Catalyst Stability Assessment in a Lab-Scale Liquid-Solid (LS)² Plug-Flow Reactor
Abstract
:1. Introduction
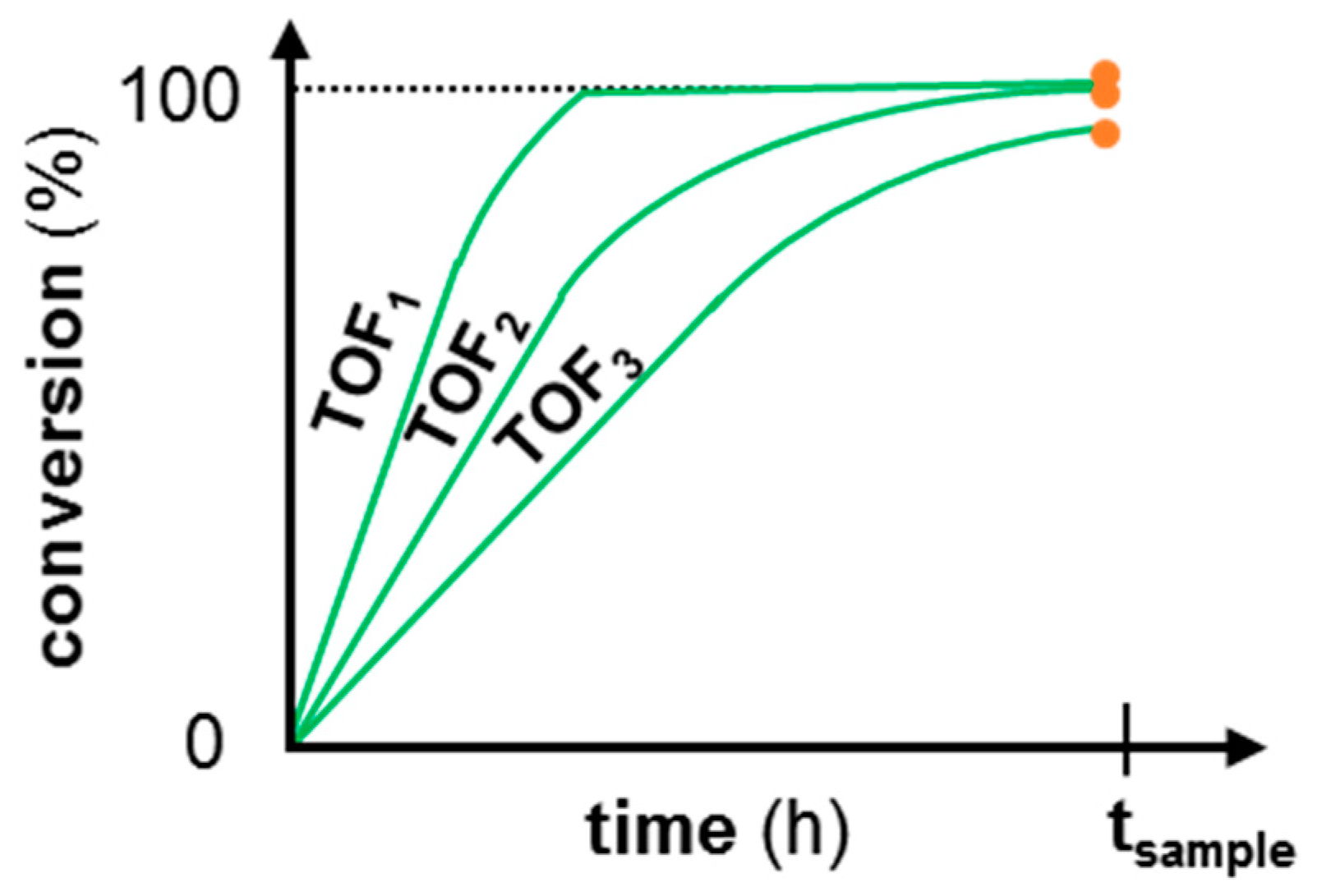
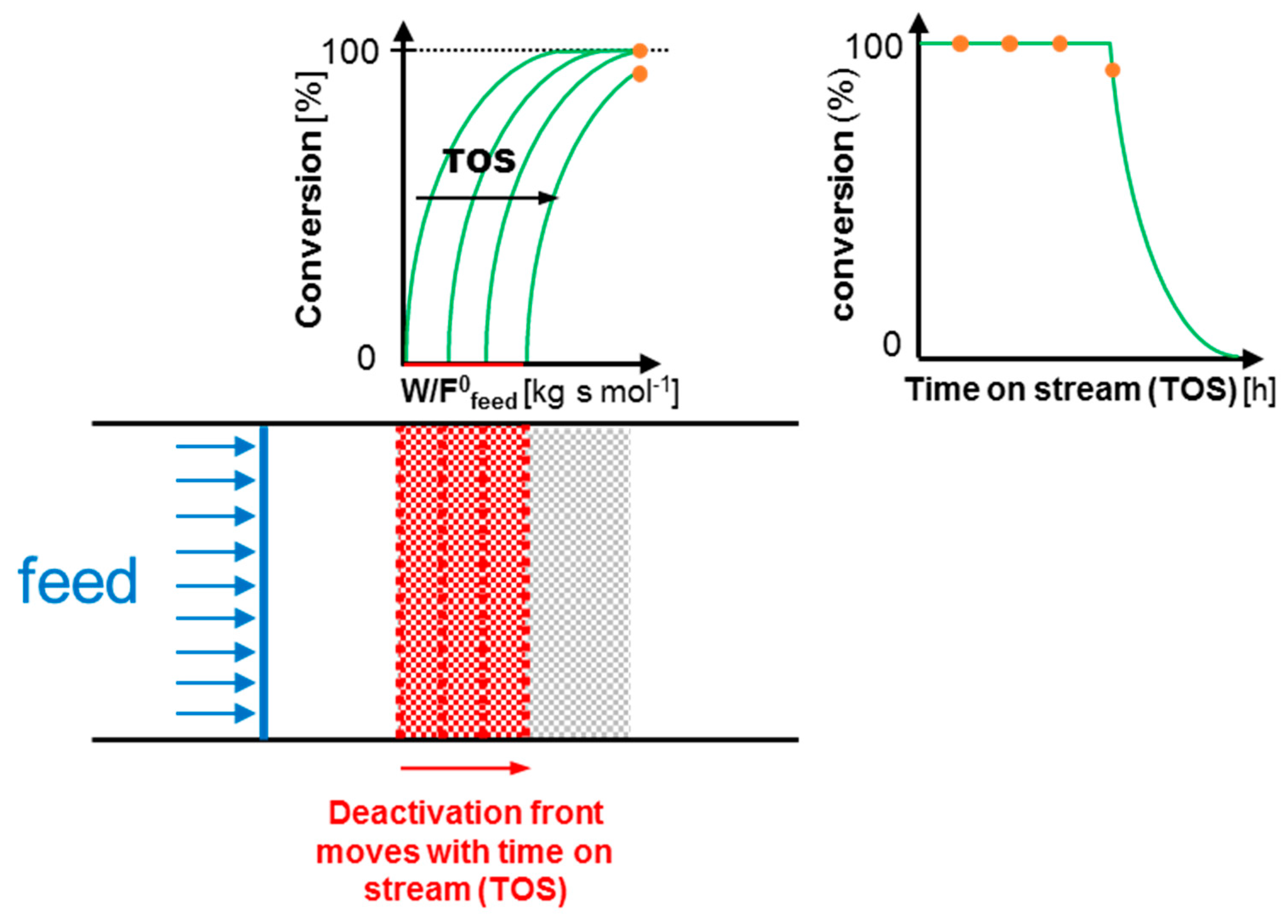
1.1. Continuous-Flow Reactor for the Assessment of Catalyst Deactivation
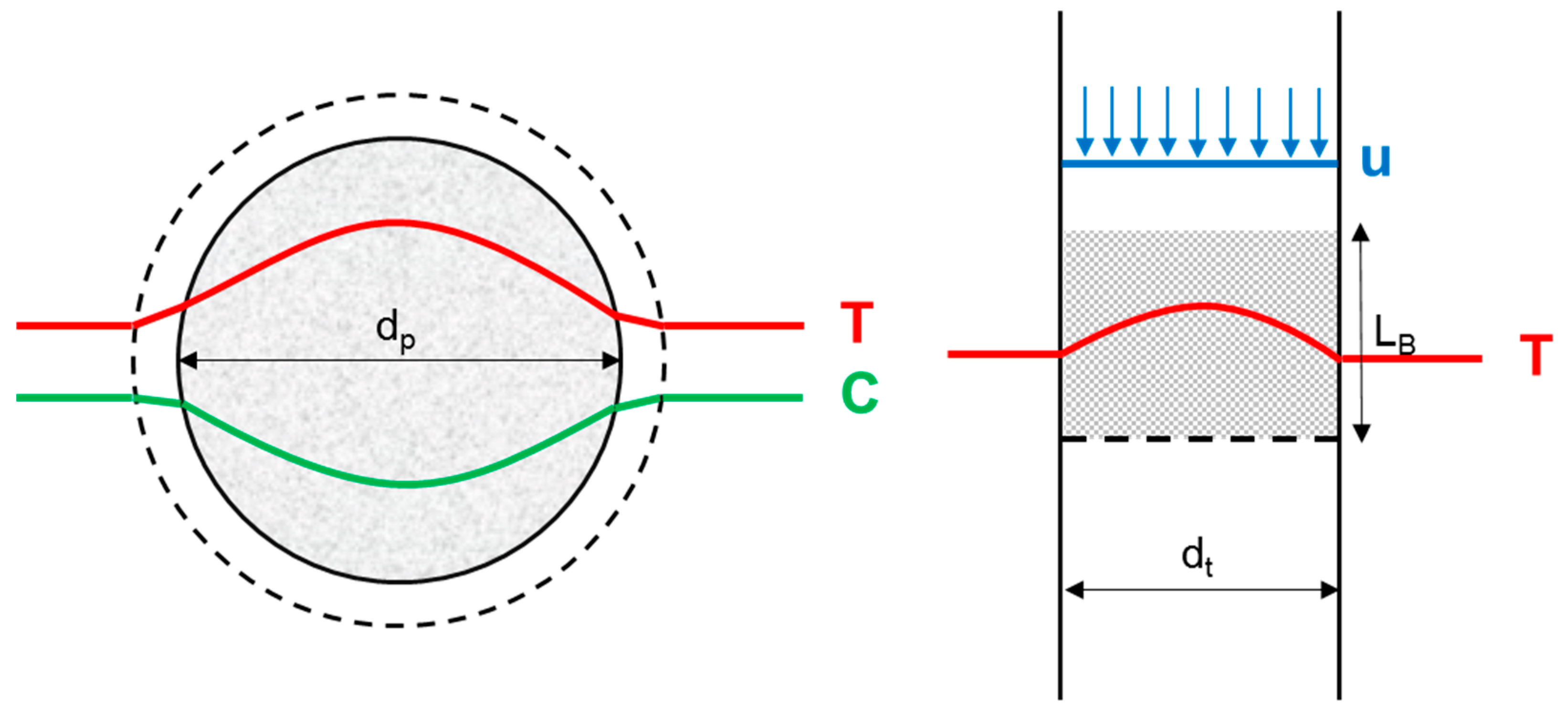
1.2. Intrinsic Kinetics Assessment
1.3. Aim of this Work
2. Results and Discussion
2.1. Case Study: Transesterification Reaction
2.1.1. Intrinsic Kinetics Verification
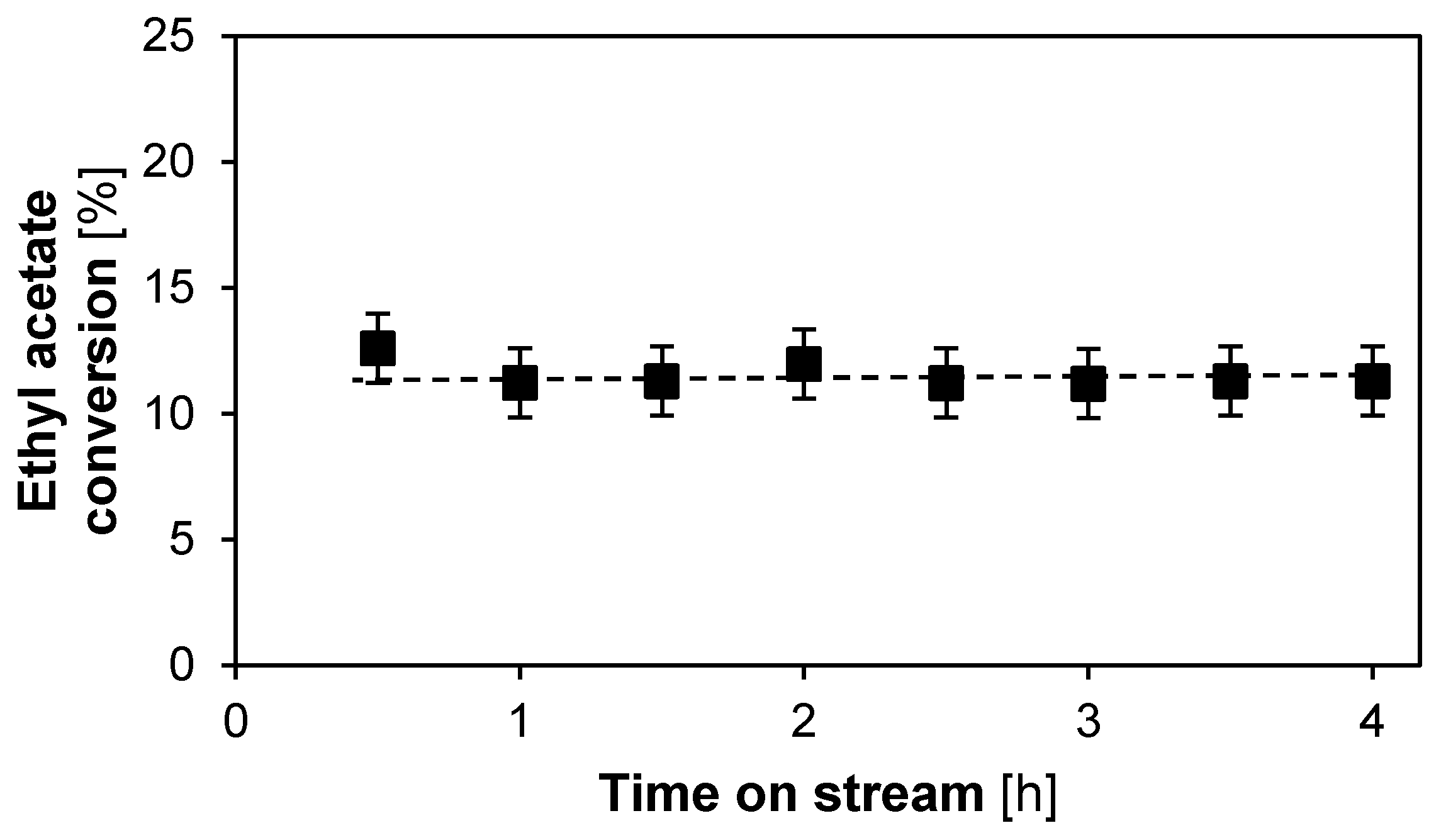
2.1.2. Catalyst Stability and Activity
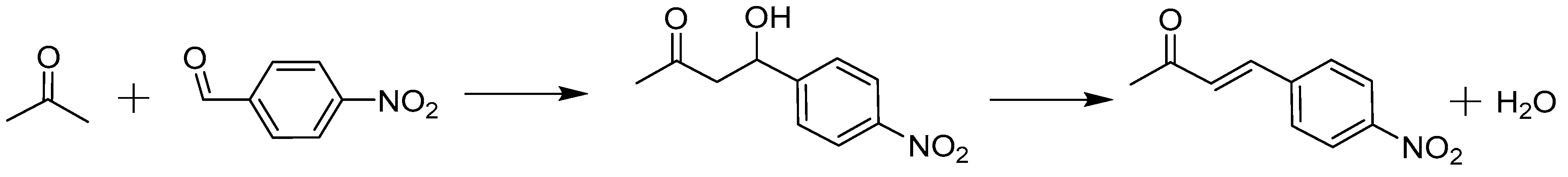
2.2. Case study: Aldol Reaction
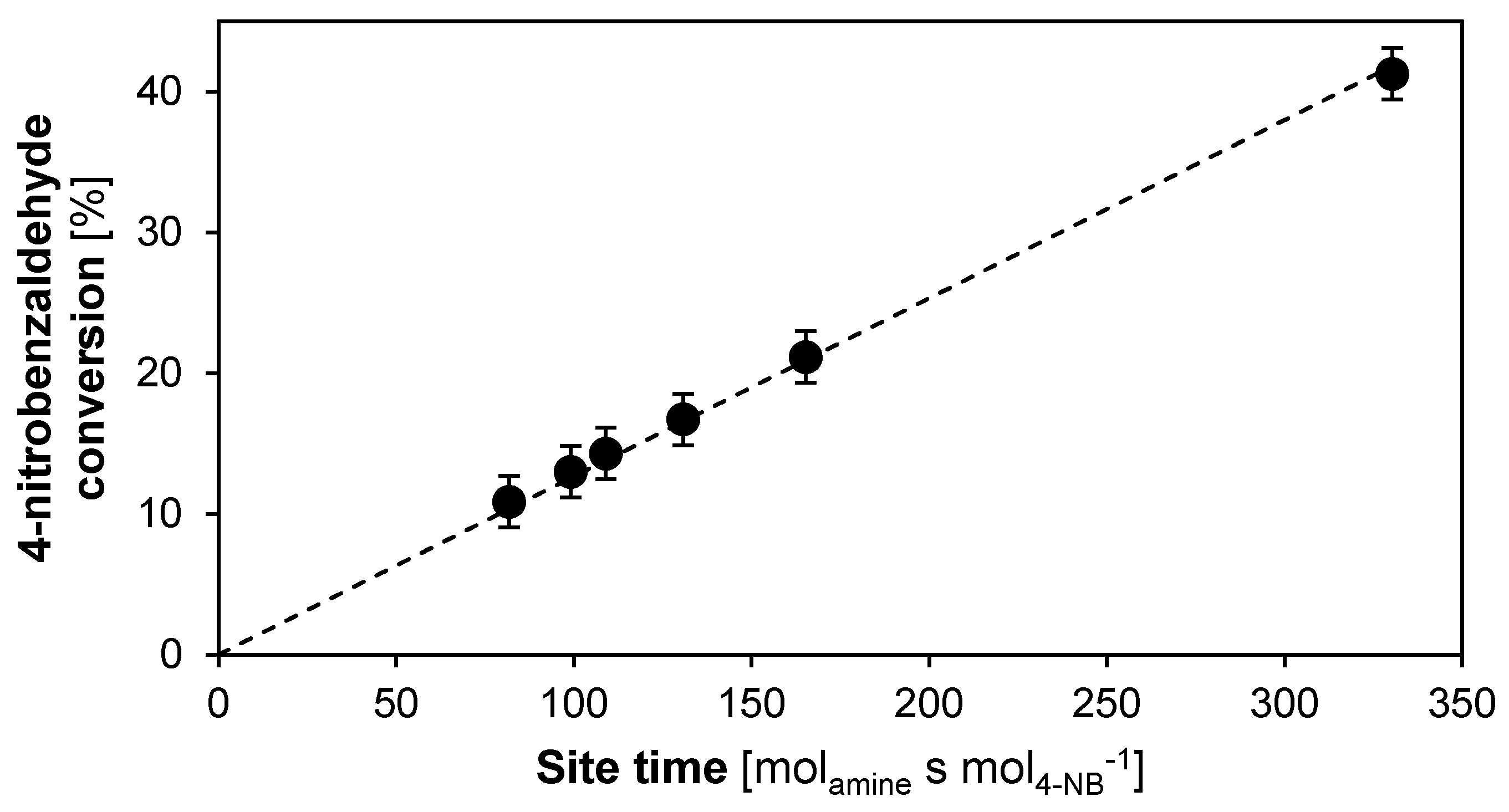
2.2.1. Intrinsic Kinetics Verification
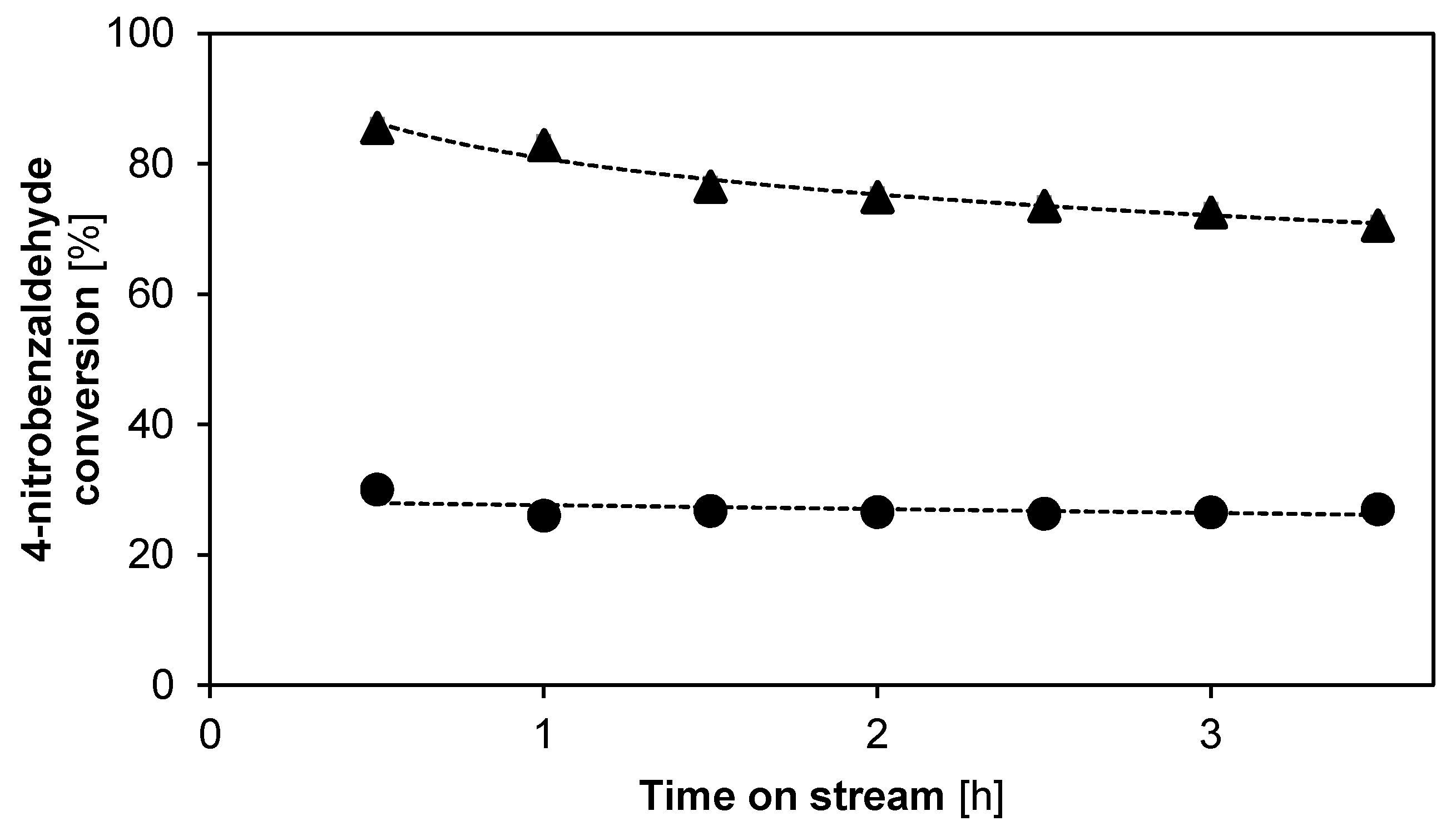
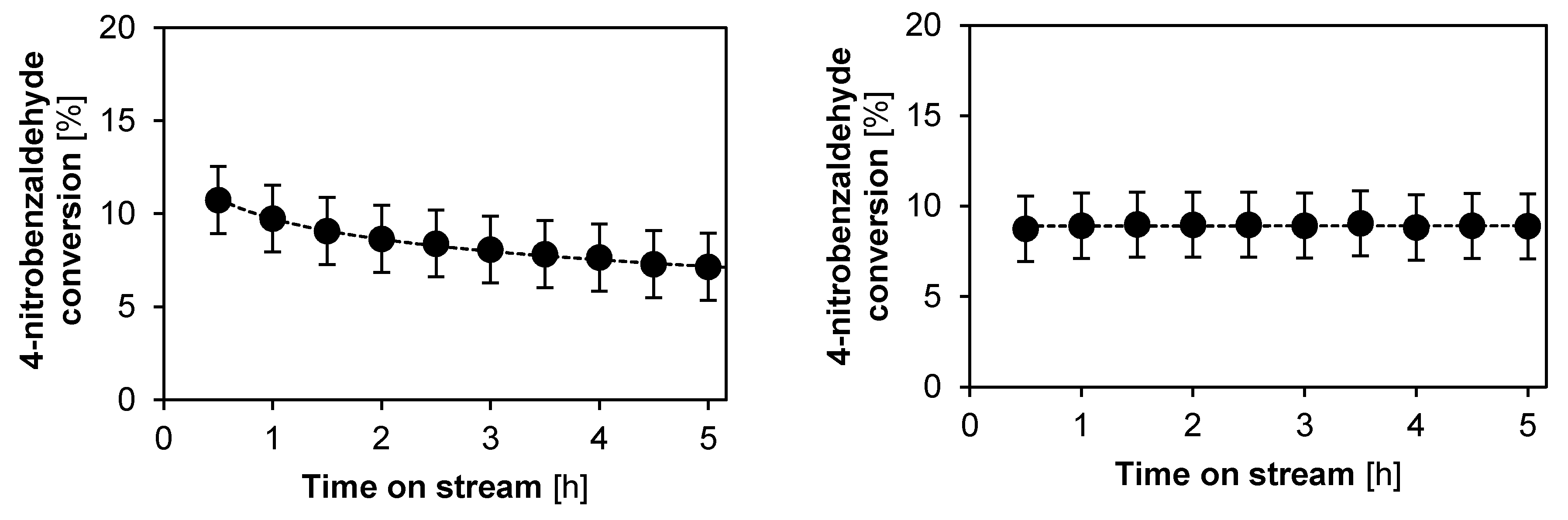
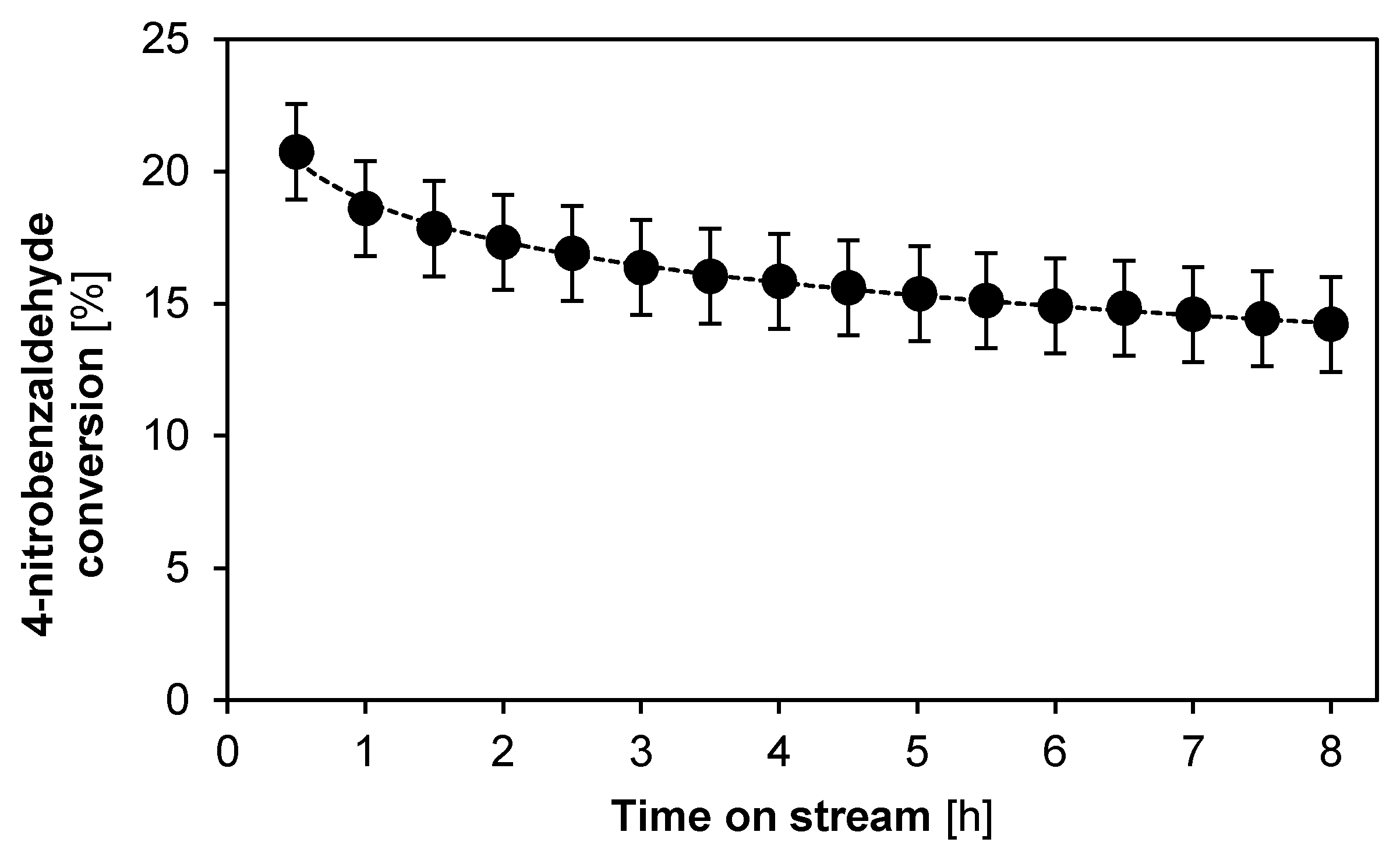
2.2.2. Catalyst Stability and Activity
3. Materials and Methods
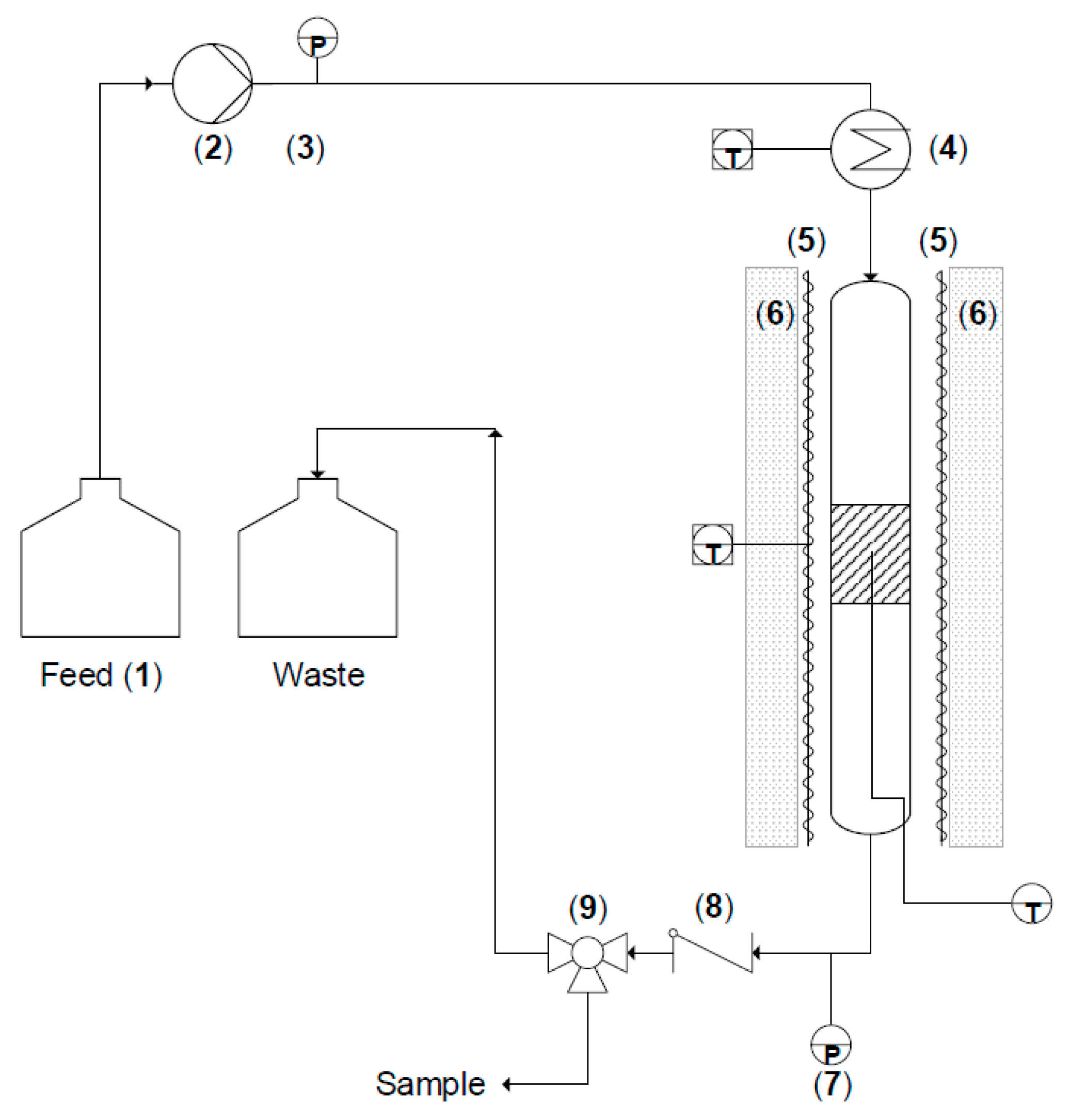
3.1. Reactor Design
3.2. Case Study: Transesterification
3.3. Case Study: Aldol Reaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van de Steene, E.; de Clercq, J.; Thybaut, J.W. Ion-Exchange Resin Catalyzed Transesterification of Ethyl Acetate with Methanol: Gel Versus Macroporous Resins. Chem. Eng. J. 2014, 242, 170–179. [Google Scholar] [CrossRef]
- Hora, L.; Kelbichová, V.; Kikhtyanin, O.; Bortnovskiy, O.; Kubička, D. Aldol Condensation of Furfural and Acetone over Mg Al Layered Double Hydroxides and Mixed Oxides. Catal. Today 2014, 223, 138–147. [Google Scholar] [CrossRef]
- Ellebracht, N.C.; Jones, C.W. Amine Functionalization of Cellulose Nanocrystals for Acid-Base Organocatalysis: Surface Chemistry, Cross-Linking, and Solvent Effects. Cellulose 2018, 25, 6495–6512. [Google Scholar] [CrossRef]
- Clerick, S.; de Canck, E.; Hendrickx, K.; Van Speybroeck, V.; van Der Voort, P. Heterogeneous Ru (Iii) Oxidation Catalysts Via ‘Click’ bidentate Ligands on A Periodic Mesoporous Organosilica Support. Green Chem. 2016, 18, 6035–6045. [Google Scholar] [CrossRef]
- De Vylder, A.; Lauwaert, J.; Esquivel, D.; Poelman, D.; De Clercq, J.; Van Der Voort, P.; Thybaut, J.W. The Role of Water in the Reusability of Aminated Silica Catalysts for Aldol Reactions. J. Catal. 2018, 361, 51–61. [Google Scholar] [CrossRef]
- Hammond, C. Intensification Studies of Heterogeneous Catalysts: Probing and Overcoming Catalyst Deactivation During Liquid Phase Operation. Green Chem. 2017, 19, 2711–2728. [Google Scholar] [CrossRef]
- Marin, G.B.; Yablonsky, G.S.; Constales, D. Kinetics of Chemical Reactions: Decoding Complexity; Wiley-Vch: Weinheim, Germany, 2019. [Google Scholar]
- Li, X.; Wang, S.; Wang, K.; Jia, X.; Hu, Z. Polymer Ionic Liquid Network: A Highly Effective Reusable Catalyst for One-Pot Synthesis of Heterocyclic Compounds. RSC Adv. 2018, 8, 42292–42299. [Google Scholar] [CrossRef]
- Li, S.; Chen, F.; Li, N.; Wang, W.; Sheng, X.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Synthesis of Renewable Triketones, Diketones, and Jet-Fuel Range Cycloalkanes with 5-Hydroxymethylfurfural and Ketones. Chemsuschem 2017, 10, 711–719. [Google Scholar] [CrossRef]
- Du, J.; Tao, M.; Zhang, W. Fiber-Supported Acid-Base Bifunctional Catalysts for Efficient Nucleophilic Addition in Water. ACS Sustain. Chem. Eng. 2016, 4, 4296–4304. [Google Scholar] [CrossRef]
- Wach, A.; Drozdek, M.; Dudek, B.; Łątka, P.; Kuśtrowski, P. Control of Availability of Poly (Vinylamine)-Derived Basic Sites, Catalytically Active in Knoevenagel Condensation, by Deposition on Various Mesoporous Silicas. Microporous Mesoporous Mater. 2016, 226, 433–443. [Google Scholar] [CrossRef]
- Touchy, A.S.; Kon, K.; Onodera, W.; Shimizu, K.I. Unprecedented Reductive Esterification of Carboxylic Acids Under Hydrogen by Reusable Heterogeneous Platinum Catalysts. Adv. Synth. Catal. 2015, 357, 1499–1506. [Google Scholar] [CrossRef]
- Pamuk, H.; Aday, B.; Şen, F.; Kaya, M. Pt Nps@ Go As A Highly Efficient And Reusable Catalyst for One-Pot Synthesis of Acridinedione Derivatives. RSC Adv. 2015, 5, 49295–49300. [Google Scholar] [CrossRef]
- Narkhede, N.; Patel, A. Efficient Synthesis of Biodiesel over A Recyclable Catalyst Comprising A Monolacunary Silicotungstate and Zeolite Hβ. RSC Adv. 2014, 4, 64379–64387. [Google Scholar] [CrossRef]
- Srivastava, V. Recyclable Hydrotalcite Clay Catalysed Baylis—Hillman Reaction. J. Chem. Sci. 2013, 125, 1207–1212. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Leung, D.Y.; Toy, P.H. Rasta Resin-Tbd as A Reusable Catalyst for Transesterification Reactions. Synlett 2013, 24, 1870–1874. [Google Scholar]
- Malyaadri, M.; Jagadeeswaraiah, K.; Prasad, P.S.; Lingaiah, N. Synthesis of Glycerol Carbonate By Transesterification of Glycerol with Dimethyl Carbonate Over Mg/Al/Zr Catalysts. Appl. Catal. A Gen. 2011, 401, 153–157. [Google Scholar] [CrossRef]
- Shanmuganathan, S.; Greiner, L.; De María, P.D. Silica-Immobilized Piperazine: A Sustainable Organocatalyst for Aldol and Knoevenagel Reactions. Tetrahedron Lett. 2010, 51, 6670–6672. [Google Scholar] [CrossRef]
- Li, H.; Pérez-Trujillo, M.; Cattoën, X.; Pleixats, R. Recyclable Mesoporous Organosilica Nanoparticles Derived from Proline-Valinol Amides for Asymmetric Organocatalysis. ACS Sustain. Chem. Eng. 2019, 7, 14815–14828. [Google Scholar] [CrossRef]
- Jones, C.W. On the Stability and Recyclability of Supported Metal-Ligand Complex Catalysts: Myths, Misconceptions and Critical Research Needs. Top. Catal. 2010, 53, 942–952. [Google Scholar] [CrossRef]
- SchüTh, F.; Ward, M.D.; Buriak, J.M. Common Pitfalls of Catalysis Manuscripts Submitted to Chemistry of Materials. Chem. Mater. 2018, 30, 3599–3600. [Google Scholar] [CrossRef]
- Scott, S.L. A Matter of Life (Time) and Death. ACS Catal. 2018, 8, 8597–8599. [Google Scholar] [CrossRef]
- Perego, C.; Peratello, S. Experimental Methods In Catalytic Kinetics. Catal. Today 1999, 52, 133–145. [Google Scholar] [CrossRef]
- Rajkhowa, T.; Marin, G.B.; Thybaut, J.W. Quantifying The Dominant Factors in Cu Catalyst Deactivation During Glycerol Hydrogenolysis. J. Ind. And Chem. 2017, 54, 270–277. [Google Scholar] [CrossRef]
- Argyle, M.; Bartholomew, C. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Levenspiel, O. Chemical Reaction Engineering. Ind. Eng. Chem. Res. 1999, 38, 4140–4143. [Google Scholar] [CrossRef]
- Forzatti, P.; Borghesi, M.; Pasquon, I.; Tronconi, E. Experimental Study on The Separability of Reaction-Deactivation Kinetics: Thermal Desorption of Alcohols from Fresh and Na-Poisoned Γ-Al2o3. AIChE J. 1986, 32, 87–95. [Google Scholar] [CrossRef]
- Janssens, T.V. A New Approach to the Modeling of Deactivation in the Conversion of Methanol on Zeolite Catalysts. J. Catal. 2009, 264, 130–137. [Google Scholar] [CrossRef]
- Govender, A.; Mahomed, A.; Friedrich, H. Water: Friend or Foe in Catalytic Hydrogenation? A Case Study Using Copper Catalysts. Catalysts 2018, 8, 474. [Google Scholar] [CrossRef]
- Ayats, C.; Henseler, A.H.; Pericàs, M.A. A Solid-Supported Organocatalyst for Continuous-Flow Enantioselective Aldol Reactions. ChemSusChem 2012, 5, 320–325. [Google Scholar] [CrossRef]
- Burguete, M.I.; Erythropel, H.; Garcia-Verdugo, E.; Luis, S.V.; Sans, V. Base Supported Ionic Liquid-Like Phases as Catalysts for The Batch and Continuous-Flow Henry Reaction. Green Chem. 2008, 10, 401–407. [Google Scholar] [CrossRef]
- Bortolini, O.; Caciolli, L.; Cavazzini, A.; Costa, V.; Greco, R.; Massi, A.; Pasti, L. Silica-Supported 5-(Pyrrolidin-2-Yl) Tetrazole: Development of Organocatalytic Processes from Batch to Continuous-Flow Conditions. Green Chem. 2012, 14, 992–1000. [Google Scholar] [CrossRef]
- Forni, L. Mass And Heat Transfer in Catalytic Reactions. Catal. Today 1999, 52, 147–152. [Google Scholar] [CrossRef]
- Dautzenberg, F.M. Ten Guidelines for Catalyst Testing. ACS Symp. Ser. 1989, 411, 99–119. [Google Scholar]
- Berger, R.J.; Stitt, E.H.; Marin, G.B.; Kapteijn, F.; Moulijn, J.A. Eurokin. Chemical Reaction Kinetics in Practice. Cattech 2001, 5, 36–60. [Google Scholar] [CrossRef]
- Froment, G. Fixed Bed Catalytic Reactors—Current Design Status. Ind. Eng. Chem. 1967, 59, 18–27. [Google Scholar] [CrossRef]
- Mears, D.E. The Role of Axial Dispersion in Trickle-Flow Laboratory Reactors. Chem. Eng. Sci. 1971, 26, 1361–1366. [Google Scholar] [CrossRef]
- Ancheyta, J. Modeling and Simulation of Catalytic Reactors for Petroleum Refining; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ergun, S. Fluid Flow Through Packed Columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Mears, D.E. Diagnostic Criteria for Heat Transport Limitations in Fixed Bed Reactors. J. Catal. 1971, 20, 127–131. [Google Scholar] [CrossRef]
- Van Den Bleek, C.; Van Der Wiele, K.; Van Den Berg, P. The Effect of Dilution on the Degree of Conversion in Fixed Bed Catalytic Reactors. Chem. Eng. Sci. 1969, 24, 681–694. [Google Scholar] [CrossRef]
- Carberry, J.J. Chemical and Catalytic Reaction Engineering; Courier Corporation: Chelmsford, MA, USA, 2001. [Google Scholar]
- Wilke, C.; Chang, P. Correlation of Diffusion Coefficients In Dilute Solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Weisz, P.; Prater, C. Interpretation of Measurements In Experimental Catalysis. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 1954; Volume 6, pp. 143–196. [Google Scholar]
- Madon, R.J.; Boudart, M. Experimental Criterion for the Absence of Artifacts in the Measurement of Rates of Heterogeneous Catalytic Reactions. Ind. Eng. Chem. Fundam. 1982, 21, 438–447. [Google Scholar] [CrossRef]
- Gupta, A.S.; Thodos, G. Mass And Heat Transfer in the Flow of Fluids Through Fixed and Fluidized Beds of Spherical Particles. AIChE J. 1962, 8, 608–610. [Google Scholar] [CrossRef]
- Barrett, C.; Chheda, J.; Huber, G.; Dumesic, J. Single-Reactor Process for Sequential Aldol-Condensation and Hydrogenation of Biomass-Derived Compounds in Water. Appl. Catal. B Environ. 2006, 66, 111–118. [Google Scholar] [CrossRef]
- Dossin, T.F.; Reyniers, M.-F.; Berger, R.J.; Marin, G.B. Simulation of Heterogeneously Mgo-Catalyzed Transesterification for Fine-Chemical and Biodiesel Industrial Production. Appl. Catal. B Environ. 2006, 67, 136–148. [Google Scholar] [CrossRef]
- Harmer, M.A.; Sun, Q. Solid Acid Catalysis Using Ion-Exchange Resins. Appl. Catal. A Gen. 2001, 221, 45–62. [Google Scholar] [CrossRef]
- Ali, S.H.; Al-Rashed, O.; Azeez, F.A.; Merchant, S.Q. Potential Biofuel Additive from Renewable Sources—Kinetic Study of Formation of Butyl Acetate by Heterogeneously Catalyzed Transesterification of Ethyl Acetate with Butanol. Bioresour. Technol. 2011, 102, 10094–10103. [Google Scholar] [CrossRef] [PubMed]
- Zielinska-Nadolska, I.; Warmuzinski, K.; Richter, J. Zeolite and other Heterogeneous Catalysts for the Transesterification Reaction of Dimethyl Carbonate with Ethanol. Catal. Today 2006, 114, 226–230. [Google Scholar] [CrossRef]
- Tesser, R.; Casale, L.; Verde, D.; Di Serio, M.; Santacesaria, E. Kinetics and Modeling of Fatty Acids Esterification on Acid Exchange Resins. Chem. Eng. J. 2010, 157, 539–550. [Google Scholar] [CrossRef]
- Bhandari, V.; Sorokhaibam, L.; Ranade, V. Ion Exchange Resin Catalyzed Reactions—An Overview. In Industrial Catalytic Processes for Fine and Specialty Chemicals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 393–426. [Google Scholar]
- Chakrabarti, A.; Sharma, M. Cationic Ion Exchange Resins As Catalyst. React. Polym. 1993, 20, 1–45. [Google Scholar] [CrossRef]
- Van De Steene, E.; De Clercq, J.; Thybaut, J.W. Adsorption and Reaction in the Transesterification of Ethyl Acetate with Methanol on Lewatit K1221. J. Mol. Catal. A Chem. 2012, 359, 57–68. [Google Scholar] [CrossRef]
- Saha, B.; Streat, M. Transesterification of Cyclohexyl Acrylate with N-Butanol and 2-Ethylhexanol: Acid-Treated Clay, Ion Exchange Resins and Tetrabutyl Titanate as Catalysts. React. Funct. Polym. 1999, 40, 13–27. [Google Scholar] [CrossRef]
- Alonso, D.M.; Granados, M.L.; Mariscal, R.; Douhal, A. Polarity of the Acid Chain of Esters and Transesterification Activity of Acid Catalysts. J. Catal. 2009, 262, 18–26. [Google Scholar] [CrossRef]
- Pappu, V.K.; Yanez, A.J.; Peereboom, L.; Muller, E.; Lira, C.T.; Miller, D.J. A Kinetic Model of the Amberlyst-15 Catalyzed Transesterification of Methyl Stearate with N-Butanol. Bioresour. Technol. 2011, 102, 4270–4272. [Google Scholar] [CrossRef] [PubMed]
- Bożek-Winkler, E.; Gmehling, J. Transesterification of Methyl Acetate and N-Butanol Catalyzed by Amberlyst 15. Ind. Eng. Chem. Res. 2006, 45, 6648–6654. [Google Scholar] [CrossRef]
- Jalilnejad Falizi, N.; Güngören Madenoğlu, T.; Yüksel, M.; Kabay, N. Biodiesel Production Using Gel-Type Cation Exchange Resin at Different Ionic Forms. Int. J. Energy Res. 2019, 43, 2188–2199. [Google Scholar] [CrossRef]
- Van De Steene, E. Kinetic Study of the (Trans) Esterification Catalyzed by Gel and Macroporous Resins; Ghent University, Faculty of Engineering and Architecture: Ghent, Belgium, 2014; p. 173. [Google Scholar]
- Alsalme, A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Heteropoly Acids as Catalysts for Liquid-Phase Esterification and Transesterification. Appl. Catal. A Gen. 2008, 349, 170–176. [Google Scholar] [CrossRef]
- Lopez, D.E.; Goodwin, J.G., Jr.; Bruce, D.A.; Lotero, E. Transesterification of Triacetin with Methanol on Solid Acid and Base Catalysts. Appl. Catal. A Gen. 2005, 295, 97–105. [Google Scholar] [CrossRef]
- Russbueldt, B.M.; Hoelderich, W.F. New Sulfonic Acid Ion-Exchange Resins for the Preesterification of Different Oils and Fats with High Content of Free Fatty Acids. Appl. Catal. A Gen. 2009, 362, 47–57. [Google Scholar] [CrossRef]
- Brunelli, N.A.; Jones, C.W. Tuning Acid-Base Cooperativity to Create next Generation Silica-Supported Organocatalysts. J. Catal. 2013, 308, 60–72. [Google Scholar] [CrossRef]
- Kandel, K.; Althaus, S.M.; Peeraphatdit, C.; Kobayashi, T.; Trewyn, B.G.; Pruski, M.; Slowing, I.I. Substrate Inhibition in the Heterogeneous Catalyzed Aldol Condensation: A Mechanistic Study of Supported Organocatalysts. J. Catal. 2012, 291, 63–68. [Google Scholar] [CrossRef]
- Singappuli-Arachchige, D.; Kobayashi, T.; Wang, Z.; Burkhow, S.J.; Smith, E.A.; Pruski, M.; Slowing, I.I. Interfacial Control of Catalytic Activity in the Aldol Condensation: Combining the Effects of Hydrophobic Environments and Water. ACS Catal. 2019. [Google Scholar] [CrossRef]
- Lauwaert, J.; De Canck, E.; Esquivel, D.; Van Der Voort, P.; Thybaut, J.W.; Marin, G.B. Effects of Amine Structure and Base Strength on Acid-Base Cooperative Aldol Condensation. Catal. Today 2015, 246, 35–45. [Google Scholar] [CrossRef]
- Kandel, K.; Althaus, S.M.; Peeraphatdit, C.; Kobayashi, T.; Trewyn, B.G.; Pruski, M.; Slowing, I.I. Solvent-Induced Reversal of Activities between Two Closely Related Heterogeneous Catalysts in the Aldol Reaction. ACS Catal. 2013, 3, 265–271. [Google Scholar] [CrossRef] [Green Version]
- De Vylder, A.; Lauwaert, J.; Sabbe, M.K.; Reyniers, M.-F.; De Clercq, J.; Van Der Voort, P.; Thybaut, J.W. Rational Design of Nucleophilic Amine Sites Via Computational Probing of Steric and Electronic Effects. Catal. Today 2019, 334, 96–103. [Google Scholar] [CrossRef]
- Lauwaert, J.; De Canck, E.; Esquivel, D.; Thybaut, J.W.; Van Der Voort, P.; Marin, G.B. Silanol-Assisted Aldol Condensation on Aminated Silica: Understanding the Arrangement of Functional Groups. Chemcatchem 2014, 6, 255–264. [Google Scholar] [CrossRef]


| Criterion | Value | Limit |
|---|---|---|
| Ca | 3.2 × 10−3 | <5 × 10−2 |
| ∅ | 1.3 × 10−2 | <8 × 10−2 |
| ∆Text (K) | 2.6 × 10−3 | <0.95 |
| ∆Trad (K) | 1.1 × 10−2 | <0.95 |
| ∆Tint (K) | 6.7 × 10−4 | <0.95 |
| ∆Tax (K) | 2.0 × 10−2 | <0.95 |
| dt/dp | 14 | >8 |
| Lr/dp | 273 | >2.2 |
| ∆P | 42.6 | <36,000 Pa |
| Vdil/Vtot | 0 | <0.995 |
| Site Time (molsite s molethyl acetate−1) | 15.8 | 15.8 |
|---|---|---|
| Flow rate (mL min−1) | 1.0 | 1.5 |
| Conversion (%) | 13 ± 1 | 12 ± 1 |
| Hexane | DMSO | Solvent-Free | Limit | |
|---|---|---|---|---|
| Ca | 6.2 × 10−3 | 4.9 × 10−3 | 2.7 × 10−3 | <5 × 10−2 |
| ϕ | 6.8 × 10−2 | 6.2 × 10−2 | 2.6 × 10−2 | <8 × 10−2 |
| ∆Text (K) | 1.6 × 10−3 | 6.0 × 10−4 | 5.3 × 10−4 | <1.32 |
| ∆Trad (K) | 7.5 × 10−2 | 2.0 × 10−2 | 2.3 × 10−2 | <1.32 |
| ∆Tint (K) | 2.4 × 10−4 | 7.7 × 10−5 | 7.0 × 10−5 | <1.32 |
| ∆Tax (K) | 7.4 × 10−2 | 1.9 × 10−2 | 3.7 × 10−2 | <1.32 |
| dt/dp | 20 | 20 | 20 | >8 |
| LB/dp | 400 | 400 | 400 | >1.6 |
| ∆P (Pa) | 106.4 | 177.8 | 78.9 | <36,000 |
| Vdil/Vtot | 0 | 0 | 0 | <0.995 |
| Average Pellet Diameter (µm) | 137 | 375 |
|---|---|---|
| Conversion (%) | 16 ± 2 | 19 ± 2 |
| Site Time (molsite s mol−1) | 165.1 | 165.1 |
|---|---|---|
| Flow rate (ml min−1) | 1.5 | 2.0 |
| Conversion (%) | 22 ± 2 | 21 ± 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vylder, A.; Lauwaert, J.; Van Auwenis, S.; De Clercq, J.; Thybaut, J.W. Catalyst Stability Assessment in a Lab-Scale Liquid-Solid (LS)² Plug-Flow Reactor. Catalysts 2019, 9, 755. https://doi.org/10.3390/catal9090755
De Vylder A, Lauwaert J, Van Auwenis S, De Clercq J, Thybaut JW. Catalyst Stability Assessment in a Lab-Scale Liquid-Solid (LS)² Plug-Flow Reactor. Catalysts. 2019; 9(9):755. https://doi.org/10.3390/catal9090755
Chicago/Turabian StyleDe Vylder, Anton, Jeroen Lauwaert, Stijn Van Auwenis, Jeriffa De Clercq, and Joris W. Thybaut. 2019. "Catalyst Stability Assessment in a Lab-Scale Liquid-Solid (LS)² Plug-Flow Reactor" Catalysts 9, no. 9: 755. https://doi.org/10.3390/catal9090755
APA StyleDe Vylder, A., Lauwaert, J., Van Auwenis, S., De Clercq, J., & Thybaut, J. W. (2019). Catalyst Stability Assessment in a Lab-Scale Liquid-Solid (LS)² Plug-Flow Reactor. Catalysts, 9(9), 755. https://doi.org/10.3390/catal9090755







