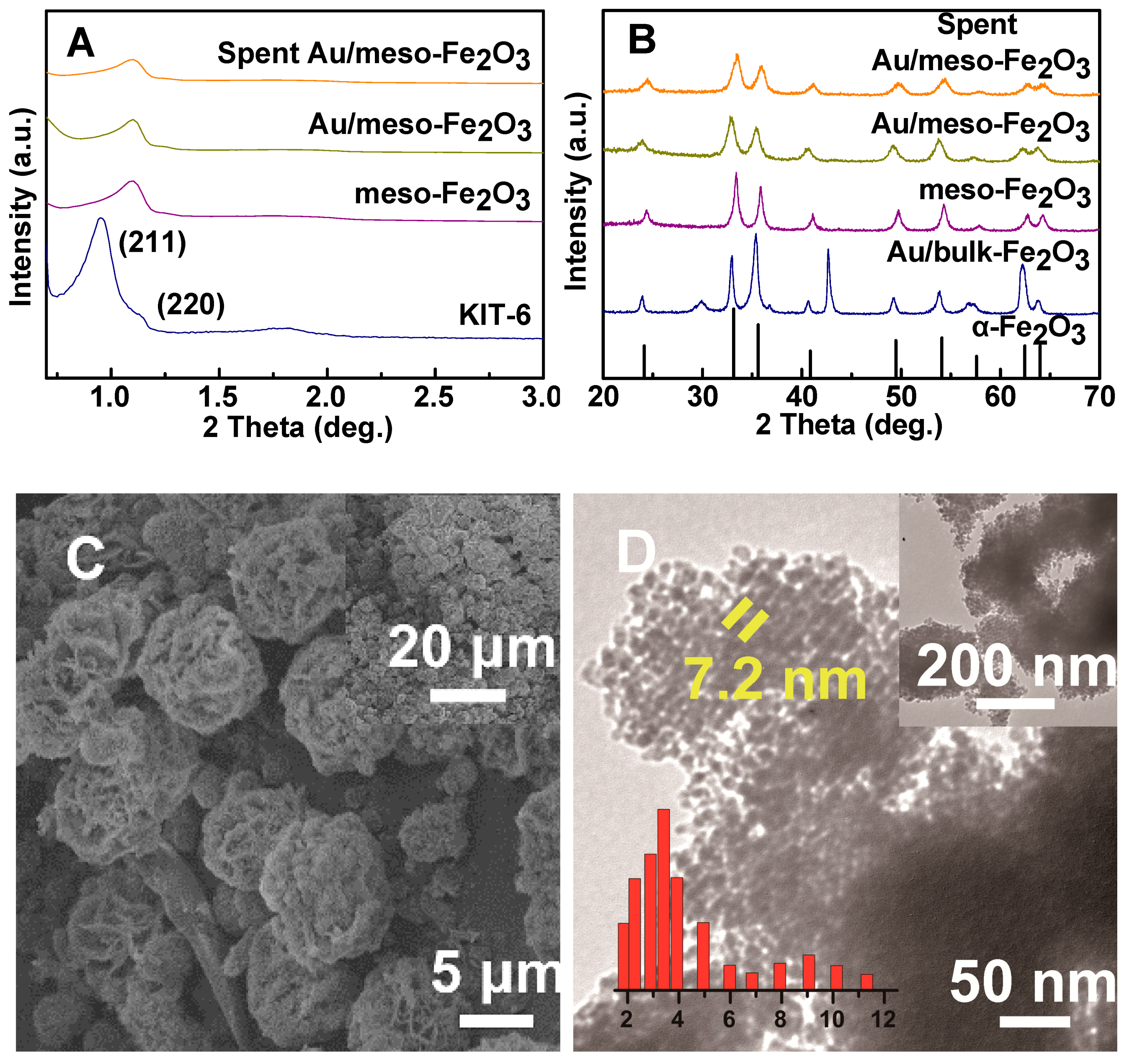
| Catalysts | SBET a (m2·g−1) | Dp b (nm) | dXRD (nm) c | Au wt% d | Na wt% | Si wt% | Au Leaching e (mg·L−1) |
|---|---|---|---|---|---|---|---|
| Au/bulk-Fe2O3 | 0.57 | - | 36.3 | 0.23 | N/D | N/D | <0.1 |
| Au/meso-Fe2O3 | 97.2 | 9.1 | 9.7 | 0.26 | 0.02 | 0.13 | <0.1 |
| Recycled 2nd Au/meso-Fe2O3 | 93.1 | 9.0 | 10.1 | 0.24 | 0.009 | 0.11 | <0.1 |
| Recycled 3rd Au/meso-Fe2O3 | 91.4 | 8.7 | 10.3 | 0.24 | 0.005 | 0.09 | <0.1 |
| Catalyst | Catalyst Dosage (mol%) | Temp. [°C] | t [h] | Conv. b [%] | Sel. b [%] |
|---|---|---|---|---|---|
| Meso Fe2O3 | 0.5 | 80 | 4 | 7 | 73 |
| Au/bulk- Fe2O3 | 0.5 | 80 | 4 | 39 | 96 |
| Au/meso- Fe2O3 | 0.5 | 80 | 4 | 99 | >99 |
| Recycled 2nd Au/meso-Fe2O3 | 0.5 | 80 | 4 | 99 | >99 |
| Recycled 3rd Au/meso-Fe2O3 | 0.5 | 80 | 4 | 99 | >99 |
| Au/meso-Fe2O3 with 1 wt% Na | 0.5 | 80 | 4 | 13 | 87 |
| Entry | Substrate a | Temp. [°C] | Time [h] | Conv. [%] b | Product | Sel. [%] c |
|---|---|---|---|---|---|---|
| 1 | 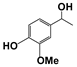 | 80 | 8 | 73 | 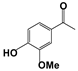 | 83 |
| 2 |  | 80 | 4 | 95 |  | 99 |
| 3 | 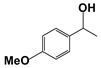 | 80 | 1 | >99 | 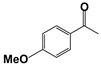 | >99 |
| 4 |  | 80 | 4 | 78 |  | >99 |
| 5 | 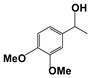 | 80 | 8 | 71 | 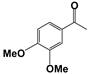 | >99 |
| 6 | 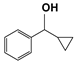 | 80 | 4 | >99 |  | >99 |
| 7 |  | 80 | 1 | >99 |  | >99 |
| 8 |  | 80 | 8 | >99 | 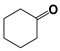 | >99 |
| 9 |  | 80 | 4 | 87 |  | >99 |
| 10 | 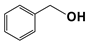 | 80 | 8 | >99 |  | >99 |
| 11 d | 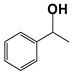 | 80 | 4 | 99 | 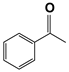 | >99 |
| 80 | 99 | >99 |
| Entry | Catalyst | Catalyst Dosage (mol%) | Time (h) | T (°C) | Conv. (%) | Sel. (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Au/NiAl-LDH | 0.01 | 1 | 160 | 19.5 | >99 | [31] |
| 2 | Au/ETS-10 | 0.03 | 6 | 160 | 42 | >99 | [34] |
| 3 | Au/CuMgAl4Ox | 0.09 | 1 | 90 | 85 | >99 | [35] |
| 4 | Au/NiAl-LDH | 0.2 | 1 | 80 | 58.7 | >99 | [24] |
| 5 | Au/γ-Fe2O3/NiAl-LDH | 0.2 | 1.5 | 80 | 95.8 | >99 | [24] |
| 6 | Au/LDH | 0.5 | 12 | 20 | 96 | >99 | [36] |
| 7 | Au/Al2O3 | 0.9 | 4 | 100 | 5.5 | >99 | [37] |
| 8 | Au/FeOx-Al2O3 | 0.9 | 4 | 100 | 52 | >99 | [37] |
| 9 | Au/TiO2 | 0.9 | 4 | 100 | 9.4 | >99 | [37] |
| 10 | Au/FeOx-Al2O3 | 0.9 | 4 | 80 | 25 | >99 | [37] |
| 11 | Au/SiO2 | 1 | 4.5 | 90 | 74 | >99 | [38] |
| 12 | Au/sPSB | 4 | 24 | 25 | 99 | >99 | [39] |
| 13 | Nanoporous Au | 10 | 10 | 60 | 88 | >99 | [40] |
| 14 a | Au/meso-Fe2O3 | 0.5 | 4 | 80 | 99 b | >99 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Chen, H. Enhanced Selective Production of Carbonyl Products for Aerobic Oxidation of Benzylic Alcohols over Mesoporous Fe2O3 Supported Gold Nanoparticles. Catalysts 2019, 9, 754. https://doi.org/10.3390/catal9090754
Jia Y, Chen H. Enhanced Selective Production of Carbonyl Products for Aerobic Oxidation of Benzylic Alcohols over Mesoporous Fe2O3 Supported Gold Nanoparticles. Catalysts. 2019; 9(9):754. https://doi.org/10.3390/catal9090754
Chicago/Turabian StyleJia, Yanjun, and Hanning Chen. 2019. "Enhanced Selective Production of Carbonyl Products for Aerobic Oxidation of Benzylic Alcohols over Mesoporous Fe2O3 Supported Gold Nanoparticles" Catalysts 9, no. 9: 754. https://doi.org/10.3390/catal9090754
APA StyleJia, Y., & Chen, H. (2019). Enhanced Selective Production of Carbonyl Products for Aerobic Oxidation of Benzylic Alcohols over Mesoporous Fe2O3 Supported Gold Nanoparticles. Catalysts, 9(9), 754. https://doi.org/10.3390/catal9090754





