Novel High Efficiency Layered Oxide Photocatalyst Li2SnO3 for Rhodamine B and Tetracycline Degradation
Abstract
:1. Introduction
2. Results and Discussion
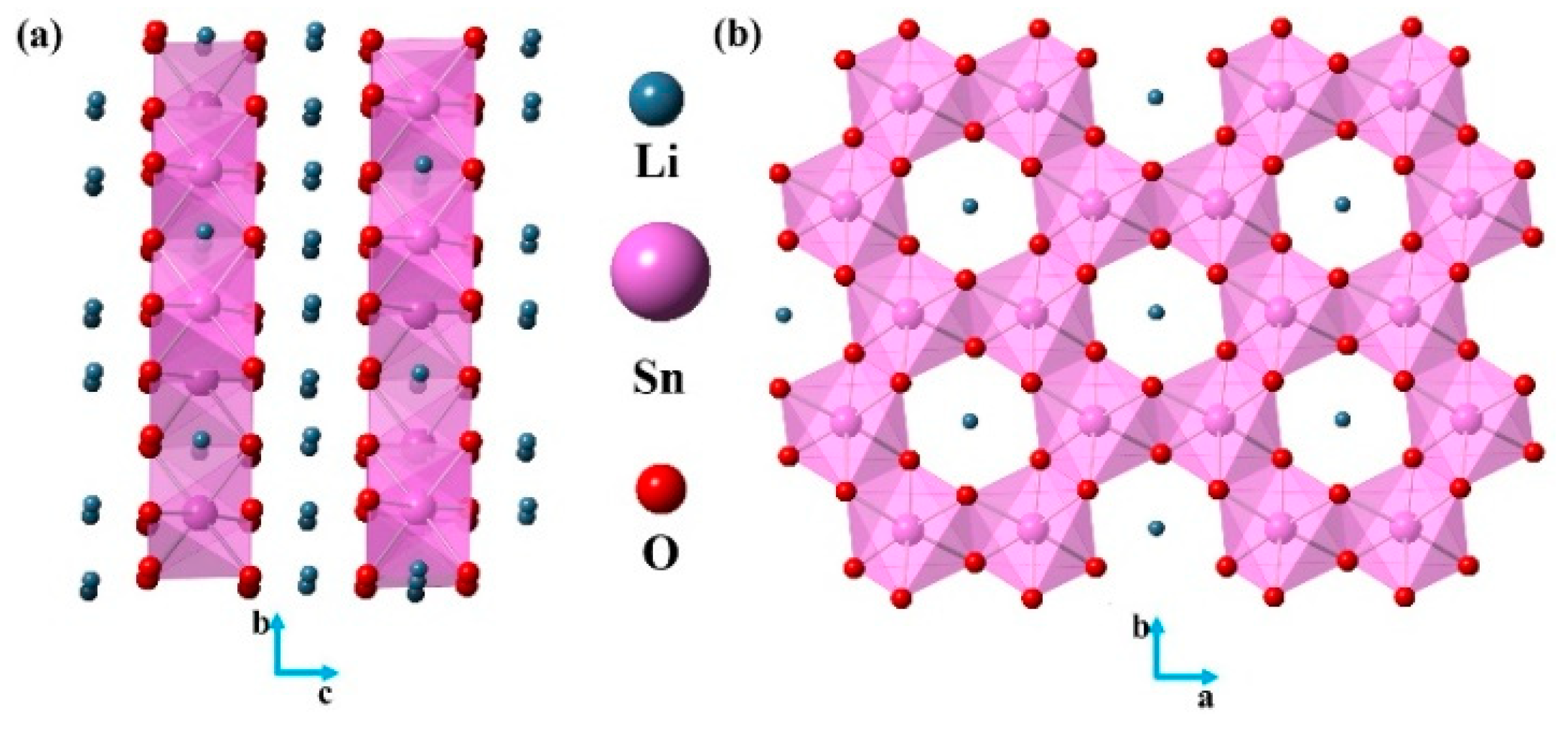
2.1. Crystal Structure and Powder XRD of Li2SnO3
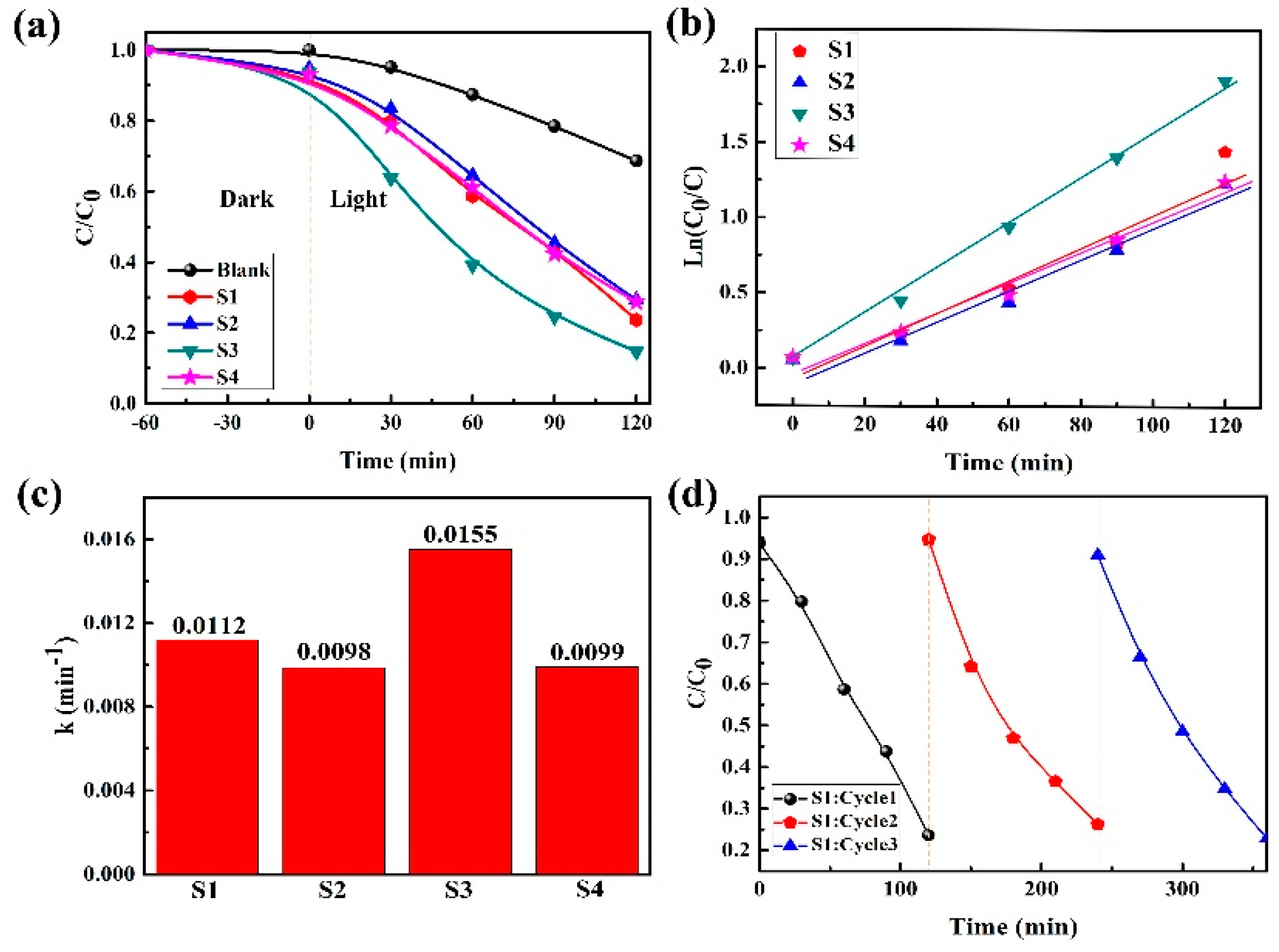
2.2. Photocatalytic Degradation Capability
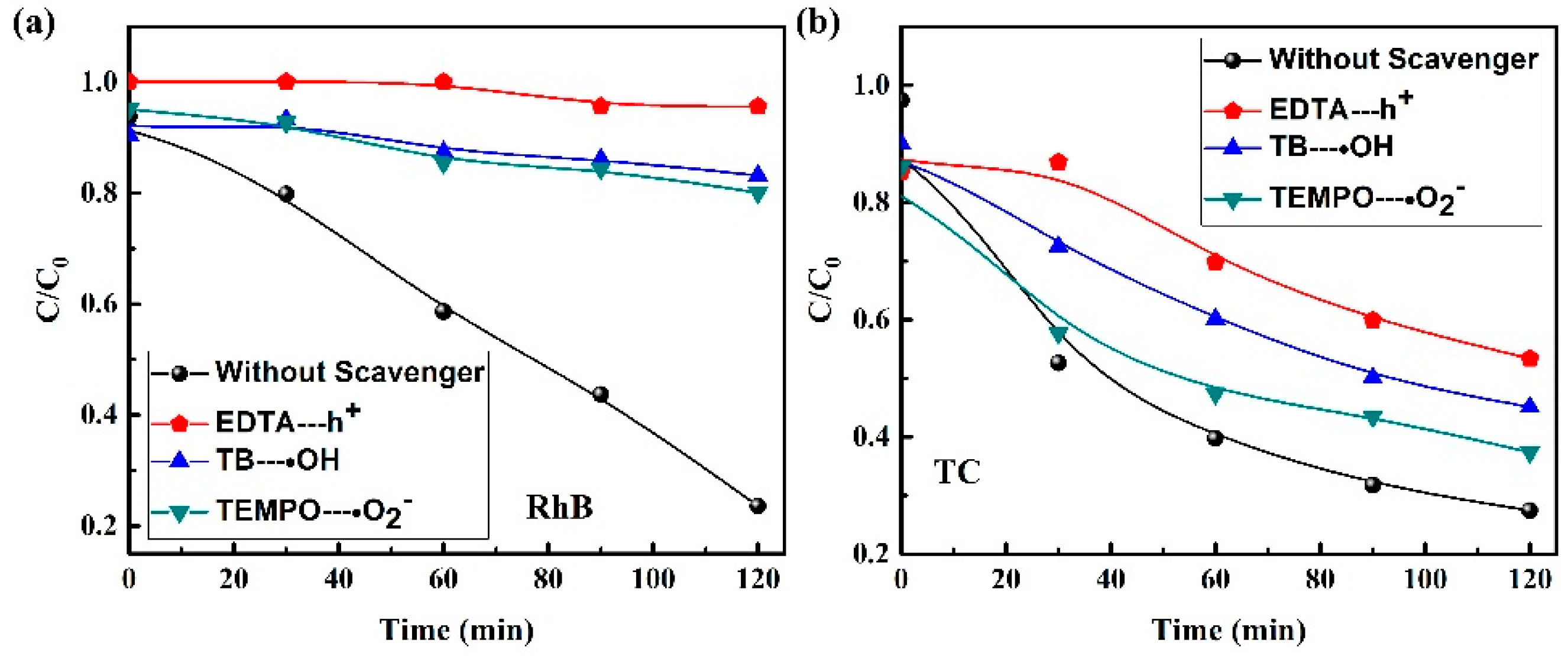
2.3. Mechanism of Photocatalytic Reaction
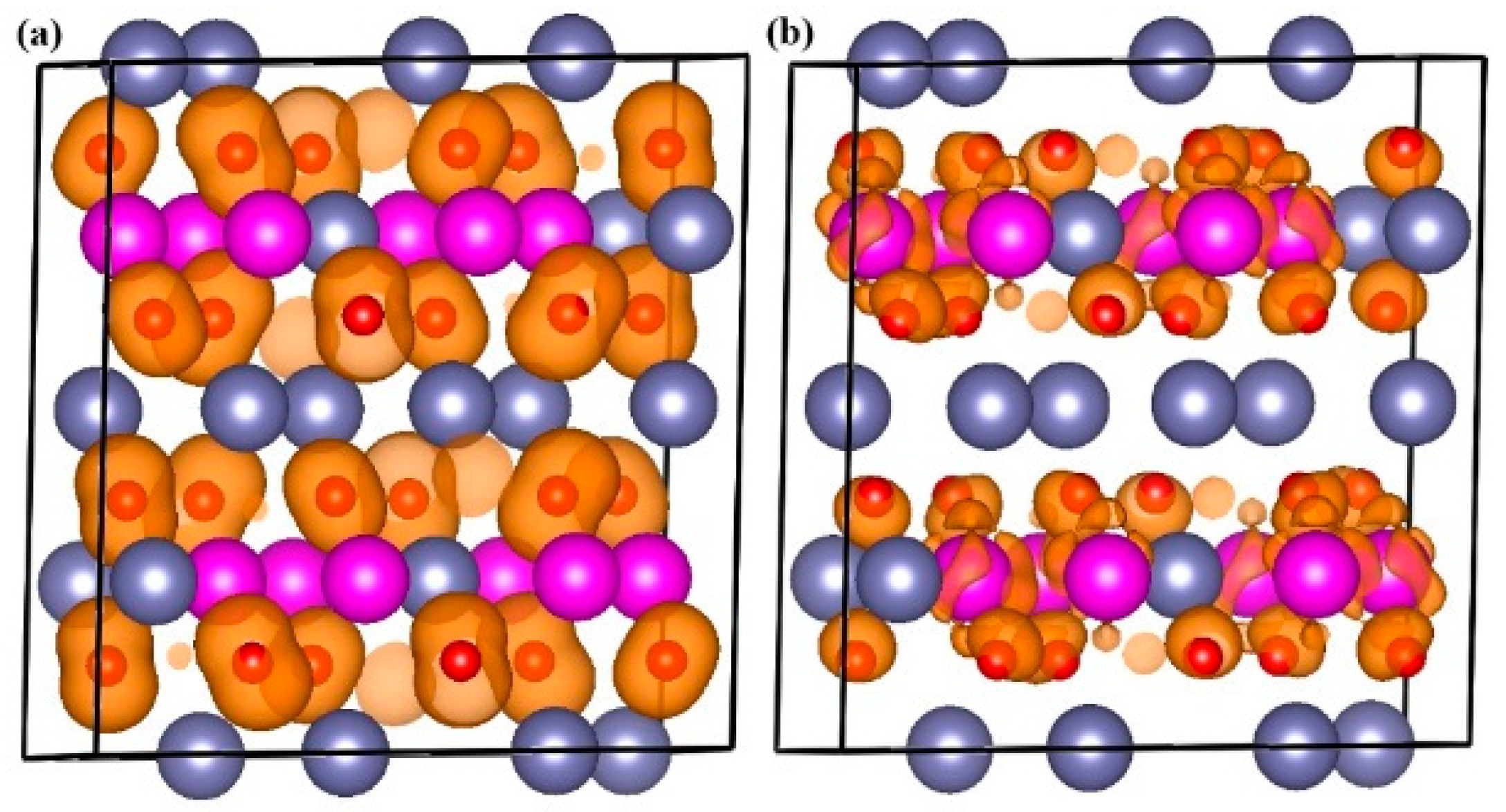
2.4. Theoretical Analysis
3. Materials and Methods
3.1. Preparation of the Photocatalysts
3.2. Characterizations and Computations
3.3. Photocatalytic Activity Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, C.C.; Ma, W.H.; Zhao, J.C. Semiconductor-mediated photo-degradation of pollutants under visible light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Daeneke, T.; Zadeh, K.K.; Ou, J.Z. Two-Dimensional Transitional Metal Oxide and Chalcogenide Based Photocatalysts. Nano-Micro Lett. 2018, 10, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Zhang, L.S.; Chen, Z.G.; Hu, J.Q.; Li, S.J.; Wang, Z.H.; Liu, J.S.; Wang, X.C. Semiconductor Heterojunction Photocatalysts: Design, Construction and Photocatalytic Performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.L.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photo-catalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Xiang, Q.J.; Liao, Y.L.; Zhang, H.W. CdS Based Photocatalyst. Energy Environ. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.D.; Xie, Y. Photoresponsive Polymeric Carbon Nitride Based Materials: Design and Application. Mater. Today 2019, 23, 72–86. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photo-catalysts for Artificial Photo-synthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.Q.; Xie, J.; Chen, X.B.; Li, X. A Review on g-C3N4 Based Photo-catalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Jin, C.H.; Lin, F.; Suenaga, K.; Lijima, S. Fabrication of a Freestanding Boron Nitride Single Layer and Its Defect Assignments. Phys. Rev. Lett. 2009, 102, 195505–195508. [Google Scholar] [CrossRef]
- Wang, Q.H.; Zadeh, K.K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and Optoelectronics of Two Dimensional Transition Metal Dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Hwang, D.W.; Lee, J.S. An Undoped Single Phase Oxide Photocatalyst Working under Visible Light. J. Am. Chem. Soc. 2004, 126, 8912–8913. [Google Scholar] [CrossRef] [PubMed]
- Hailili, R.; Dong, G.H.; Ma, Y.C.; Wang, S.J.C.Y.; Xu, T. Layered Perovskite Pb2Bi4Ti5O18 for Excellent Visible Light Driven Photocatalytic No Removal. Ind. Eng. Chem. Res. 2017, 56, 2908–2916. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Meng, D.C.; Yang, H.S.; Ruan, K.Q.; Xu, X.L. Multiferroic and Visible Light Photo-catalytic Properties of Six-layered Perovskite Oxide Nd6Ti4Fe2O20. Appl. Phys. A 2017, 123, 301–306. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Zvereva, I.A. Photocatalytic Activity of Layered Perovskite Like Oxides in Practically Valuable Chemical Reactions. Russ. Chem. Rev. 2016, 85, 248–279. [Google Scholar] [CrossRef]
- Muktha, B.; Priya, M.H.; Madras, G.; Row, T.N.G. Synthesis, Structure and Photo-catalysis in a New Structural Variant of the Aurivillius Phase: LiBi4M3O14 (M = Nb, Ta). J. Phys. Chem. B 2005, 109, 11442–11449. [Google Scholar] [CrossRef]
- Watanabe, K.; Iwase, A.; Nozawa, S.; Adachi, S.I.; Kudo, A. Effects of Coapplication of Rh-doping and Ag-substitution on Band Structure of Li2TiO3 and the Photocatalytic Property. ACS Sustain. Chem. Eng. 2019, 7, 9881–9887. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Zhang, Y.F.; Lin, M.S.; Long, J.L.; Zhang, Z.Z.; Lin, H.X.; Wu, J.C.S.; Wang, X.X. Monolayered Bi2WO6 Nanosheets Mimicking Heterojunction Interface with Open Surfaces for Photocatalysis. Nat. Commun. 2015, 6, 8340–8347. [Google Scholar] [CrossRef]
- Shein, I.R.; Denisova, T.A.; Baklanova, Y.V.; Ivanovskii, A.L. Structural, Electronic Properties and Chemical Bonding in Protonated Lithium Metallates Li2-xHxMO3 (M = Ti, Zr, Sn). J. Struct. Chem. 2011, 52, 1043–1050. [Google Scholar] [CrossRef]
- Wang, Q.F.; Huang, Y.; Miao, J.; Zhao, Y.; Wang, Y. Synthesis and Properties of Li2SnO3/polyaniline Nanocomposites as Negative Electrode Material for Lithium-ion Batteries. Appl. Surf. Sci. 2012, 258, 9896–9901. [Google Scholar] [CrossRef]
- Liu, C.W.; Wu, N.X.; Mao, Y.L.; Bian, J.J. Phase Formation, Microstructure and Microwave Dielectric Properties of Li2SnO3-MO (Mg, Zn) Ceramics. J. Electroceramics 2014, 32, 199–204. [Google Scholar] [CrossRef]
- Sathiya, M.; Rousse, G.; Ramesha, K.; Laisa, C.P.; Vezin, H.; Sougrati, M.T.; Doublet, M.L.; Foix, D.; Gonbeau, D.; Walker, W.; et al. Reversible Anionic Redox Chemistry in High Capacity Layered Oxide Electrodes. Nat. Mater. 2013, 12, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.; Holzwarth, N.A.W. First Principles Simulations of the Porous Layered Calcogenides Li2+xSnO3 and Li2+xSnS3. Phys. Rev. B 2016, 94, 064108–064120. [Google Scholar] [CrossRef]
- Ben, Y.M.; Vergnet, J.; Saubanere, M.; Doublet, M.L. Unified Picture of Anionic Redox in Li/Na-ion Batteries. Nat. Mater. 2019, 18, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, M.L.; Qiao, R.M.; Renfrew, S.E.; Ma, L.; Wu, T.P.; Hwang, S.; Nordlund, D.; Su, D.; Amine, K.; et al. Elucidating Anionic Oxygen Activity in Lithium Rich Layered Oxides. Nat. Commun. 2018, 9, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Tecedor, M.G.; Bartolome, J.; Maestre, D.; Trampert, A.; Cremades, A. Li2SnO3 Branched Nano- and Microstructures with Intense and Broadband White Light Emission. Nano Res. 2019, 12, 441–448. [Google Scholar] [CrossRef]
- Kreuzburg, G.; Stewner, F.; Hoppe, R. Die Kristallstruktur von Li2SnO3. Z. Fuer Anorg. Und Allg. Chem. 1971, 379, 242–254. [Google Scholar] [CrossRef]
- Zhao, H.P.; Li, G.F.; Tian, F.; Jia, Q.T.; Liu, Y.L. Rg-C3N4 Surface-decorated Bi2O2CO3 for Improved Photocatalytic Performance: Theoretical Calculation and Photo-degradation of Antibiotics in Actual Water Matrix. Chem. Chem. Eng. J. 2019, 366, 468–479. [Google Scholar] [CrossRef]
- Li, H.P.; Liu, J.Y.; Hou, W.G.; Du, N.; Zhang, R.J.; Tao, X.T. Synthesis and Characterization of g-C3N4/Bi2MoO6 Heterojunctions with Enhanced Visible Light Photocatalytic Activity. Appl. Catal. B Environ. 2014, 160, 89–97. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, H.J.; Zhu, Y.S.; Dong, P.Y.; Hou, H.J.; Xu, Q.X.; Chen, X.W.; Xi, X.G.; Hou, W.H. Ordered Layered N-doped KTiNbO5/g-C3N4 heterojunction with Enhanced Visible Light Photocatalytic Activity. Appl. Catal. B Environ. 2018, 228, 54–63. [Google Scholar] [CrossRef]
- Zhuang, H.L.; Hennig, R.G. Theoretical Perspective of Photocatalytic Porperties of Single Layer SnS2. Phys. Rev. B 2013, 88, 115314–115318. [Google Scholar] [CrossRef]
- Harb, M.; Cavallo, L. Suitable Fundamental Properties of Ta0.75V0.25ON Material for Visible Light Driven Photo-catalysis: A DFT Study. ACS Omega 2016, 1, 1041–1048. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yang, Q.M.; Wang, Z.M.; Wang, G.Y.; Zhang, B.; Zhang, Q.; Yang, D.F. Rapid Fabrication of SnO2 Nanoparticle Photo-catalyst: Computational Understanding and Photo-catalytic Degradation of Organic Dye. Inorg. Chem. Front. 2018, 5, 3005–3014. [Google Scholar] [CrossRef]
- Ma, Z.J.; Yi, Z.G.; Sun, J.; Wu, K.C. Electronic and Photocatalytic Properties of Ag3PC4 (C = O, S, Se): A Systemic Hybrid DFT Study. J. Phys. Chem. C 2012, 116, 25074–25080. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector Augmented Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, M.; Yang, D.; Zeng, H.; Zhang, T.; Shen, J.; Zhang, B.; Li, Q. Novel High Efficiency Layered Oxide Photocatalyst Li2SnO3 for Rhodamine B and Tetracycline Degradation. Catalysts 2019, 9, 712. https://doi.org/10.3390/catal9090712
Li Y, Wu M, Yang D, Zeng H, Zhang T, Shen J, Zhang B, Li Q. Novel High Efficiency Layered Oxide Photocatalyst Li2SnO3 for Rhodamine B and Tetracycline Degradation. Catalysts. 2019; 9(9):712. https://doi.org/10.3390/catal9090712
Chicago/Turabian StyleLi, Yuanyuan, Meijun Wu, Dingfeng Yang, Hanlu Zeng, Tao Zhang, Jinfeng Shen, Bin Zhang, and Qiaoqi Li. 2019. "Novel High Efficiency Layered Oxide Photocatalyst Li2SnO3 for Rhodamine B and Tetracycline Degradation" Catalysts 9, no. 9: 712. https://doi.org/10.3390/catal9090712
APA StyleLi, Y., Wu, M., Yang, D., Zeng, H., Zhang, T., Shen, J., Zhang, B., & Li, Q. (2019). Novel High Efficiency Layered Oxide Photocatalyst Li2SnO3 for Rhodamine B and Tetracycline Degradation. Catalysts, 9(9), 712. https://doi.org/10.3390/catal9090712





