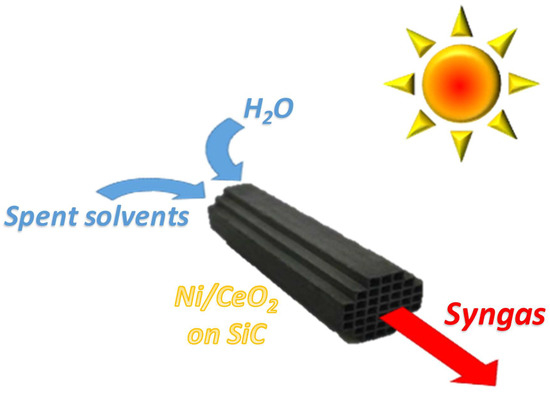
Ni/CeO2 Structured Catalysts for Solar Reforming of Spent Solvents
Abstract
1. Introduction
2. Results
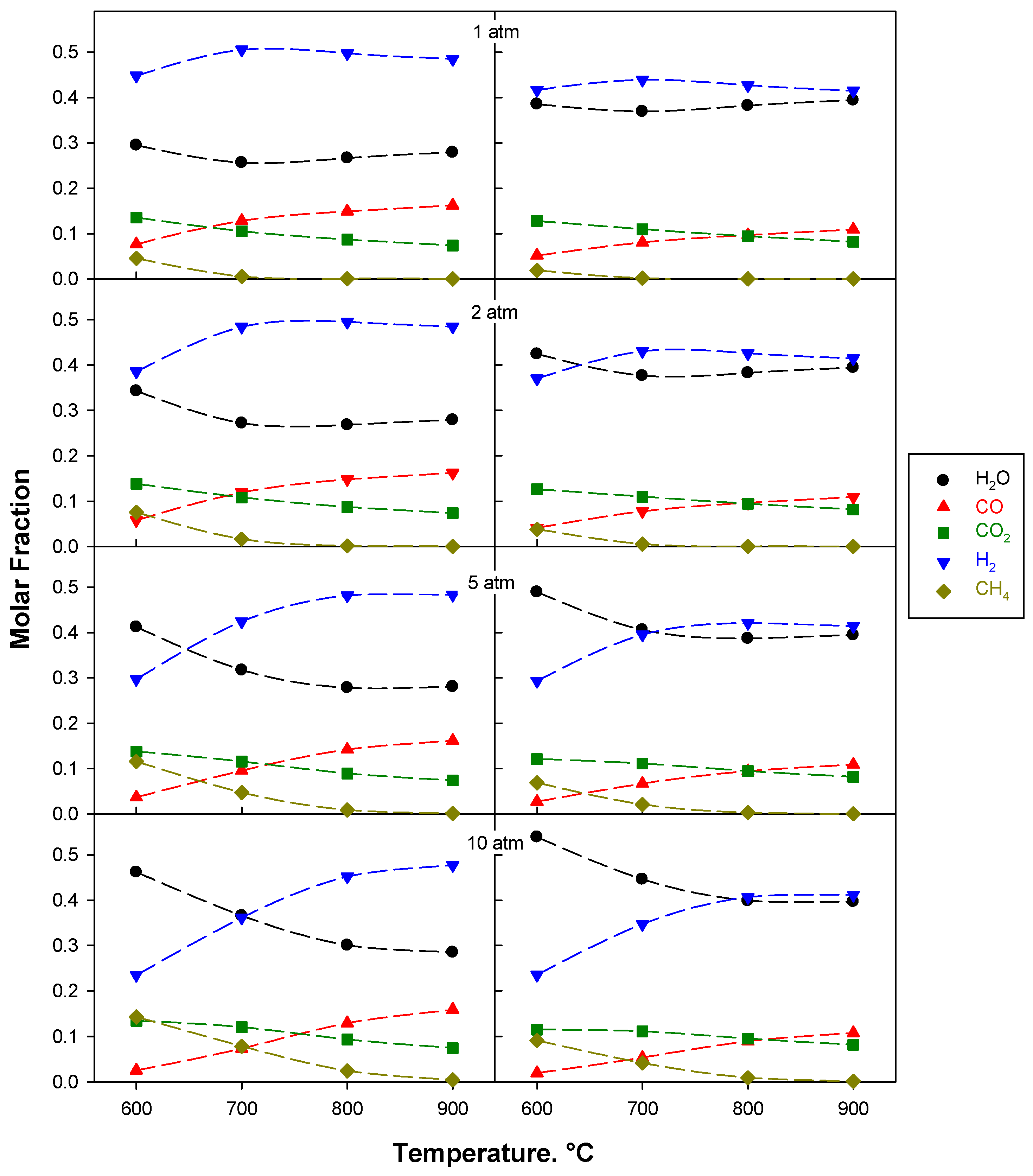
2.1. Thermodynamic Analysis
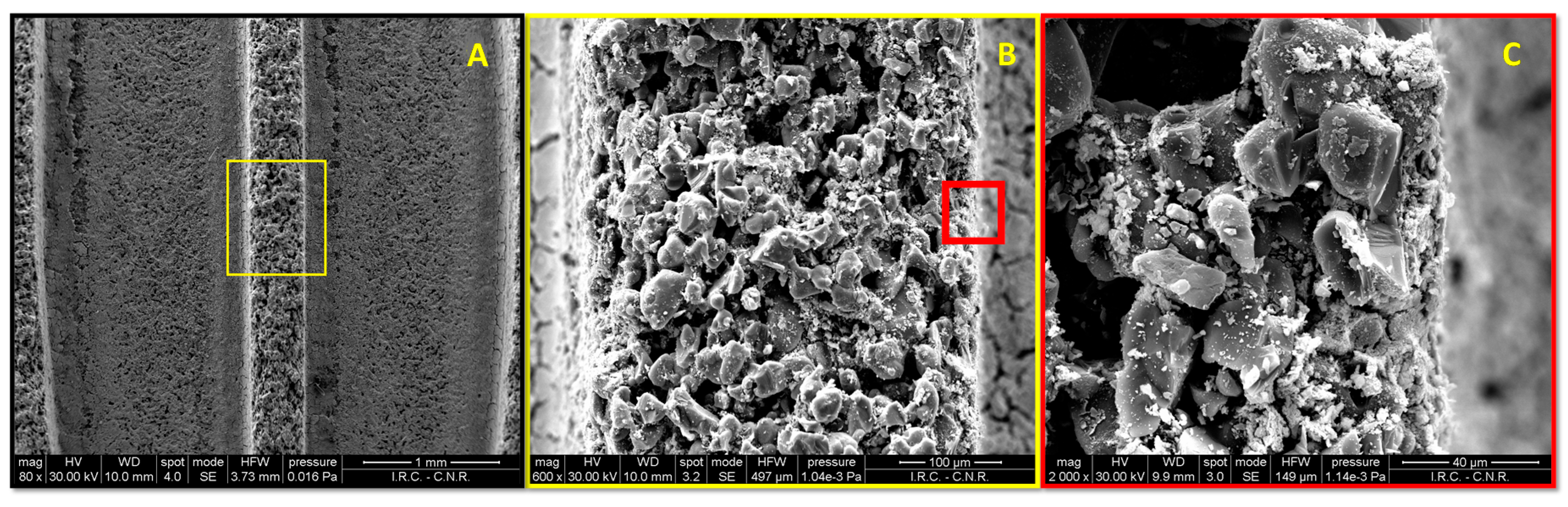
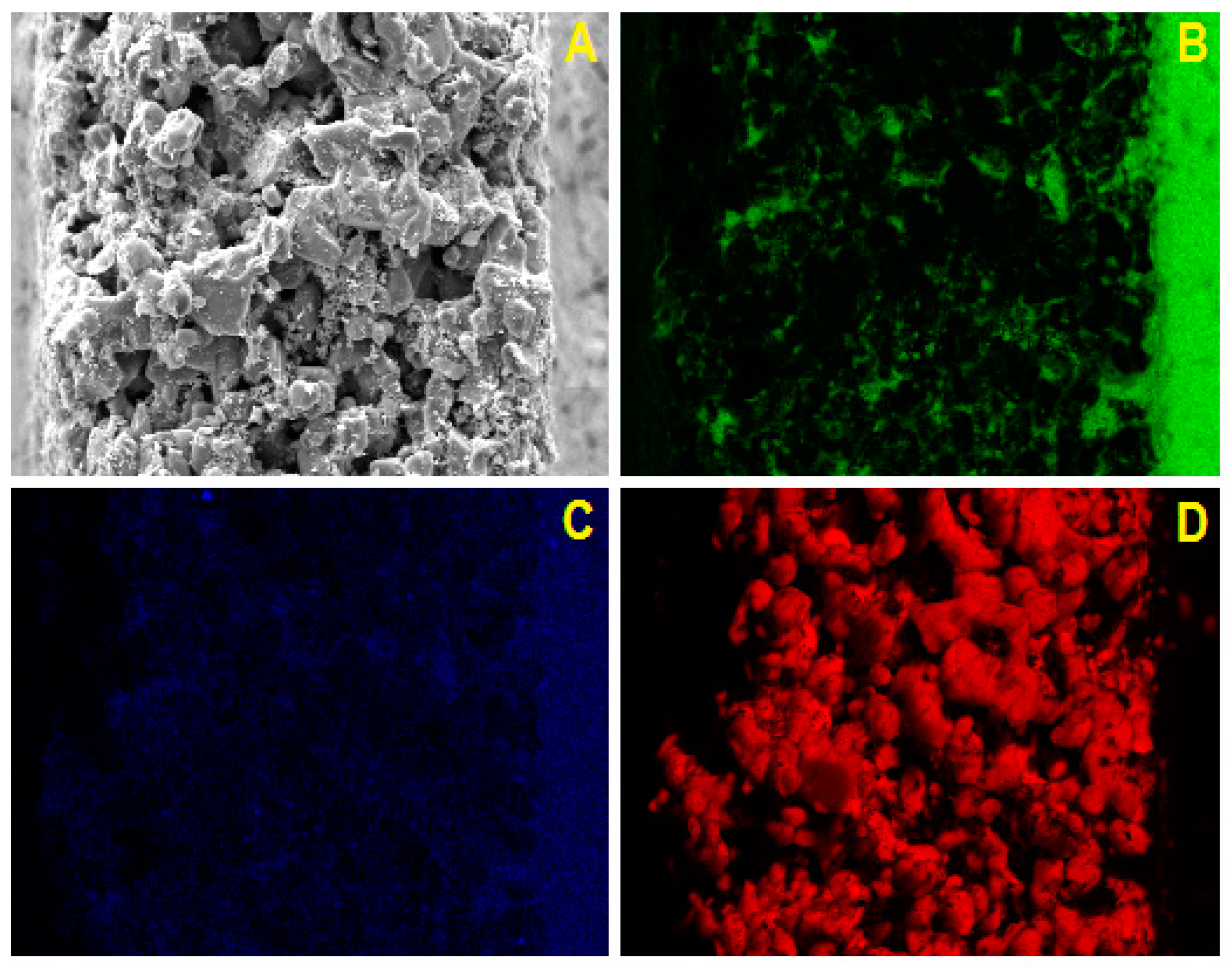
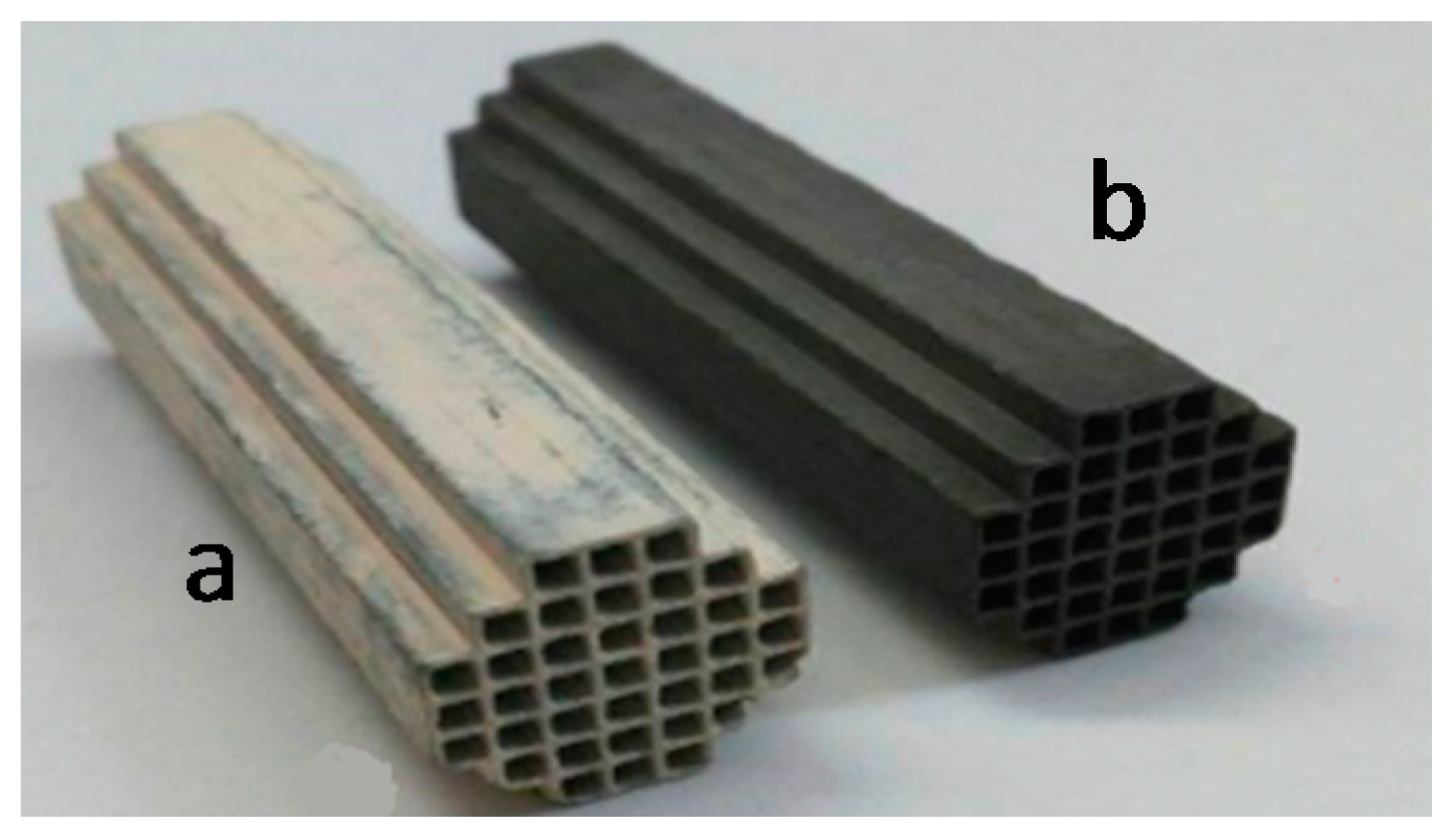
2.2. SEM/EDX Characterization
2.3. Catalytic Tests
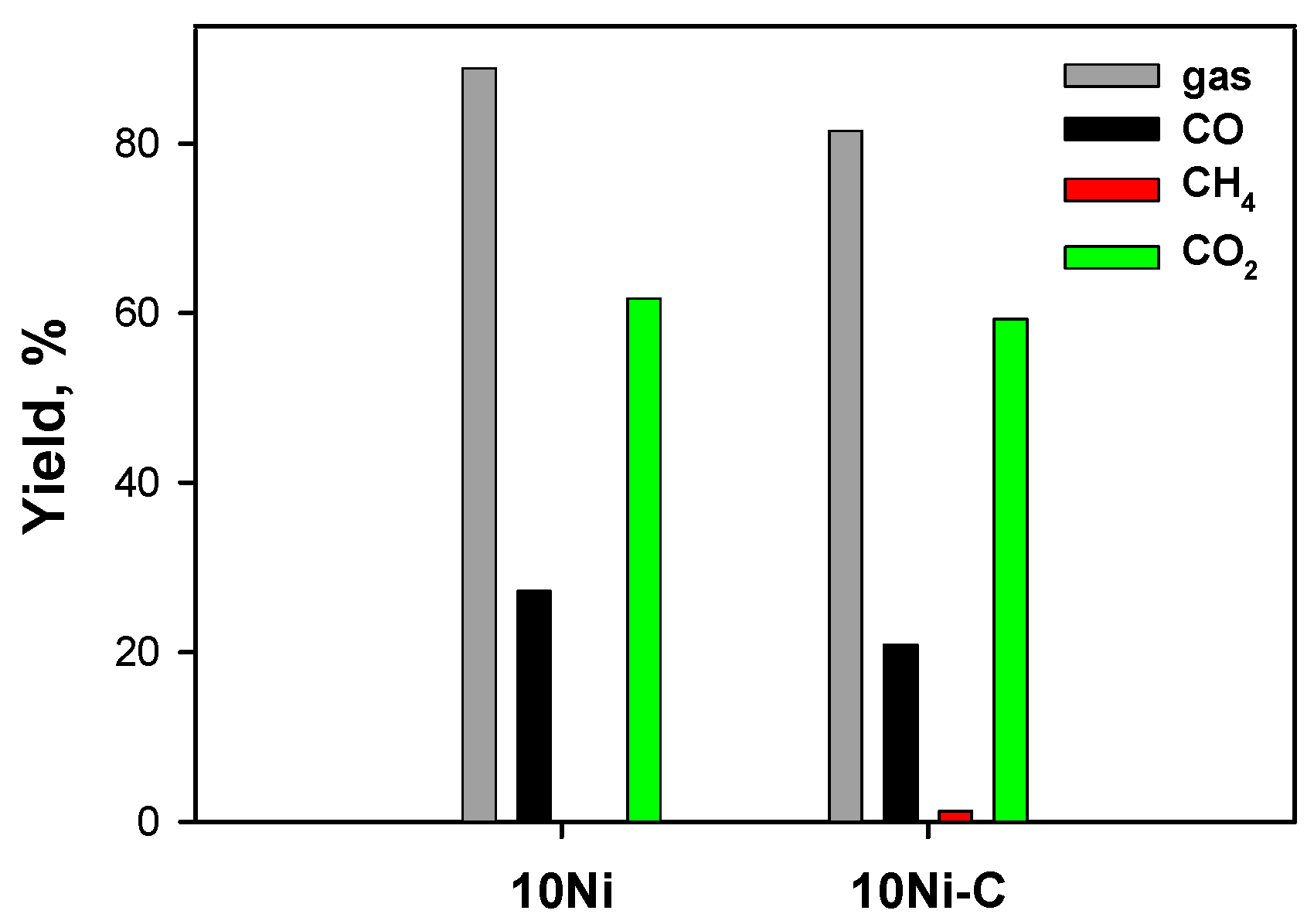
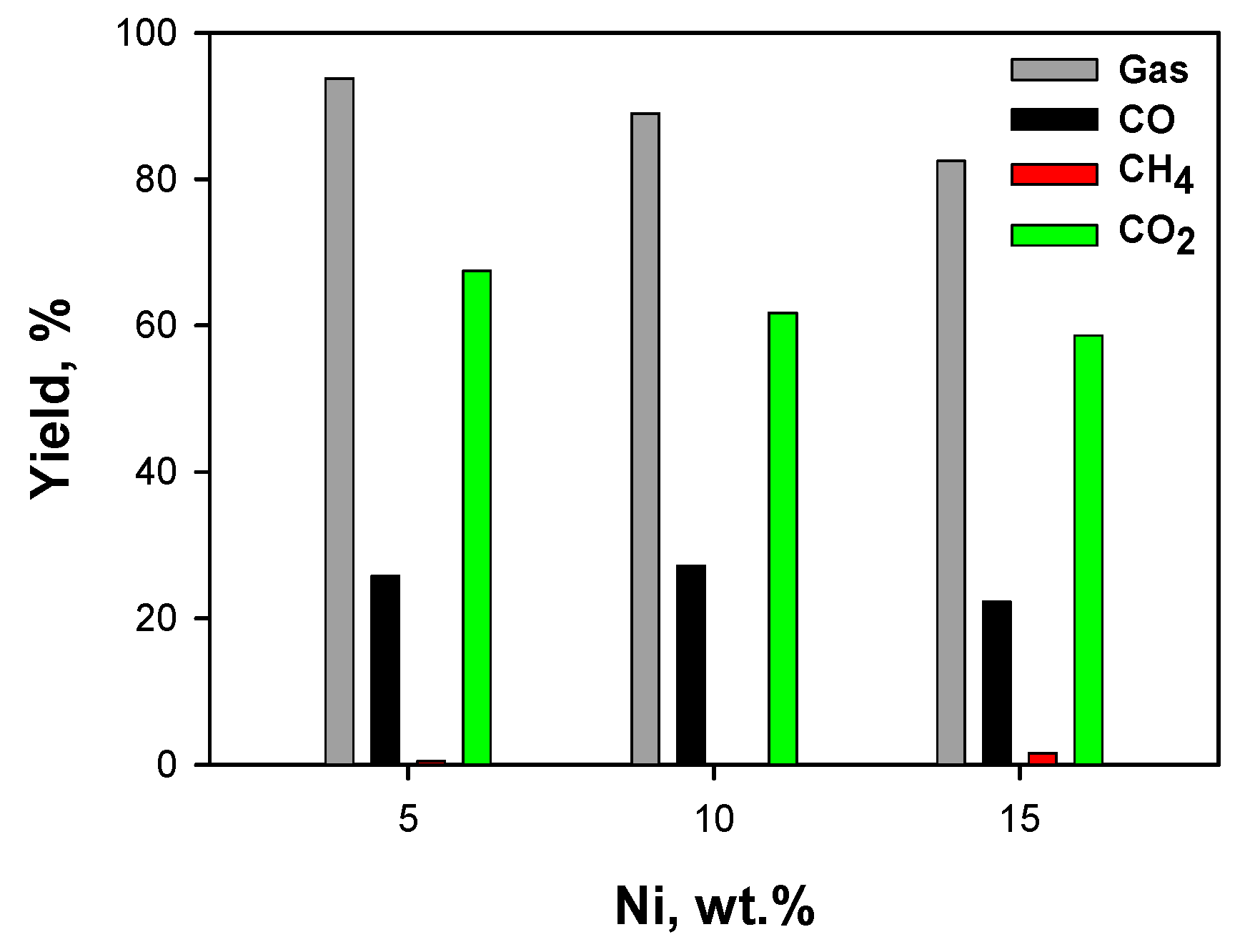
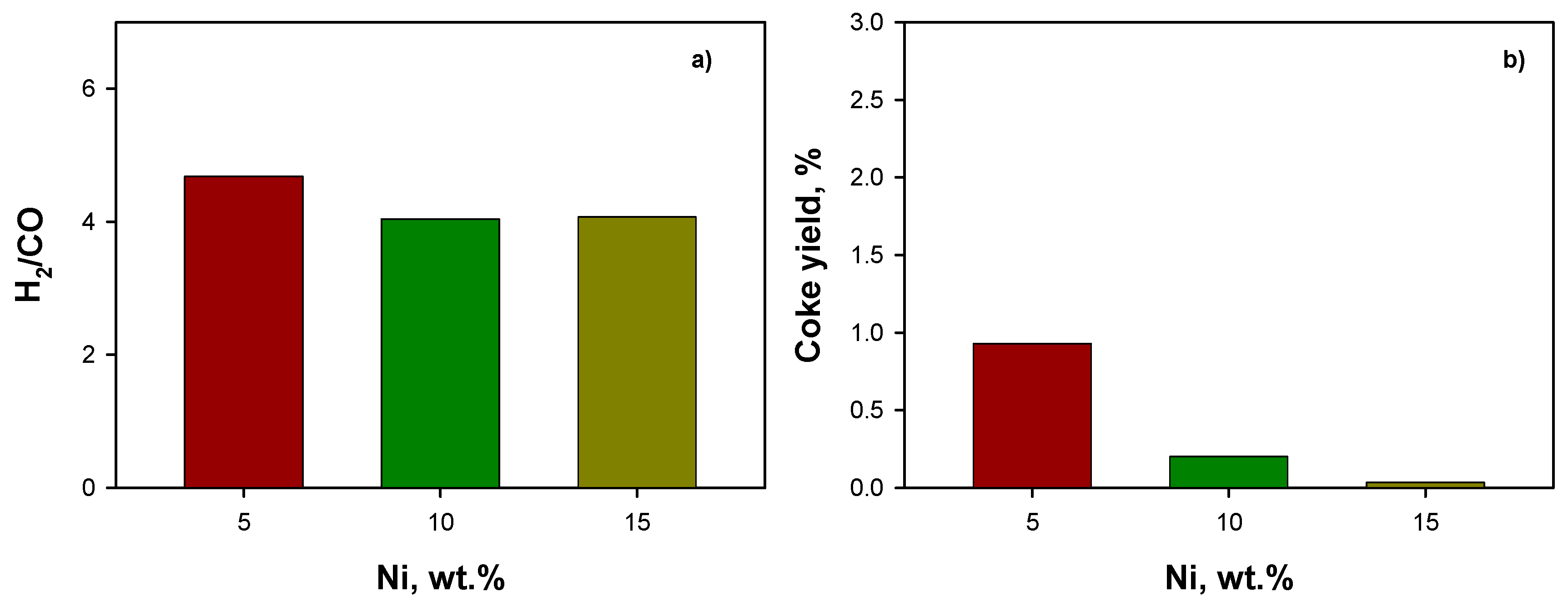
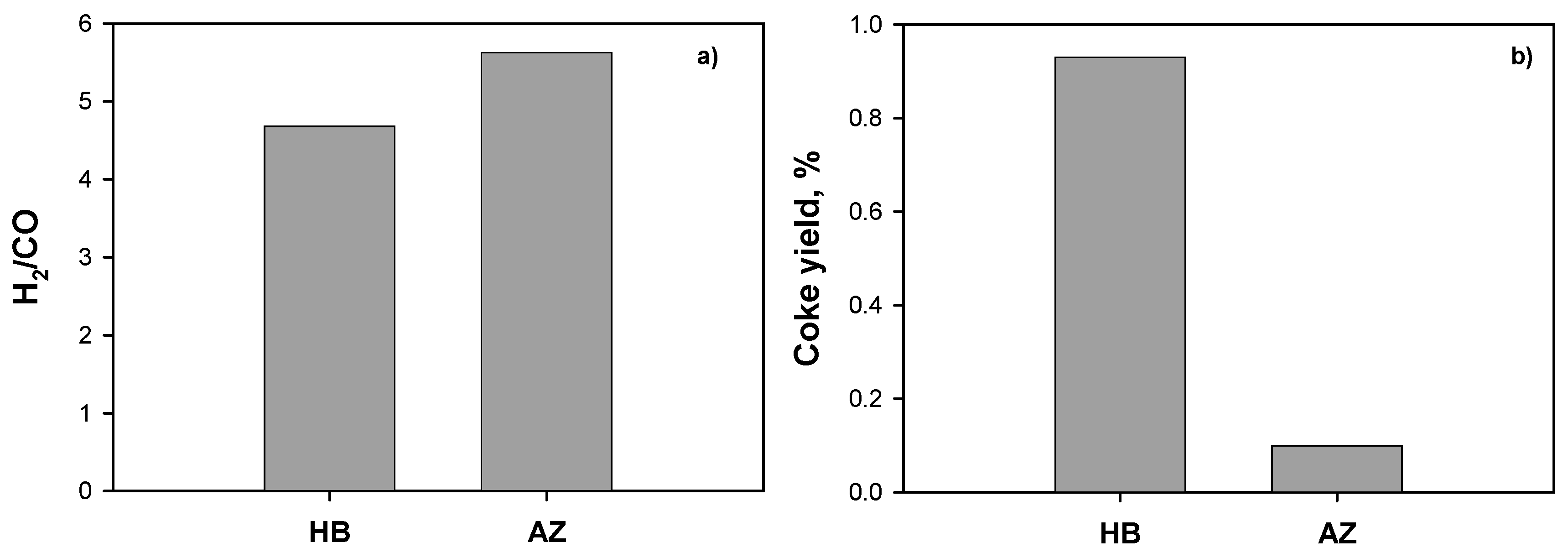
2.3.1. Effect of Ceria Source and Ni Loading: HB Mixture
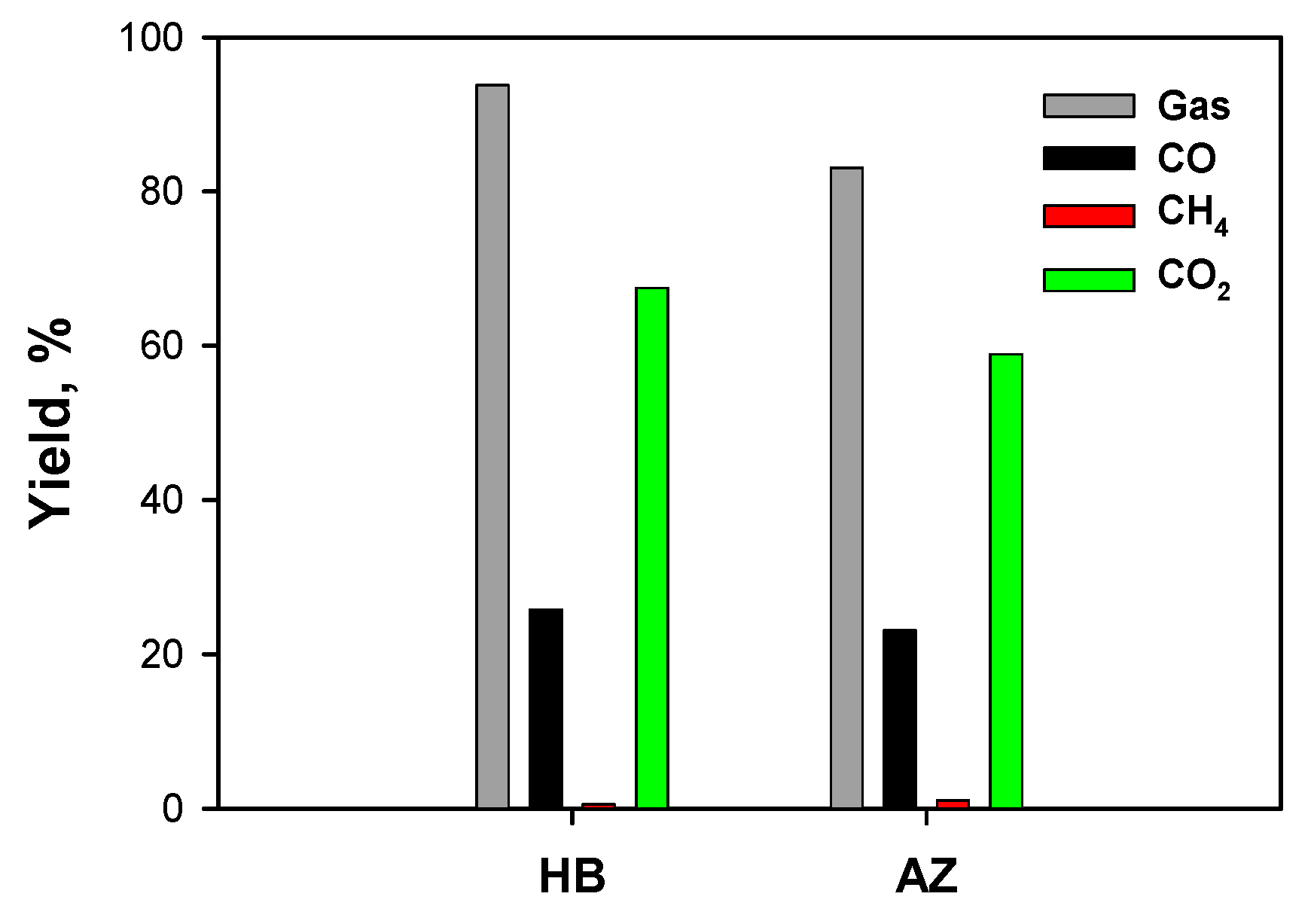
2.3.2. Effect of Solvents Mixture
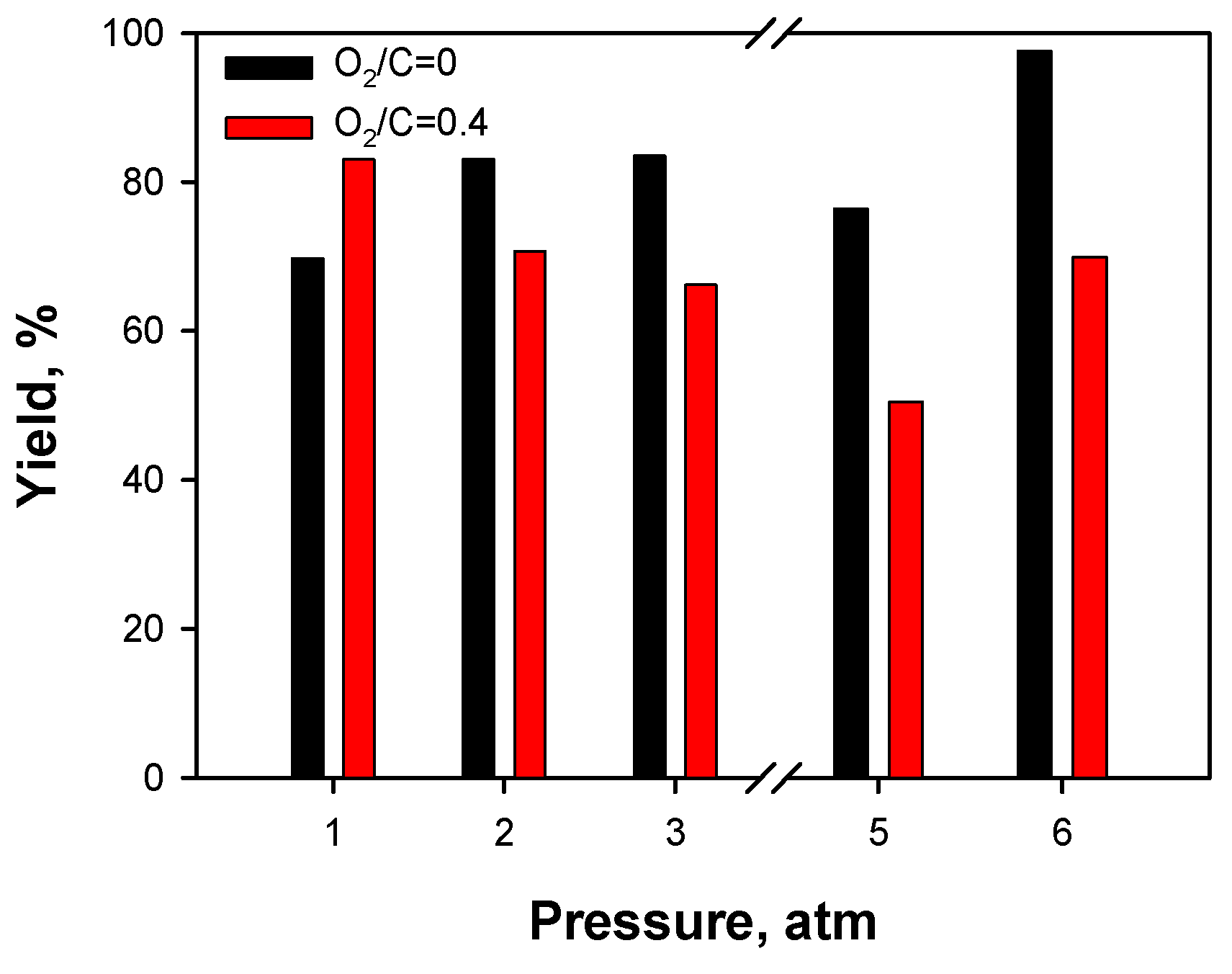
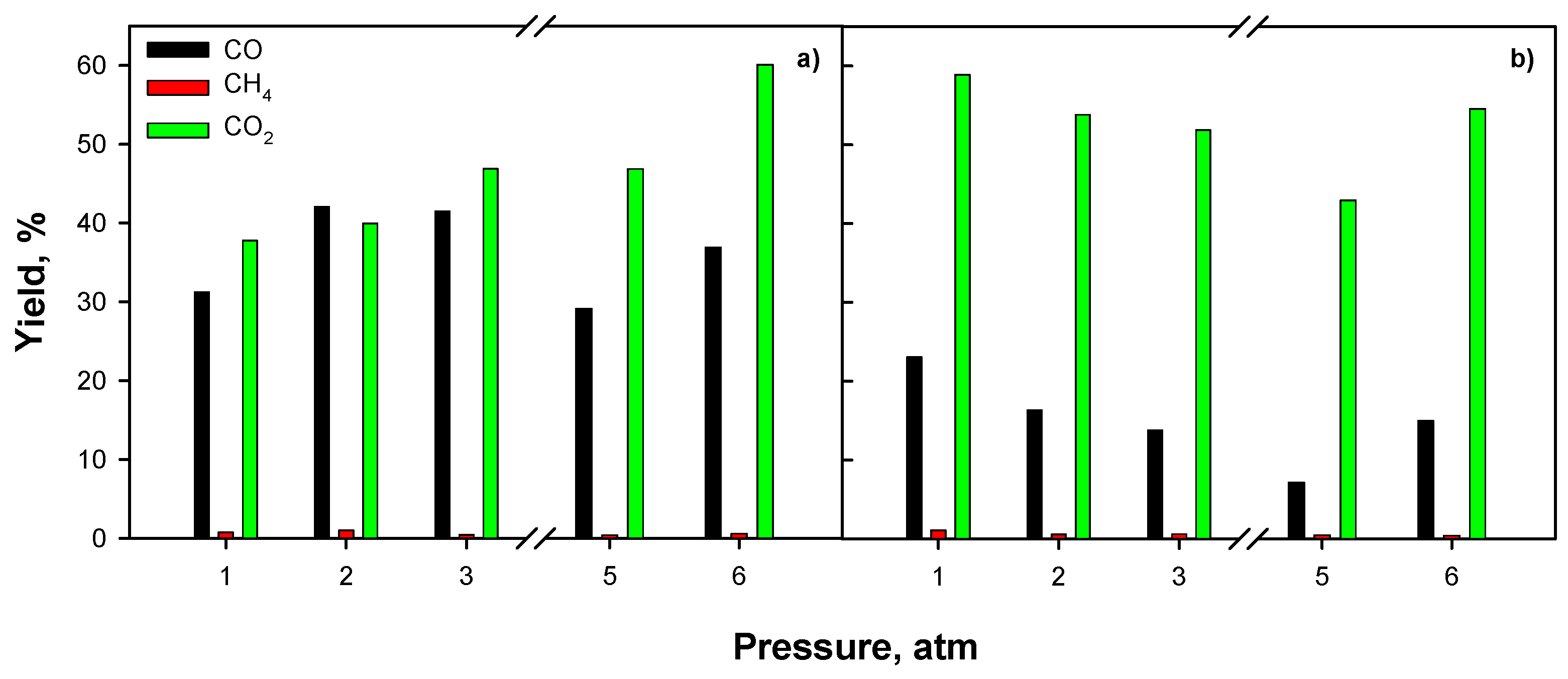
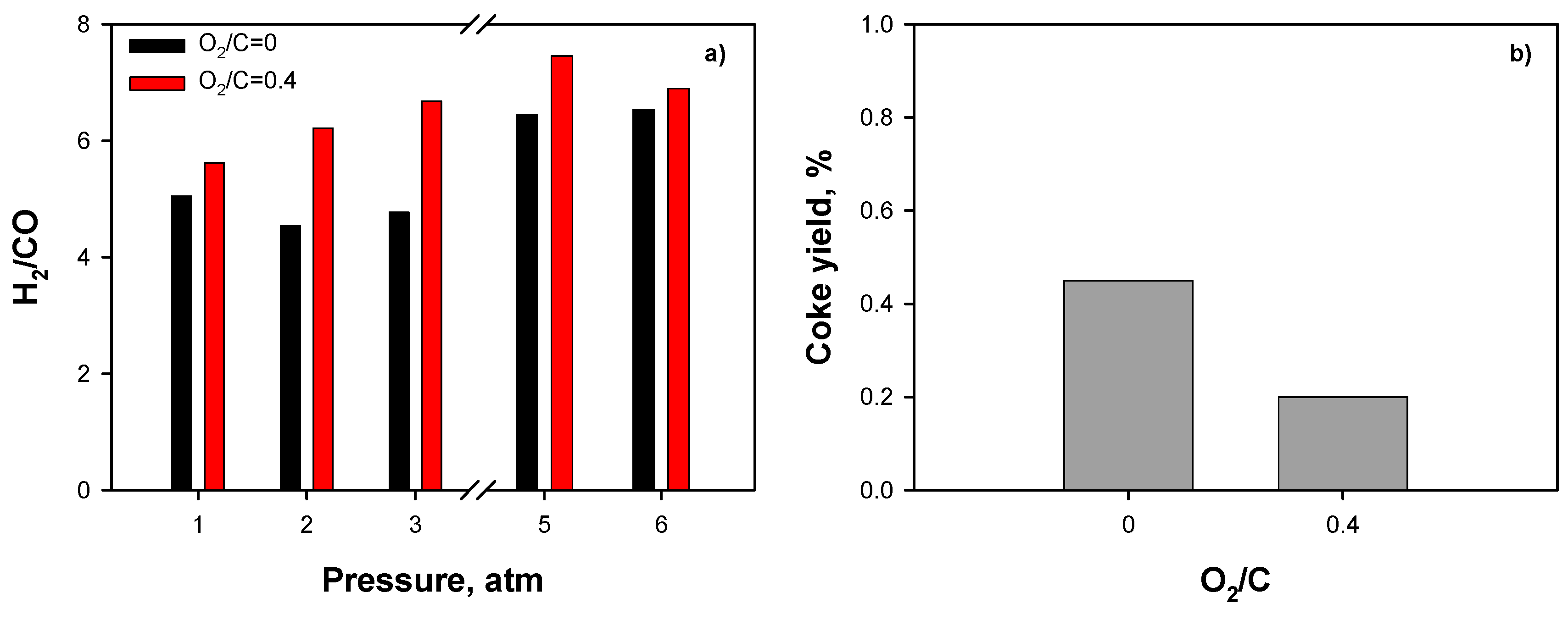
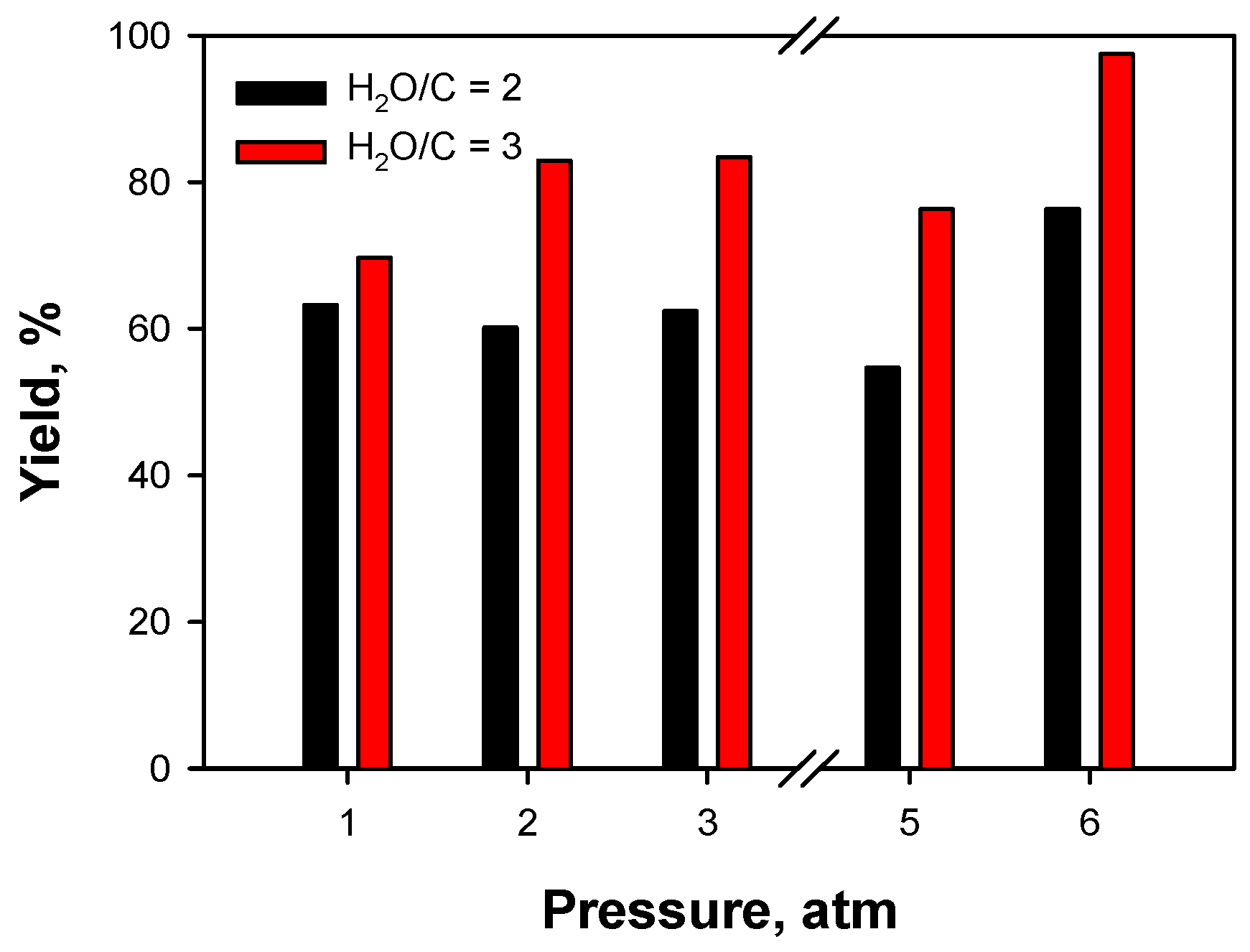
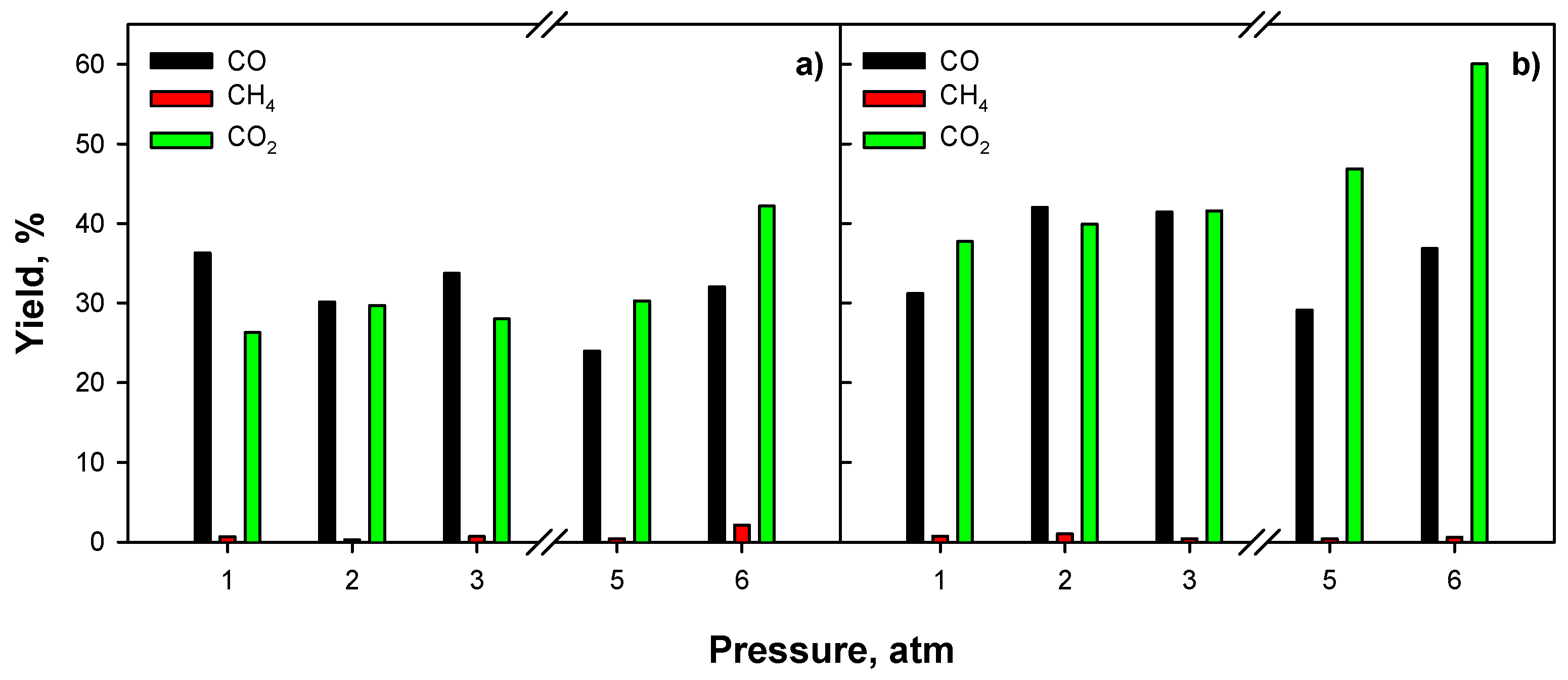
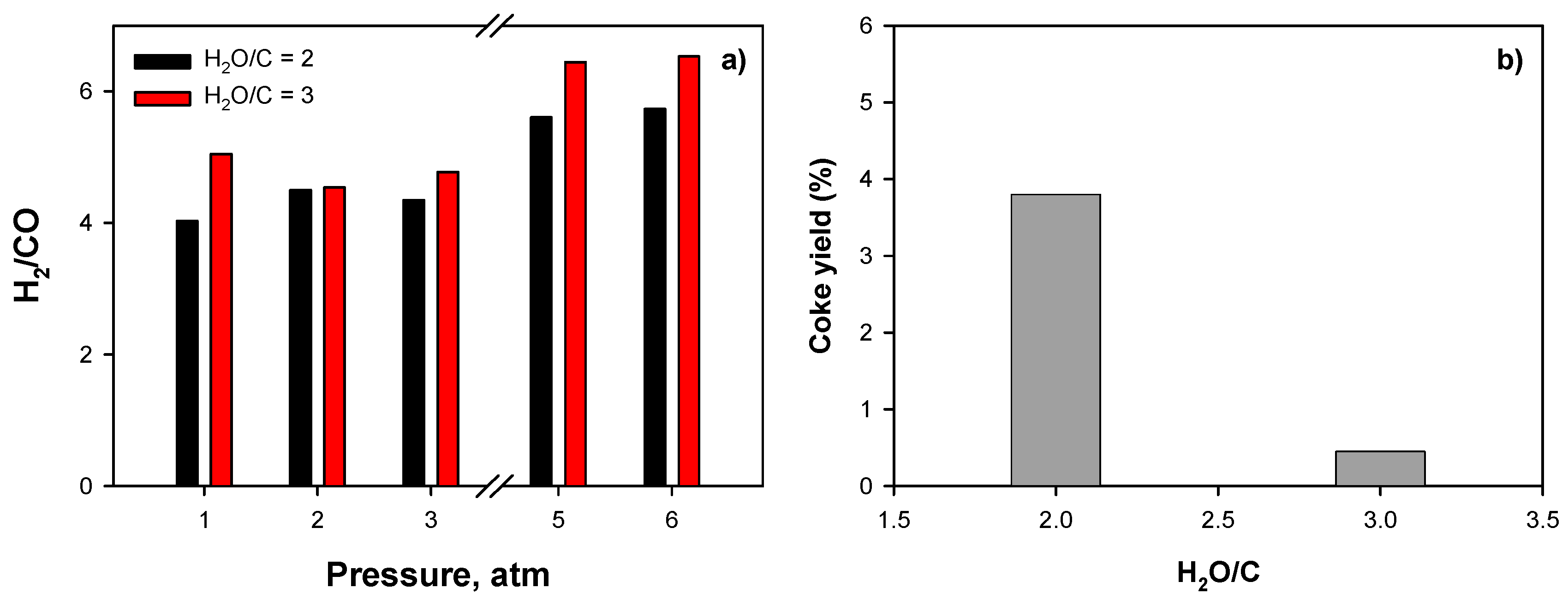
2.3.3. Effect of Operating Conditions: AZ Mixture
3. Discussion
| Ethanol steam reforming | C2H5OH + H2O → 2CO + 4H2 | (1) |
| Water gas shift | CO +H2O ↔ CO2 + H2 | (2) |
| Ethanol dehydrogenation | C2H5OH → C2H4O + H2 | (3) |
| Ethanol dehydration to ethylene | C2H5OH → C2H4 + H2O | (4) |
| Ethylene decomposition | C2H4→ 2C + 2H2 | (5) |
| Acetaldehyde decomposition | C2H4O → CH4 + CO | (6) |
| Acetaldehyde steam reforming | CH3CHO + H2O → 2CO + 3H2 | (7) |
| Ethanol water oxidation | C2H5OH + H2O → CH3COOH + 2H2 | (8) |
| Ethanol decomposition | C2H5OH → CH4 + CO + H2 | (9) |
| Acetadehyde recombination | 2CH3CHO→ CH3COCH3 + CO + H2 | (10) |
| Acetone steam reforming | CH3COCH3 + 3H2O → 3CO + 6H2 | (11) |
| Methane steam reforming | CH4 + H2O ↔ CO + 3H2 | (12) |
| Methane dry reforming | CH4 + CO2 ↔ 2CO + 2H2 | (13) |
| CO methanation | CO + 3H2 ↔ CH4 + H2O | (14) |
| Acetic acid steam reforming | CH3COOH + H2O → 2CO + 3H2 | (15) |
| Ketonization | 2CH3COOH → CH3COCH3 + CO2 + H2O | (16) |
| Acetic acid decomposition (1) | CH3COOH → CH4 + CO2 | (17) |
| Acetic acid decomposition (2) | CH3COOH → 2H2 + CO2 + C | (18) |
| Dehydration | CH3COOH → CH2CO + H2O | (19) |
| Ketene steam reforming | CH2CO + H2O → 2CO + 2H2 | (20) |
| Ketene coupling | 2CH2CO → C2H4 + 2CO | (21) |
| Acetone aldol condensation | 2CH3COCH3 → H2O + (CH3)2CCHCOCH3 | (22) |
| Condensation | (CH3)2CCHCOCH3 + CH3COCH3 → C9H12 + 2H2O | (23) |
| Coke formation | C9H12 → Coke | (24) |
| Ethyl acetate reforming | C4H8O2 + 2H2O → 4 CO + 6 H2 | (25) |
| Ethyl acetate hydrogenation | C4H8O2 + H2 → CH3CHO + CH3CH2OH | (26) |
| Ethyl acetate decomposition | C4H8O2 → 2 CH4 + 2 CO | (27) |
| Boudouard reaction | 2CO ↔ CO2 + C | (28) |
| Reverse gasification | CO + H2 ↔ H2O + C | (29) |
| Methane decomposition | CH4 ↔ C+ 2H2 | (30) |
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smithers Pira Packaging Industry Reports|Market Trends Analysis|Smithers Pira. Available online: https://www.smitherspira.com/industry-market-reports/packaging/the-future-of-global-packaging-to-2022 (accessed on 19 June 2019).
- Schlegelmilch, M.; Streese, J.; Stegmann, R. Odour management and treatment technologies: An overview. Waste Manag. 2005, 25, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Henning, K.D. Solvent Recycling, Removal and Degradation. Handb. Solvents 2014, 2, 787–861. [Google Scholar]
- Landi, G.; Sarli, V.; Di Benedetto, A.; Di Berardini, F.; Mensitieri, M. Catalytic Combustion of Waste Streams Coming from the Solvent Recovery Stage of a Packaging Industry. J. Appl. Packag. Res. 2016, 8, 3. [Google Scholar]
- Compagnoni, M.; Tripodi, A.; Rossetti, I. Parametric study and kinetic testing for ethanol steam reforming. Appl. Catal. B Environ. 2017, 203, 899–909. [Google Scholar] [CrossRef]
- Konsolakis, M.; Ioakimidis, Z.; Kraia, T.; Marnellos, G. Hydrogen Production by Ethanol Steam Reforming (ESR) over CeO2 Supported Transition Metal (Fe, Co, Ni, Cu) Catalysts: Insight into the Structure-Activity Relationship. Catalysts 2016, 6, 39. [Google Scholar] [CrossRef]
- Moraes, T.S.; Rabelo Neto, R.C.; Ribeiro, M.C.; Mattos, L.V.; Kourtelesis, M.; Ladas, S.; Verykios, X.; Noronha, F.B. Ethanol conversion at low temperature over CeO2-Supported Ni-based catalysts. Effect of Pt addition to Ni catalyst. Appl. Catal. B Environ. 2016, 181, 754–768. [Google Scholar] [CrossRef]
- Goicoechea, S.; Ehrich, H.; Arias, P.L.; Kockmann, N. Thermodynamic analysis of acetic acid steam reforming for hydrogen production. J. Power Sources 2015, 279, 312–322. [Google Scholar] [CrossRef]
- Palma, V.; Castaldo, F.; Ciambelli, P.; Iaquaniello, G. CeO2-supported Pt/Ni catalyst for the renewable and clean H2 production via ethanol steam reforming. Appl. Catal. B Environ. 2014, 145, 73–84. [Google Scholar] [CrossRef]
- Yang, H.C.; Lee, M.W.; Hwang, H.S.; Moon, J.K.; Chung, D.Y. Study of cerium-promoted rhodium alumina catalyst as a steam reforming catalyst for treatment of spent solvents. J. Rare Earths 2014, 32, 831–836. [Google Scholar] [CrossRef]
- Le Valant, A.; Garron, A.; Bion, N.; Duprez, D.; Epron, F. Effect of higher alcohols on the performances of a 1%Rh/MgAl2O4/Al2O3 catalyst for hydrogen production by crude bioethanol steam reforming. Int. J. Hydrogen Energy 2011, 36, 311–318. [Google Scholar] [CrossRef]
- Hu, X.; Lu, G. Investigation of the steam reforming of a series of model compounds derived from bio-oil for hydrogen production. Appl. Catal. B Environ. 2009, 88, 376–385. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Lei, J.; Jin, H. Numerical investigation of the thermophysical characteristics of the mid-and-low temperature solar receiver/reactor for hydrogen production. Int. J. Heat Mass Transf. 2016, 97, 379–390. [Google Scholar] [CrossRef]
- Sheu, E.J.; Mokheimer, E.M.A.; Ghoniem, A.F. A review of solar methane reforming systems. Int. J. Hydrogen Energy 2015, 40, 12929–12955. [Google Scholar] [CrossRef]
- Yadav, D.; Banerjee, R. A review of solar thermochemical processes. Renew. Sustain. Energy Rev. 2016, 54, 497–532. [Google Scholar] [CrossRef]
- Agrafiotis, C.C.; Pagkoura, C.; Lorentzou, S.; Kostoglou, M.; Konstandopoulos, A.G. Hydrogen production in solar reactors. Catal. Today 2007, 127, 265–277. [Google Scholar] [CrossRef]
- Fend, T.; Pitz-Paal, R.; Reutter, O.; Bauer, J.; Hoffschmidt, B. Two novel high-porosity materials as volumetric receivers for concentrated solar radiation. Sol. Energy Mater. Sol. Cells 2004, 84, 291–304. [Google Scholar] [CrossRef]
- Wang, F.; Tan, J.; Shuai, Y.; Gong, L.; Tan, H. Numerical analysis of hydrogen production via methane steam reforming in porous media solar thermochemical reactor using concentrated solar irradiation as heat source. Energy Convers. Manag. 2014, 87, 956–964. [Google Scholar] [CrossRef]
- Bai, F. One dimensional thermal analysis of silicon carbide ceramic foam used for solar air receiver. Int. J. Therm. Sci. 2010, 49, 2400–2404. [Google Scholar] [CrossRef]
- Fend, T.; Hoffschmidt, B.; Pitz-Paal, R.; Reutter, O.; Rietbrock, P. Porous materials as open volumetric solar receivers: Experimental determination of thermophysical and heat transfer properties. Energy 2004, 29, 823–833. [Google Scholar] [CrossRef]
- Arellano-López, A.R.; Martínez-Fernández, J.; González, P.; Domínguez, C.; Fernández-Quero, V.; Singh, M. Biomorphic SiC: A New Engineering Ceramic Material. Int. J. Appl. Ceram. Technol. 2005, 1, 56–67. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Zhang, H. Computation of radar absorbing silicon carbide foams and their silica matrix composites. Comput. Mater. Sci. 2007, 38, 857–864. [Google Scholar] [CrossRef]
- Agrafiotis, C.C.; Mavroidis, I.; Konstandopoulos, A.G.; Hoffschmidt, B.; Stobbe, P.; Romero, M.; Fernandez-Quero, V. Evaluation of porous silicon carbide monolithic honeycombs as volumetric receivers/collectors of concentrated solar radiation. Sol. Energy Mater. Sol. Cells 2007, 91, 474–488. [Google Scholar] [CrossRef]
- Basagiannis, A.C.; Verykios, X.E. Catalytic steam reforming of acetic acid for hydrogen production. Int. J. Hydrogen Energy 2007, 32, 3343–3355. [Google Scholar] [CrossRef]
- Vagia, E.C.; Lemonidou, A.A. Hydrogen production via steam reforming of bio-oil components over calcium aluminate supported nickel and noble metal catalysts. Appl. Catal. A Gen. 2008, 351, 111–121. [Google Scholar] [CrossRef]
- Fatsikostas, A.N.; Verykios, X.E. Reaction network of steam reforming of ethanol over Ni-based catalysts. J. Catal. 2004, 225, 439–452. [Google Scholar] [CrossRef]
- Barattini, L.; Ramis, G.; Resini, C.; Busca, G.; Sisani, M.; Costantino, U. Reaction path of ethanol and acetic acid steam reforming over Ni-Zn-Al catalysts. Flow reactor studies. Chem. Eng. J. 2009, 153, 43–49. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Spadaro, L.; Chiodo, V.; Bonura, G.; Donato, S.; Cavallaro, S. H2 production for MC fuel cell by steam reforming of ethanol over MgO supported Pd, Rh, Ni and Co catalysts. Catal. Commun. 2004, 5, 611–615. [Google Scholar] [CrossRef]
- Comas, J.; Marino, F.; Laborde, M.; Amadeo, N. Bio-ethanol steam reforming on Ni/Al2O3 catalyst. Chem. Eng. J. 2004, 98, 61–68. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Chiodo, V.; Spadaro, L.; Di Blasi, O.; Bonura, G.; Cavallaro, S. Steam reforming of bio-ethanol on alkali-doped Ni/MgO catalysts: Hydrogen production for MC fuel cell. Appl. Catal. A Gen. 2004, 270, 1–7. [Google Scholar] [CrossRef]
- Moraes, T.S.; Neto, R.C.R.; Ribeiro, M.C.; Mattos, L.V.; Kourtelesis, M.; Verykios, X.; Noronha, F.B. Effects of ceria morphology on catalytic performance of Ni/CeO2 catalysts for low temperature steam reforming of ethanol. Top. Catal. 2015, 58, 281–294. [Google Scholar] [CrossRef]
- Italiano, C.; Bizkarra, K.; Barrio, V.L.; Cambra, J.F.; Pino, L.; Vita, A. Renewable hydrogen production via steam reforming of simulated bio-oil over Ni-based catalysts. Int. J. Hydrogen Energy 2019, 44, 14671–14682. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Vita, A.; Italiano, C.; Fabiano, C.; Huang, Y.; Basile, A. The oncoming energy vector: Hydrogen produced in Pd-composite membrane reactor via bioethanol reforming over Ni/CeO2 catalyst. Catal. Today 2016, 259, 368–375. [Google Scholar] [CrossRef]
- Basagiannis, A.C.; Verykios, X.E. Influence of the carrier on steam reforming of acetic acid over Ru-based catalysts. Appl. Catal. B Environ. 2008, 82, 77–88. [Google Scholar] [CrossRef]
- Matas Güell, B.; Babich, I.; Nichols, K.P.; Gardeniers, J.G.E.; Lefferts, L.; Seshan, K. Design of a stable steam reforming catalyst-A promising route to sustainable hydrogen from biomass oxygenates. Appl. Catal. B Environ. 2009, 90, 38–44. [Google Scholar] [CrossRef]
- Takanabe, K.; Aika, K.I.; Seshan, K.; Lefferts, L. Sustainable hydrogen from bio-oil—Steam reforming of acetic acid as a model oxygenate. J. Catal. 2004, 227, 101–108. [Google Scholar] [CrossRef]
- Liguras, D.K.; Kondarides, D.I.; Verykios, X.E. Production of hydrogen for fuel cells by steam reforming of ethanol over supported noble metal catalysts. Appl. Catal. B Environ. 2003, 43, 345–354. [Google Scholar] [CrossRef]
- Casanovas, A.; Llorca, J.; Homs, N.; Fierro, J.L.G.; Ramírez de la Piscina, P. Ethanol reforming processes over ZnO-supported palladium catalysts: Effect of alloy formation. J. Mol. Catal. A Chem. 2006, 250, 44–49. [Google Scholar] [CrossRef]
- Cavallaro, S.; Chiodo, V.; Freni, S.; Mondello, N.; Frusteri, F. Performance of Rh/Al2O3 catalyst in the steam reforming of ethanol: H2 production for MCFC. Appl. Catal. A Gen. 2003, 249, 119–128. [Google Scholar] [CrossRef]
- Barbato, P.S.; Di Benedetto, A.; Landi, G.; Lisi, L. CuO/CeO2 based monoliths for CO preferential oxidation in H2-rich streams. Chem. Eng. J. 2015, 279, 983–993. [Google Scholar] [CrossRef]
- Landi, G.; Barbato, P.S.; Di Benedetto, A.; Lisi, L. Optimization of the preparation method of CuO/CeO2 structured catalytic monolith for CO preferential oxidation in H2-rich streams. Appl. Catal. B Environ. 2016, 181, 727–737. [Google Scholar] [CrossRef]
- Bimbela, F.; Oliva, M.; Ruiz, J.; García, L.; Arauzo, J. Hydrogen production by catalytic steam reforming of acetic acid, a model compound of biomass pyrolysis liquids. J. Anal. Appl. Pyrolysis 2007, 79, 112–120. [Google Scholar] [CrossRef]
- Trane, R.; Dahl, S.; Skjøth-Rasmussen, M.S.; Jensen, A.D. Catalytic steam reforming of bio-oil. Int. J. Hydrogen Energy 2012, 37, 6447–6472. [Google Scholar] [CrossRef]
- Xie, H.; Yu, Q.; Yao, X.; Duan, W.; Zuo, Z.; Qin, Q. Hydrogen production via steam reforming of bio-oil model compounds over supported nickel catalysts. J. Energy Chem. 2015, 24, 299–308. [Google Scholar] [CrossRef]
- Fierro, V.; Klouz, V.; Akdim, O.; Mirodatos, C. Oxidative reforming of biomass derived ethanol for hydrogen production in fuel cell applications. Catal. Today 2002, 75, 141–144. [Google Scholar] [CrossRef]
- Fierro, V.; Akdim, O.; Provendier, H.; Mirodatos, C. Ethanol oxidative steam reforming over Ni-based catalysts. J. Power Sources 2005, 145, 659–666. [Google Scholar] [CrossRef]
- Xue, Z.; Shen, Y.; Li, P.; Zhang, Y.; Li, J.; Qin, B.; Zhang, J.; Zeng, Y.; Zhu, S. Key Role of Lanthanum Oxychloride: Promotional Effects of Lanthanum in NiLaOy/NaCl for Hydrogen Production from Ethyl Acetate and Water. Small 2018, 14, 1800927. [Google Scholar] [CrossRef] [PubMed]
- Ruhswurmova, N.; Kim, S.; Yoo, J.; Chun, D.; Rhim, Y.; Lim, J.; Kim, S.; Choi, H.; Lee, S. Nickel supported on low-rank coal for steam reforming of ethyl acetate. Int. J. Hydrogen Energy 2018, 43, 15880–15890. [Google Scholar] [CrossRef]
- Xue, Z.; Shen, Y.; Li, P.; Zhang, Y.; Zeng, Y.; Zhu, S. Controllable synthesis of carbon nanotubes via autothermal reforming of ethyl acetate. Mater. Des. 2018, 141, 150–158. [Google Scholar] [CrossRef]
- Baviskar, C.V.; Vaidya, P.D. Steam reforming of model bio-oil compounds 2-butanone, 1-methoxy-2-propanol, ethyl acetate and butyraldehyde over Ni/Al2O. Int. J. Hydrogen Energy 2017, 42, 21667–21676. [Google Scholar] [CrossRef]
- Xue, Z.; Shen, Y.; Zhu, S.; Li, P.; Zeng, Y.; Xi, Z.; Cai, Y. Autothermal reforming of ethyl acetate for hydrogen production over Ni3La7Oy/Al2O3 catalyst. Energy Convers. Manag. 2017, 146, 34–42. [Google Scholar] [CrossRef]
- Fishtik, I.; Alexander, A.; Datta, R.; Geana, D. Thermodynamic analysis of hydrogen production by steam reforming of ethanol via response reactions. Int. J. Hydrogen Energy 2000, 25, 31–45. [Google Scholar] [CrossRef]
- Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. Production of Hydrogen from Ethanol: Review of Reaction Mechanism and Catalyst Deactivation. Chem. Rev. 2012, 112, 4094–4123. [Google Scholar] [CrossRef] [PubMed]
- Barbato, P.S.; Di Benedetto, A.; Di Sarli, V.; Landi, G.; Pirone, R. High-pressure methane combustion over a perovskyte catalyst. Ind. Eng. Chem. Res. 2012, 51, 7547–7558. [Google Scholar] [CrossRef]

| Sample | Nominal Ni Content | Ceria Source |
|---|---|---|
| 5 Ni | 5 | Powder + colloidal |
| 10 Ni | 10 | Powder + colloidal |
| 15 Ni | 15 | Powder + colloidal |
| 10 Ni-C | 10 | Colloidal |
| Compound | Azeotropic | High-Boiling Point |
|---|---|---|
| Cyclohexane | 0.35 | - |
| Acetone | 0.61 | - |
| Ethyl acetate | 52.88 | 8.15 |
| Isopropyl acetate | 0.04 | 0.43 |
| Isopropanol | 1.39 | - |
| Ethanol | 44.29 | 0.04 |
| n-propyl acetate | 0.38 | 86.97 |
| n-propanol | 0.05 | 0.20 |
| Acetic acid | - | 2.92 |
| 1-metoxy 2-propanol | - | 0.57 |
| 1-metoxy propyl acetate | - | 0.53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landi, G.; Di Benedetto, A. Ni/CeO2 Structured Catalysts for Solar Reforming of Spent Solvents. Catalysts 2019, 9, 688. https://doi.org/10.3390/catal9080688
Landi G, Di Benedetto A. Ni/CeO2 Structured Catalysts for Solar Reforming of Spent Solvents. Catalysts. 2019; 9(8):688. https://doi.org/10.3390/catal9080688
Chicago/Turabian StyleLandi, Gianluca, and Almerinda Di Benedetto. 2019. "Ni/CeO2 Structured Catalysts for Solar Reforming of Spent Solvents" Catalysts 9, no. 8: 688. https://doi.org/10.3390/catal9080688
APA StyleLandi, G., & Di Benedetto, A. (2019). Ni/CeO2 Structured Catalysts for Solar Reforming of Spent Solvents. Catalysts, 9(8), 688. https://doi.org/10.3390/catal9080688