Catalytic Behaviors of Supported Cu, Ni, and Co Phosphide Catalysts for Deoxygenation of Oleic Acid
Abstract
:1. Introduction
2. Results and Discussion
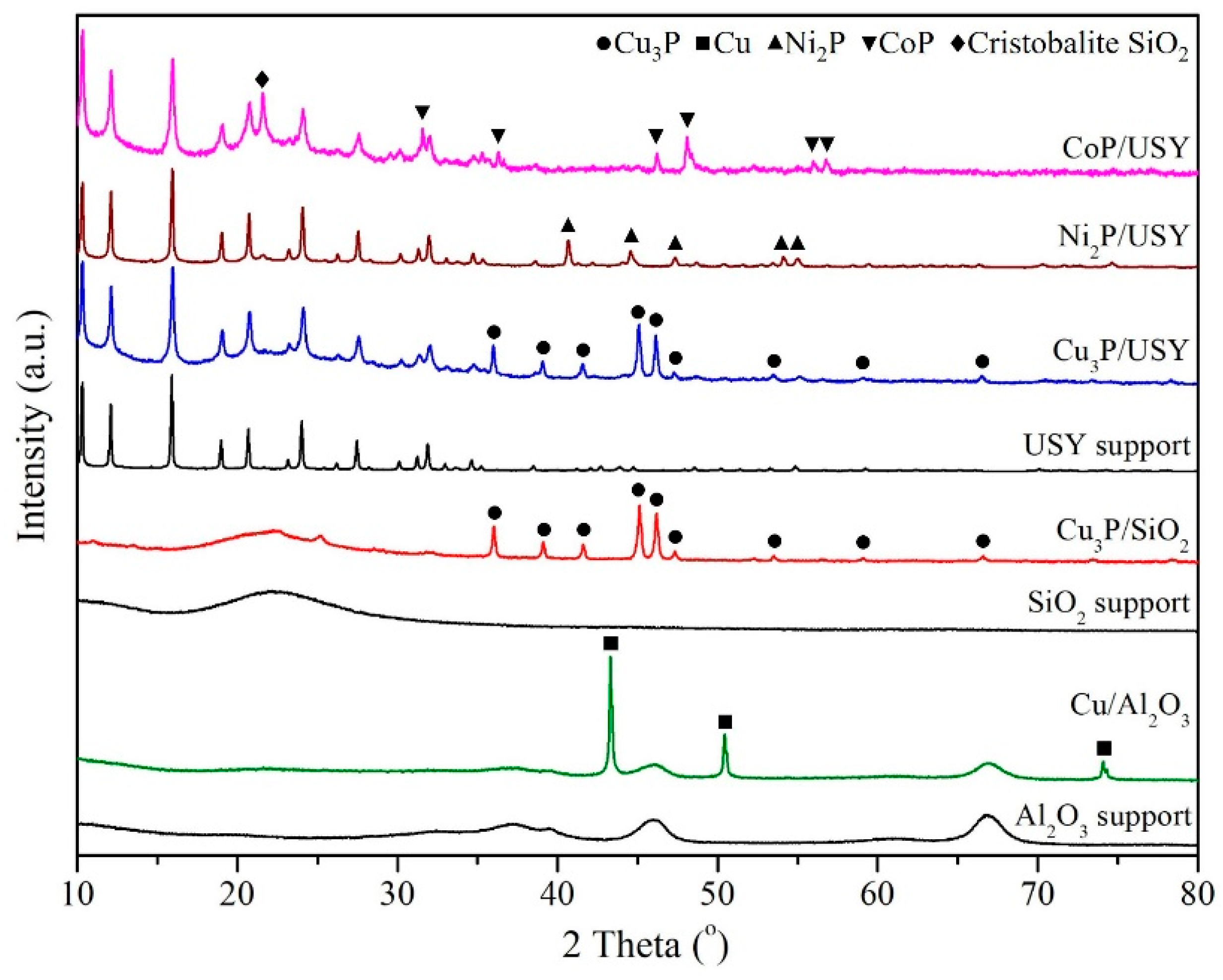
2.1. Crystal Structures
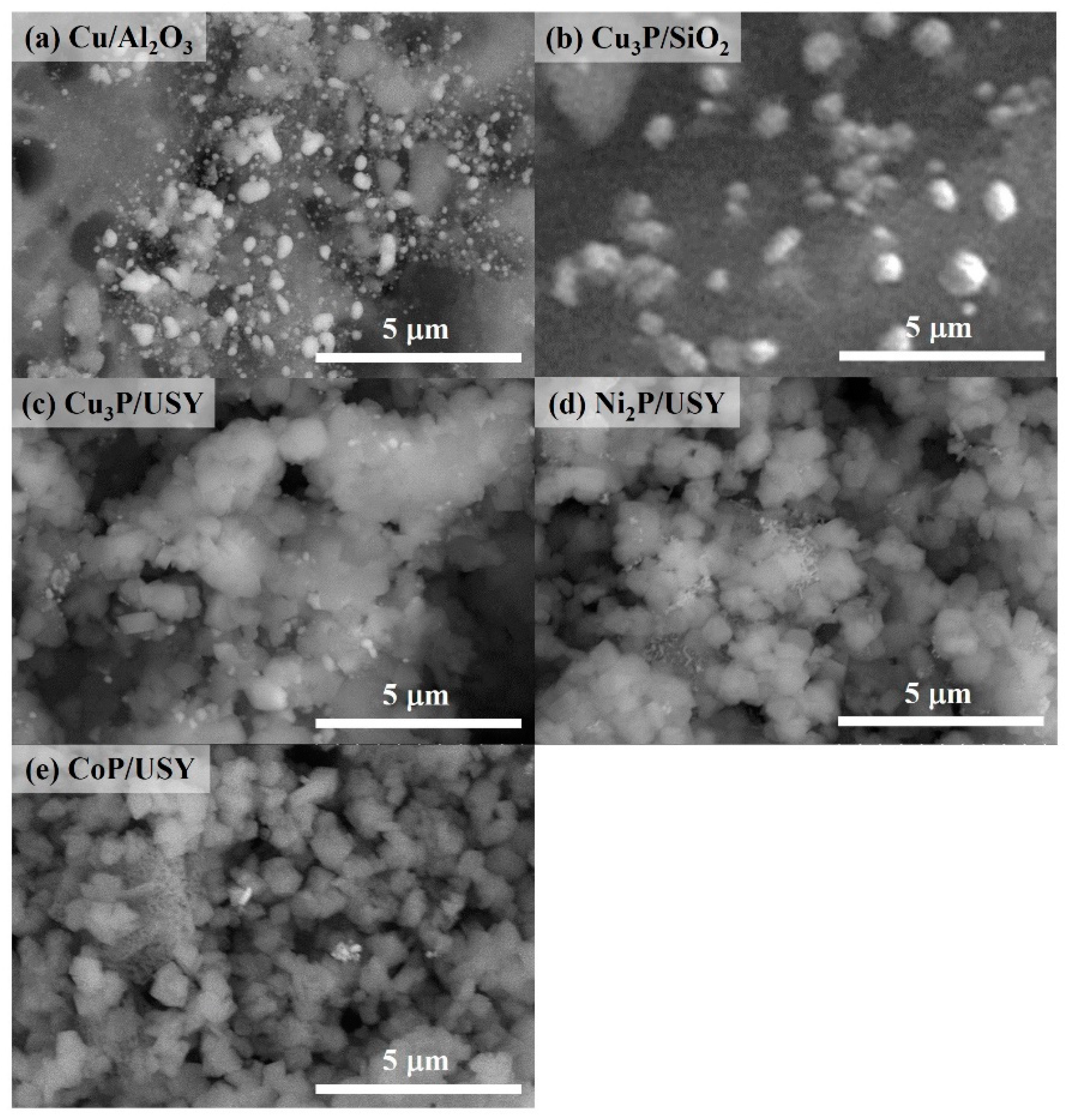
2.2. Morphology and Surface Properties
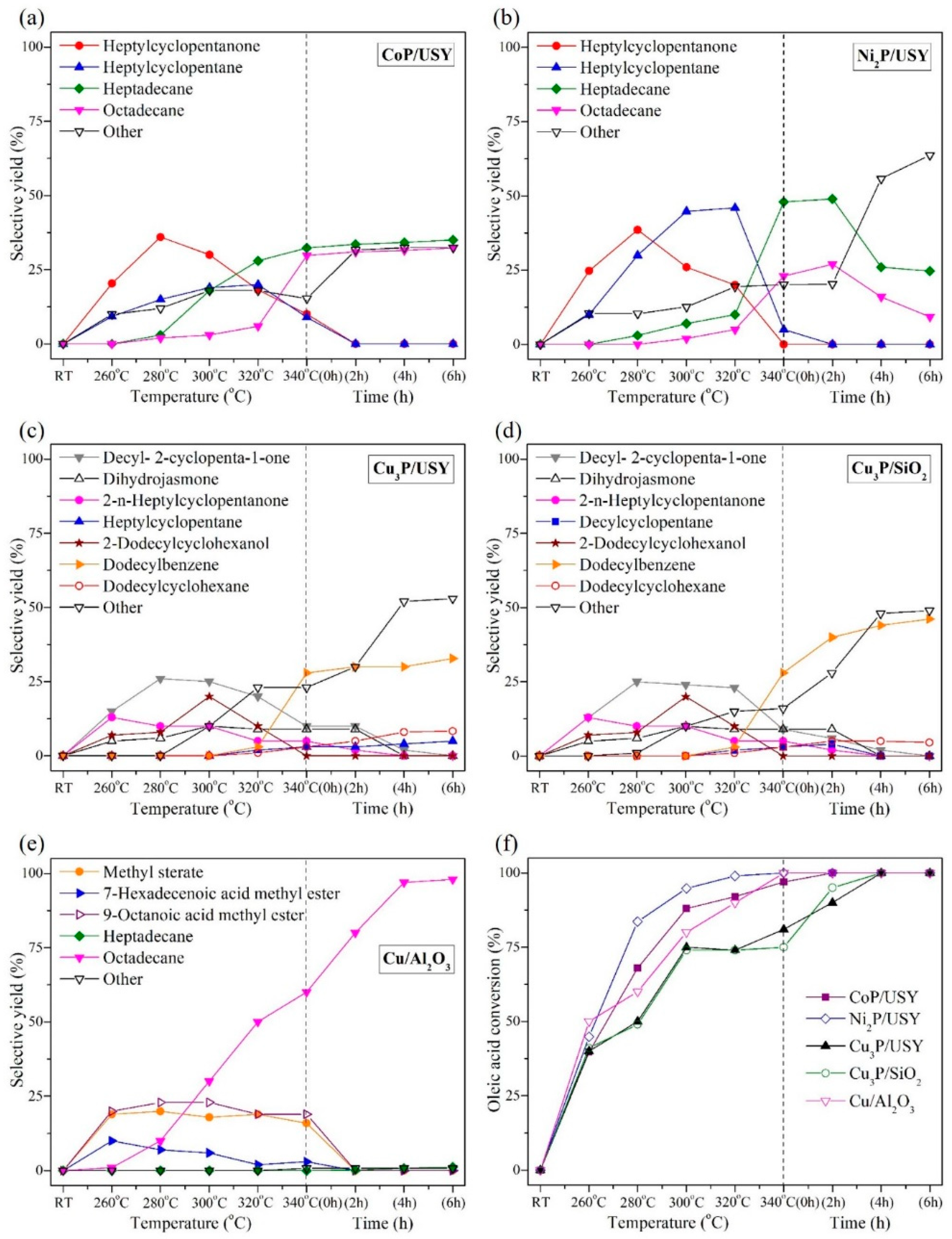
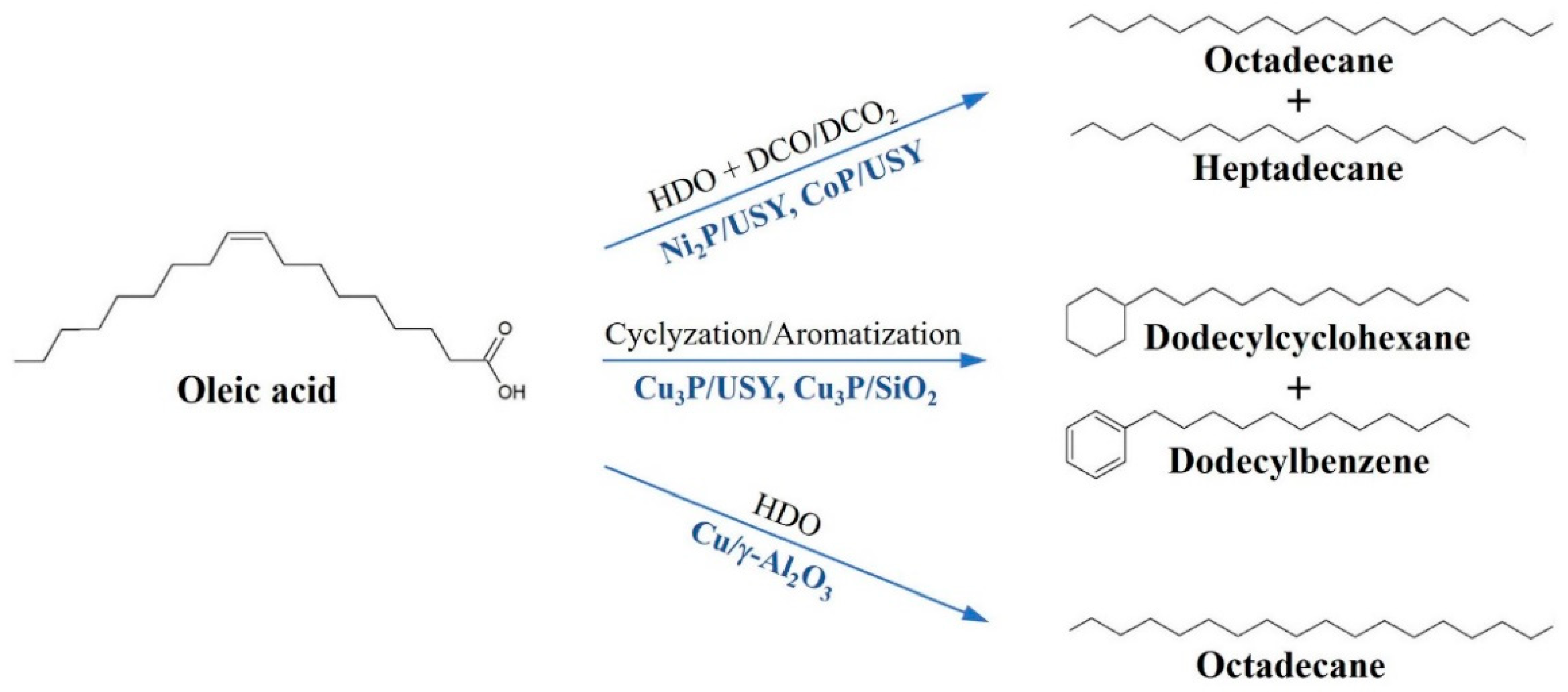
2.3. Deoxygenation of Oleic Acid
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Supported Metal Phosphide Catalysts
3.3. Characterization of Supported Metal Phosphide Catalysts
3.4. Deoxygenation of Oxygenated Hydrocarbon Compounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meier, D.; van de Beld, B.; Bridgwater, A.V.; Elliott, D.C.; Oasmaa, A.; Preto, F. State-of-the-art of fast pyrolysis in IEA bioenergy member countries. Renew. Sustain. Energy Rev. 2013, 20, 619–641. [Google Scholar] [CrossRef]
- Furimsky, E. Hydroprocessing challenges in biofuels production. Catal. Today 2013, 217, 13–56. [Google Scholar] [CrossRef]
- Zuo, H.; Liu, Q.; Wang, T.; Ma, L.; Zhang, Q.; Zhang, Q. Hydrodeoxygenation of Methyl Palmitate over Supported Ni Catalysts for Diesel-like Fuel Production. Energy Fuels 2012, 26, 3747–3755. [Google Scholar] [CrossRef]
- Arun, N.; Sharma, R.V.; Dalai, A.K. Green diesel synthesis by hydrodeoxygenation of bio-based feedstocks: Strategies for catalyst design and development. Renew. Sustain. Energy Rev. 2015, 48, 240–255. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Zhao, X.; Julson, J. Application, Deactivation, and Regeneration of Heterogeneous Catalysts in Bio-Oil Upgrading. Catalysts 2016, 6, 195. [Google Scholar] [CrossRef]
- Si, Z.; Zhang, X.; Wang, C.; Ma, L.; Dong, R. An Overview on Catalytic Hydrodeoxygenation of Pyrolysis Oil and Its Model Compounds. Catalysts 2017, 7, 169. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Wang, Y.; He, T.; Liu, K.; Wu, J.; Fang, Y. From biomass to advanced bio-fuel by catalytic pyrolysis/hydro-processing: Hydrodeoxygenation of bio-oil derived from biomass catalytic pyrolysis. Bioresour. Technol. 2012, 108, 280–284. [Google Scholar] [CrossRef]
- Kim, T.S.; Oh, S.Y.; Kim, J.Y.; Choi, I.G.; Choi, J.W. Study on the hydrodeoxygenative upgrading of crude bio-oil produced from woody biomass by fast pyrolysis. Energy 2014, 68, 437–443. [Google Scholar] [CrossRef]
- Yao, G.; Wu, G.; Dai, W.; Guan, N.; Li, L. Hydrodeoxygenation of lignin-derived phenolic compounds over bi-functional Ru/H-Beta under mild conditions. Fuel 2015, 150, 175–183. [Google Scholar] [CrossRef]
- Lee, H.W.; Jun, B.R.; Kim, H.; Kim, D.H.; Jeon, J.K.; Park, S.H.; Ko, C.H.; Kim, T.W.; Park, Y.K. Catalytic hydrodeoxygenation of 2-methoxy phenol and dibenzofuran over Pt/mesoporous zeolites. Energy 2015, 81, 33–40. [Google Scholar] [CrossRef]
- Itthibenchapong, V.; Ratanatawanate, C.; Oura, M.; Faungnawakij, K. A facile and low-cost synthesis of MoS2 for hydrodeoxygenation of phenol. Catal. Commun. 2015, 68, 31–35. [Google Scholar] [CrossRef]
- Leiva, K.; Sepulveda, C.; García, R.; Laurenti, D.; Vrinat, M.; Geantet, C.; Escalona, N. Kinetic study of the conversion of 2-methoxyphenol over supported Re catalysts: Sulfide and oxide state. Appl. Catal. A 2015, 505, 302–308. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Luo, H.; Liu, W. Effect of additive (Co, La) for Ni–Mo–B amorphous catalyst and its hydrodeoxygenation properties. Catal. Commun. 2010, 11, 803–807. [Google Scholar] [CrossRef]
- Wang, W.; Yang, S.; Qiao, Z.; Liu, P.; Wu, K.; Yang, Y. Preparation of Ni–W–P–B amorphous catalyst for the hydrodeoxygenation of p-cresol. Catal. Commun. 2015, 60, 50–54. [Google Scholar] [CrossRef]
- Santillan-Jimenez, E.; Perdu, M.; Pace, R.; Morgan, T.; Crocker, M. Activated Carbon, Carbon Nanofiber and Carbon Nanotube Supported Molybdenum Carbide Catalysts for the Hydrodeoxygenation of Guaiacol. Catalysts 2015, 5, 424–441. [Google Scholar] [CrossRef]
- Monnier, J.; Sulimma, H.; Dalai, A.; Caravaggio, G. Hydrodeoxygenation of oleic acid and canola oil over alumina-supported metal nitrides. Appl. Catal. A 2010, 382, 176–180. [Google Scholar] [CrossRef]
- Afanasiev, P.; Laurenti, D. CCl4-Assisted Preparation of Highly Dispersed Molybdenum and Tungsten Nitrides. Top. Catal. 2012, 55, 940–949. [Google Scholar] [CrossRef]
- Prins, R.; Bussell, M.E. Metal phosphides: Preparation, characterization and catalytic reactivity. Catal. Lett. 2012, 142, 1413–1436. [Google Scholar] [CrossRef]
- Consuelo Alvarez-Galvan, M.; Campos-Martin, J.M.; Fierro, J.L.G. Transition Metal Phosphides for the Catalytic Hydrodeoxygenation of Waste Oils into Green Diesel. Catalysts 2019, 9, 293. [Google Scholar] [CrossRef]
- Iino, A.; Cho, A.; Takagaki, A.; Kikuchi, R.; Oyama, S.T. Kinetic studies of hydrodeoxygenation of 2-methyltetrahydrofuran on a Ni2P/SiO2 catalyst at medium pressure. J. Catal. 2014, 311, 17–27. [Google Scholar] [CrossRef]
- Moon, J.S.; Kim, E.G.; Lee, Y.K. Active sites of Ni2P/SiO2 catalyst for hydrodeoxygenation of guaiacol: A joint XAFS and DFT study. J. Catal. 2014, 311, 144–152. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Jiménez-López, A.; Oyama, S.T. Oxygen-removal of dibenzofuran as a model compound in biomass derived bio-oil on nickel phosphide catalysts: Role of phosphorus. Appl. Catal. B 2013, 136, 140–149. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Li, D.; Bui, P.; Oyama, S.T. Hydrodeoxygenation of guaiacol as model compound for pyrolysis oil on transition metal phosphide hydroprocessing catalysts. Appl. Catal. A 2011, 391, 305–310. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ma, L.; Zhang, Q.; Yu, Y.; Liu, Q. Characterization and catalytic properties of Ni and NiCu catalysts supported on ZrO2–SiO2 for guaiacol hydrodeoxygenation. Catal. Commun. 2013, 33, 15–19. [Google Scholar] [CrossRef]
- Soták, T.; Schmidt, T.; Hronec, M. Hydrogenolysis of polyalcohols in the presence of metal phosphide catalysts. Appl. Catal. A 2013, 459, 26–33. [Google Scholar] [CrossRef]
- Shi, H.; Chen, J.; Yang, Y.; Tian, S. Catalytic deoxygenation of methyl laurate as a model compound to hydrocarbons on nickel phosphide catalysts: Remarkable support effect. Fuel Process. Technol. 2014, 118, 161–170. [Google Scholar] [CrossRef]
- Burns, A.W.; Layman, K.A.; Bale, D.H.; Bussel, M.E. Understanding the relationship between composition and hydrodesulfurization properties for cobalt phosphide catalysts. Appl. Catal. A 2008, 343, 68–76. [Google Scholar] [CrossRef]
- Weidenthaler, C.; Schmidt, W. Thermal Stability and Thermal Transformations of Co2+- or Ni2+-Exchanged Zeolites A, X, and Y. Chem. Mater. 2000, 12, 3811–3820. [Google Scholar] [CrossRef]
- Möller, K.; Bein, T. Mesoporosity—A new dimension for zeolites. Chem. Soc. Rev. 2013, 42, 3689–3707. [Google Scholar] [CrossRef]
- Huang, C.; Lia, A.; Chao, Z.S. Heterogeneous catalytic synthesis of quinoline compounds from aniline and C1–C4 alcohols over zeolite-based catalysts. RSC Adv. 2017, 7, 48275–48285. [Google Scholar] [CrossRef]
- Li, K.; Wang, R.; Chen, J. Hydrodeoxygenation of Anisole over Silica-Supported Ni2P, MoP, and NiMoP Catalysts. Energy Fuels 2011, 25, 854–863. [Google Scholar] [CrossRef]
- Gafurov, M.R.; Mukhambetov, I.N.; Yavkin, B.V.; Mamin, G.V.; Lamberov, A.A.; Orlinskii, S.B. Quantitative Analysis of Lewis Acid Centers of γ-Alumina by Using EPR of the Adsorbed Anthraquinone as a Probe Molecule: Comparison with the Pyridine, Carbon Monoxide IR, and TPD of Ammonia. J. Phys. Chem. C 2015, 119, 27410–27415. [Google Scholar] [CrossRef]
- Tian, Q.; Qiao, K.; Zhou, F.; Chen, K.; Wang, T.; Fu, J.; Lu, X.; Ouyang, P. Direct Production of Aviation Fuel Range Hydrocarbons and Aromatics from Oleic Acid without an Added Hydrogen Donor. Energy Fuels 2016, 30, 7291–7297. [Google Scholar] [CrossRef]
- Peroni, M.; Lee, I.; Huang, X.; Baráth, E.; Gutierrez, O.Y.; Lercher, J.A. Deoxygenation of Palmitic Acid on Unsupported Transition-Metal Phosphides. ACS Catal. 2017, 7, 6331–6341. [Google Scholar] [CrossRef]
- Oyama, S.T.; Wang, X.; Lee, Y.K.; Bando, K.; Requejo, F.G. Effect of phosphorus content in nickel phosphide catalysts studied by XAFS and other techniques. J. Catal. 2002, 210, 207–217. [Google Scholar] [CrossRef]
- Bui, P.; Cecilia, J.A.; Oyama, S.T.; Takagaki, A.; Infantes-Molina, A.; Zhao, H.; Li, D.; Rodríguez-Castellón, E.; López, A.J. Studies of the synthesis of transition metal phosphides and their activity in the hydrodeoxygenation of a biofuel model compound. J. Catal. 2012, 294, 184–198. [Google Scholar] [CrossRef]
- Wang, X.; Clark, P.; Oyama, S.T. Synthesis, Characterization, and Hydrotreating Activity of Several Iron Group Transition Metal Phosphides. J. Catal. 2002, 208, 321–331. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Developments of the Program FULLPROF. In Newsletter; Commission on Powder Diffraction (IUCr): Chester, UK, 2001; Volume 26, pp. 12–19. [Google Scholar]
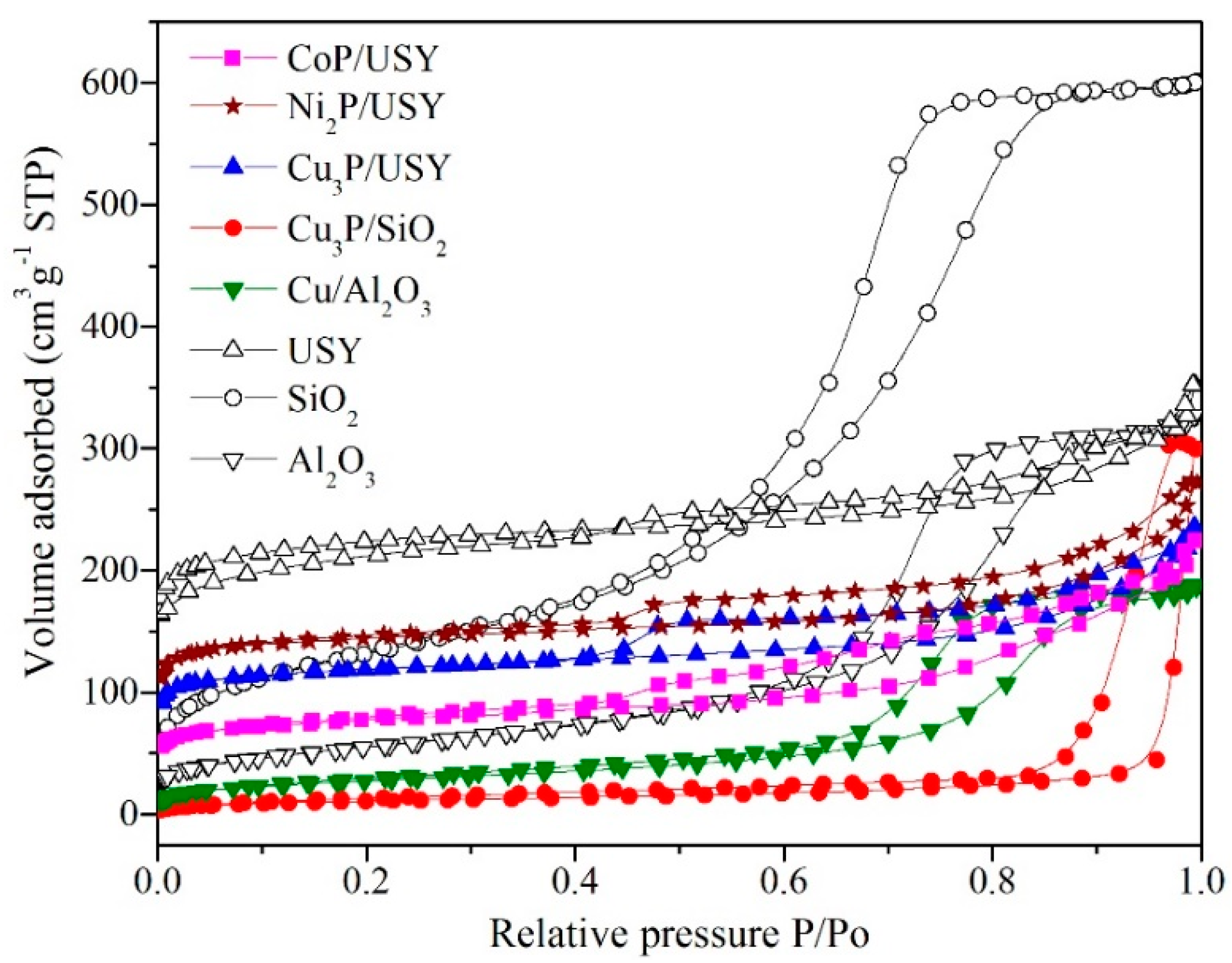


| Catalyst | Acid Site (mmol/g) a | SBET (m2/g) | Smicro (m2/g) | Total Pore Volume (cc/g) | Micropore Volume (cc/g) | Pore Diameter (nm) | Crystallite Size (nm) b |
|---|---|---|---|---|---|---|---|
| CoP/USY | 0.19 | 247 | 95 | 0.28 | 0.06 | 3.7 | 58.4 |
| Ni2P/USY | 0.60 | 446 | 321 | 0.24 | 0.17 | 3.7 | 39.2 |
| Cu3P/USY | 0.34 | 371 | 253 | 0.24 | 0.13 | 3.7 | 33.2 |
| Cu3P/SiO2 | 0.04 | 38 | - | 0.50 | - | 27.2 | 33.2 |
| Cu/γ-Al2O3 | 0.50 | 101 | - | 0.31 | - | 7.8 | 46.0 |
| USY | 0.52 | 696 | 558 | 0.27 | 0.26 | 3.7 | - |
| SiO2 | - | 471 | - | 0.99 | - | 6.9 | - |
| γ-Al2O3 | 0.43 | 198 | - | 0.54 | - | 7.8 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochaputi, N.; Kongmark, C.; Khemthong, P.; Butburee, T.; Kuboon, S.; Worayingyong, A.; Faungnawakij, K. Catalytic Behaviors of Supported Cu, Ni, and Co Phosphide Catalysts for Deoxygenation of Oleic Acid. Catalysts 2019, 9, 715. https://doi.org/10.3390/catal9090715
Kochaputi N, Kongmark C, Khemthong P, Butburee T, Kuboon S, Worayingyong A, Faungnawakij K. Catalytic Behaviors of Supported Cu, Ni, and Co Phosphide Catalysts for Deoxygenation of Oleic Acid. Catalysts. 2019; 9(9):715. https://doi.org/10.3390/catal9090715
Chicago/Turabian StyleKochaputi, Nopparuj, Chanapa Kongmark, Pongtanawat Khemthong, Teera Butburee, Sanchai Kuboon, Attera Worayingyong, and Kajornsak Faungnawakij. 2019. "Catalytic Behaviors of Supported Cu, Ni, and Co Phosphide Catalysts for Deoxygenation of Oleic Acid" Catalysts 9, no. 9: 715. https://doi.org/10.3390/catal9090715
APA StyleKochaputi, N., Kongmark, C., Khemthong, P., Butburee, T., Kuboon, S., Worayingyong, A., & Faungnawakij, K. (2019). Catalytic Behaviors of Supported Cu, Ni, and Co Phosphide Catalysts for Deoxygenation of Oleic Acid. Catalysts, 9(9), 715. https://doi.org/10.3390/catal9090715







