SBA-15 as a Support for Effective Olefin Metathesis Catalysts
Abstract
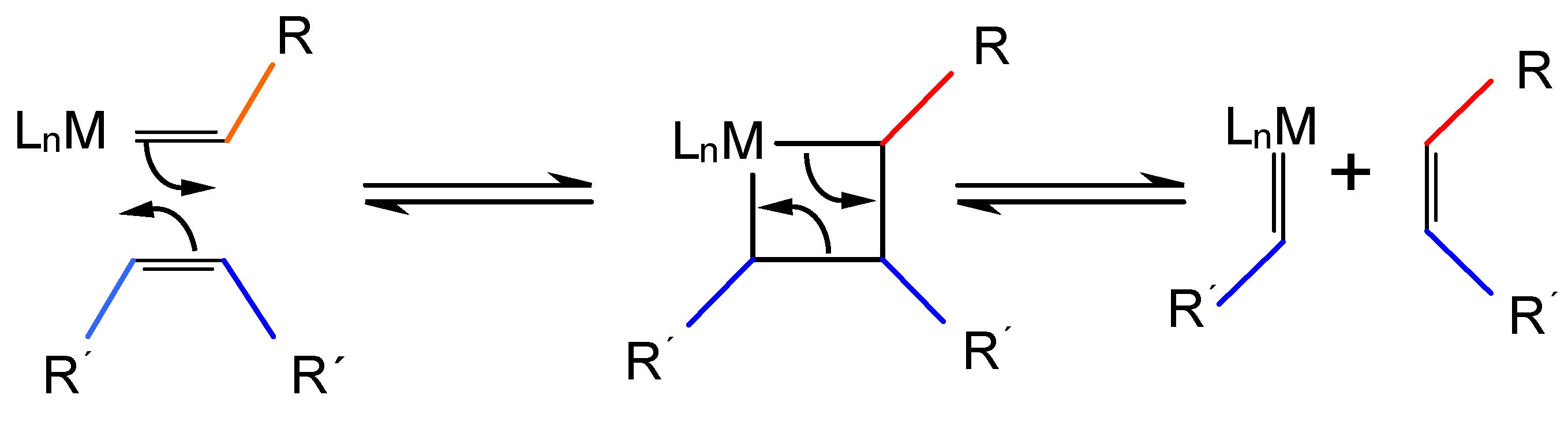
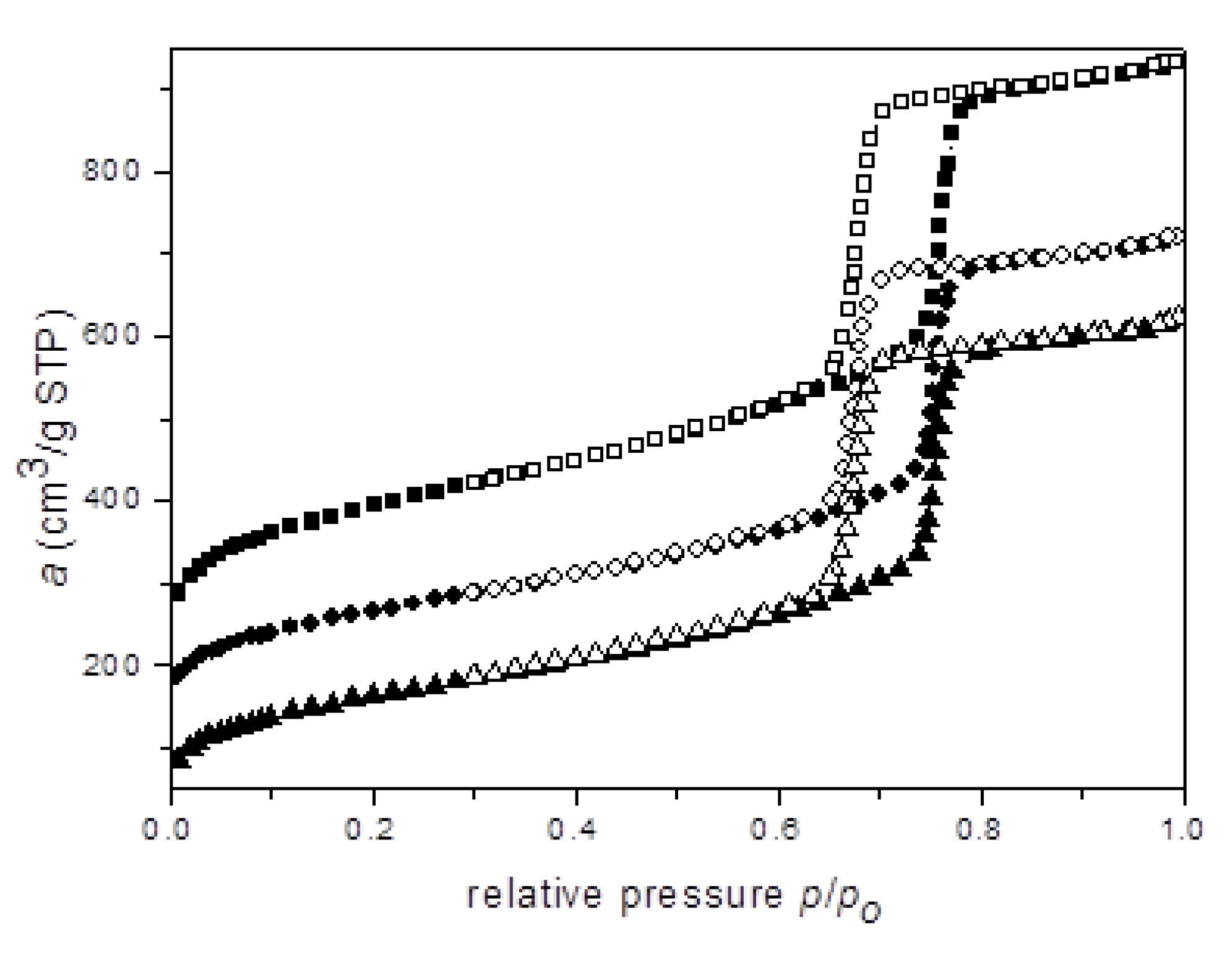
:1. Introduction: Olefin Metathesis and the Basic Properties of Santa Barbara Amorphous (SBA-15)
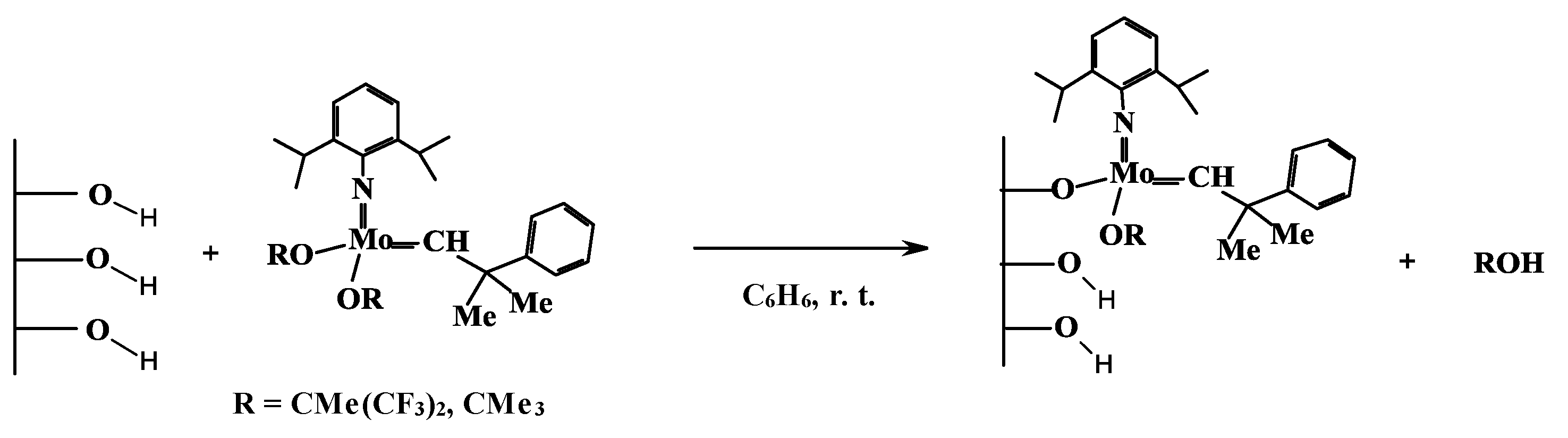
2. Well-Defined Heterogeneous Olefin Metathesis Catalysts Prepared by Immobilization of Mo and Ru Organometallic Complexes on SBA-15
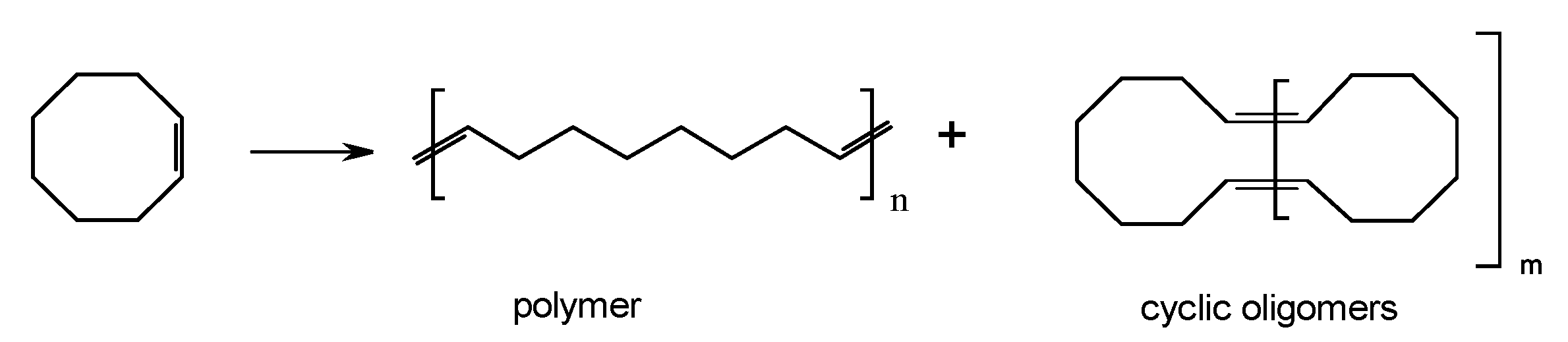
3. Application of SBA-15-Based Hybrid Catalysts in Metathesis Polymerization Reactions
4. Heterogeneous Olefin Metathesis Catalysts Consisting of Mo and W Oxides on SBA-15
5. Comparison of SBA-Based Olefin Metathesis Catalyst with Catalysts using other Advanced Supports (Mesoporous Molecular Sieves and Zeolites)
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ivin, K.J.; Mol, J.C. Olefin Metathesis and Metathesis Polymerization; Academic Press: London, UK, 1997. [Google Scholar]
- Grubbs, R.H.; Wenzel, A.G.; O´Leary, D.J.; Khosravi, E. (Eds.) Handbook of Metathesis; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Grela, K. (Ed.) Olefin metathesis: Theory and Practice; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Cossy, J.; Arseniyadis, S.; Meyer, C. Metathesis in Natural Product Synthesis; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Grubbs, R.H. The role of the “Tebbe complex” in olefin metathesis. In Handbook of Metathesis; Grubbs, R.H., Ed.; Wiley-VCH: Weinheim, Germany, 2003; pp. 4–7. [Google Scholar]
- Wallace, K.C.; Liu, A.H.; Dewan, J.C.; Schrock, R.R. Preparation and reactions of tantalum alkylidene complexes containing bulky phenoxide or thiolate ligands. Controlling ring-opening metathesis polymerization activity and mechanism through choice of anionic ligand. J. Am. Chem. Soc. 1988, 110, 4964–4977. [Google Scholar] [CrossRef]
- Hérisson, P.J.; Chauvin, Y. Catalyse de transformation des oléfines par les complexes du tungstène. II. Télomérisation des oléfines cycliques en présence d’oléfines acycliques. Die Makromol. Chem. 1971, 141, 161–176. [Google Scholar] [CrossRef]
- Copéret, C.; Lefebvre, F.; Basset, J.-M. From ill-defined to well-defined W alkylidene complexes. In Handbook of Metathesis; Grubbs, R.H., Ed.; Wiley-VCH: Weinheim, Germany, 2003; pp. 33–46. [Google Scholar]
- Lwin, S.; Wachs, I.E. Olefin Metathesis by Supported Metal Oxide Catalysts. ACS Catal. 2014, 4, 2505–2520. [Google Scholar] [CrossRef]
- Schrock, R.R.; Hoveyda, A.H. Molybdenum and Tungsten Imido Alkylidene Complexes as Efficient Olefin-Metathesis Catalysts. Angew. Chem. Int. Ed. 2003, 42, 4592–4633. [Google Scholar] [CrossRef]
- Trnka, T.M.; Grubbs, R.H. The Development of L2X2RuCHR Olefin Metathesis Catalysts: An Organometallic Success Story. Acc. Chem. Res. 2001, 34, 18–29. [Google Scholar] [CrossRef]
- Melis, K.; De Vos, D.; Jacobs, P.; Verpoort, F. ROMP and RCM catalysed by (R3P)2Cl2Ru=CHPh immobilised on a mesoporous support. J. Mol. Catal. A Chem. 2001, 169, 47–56. [Google Scholar] [CrossRef]
- Balcar, H.; Žilková, N.; Sedláček, J.; Zedník, J. MCM-41 anchored Schrock catalyst Mo(=CHCMe2Ph)(=N-2, 6-i-Pr2C6H3)[OCMe(CF3)2] 2-activity in 1-heptene metathesis and cross-metathesis reactions. J. Mol. Catal. A Chem. 2005, 232, 53–58. [Google Scholar] [CrossRef]
- Ookoshi, T.; Onaka, M. A remarkable Mo catalyst for olefin metathesis: Hexagonal mesoporous silica-supported molybdenum oxide (MoO3/HMS). Chem. Commun. 1998, 21, 2399–2400. [Google Scholar] [CrossRef]
- Topka, P.; Balcar, H.; Rathouský, J.; Žilková, N.; Verpoort, F.; Čejka, J. Metathesis of 1-octene over MoO3 supported on mesoporous molecular sieves: The influence of the support architecture. Microporous Mesoporous Mater. 2006, 96, 44–54. [Google Scholar] [CrossRef]
- Balcar, H.; Čejka, J. Mesoporous molecular sieves as advanced supports for olefin metathesis catalysts. Coord. Chem. Rev. 2013, 257, 3107–3124. [Google Scholar] [CrossRef]
- Dewaele, A.; Verpoort, F.; Sels, B. Opportunities of Immobilized Homogeneous Metathesis Complexes as Prominent Heterogeneous Catalysts. ChemCatChem 2016, 8, 3010–3030. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Meynen, V.; Cool, P.; VanSant, E. Verified syntheses of mesoporous materials. Microporous Mesoporous Mater. 2009, 125, 170–223. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the porous structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Kruk, M.; Cao, L. Pore Size Tailoring in Large-Pore SBA-15 Silica Synthesized in the Presence of Hexane. Langmuir 2007, 23, 7247–7254. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ma, Z.; Wang, Y.; Wang, Y.; Fang, L. Hoveyda–Grubbs catalyst confined in the nanocages of SBA-1: Enhanced recyclability for olefin metathesis. Chem. Commun. 2010, 46, 8659–8661. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.H.; Van Der Voort, P.; Koster, A.J.; De Jong, K.P. A 3D-TEM study of the shape of mesopores in SBA-15 and modified SBA-15 materials. Chem. Commun. 2002, 15, 1632–1633. [Google Scholar] [CrossRef]
- Shenderovich, I.G.; Buntkowsky, G.; Schreiber, A.; Gedat, E.; Sharif, S.; Albrecht, J.; Golubev, N.S.; Findenegg, G.H.; Limbach, H.-H. Pyridine-15N A Mobile NMR Sensor for Surface Acidity and Surface Defects of Mesoporous Silica. J. Phys. Chem. B 2003, 107, 11924–11939. [Google Scholar] [CrossRef]
- Ide, M.; El-Roz, M.; De Canck, E.; Vicente, A.; Planckaert, T.; Bogaerts, T.; Van Driessche, I.; Lynen, F.; Van Speybroeck, V.; Thybault-Starzyk, F.; et al. Quantification of silanol sites for the most common mesoporous ordered silicas and organosilicas: Total versus accessible silanols. Phys. Chem. Chem. Phys. 2013, 15, 642–650. [Google Scholar] [CrossRef]
- Le Roux, E.; Liang, Y.; Törnroos, K.W.; Nief, F.; Anwander, R. Heterogenization of Lanthanum and Neodymium Monophosphacyclopentadienyl Bis(tetramethylaluminate) Complexes onto Periodic Mesoporous Silica SBA-15. Organometallics 2012, 31, 6526–6537. [Google Scholar] [CrossRef]
- El Eter, M.; Hamzaoui, B.; Abou-Hamad, E.; Pelletier, J.D.A.; Basset, J.-M. Well-defined azazirconacyclopropane complexes supported on silica structurally determined by 2D NMR comparative elucidation. Chem. Commun. 2013, 49, 4616–4618. [Google Scholar] [CrossRef] [PubMed]
- Copéret, C.; Fedorov, A.; Zhizhko, P.A. Surface Organometallic Chemistry: Paving the Way Beyond Well-Defined Supported Organometallics and Single-Site Catalysis. Catal. Lett. 2017, 147, 2247–2259. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Čejka, J. Two-Dimensional Zeolites: Current Status and Perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef] [PubMed]
- Opanasenko, M.V.; Roth, W.J.; Čejka, J. Two-dimensional zeolites in catalysis: Current status and perspectives. Catal. Sci. Technol. 2016, 6, 2467–2484. [Google Scholar] [CrossRef]
- Přech, J.; Pizarro, P.; Serrano, D.P.; Čejka, J. From 3D to 2D zeolite catalytic materials. Chem. Soc. Rev. 2018, 47, 8263–8306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copéret, C.; Comas-Vives, A.; Conley, M.P.; Estes, D.P.; Fedorov, A.; Mougel, V.; Nagae, H.; Nunez-Zarur, F.; Zhizhko, P.A. Surface Organometallic and Coordination Chemistry toward Single-Site Heterogeneous Catalysts: Strategies, Methods, Structures, and Activities. Chem. Rev. 2016, 116, 323–421. [Google Scholar] [CrossRef] [PubMed]
- Balcar, H.; Roth, W.J. Hybrid Catalysts for Olefin Metathesis and Related Polymerizations. In New and Future Developments in Catalysis; Suib, S.L., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 1–26. [Google Scholar]
- Allen, D.P. Supported Catalysts and Nontraditional Reaction Media. In Handbook of Metathesis; Grubbs, R.H., Wenzel, A.G., Eds.; Wiley: Hoboken, NJ, USA, 2015; Volume 1, pp. 97–158. [Google Scholar]
- Buchmeiser, M.R. Immobilization of Olefin Metathesis Catalysts. In Olefin Metathesis: Theory and Practice; Grela, K., Ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 495–514. [Google Scholar]
- Drăguţan, I.; Dragutan, V. Practical New Strategies for Immobilising Ruthenium Alkylidene Complexes: Part I. Platin. Met. Rev. 2008, 52, 71–82. [Google Scholar] [CrossRef]
- Staub, H.; Kleitz, F.; Fontaine, F.-G. Confinement of the Grubbs catalyst in alkene-functionalized mesoporous silica. Microporous Mesoporous Mater. 2013, 175, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Balcar, H.; Čejka, J. Mesoporous molecular sieves as advanced supports for olefin metathesis catalysts. Macromol. Symp. 2010, 293, 43–47. [Google Scholar] [CrossRef]
- Rendón, N.; Berthoud, R.; Blanc, F.; Gajan, D.; Maishal, T.; Copéret, C.; Lesage, A.; Emsley, L.; Marinescu, S.C.; Singh, R.; et al. Well-Defined Silica-Supported Mo-Alkylidene Catalyst Precursors Containing One OR Substituent: Methods of Preparation and Structure-Reactivity Relationship in Alkene Metathesis. Chem. Eur. J. 2009, 15, 5083–5089. [Google Scholar] [CrossRef]
- Hübner, S.; de Vries, J.G.; Farina, V. Why does industry not use immobilized transition metal complexes as catalysts? Adv. Synth. Catal. 2016, 358, 3–25. [Google Scholar] [CrossRef]
- Zhong, R.; Lindhorst, A.C.; Groche, F.J.; Kühn, F.E. Immobilization of N‑heterocyclic carbene compounds: A Synthetic Perspective. Chem. Rev. 2017, 117, 1970–2058. [Google Scholar] [CrossRef] [PubMed]
- Bek, D.; Žilková, N.; Dědeček, J.; Sedláček, J.; Balcar, H. SBA-15 immobilized ruthenium carbenes as catalysts for ring closing metathesis and ring opening metathesis polymerization. Top. Catal. 2010, 53, 200–209. [Google Scholar] [CrossRef]
- Bek, D.; Balcar, H.; Žilková, N.; Zukal, A.; Horáček, M.; Čejka, J. Grubbs catalysts immobilized on mesoporous molecular sieves via phosphine and pyridine linkers. ACS Catal. 2011, 1, 709–718. [Google Scholar] [CrossRef]
- Bek, D.; Gawin, R.; Grela, K.; Balcar, H. Ruthenium metathesis catalyst bearing chelating carboxylate ligand immobilized on mesoporous molecular sieve SBA-15. Catal. Commun. 2012, 21, 42–45. [Google Scholar] [CrossRef]
- Pastva, J.; Čejka, J.; Žilková, N.; Mestek, O.; Rangusc, M.; Balcar, H. Hoveyda–Grubbs first generation type catalyst immobilized on mesoporous molecular sieves. J. Mol. Catal. A Chem. 2013, 378, 184–192. [Google Scholar] [CrossRef]
- Shinde, T.; Varga, V.; Polášek, M.; Horáček, M.; Žilková, N.; Balcar, H. Metathesis of cardanol over Ru catalysts supported on mesoporous molecular sieve SBA-15. Appl. Catal. A Gen. 2014, 478, 138–145. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Shao, S.; Wu, H.; Wu, P. Grubbs-type catalysts immobilized on SBA-15: A novel heterogeneous catalyst for olefin metathesis. J. Mol. Catal. A Chem. 2013, 372, 35–43. [Google Scholar] [CrossRef]
- Li, L.; Shi, J.-L. A Highly Active and Reusable Heterogeneous Ruthenium Catalyst for Olefin Metathesis. Adv. Synth. Catal. 2005, 347, 1745–1749. [Google Scholar] [CrossRef]
- Van Berlo, B.; Houthoofd, K.; Sels, B.F.; Jacobs, P.A. Silica Immobilized Second Generation Hoveyda-Grubbs: A Convenient, Recyclable and Storageable Heterogeneous Solid Catalyst. Adv. Synth. Catal. 2008, 350, 1949–1953. [Google Scholar] [CrossRef]
- Balcar, H.; Shinde, T.; Žilková, N.; Bastl, Z. Hoveyda–Grubbs type metathesis catalyst immobilized on mesoporous molecular sieves MCM-41 and SBA-15. Beilstein J. Org. Chem. 2011, 7, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Shinde, T.; Žilková, N.; Hanková, V.; Balcar, H. Hoveyda—Grubbs type metathesis catalyst immobilized on mesoporous molecular sieves—The influence of pore size on the catalyst activity. Catal. Today 2012, 179, 123–129. [Google Scholar] [CrossRef]
- Bru, M.; Dehn, R.; Teles, J.H.; Deuerlein, S.; Danz, M.; Müller, I.B.; Teles, H.; Limbach, M. Ruthenium Carbenes Supported on Mesoporous Silicas as Highly Active and Selective Hybrid Catalysts for Olefin Metathesis Reactions under Continuous Flow. Chem. Eur. J. 2013, 19, 11661–11671. [Google Scholar] [CrossRef] [PubMed]
- Dewaele, A.; Van Berlo, B.; Dijkmans, J.; Jacobs, P.A.; Sels, B.F. Immobilized Grubbs catalysts on mesoporous silica materials: Insight into support characteristics and their impact on catalytic activity and product selectivity. Catal. Sci. Technol. 2016, 6, 2580–2597. [Google Scholar] [CrossRef]
- Werghi, B.; Pump, E.; Tretiakov, M.; Abou-Hamad, E.; Gurinov, A.; Doggali, P.; Anjum, D.H.; Cavallo, L.; Bendjeriou-Sedjerari, A.; Basset, J.-M. Exploiting the interactions between the ruthenium Hoveyda–Grubbs catalyst and Al-modified mesoporous silica: The case of SBA15 vs. KCC-1. Chem. Sci. 2018, 9, 3531–3537. [Google Scholar] [CrossRef]
- Pastva, J.; Skowerski, K.; Czarnocki, S.J.; Žilková, N.; Čejka, J.; Bastl, Z.; Balcar, H. Ru-based complexes with quaternary ammonium tags immobilized on mesoporous silica as olefin metathesis catalysts. ACS Catal. 2014, 4, 3227–3236. [Google Scholar] [CrossRef]
- Skowerski, K.; Pastva, J.; Czarnocki, S.J.; Janoscova, J. Exceptionally Stable and Efficient Solid Supported Hoveyda-Type Catalyst. Org. Process. Res. Dev. 2015, 19, 872–877. [Google Scholar] [CrossRef]
- Balcar, H.; Žilková, N.; Kubů, M.; Polášek, M.; Zedník, J. Metathesis of cardanol over ammonium tagged Hoveyda-Grubbs type catalyst supported on SBA-15. Catal. Today 2018, 304, 127–134. [Google Scholar] [CrossRef]
- Webb, J.D.; Seki, T.; Goldston, J.F.; Pruski, M.; Crudden, C.M. Selective functionalization of the mesopores of SBA-15. Microporous Mesoporous Mater. 2015, 203, 123–131. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Van Der Sluys, M.; Jones, C.W. On the Nature of the Active Species in Palladium Catalyzed Mizoroki–Heck and Suzuki–Miyaura Couplings–Homogeneous or Heterogeneous Catalysis, A Critical Review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Balcar, H.; Čejka, J. Mesoporous Molecular Sieves Based Catalysts for Olefin Metathesis and Metathesis Polymerization. In Green Metathesis Chemistry: Great Challenges in Synthesis, Catalysis and Nanotechnology, NATO Science for Peace and Security Series A: Chemistry and Biology; Dragutan, V., Demonceau, A., Dragutan, I., Finkelshtein, E.S., Eds.; Springer Science and Business Media LLC: Berlin, Germany, 2010; pp. 101–114. [Google Scholar]
- Bek, D.; Balcar, H.; Sedláček, J. [RuCl2(p-Cymene)]2 Immobilized on Mesoporous Molecular Sieves SBA-15 as Catalyst for ROMP of Norbornene. In Green Metathesis Chemistry: Great Challenges in Synthesis, Catalysis and Nanotechnology, NATO Science for Peace and Security Series A: Chemistry and Biology; Dragutan, V., Demonceau, A., Dragutan, I., Finkelshtein, E.S., Eds.; Springer Science and Business Media LLC: Berlin, Germany, 2010; pp. 391–400. [Google Scholar]
- Balcar, H.; Bek, D.; Sedláček, J.; Dědeček, J.; Bastl, Z.; Lamač, M. RuCl2(p-cymene)(PCy3) immobilized on mesoporous molecular sieves as catalyst for ROMP of norbornene and its derivatives. J. Mol. Catal. A Chem. 2010, 332, 19–24. [Google Scholar] [CrossRef]
- Gholampour, N.; Yusubov, M.; Verpoort, F. Investigation of the preparation and catalytic activity of supported Mo, W, and Re oxides as heterogeneous catalysts in olefin metathesis. Catal. Rev. 2016, 58, 113–156. [Google Scholar] [CrossRef]
- Bhuiyan, T.I.; Arudra, P.; Akhtar, M.N.; Aitani, A.M.; Abudawoud, R.H.; Al-Yami, M.A.; Al-Khattaf, S.S. Metathesis of 2-butene to propylene over W-mesoporous molecular sieves: A comparative study between tungsten containing MCM-41 and SBA-15. Appl. Catal. A Gen. 2013, 467, 224–234. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Akhtar, M.N.; Čejka, J.; Montanari, E.; Balcar, H.; Kubů, M.; Al-Khattaf, S.S. Metathesis of 2-pentene over Mo and W supported mesoporous molecular sieves MCM-41 and SBA-15. J. Ind. Eng. Chem. 2017, 53, 119–126. [Google Scholar] [CrossRef]
- Liu, H.; Tao, K.; Zhang, P.; Xu, W.; Zhou, S. Enhanced catalytic performance for metathesis reactions over ordered tungsten and aluminum co-doped mesoporous KIT-6 catalysts. New J. Chem. 2015, 39, 7971–7978. [Google Scholar] [CrossRef]
- Balcar, H.; Kubů, M.; Žilková, N.; Shamzhy, M. MoO3 on zeolites MCM-22, MCM-56 and 2D-MFI as catalysts for 1-octene metathesis. Beilstein J. Org. Chem. 2018, 14, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Consoli, D.F.; Zhang, S.; Shaikh, S.; Román-Leshkov, Y. Influence of framework heteroatoms on olefin metathesis activity using MoO3‑MFI catalysts. Org. Process. Res. Dev. 2018, 22, 1683–1686. [Google Scholar] [CrossRef]
- Thielemann, J.P.; Hess, C. Structure of silica-supported molybdenum oxide studied by in situ spectroscopy under reactive and non-reactive conditions. J. Catal. 2012, 288, 124–126. [Google Scholar] [CrossRef]
- Amakawa, K.; Wrabetz, S.; Kröhnert, J.; Tzolova-Müller, G.; Schlögl, R.; Trunschke, A. In Situ Generation of Active Sites in Olefin Metathesis. J. Am. Chem. Soc. 2012, 134, 11462–11473. [Google Scholar] [CrossRef]
- Lin, C.; Tao, K.; Yu, H.; Hua, D.; Zhou, S. Enhanced catalytic performance of molybdenum-doped mesoporous SBA-15 for metathesis of 1-butene and ethene to propene. Catal. Sci. Technol. 2014, 4, 4010–4019. [Google Scholar] [CrossRef]
- Balcar, H.; Mishra, D.; Marceau, E.; Carrier, X.; Žilková, N.; Bastl, Z. Molybdenum oxide catalysts for metathesis of higher 1-alkenes via supporting MoO2(acetylacetonate)2 and MoO2(glycolate)2 on SBA-15 mesoporous molecular sieves. Appl. Catal. A Gen. 2009, 359, 129–135. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Liu, S.; Zhu, X.; Chen, F.; Xu, L. 1-Butene Metathesis to Propene over Mo/Al2O3@SBA-15: Influences of Alumina Introduction Methods on the Catalytic Performance. Chem. Asian J. 2015, 10, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, T.I.; Arudra, P.; Hossain, M.M.; Akhtar, M.N.; Aitani, A.M.; Abudawoud, R.H.; Al-Khattaf, S.S. Kinetics modelling of 2-butene metathesis over tungsten oxide containing mesoporous silica catalyst. Can. J. Chem. Eng. 2014, 92, 1271–1282. [Google Scholar] [CrossRef]
- Chen, L.-F.; Hu, J.-C.; Wang, Y.-D.; Zhu, K.; Richards, R.; Yang, W.-M.; Liu, Z.-C.; Xu, W. Highly efficient tungsten-substituted mesoporous SBA-15 catalysts for 1-butene metathesis. Mater. Lett. 2006, 60, 3059–3062. [Google Scholar] [CrossRef]
- Hu, J.-C.; Wang, Y.-D.; Chen, L.-F.; Richards, R.; Yang, W.-M.; Liu, Z.-C.; Xu, W. Synthesis and characterization of tungsten-substituted SBA-15: An enhanced catalyst for 1-butene metathesis. Microporous Mesoporous Mater. 2006, 93, 158–163. [Google Scholar] [CrossRef]
- Thielemann, J.P.; Ressler, T.; Walter, A.; Tzolova-Müller, G.; Hess, C. Structure of molybdenum oxide supported on silica SBA-15 studied by Raman, UV–Vis and X-ray absorption spectroscopy. Appl. Catal. A Gen. 2011, 399, 28–34. [Google Scholar] [CrossRef]
- Amakawa, K.; Sun, L.; Guo, C.; Hävecker, M.; Kube, P.; Wachs, I.E.; Lwin, S.; Frenkel, A.I.; Patlolla, A.; Hermann, K.; et al. How Strain Affects the Reactivity of Surface Metal Oxide Catalysts. Angew. Chem. Int. Ed. 2013, 52, 13553–13557. [Google Scholar] [CrossRef]
- Handzlik, J.; Kurleto, K. Theoretical investigation of heterogeneous olefin metathesis catalysts. Curr. Org. Chem. 2013, 17, 2796–2813. [Google Scholar] [CrossRef]
- Handzlik, J. Metathesis Activity and Properties of Mo−Alkylidene Sites Differently Located on Silica. A Density Functional Theory Study. J. Phys. Chem. B 2005, 109, 20794–20804. [Google Scholar] [CrossRef]
- Handzlik, J. Theoretical Investigations of Isolated Mo (VI) and Mo (IV) Centers of a Molybdena− Silica Catalyst for Olefin Metathesis. J. Phys. Chem. C 2007, 111, 9337–9348. [Google Scholar] [CrossRef]
- Handzlik, J. DFT study of molybdena–silica system–A selection of density functionals based on their performance in thermochemistry of molybdenum compounds. Chem. Phys. Lett. 2009, 469, 140–144. [Google Scholar] [CrossRef]
- Balcar, H.; Žilková, N.; Kubů, M.; Mazur, M.; Bastl, Z.; Čejka, J. Ru complexes of Hoveyda–Grubbs type immobilized on lamellar zeolites: Activity in olefin metathesis reactions. Beilstein J. Org. Chem. 2015, 11, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Spekreijse, J.; Őhrstrőm, L.; Sanders, J.P.M.; Bitter, J.H.; Scott, E.L. Mechanochemical immobilisation of metathesis catalysts in a metal–organic framework. Chem. Eur. J. 2016, 22, 15437–15443. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, H.; Germain, S.; Queval, P.; Bouvier, C.; Mauduit, M.; Crévisy, C.; Schulz, E. Non covalent immobilization of pyrene-tagged ruthenium complexes onto graphene surfaces for recycling in olefin metathesis reactions. J. Mol. Catal. A Chem. 2016, 425, 136–146. [Google Scholar] [CrossRef]
- Kaczanowska, K.; Chwalba, M.; Pastva, J.; Kubů, M.; Ruszczyńska, A.; Bulska, E.; Balcar, H.; Skowerski, K. Carboxyl graphene as a superior support for bulky ruthenium-based olefin metathesis catalyst. Organometallics 2018, 37, 1837–1844. [Google Scholar] [CrossRef]
- Skowerski, K.; Białecki, J.; Czarnocki, S.J.; Żukowska, K.; Grela, K. Effective immobilisation of a metathesis catalyst bearing an ammonium-tagged NHC ligand on various solid supports. Beilstein J. Org. Chem. 2016, 12, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahane, S.; Bruneau, C.; Fischmeister, C. Z Selectivity: Recent Advances in one of the Current Major Challenges of Olefin Metathesis. ChemCatChem 2013, 5, 3436–3459. [Google Scholar] [CrossRef]
- Stenne, B.; Collins, S.K. Enantioselective olefin metathesis. In Olefin metathesis: Theory and Practice; Grela, K., Ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 233–267. [Google Scholar]
- Hauser, P.M.; Hunger, M.; Buchmeiser, M.R. Silica-Supported Molybdenum Alkylidyne N-Heterocyclic Carbene Catalysts: Relevance of Site Isolation to Catalytic Performance. ChemCatChem 2018, 10, 1829–1834. [Google Scholar] [CrossRef]


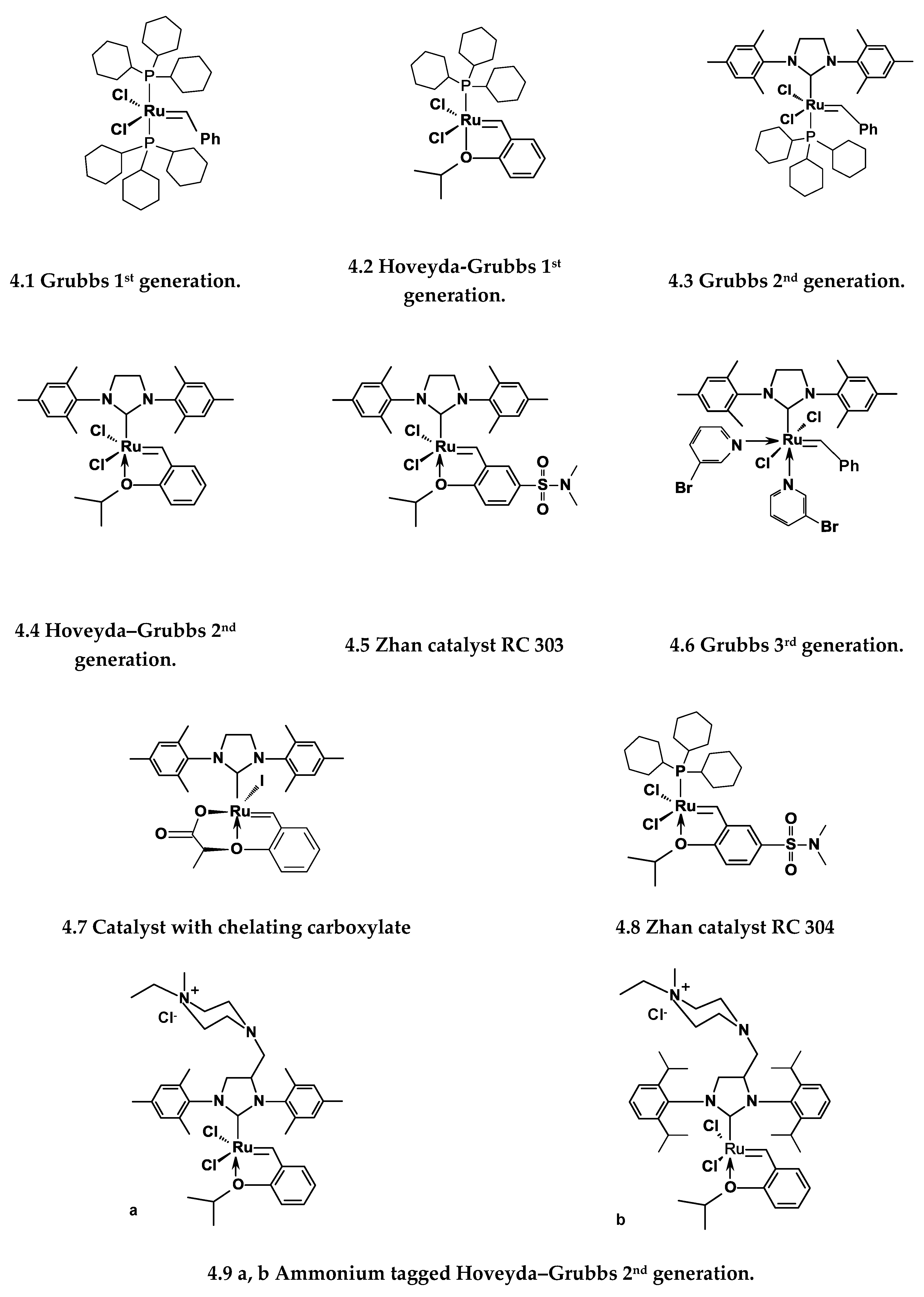
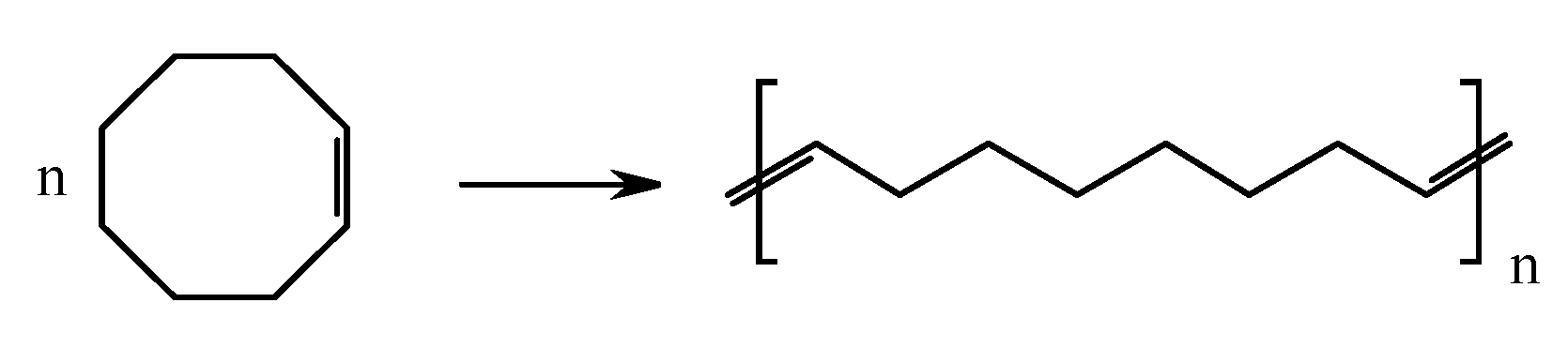
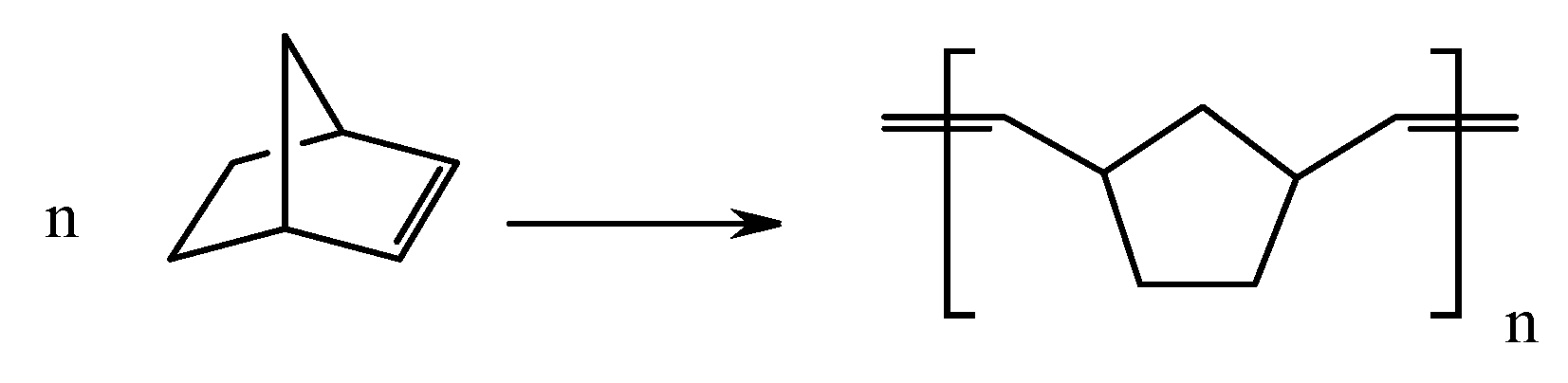
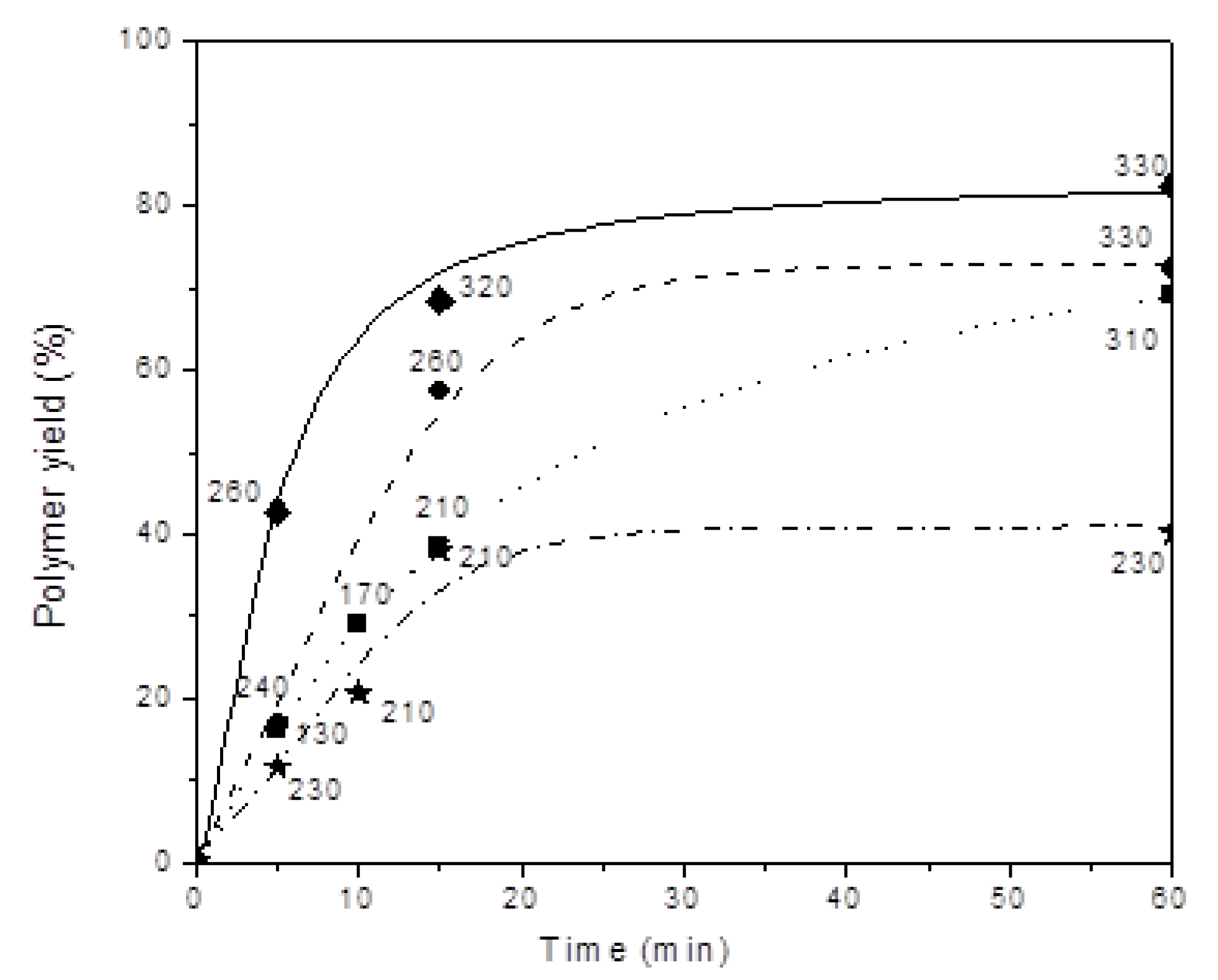
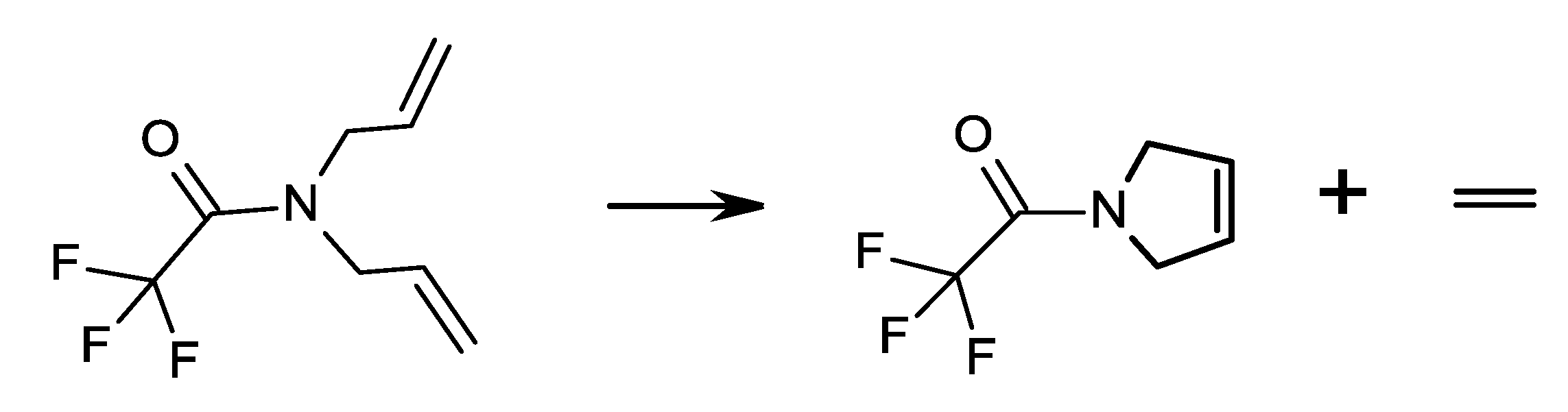
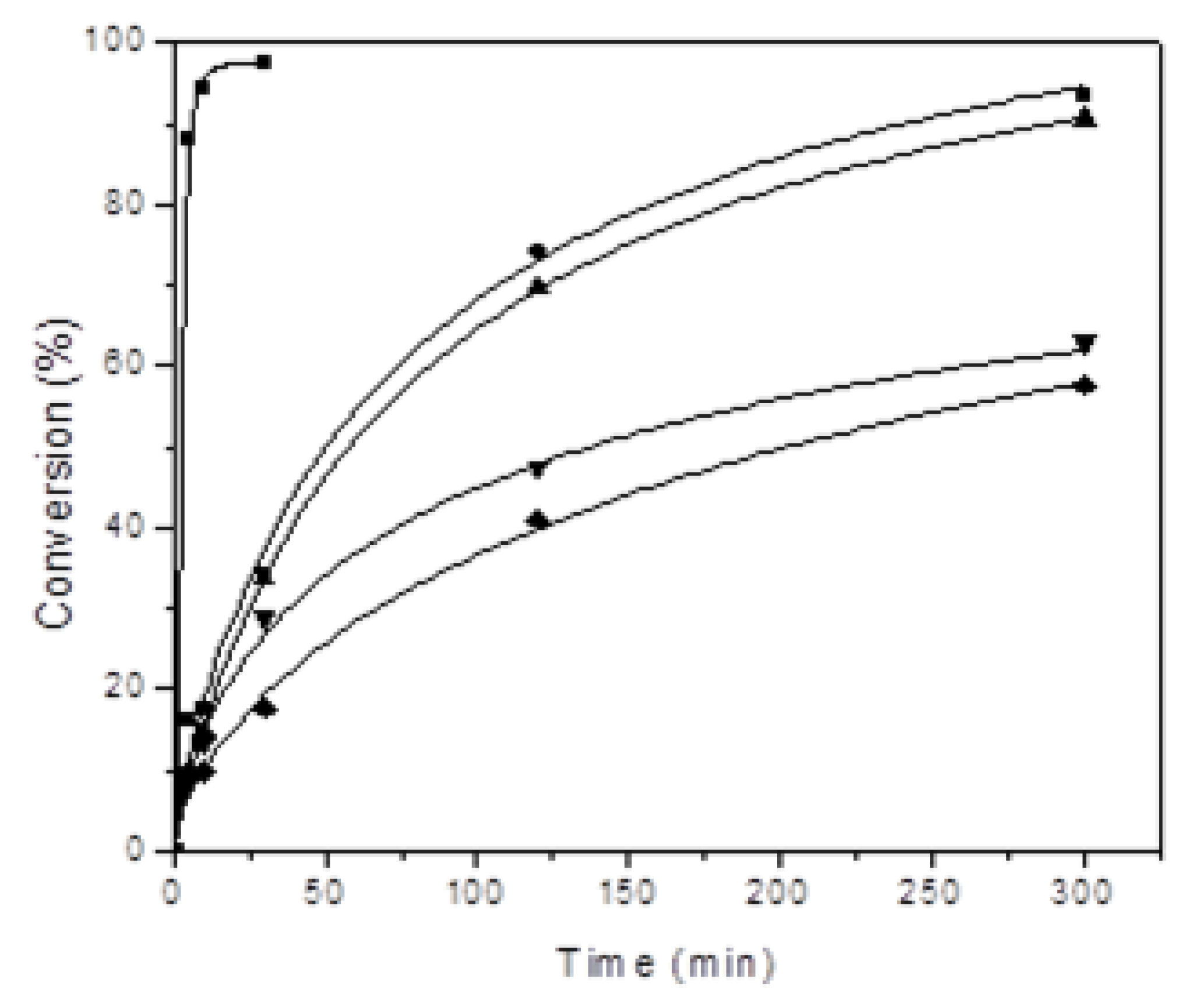
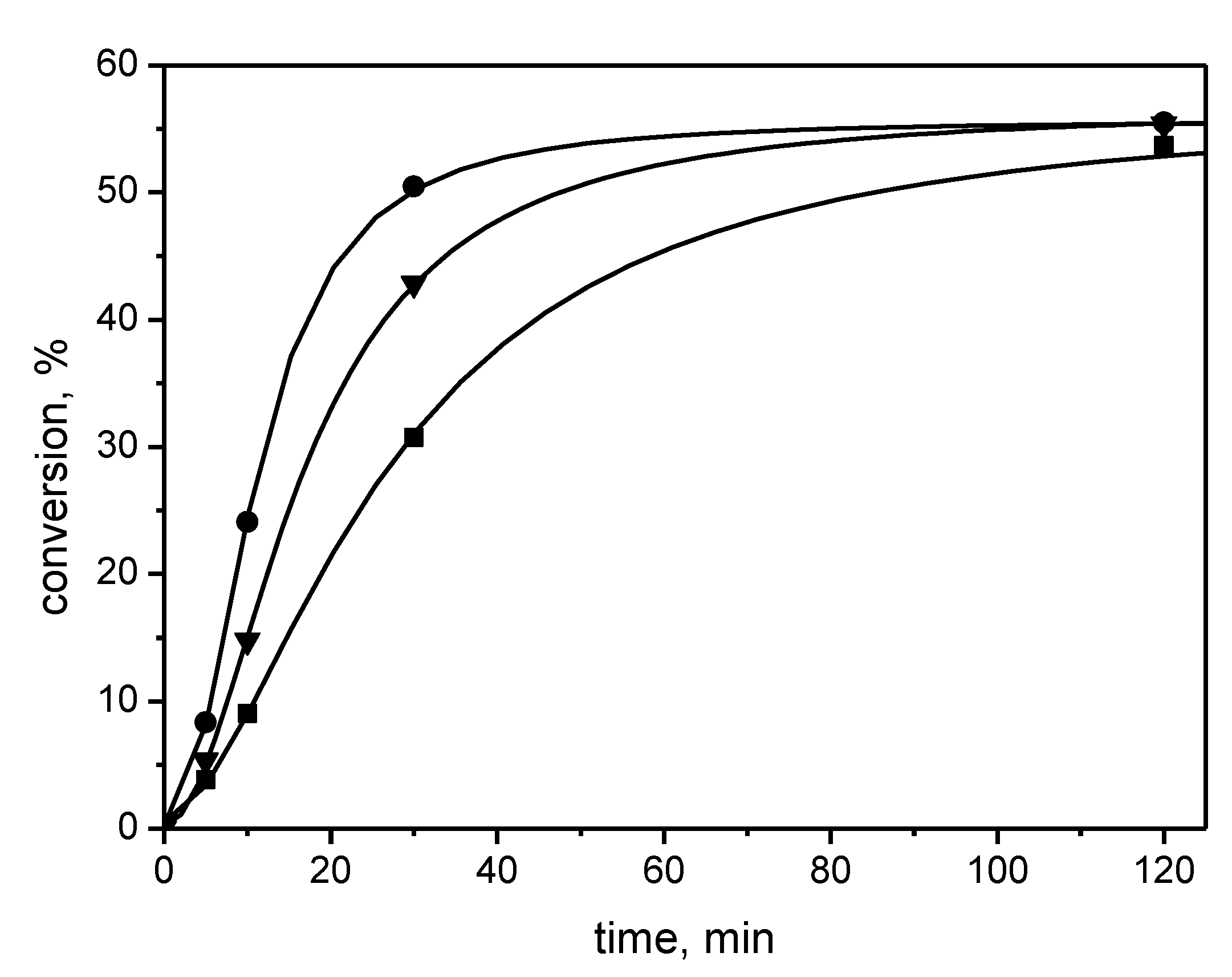
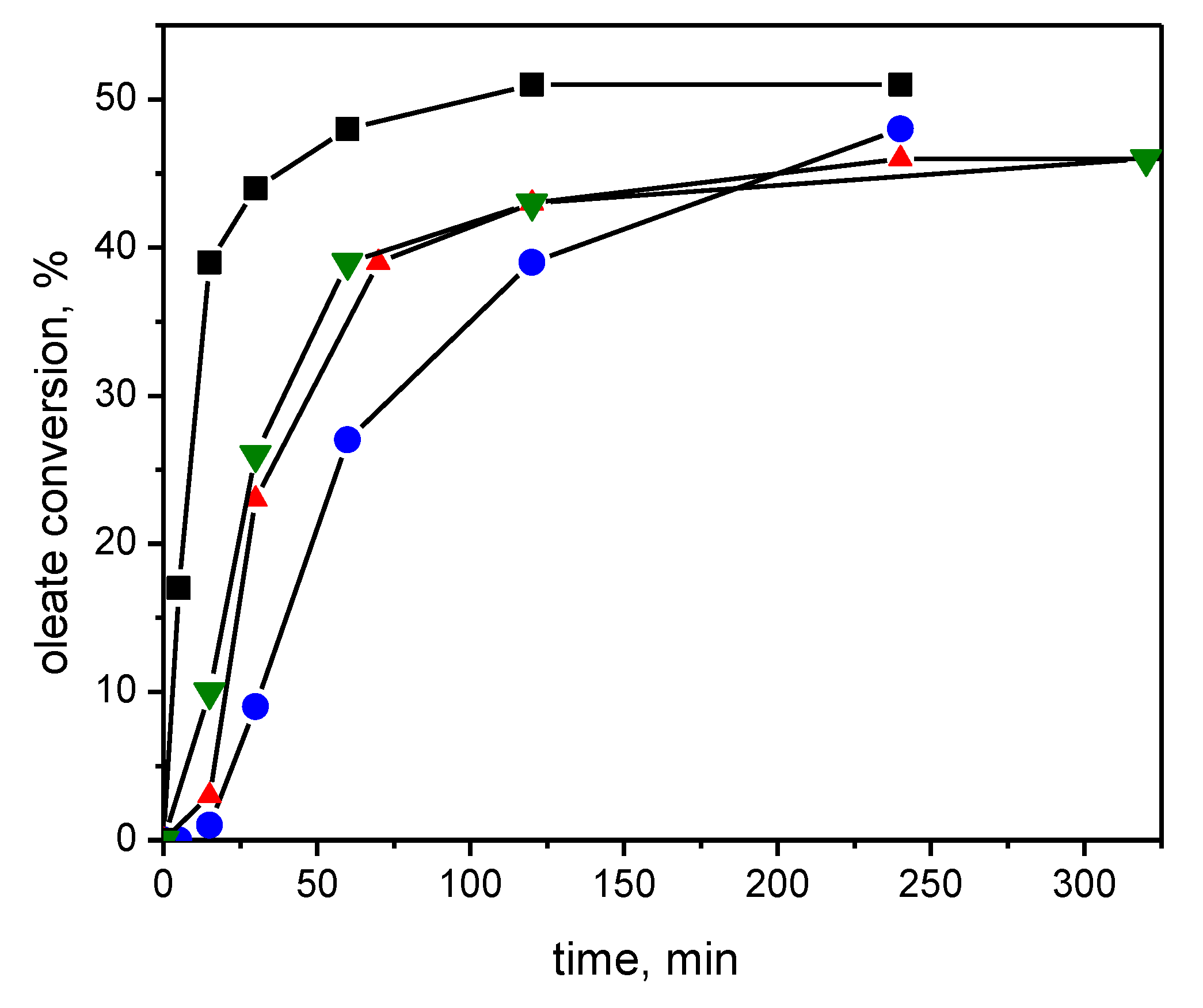
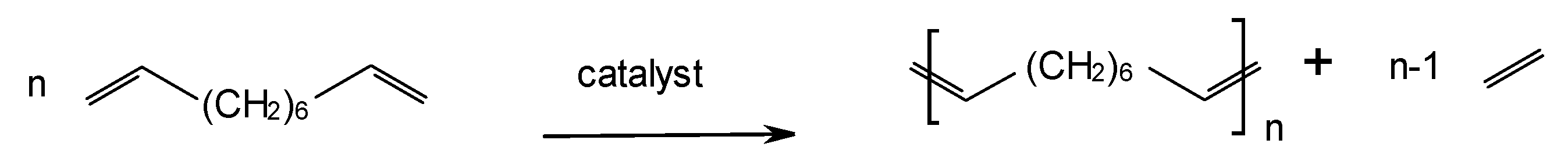
| Number | Carbene Complex | Immobilization Mode | Loading, in wt % Ru | Activity | Leaching in % of Starting Amount of Ru | Reusing | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 4.4 4.5 | By the linker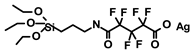 | 0.85% 0.65% | TON in RCM = 2900 | 2.3% | 3 times | [42] |
| 2 | 4.3 4.6 | By the linkers | 0.3–1.0% | TON in RCM = 2000 | 0.18–7.7% | 6 times | [43] |
| 3 | 4.7 | By the linker | 0.27% | TON in RCM = 2100 | 2.7% | 5 times | [44] |
| 4 | 4.8 | By the linker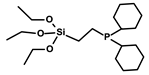 | 1.07% | RCM, ROMP | 0.1–0.38% | 6 times | [45] |
| 5 | 4.8 | By the linker | 0.3% | Metathesis and cross-metathesis of cardanol, TON up to 330 | 0.5% | 5 timescummulative TON = 1125 | [46] |
| 6 | 4.2 4.4 | 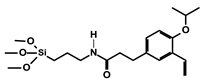 | 0.5–1.7% | RCM TON = 20 in 1 cycle | 6–30% according to pore size | 8 times | [47] |
| 7 | 4.1 | 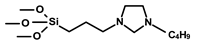 | - | RCM TON = 20 in 1 cycle. | below detection limit | 5 times | [48] |
| 8 | 4.4 4.5 | Linker free | 0.29–1.0% | TON up to 600 batch reactor | 0.04% in C6H12, 14% in DCM | Flow conditions possible | [49,50,51,52,53] |
| 9 | 4.4 | By means of Al modification of the surface | 1.5% | Cumulative TON about 3000 (propene metathesis) | Catalyst leaches in polar medium | - | [54] |
| 10 | 4.9a | Linker free | 1.2% | TON up to 3300 in RCM | About 1% in DCM and EtOAc | 5 times | [55] |
| 11 | 4.9b | Linker free | 0.1–1% | TON up to 35000 in RCM, TON = 1600 in cardanol metathesis | About 1% | More than 10 times suitable for flow condition | [56,57] |
| Number | WOx,MoOx Species Source | Method of Catalyst Preparation | W, Mo Loading Wt % | Substrate | Activity | Reference |
|---|---|---|---|---|---|---|
| 1 | MoO3 | thermal spreading | 4–12 | 1-octene | TOF = 0.003 mol/molMo.s at 40 °C | [15] |
| 2 | ammonium heptamolybdate (NH4)6Mo7O24.4H2O | ion exchange | 10.1 | - | - | [69] |
| 3 | ammonium heptamolybdate (NH4)6Mo7O24.4H2O | ion exchange | 9.7 | propene | TOF = 2.06 mmol/molMo.s at 50 °C | [70] |
| 4 | ammonium heptamolybdate (NH4)6Mo7O24.4H2O | wet impregnation | 3 | 1-butene | 60% conversion at 450 °C | [71] |
| 5 | MoO2(acac)2 MoO2(gly)2 | thermal spreading, wet impregnation | 4–12 | 1-octene, 1-dodecene, 1-tetradecene | TOF = 0.008 mol1-oct/molMo.s at 40 °C | [72] |
| 6 | MoO3, MoO2(acac)2 | thermal spreading | 6 | 2-pentene | 40% conversion at 500 °C | [65] |
| 7 | ammonium heptamolybdate (NH4)6Mo7O24.4H2O | direct co- condensation | 1.3–10.8 | 1-butene | 65% conversion at 450 °C | [71] |
| 8 | ammonium heptamolybdate (NH4)6Mo7O24.4H2O | wet impregnation on alumina- modified surface | ca. 6 | 1-butene | 65% conversion at 90 °C | [73] |
| 9 | ammonium metatungstate (NH4)6H2W12O40·xH2O | wet impregnation | 10 wt% of WO3 (8% of W) | 2-butene | 75% conversion at 550 °C | [64] |
| 10 | Sodium tungstate NaWO4·2H2O | direct co- condensation | 5% and 9% of WO3 | 2-butene | 87% conversion at 550 °C | [64,74] |
| 11 | ammonium metatungstate (NH4)6H2W12O40·xH2O | wet impregnation | 6 | 2-pentene | 56.5% conversion at 550 °C | [65] |
| 12 | Sodium tungstate NaWO4·2H2O | direct co- condensation | Si/W=35 | 1-butene | 92% conversion at 350 °C | [75,76] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balcar, H.; Čejka, J. SBA-15 as a Support for Effective Olefin Metathesis Catalysts. Catalysts 2019, 9, 743. https://doi.org/10.3390/catal9090743
Balcar H, Čejka J. SBA-15 as a Support for Effective Olefin Metathesis Catalysts. Catalysts. 2019; 9(9):743. https://doi.org/10.3390/catal9090743
Chicago/Turabian StyleBalcar, Hynek, and Jiří Čejka. 2019. "SBA-15 as a Support for Effective Olefin Metathesis Catalysts" Catalysts 9, no. 9: 743. https://doi.org/10.3390/catal9090743
APA StyleBalcar, H., & Čejka, J. (2019). SBA-15 as a Support for Effective Olefin Metathesis Catalysts. Catalysts, 9(9), 743. https://doi.org/10.3390/catal9090743




