Synthesis of Au–Ag Alloy Nanoparticle-Incorporated AgBr Crystals
Abstract
:1. Introduction
2. Results and Discussion
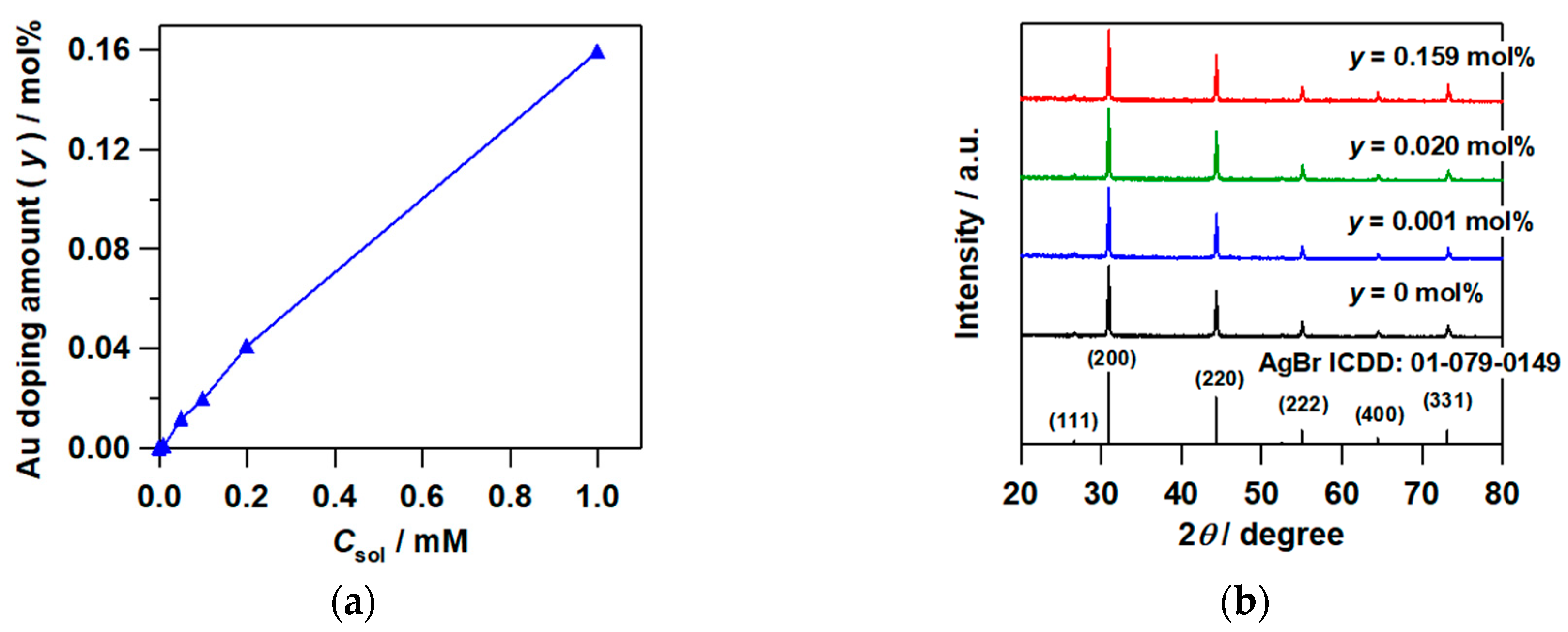
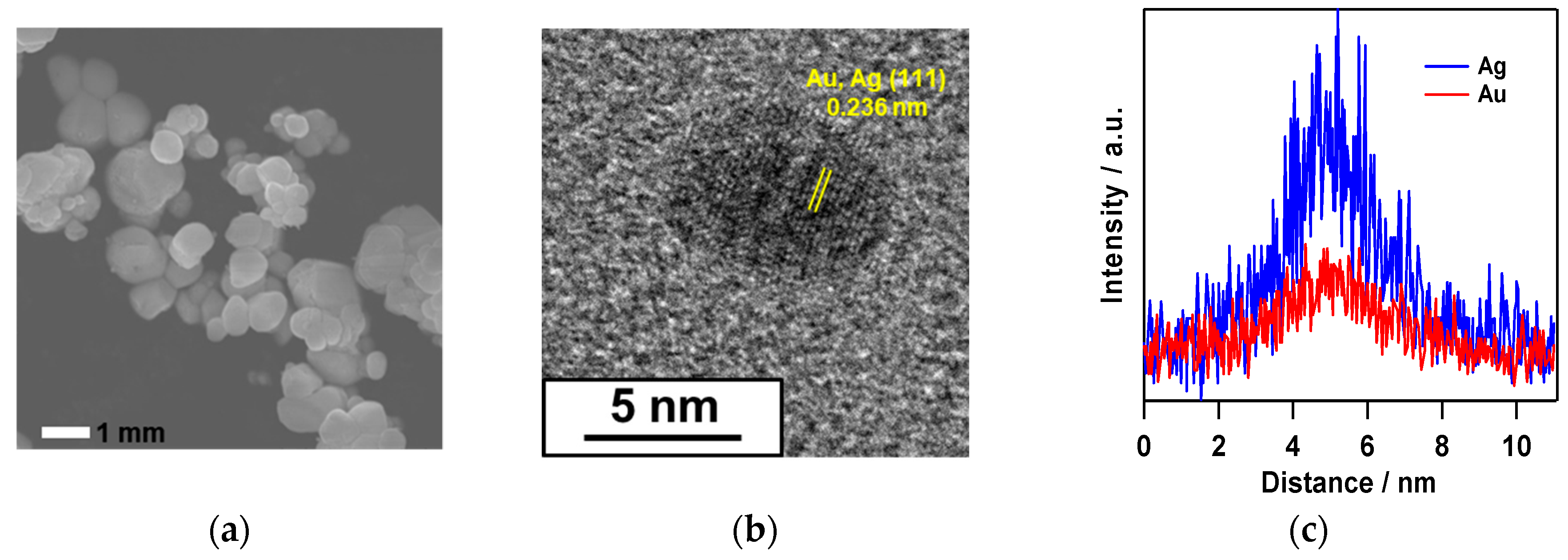
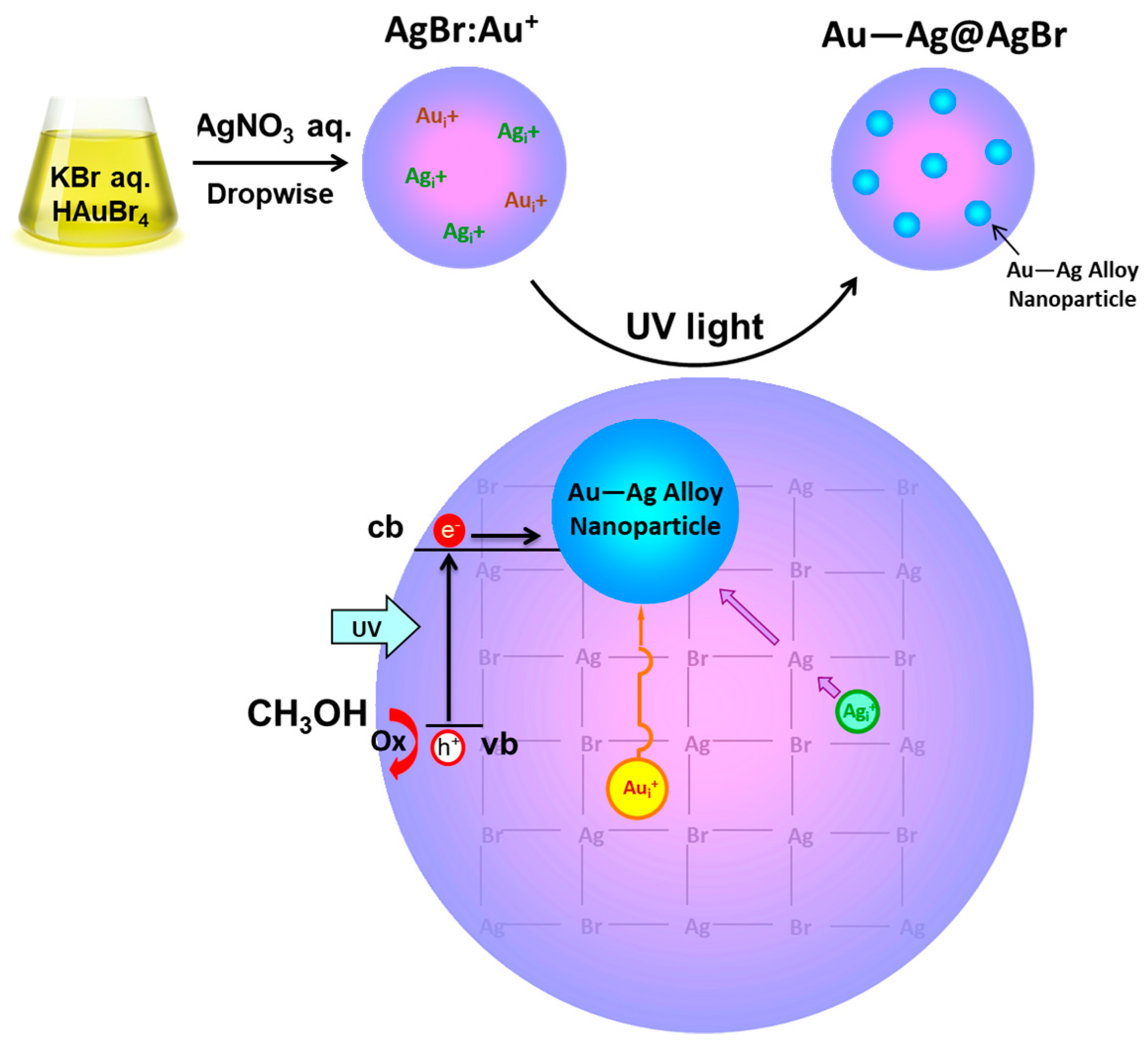
2.1. Synthesis of Au–Ag@AgBr
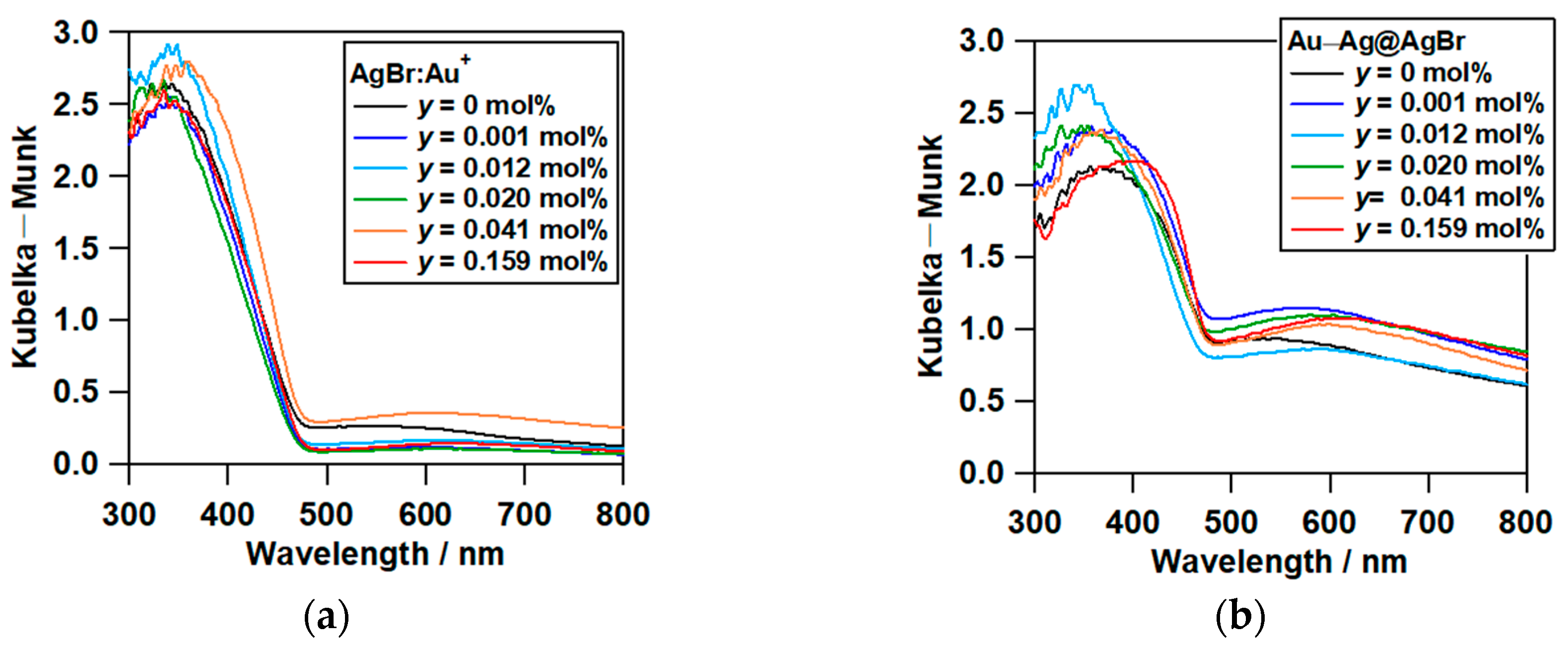
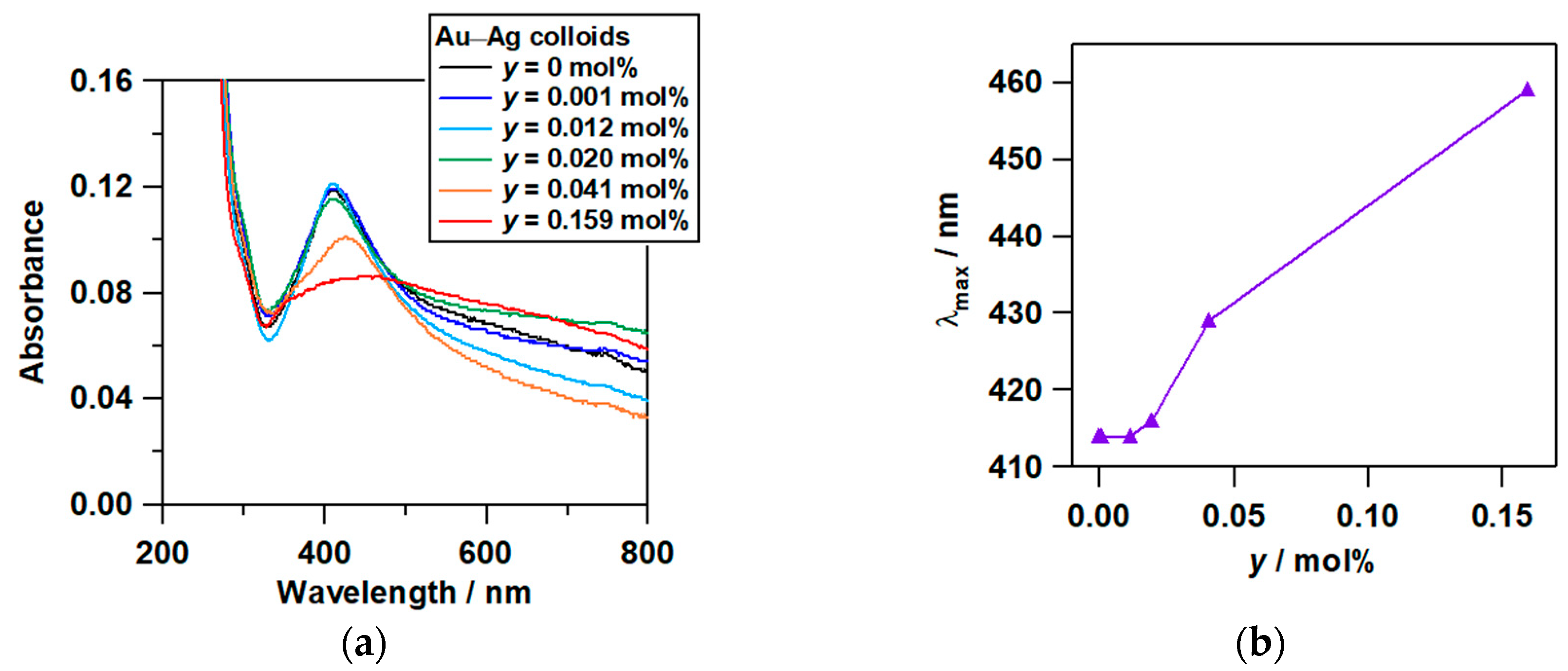
2.2. Optical Properties of Au–Ag@AgBr
2.3. Mechanism on the Formation of Au–Ag@AgBr
3. Experimental Section
3.1. Catalyst Preparation and Characterization
3.2. FDTD Calculaitons
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panayotov, D.A.; Morris, J.R. Surface chemistry of Au/TiO2: Thermally and photolytically activated reactions. Surf. Sci. Rep. 2016, 71, 77–271. [Google Scholar] [CrossRef]
- Tada, H. Size, shape and interface control in gold nanoparticle-based plasmonic photocatalysts for solar-to-chemical transformations. Dalton Trans. 2019, 48, 6308–6313. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, N.; Goto, N.; Ohkita, H.; Mizushima, T. Silver Bromide as a Photocatalyst for Hydrogen Generation from CH3OH/H2O Solution. J. Phys. Chem. B 1999, 103, 5917–5919. [Google Scholar] [CrossRef]
- An, C.; Wang, J.; Wang, S.; Zhang, Q.-H. Plasmonic enhancement of photocatalysis over Ag incorporated AgI hollow nanostructures. RSC Adv. 2014, 4, 2409–2413. [Google Scholar] [CrossRef]
- An, C.; Wang, J.; Qin, C.; Jiang, W.; Wang, S.; Li, Y.; Zhang, Q. Synthesis of Ag@AgBr/AgCl heterostructured nanocashews with enhanced photocatalytic performance via anion exchange. J. Mater. Chem. 2012, 22, 13153. [Google Scholar] [CrossRef]
- Asi, M.A.; Zhu, L.; He, C.; Sharma, V.K.; Shu, D.; Li, S.; Yang, J.; Xiong, Y. Visible-light-harvesting reduction of CO2 to chemical fuels with plasmonic Ag@AgBr/CNT nanocom-posites. Catal. Today 2013, 216, 268–275. [Google Scholar] [CrossRef]
- Marszewski, M.; Cao, S.; Yu, J.; Jaroniec, M. Semiconductor-based photocatalytic CO2 conversion. Mater. Horiz. 2015, 2, 261–278. [Google Scholar] [CrossRef]
- Hayashido, Y.; Naya, S.-I.; Tada, H. Local Electric Field-Enhanced Plasmonic Photocatalyst: Formation of Ag Cluster-Incorporated AgBr Nanoparticles on TiO2. J. Phys. Chem. C 2016, 120, 19663–19669. [Google Scholar] [CrossRef]
- Tada, H.; Naya, S.-I.; Fujishima, M. Water splitting by plasmonic photocatalysts with a gold nanoparticle/cadmium sulfide heteroepitaxial junction: A mini review. Electrochem. Commun. 2018, 97, 22–26. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Physical Chemistry, 8th ed.; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Gao, C.; Hu, Y.; Wang, M.; Chi, M.; Yin, Y. Fully Alloyed Ag/Au Nanospheres: Combining the Plasmonic Property of Ag with the Stability of Au. J. Am. Chem. Soc. 2014, 136, 7474–7479. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, M.J.; Zhong, C.-J.; Yen, B.K.H.; Anderegg, J.; Gross, S.M.; Evans, N.D.; Porter, M.; Murray, R.W. Stable, Monolayer-Protected Metal Alloy Clusters. J. Am. Chem. Soc. 1998, 120, 9396–9397. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Kowalska, E.; Sobczak, J.W.; Lisowski, W.; Ohtani, B.; Zaleska, A. Preparation and characterization of monometallic (Au) and bimetallic (Ag/Au) modified-titania photocatalysts activated by visible light. Appl. Catal. B Environ. 2011, 101, 504–514. [Google Scholar] [CrossRef]
- Link, S.; Wang, Z.L.; El-Sayed, M.A. Alloy Formation of Gold−Silver Nanoparticles and the Dependence of the Plasmon Absorption on Their Composition. J. Phys. Chem. B 1999, 103, 3529–3533. [Google Scholar] [CrossRef]
- Sun, L.; Lv, P.; Li, H.; Wang, F.; Su, W.; Zhang, L. One-step synthesis of Au–Ag alloy nanoparticles using soluble starch and their photocatalytic performance for 4-nitrophenol degradation. J. Mater. Sci. 2018, 53, 15895–15906. [Google Scholar] [CrossRef]
- Sun, L.; Yin, Y.; Lv, P.; Su, W.; Zhang, L. Green controllable synthesis of Au–Ag alloy nanoparticles using Chinese wolfberry fruit extract and their tunable photocatalytic activity. RSC Adv. 2018, 8, 3964–3973. [Google Scholar] [CrossRef]
- Ray, P.; Clément, M.; Martini, C.; Abdellah, I.; Beaunier, P.; Rodriguez-Lopez, J.L.; Huc, V.; Remita, H.; Lampre, I. Stabilisation of small mono- and bimetallic gold–silver nanoparticles using calix [8] arene derivatives. New J. Chem. 2018, 42, 14128–14137. [Google Scholar] [CrossRef]
- Naya, S.-I.; Hayashido, Y.; Akashi, R.; Kitazono, K.; Soejima, T.; Fujishima, M.; Kobayashi, H.; Tada, H. Solid-Phase Photochemical Growth of Composition-Variable Au–Ag Alloy Nanoparticles in AgBr Crystal. J. Phys. Chem. C 2017, 121, 20763–20768. [Google Scholar] [CrossRef]
- Negishi, R.; Naya, S.-I.; Kobayashi, H.; Tada, H. Gold(Core)-Lead(Shell) Nanoparticle-Loaded Titanium(IV) Oxide Prepared by Underpotential Photodeposition: Plasmonic Water Oxidation. Angew. Chem. Int. Ed. 2017, 56, 10347–10351. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Kiyonaga, T.; Naya, S.-I. Rational design and applications of highly efficient reaction systems photocatalyzed by noble metal nanoparticle-loaded titanium(iv) dioxide. Chem. Soc. Rev. 2009, 38, 1849. [Google Scholar] [CrossRef] [PubMed]
- Tani, T. Silver Nanoparticles from Silver Halide Photography to Plasmonics; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Rodríguez, O.P.; Caro, M.; Rivera, A.; Olivares, J.; Perlado, J.M.; Caro, A. Optical properties of Au-Ag alloys: An ellipsometric study. Opt. Mater. Express 2014, 4, 403. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naya, S.-i.; Fujishima, M.; Tada, H. Synthesis of Au–Ag Alloy Nanoparticle-Incorporated AgBr Crystals. Catalysts 2019, 9, 745. https://doi.org/10.3390/catal9090745
Naya S-i, Fujishima M, Tada H. Synthesis of Au–Ag Alloy Nanoparticle-Incorporated AgBr Crystals. Catalysts. 2019; 9(9):745. https://doi.org/10.3390/catal9090745
Chicago/Turabian StyleNaya, Shin-ichi, Musashi Fujishima, and Hiroaki Tada. 2019. "Synthesis of Au–Ag Alloy Nanoparticle-Incorporated AgBr Crystals" Catalysts 9, no. 9: 745. https://doi.org/10.3390/catal9090745
APA StyleNaya, S.-i., Fujishima, M., & Tada, H. (2019). Synthesis of Au–Ag Alloy Nanoparticle-Incorporated AgBr Crystals. Catalysts, 9(9), 745. https://doi.org/10.3390/catal9090745






