Zinc-Based Curing Activators: New Trends for Reducing Zinc Content in Rubber Vulcanization Process
Abstract
1. Introduction
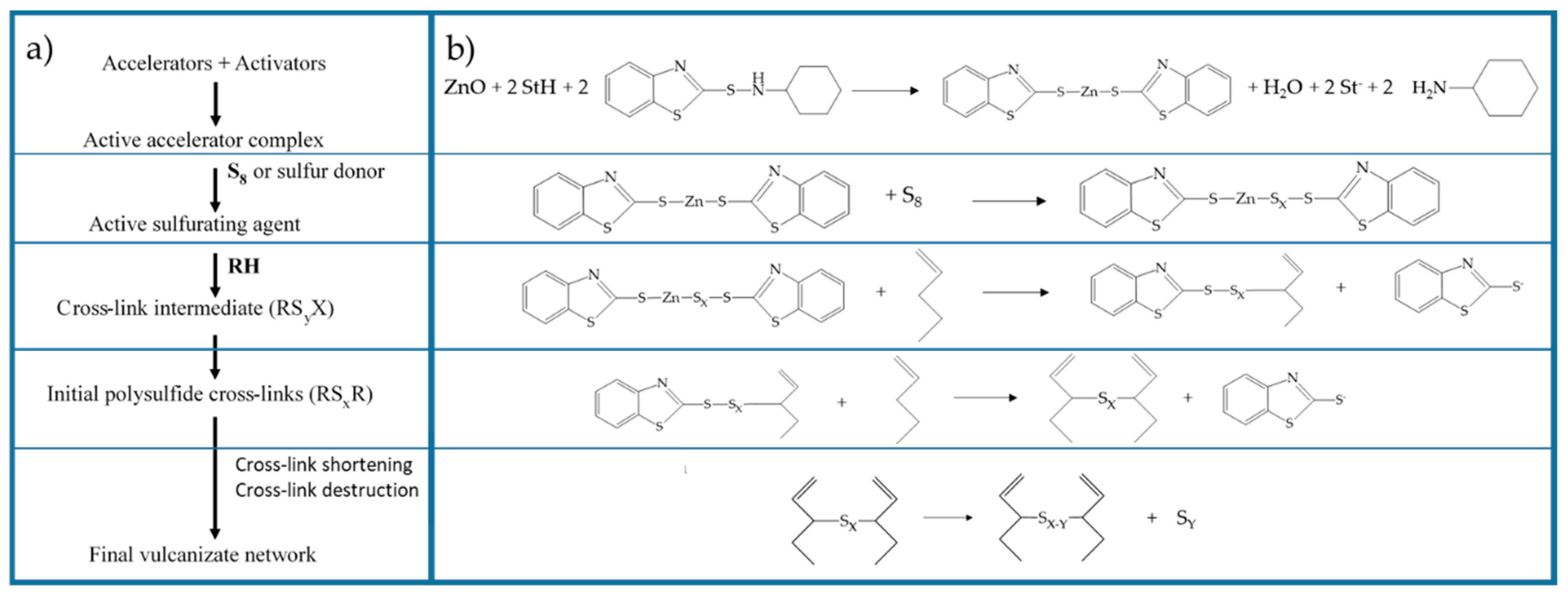
2. Rubber Vulcanization
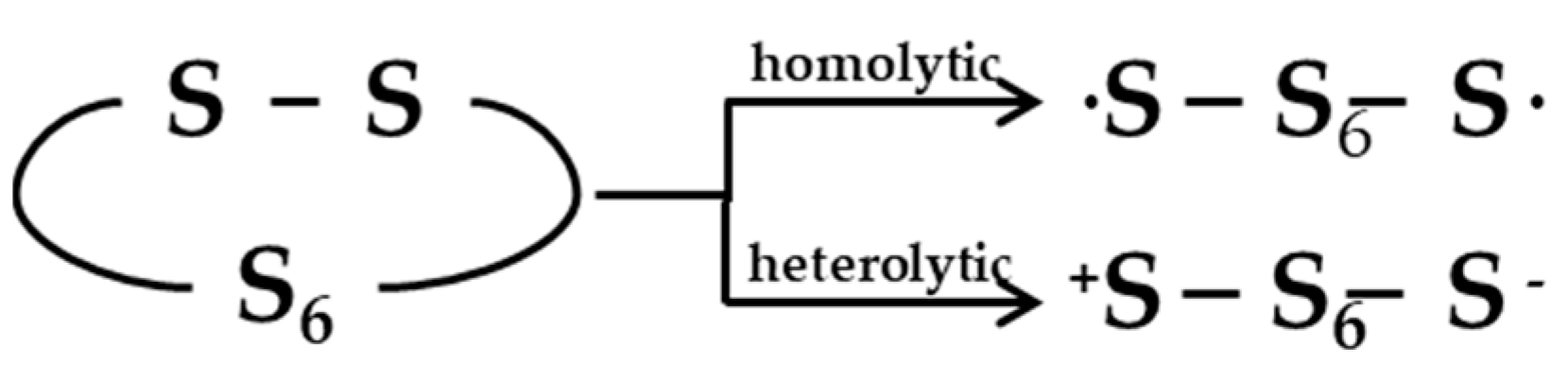
2.1. Vulcanization Mechanism
2.2. Activator and Co-Activator
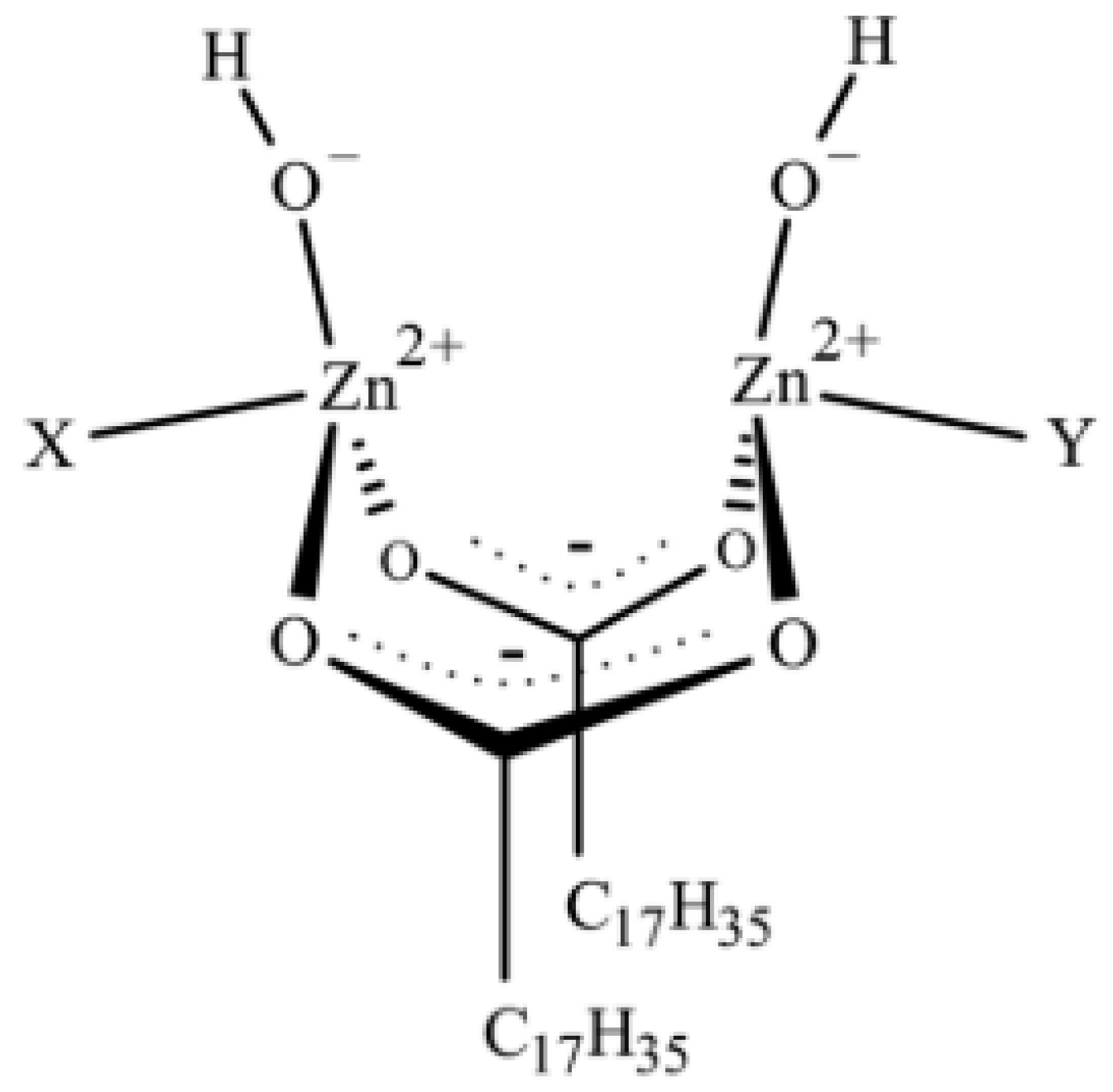
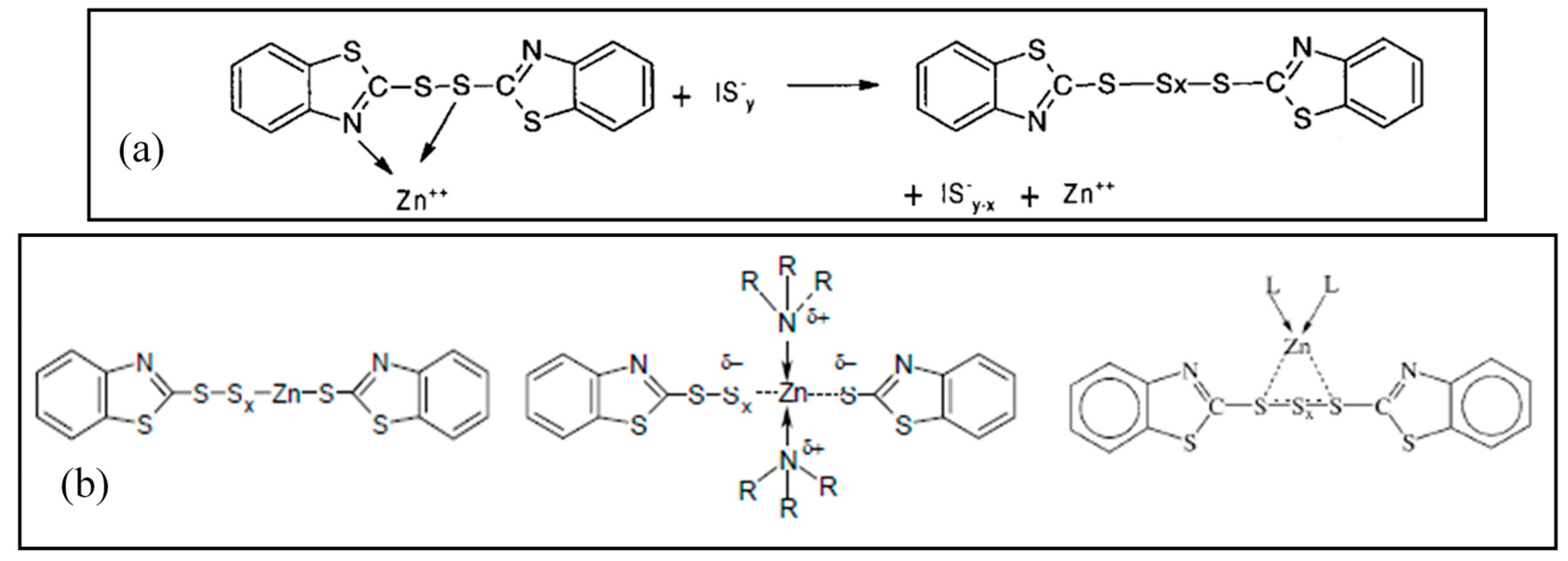
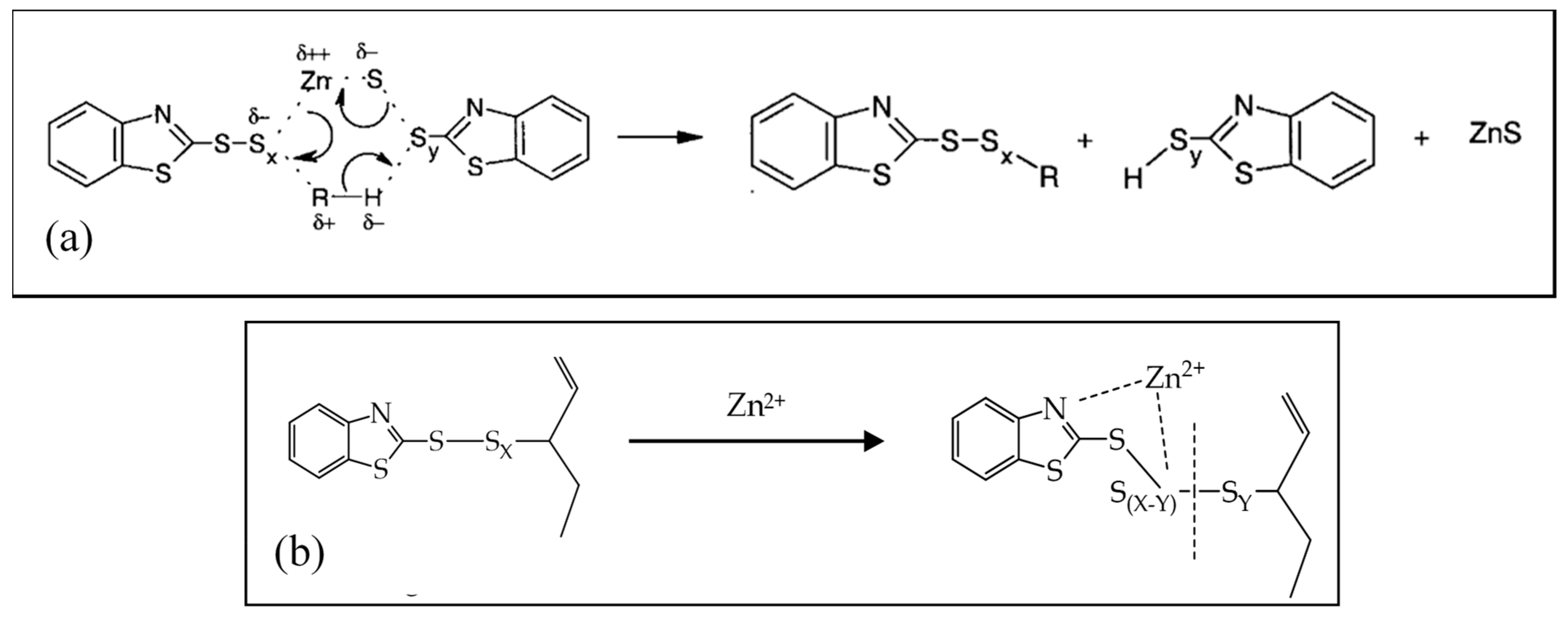
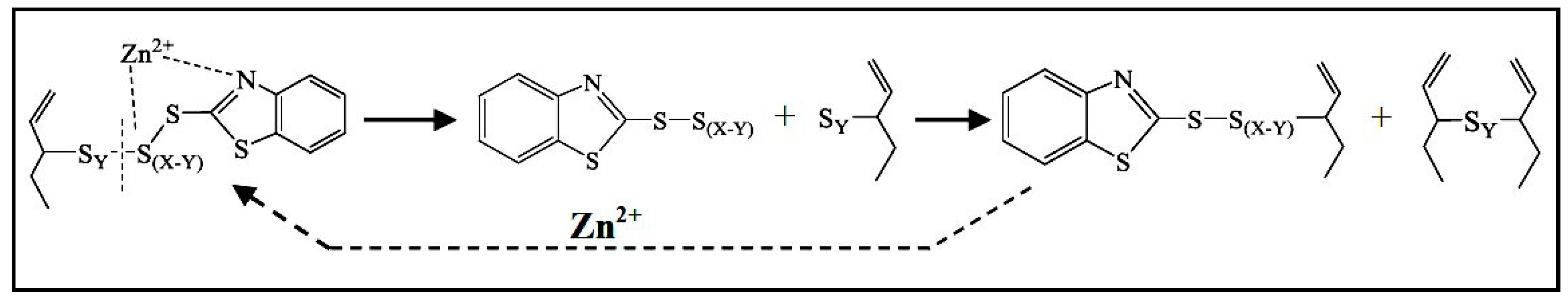
2.3. Active Accelerator Complex and Active Sulfurating Agent
2.4. Cross-Link Intermediates
2.5. Cross-Linked Products
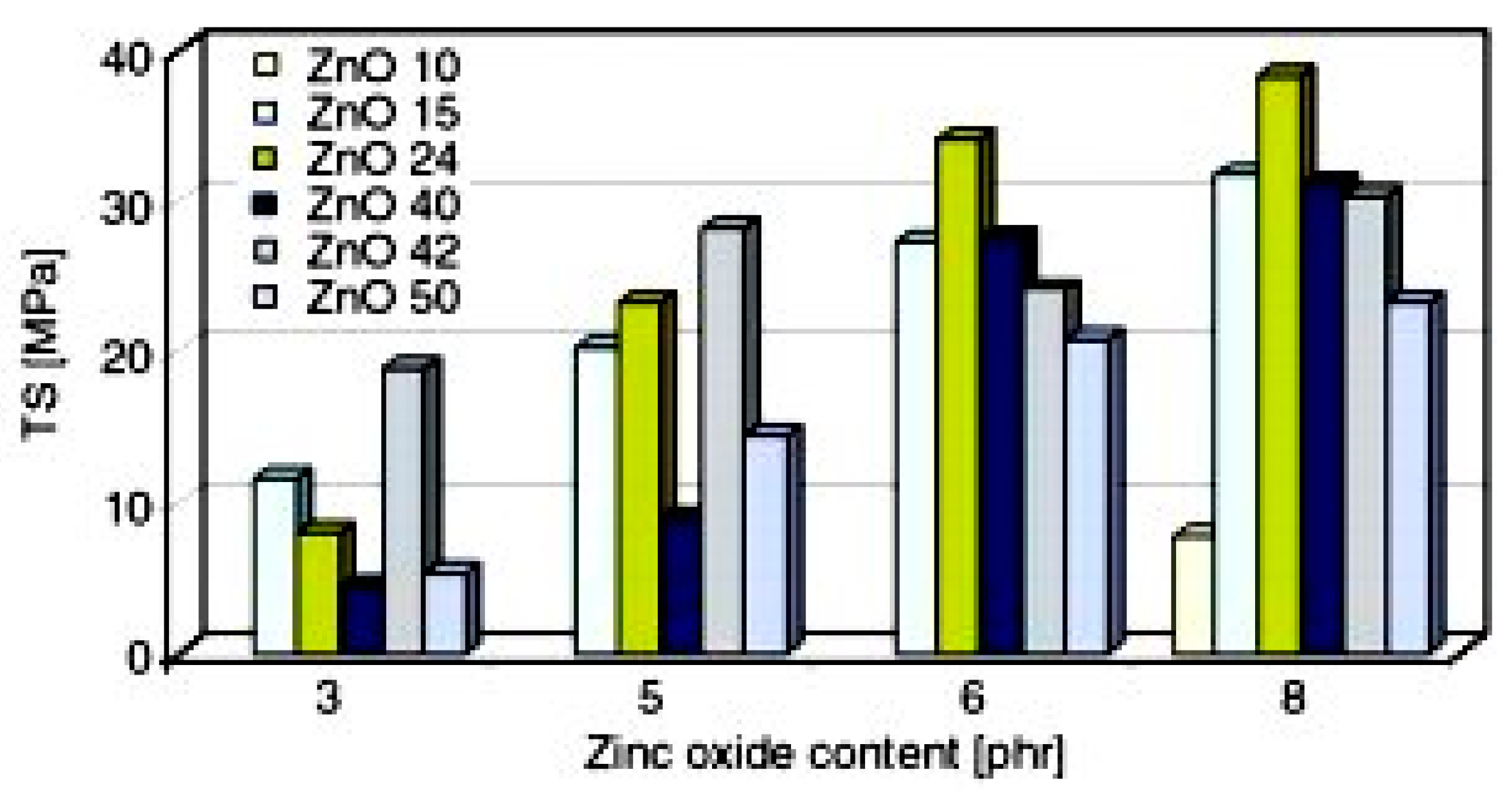
3. Nano vs. Micro Zinc Oxide
4. Zinc(II) Complexes
5. Homogeneous Distribution of Curing Agents
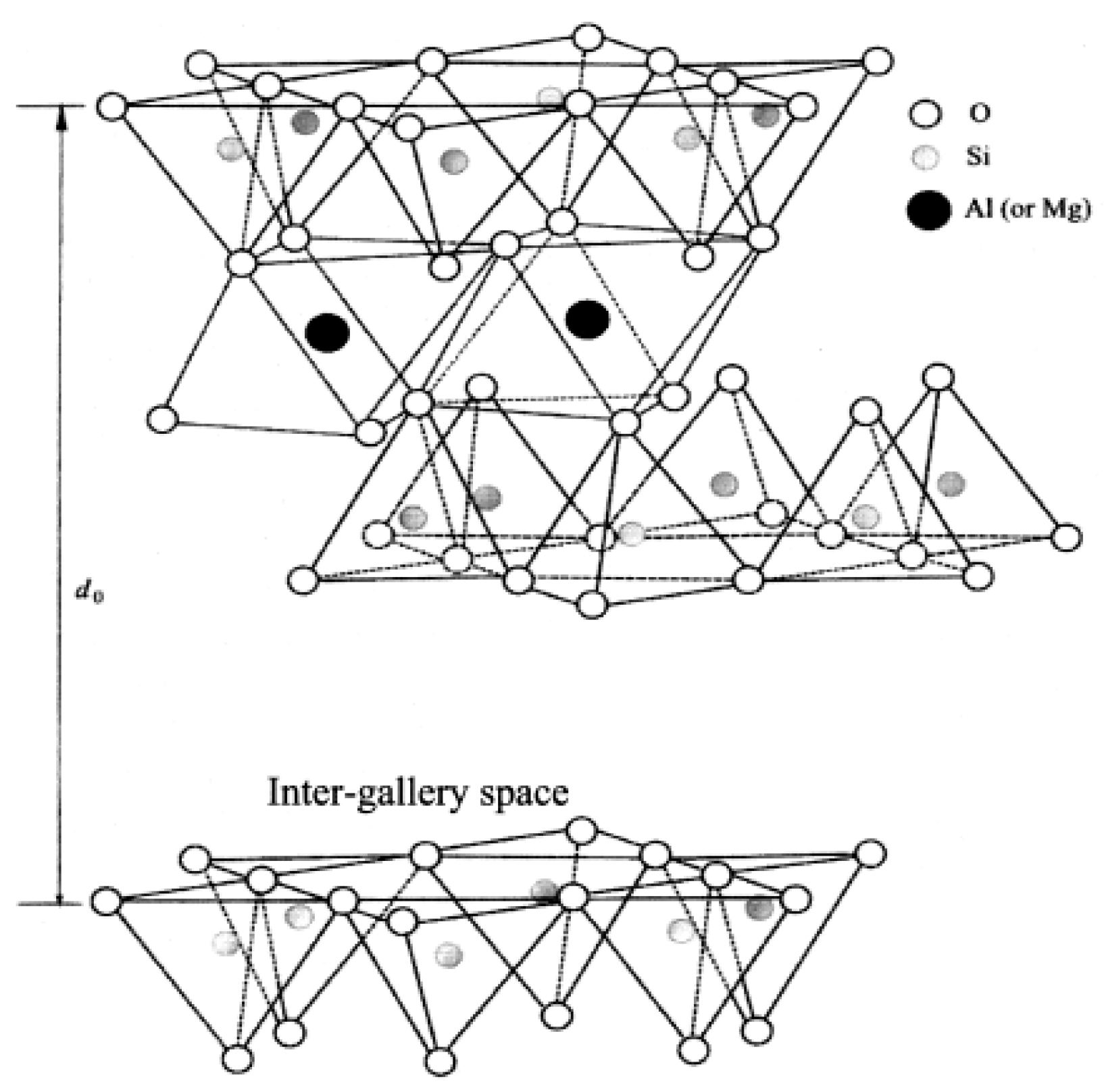
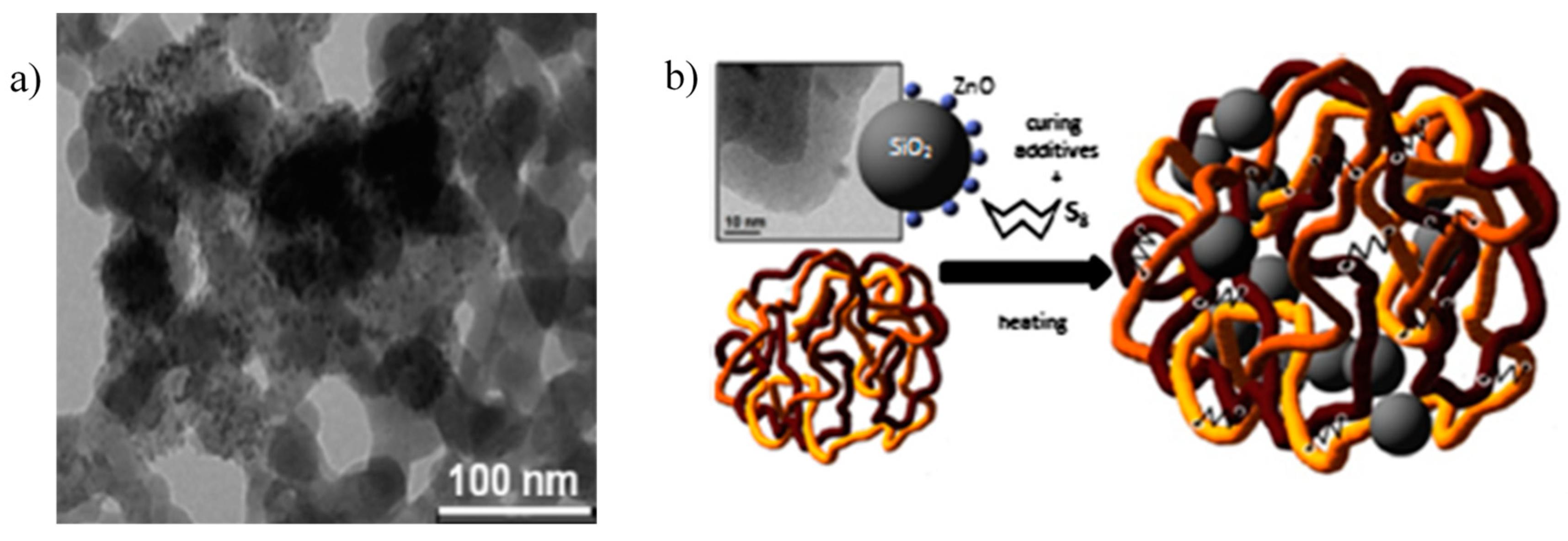
5.1. Zinc-Based Activators Inserted in Porous Materials or Supported on a Substrate
6. Conclusions
- -
- tailored nanosized ZnO particles to achieve a better distribution and availability of the activator in rubber, thanks to their high surface area;
- -
- Zn(II) complexes to favor the interaction of already formed zinc centers with the curing agents, and thus to generate the sulfurating intermediate complexes active into vulcanization process;
- -
- zinc-based activators constituted by Zn2+ ions inserted in porous materials (e.g., clays) or ZnO NPs distributed through the addition of dispersing agents, to improve the availability of the zinc centers to react with the curatives; and,
- -
- supported Zn-based activators, consisting of ZnO NPs onto the surface of different supports (e.g., silica, graphene) and in particular ZnO/SiO2 double function filler, simultaneously behaving as rubber reinforcing filler and curing activator.
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles—A review. Environ. Pollut. 2013, 172, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Vaseem, M.; Umar, A.; Hahn, Y. ZnO Nanoparticles: Growth, Properties, and Applications. J. Mater. Chem. 1988, 22, 6526–6535. [Google Scholar]
- Carotta, M.C.; Cervi, A.; Natale, V.; Gherardi, S.; Giberti, A.; Guidi, V.; Puzzovio, D.; Vendemiati, B.; Martinelli, G.; Sacerdoti, M.; et al. ZnO gas sensors: A comparison between nanoparticles and nanotetrapods-based thick films. Sens. Actuators B Chem. 2009, 137, 164–169. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Sensors and Actuators a: Physical Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Haarindraprasad, R.; Hashim, U.; Gopinath, S.C.B.; Kashif, M.; Veeradasan, P.; Balakrishnan, S.R.; Foo, K.L.; Poopalan, P. Low Temperature Annealed Zinc Oxide Nanostructured Thin Film-Based Transducers: Characterization for Sensing Applications. PLoS ONE 2015, 10, e0132755. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- French, S.A.; Sokol, A.A.; Bromley, S.T.; Catlow, C.R.A.; Rogers, S.C.; King, F.; Sherwood, P. From CO2 to methanol by hybrid QM/MM embedding. Angew. Chem. Int. Ed. 2001, 40, 4437–4440. [Google Scholar] [CrossRef]
- Natta, G. Synthesis of methanol. Catalysis 1955, 3, 349–411. [Google Scholar]
- Park, S.W.; Joo, O.S.; Jung, K.D.; Kim, H.; Han, S.H. Development of ZnO/Al2O3 catalyst for reverse-water-gas-shift reaction of CAMERE (carbon dioxide hydrogenation to form methanol via a reverse-water-gas-shift reaction) process. Appl. Catal. A Gen. 2001, 211, 81–90. [Google Scholar] [CrossRef]
- Shido, T.; Yamaguchi, A.; Asakura, K.; Iwasawa, Y. Surface catalytic reactions assisted by gas phase molecules: Activation of reaction intermediates. J. Mol. Catal. A Chem. 2000, 163, 67–77. [Google Scholar] [CrossRef]
- Shido, T.; Iwasawa, Y. Reactant-promoted reaction mechanism for water-gas shift reaction on ZnO, as the genesis of surface catalysis. J. Catal. 1991, 129, 343–355. [Google Scholar] [CrossRef]
- Ueno, A.; Yamamoto, T.; Onishi, T.; Tamaru, K. The Behaviour of Chemisorbed Species under the Reaction Conditions and the Mechanism of the Water-gas Shift Reaction on ZnO and MgO. Bull. Chem. Soc. Jpn. 1969, 42, 3040. [Google Scholar] [CrossRef]
- Shishido, T.; Yamamoto, Y.; Morioka, H.; Takaki, K.; Takehira, K. Active Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation method in steam reforming of methanol. Appl. Catal. A Gen. 2004, 263, 249–253. [Google Scholar] [CrossRef]
- Chin, Y.; Dagle, R.; Hu, J.; Dohnalkova, A.C.; Wang, Y. Steam reforming of methanol over highly active Pd/ZnO catalyst. Catal. Today 2002, 77, 79–88. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Pargoletti, E.; Mostoni, S.; Rassu, G.; Pifferi, V.; Meroni, D.; Falciola, L.; Davoli, E.; Marelli, M.; Cappelletti, G. Zn- vs Bi-based oxides for o-toluidine photocatalytic treatment under solar light. Env. Sci. Pollut. Res. 2017, 24, 8287–8296. [Google Scholar] [CrossRef]
- IRSG. International rubber study group Statistical summary of world rubber situation. Rubber Stat. Bull. 2017. Available online: http://www.rubberstudy.com/documents/Sample%20Report/Sample%20RSB%20Report.pdf (accessed on 1 June 2017).
- Perl, A. Annual Minerals Review. Am. Ceram. Soc. Bull. 1997, 76, 140. [Google Scholar]
- International Zinc Association-Zinc Oxide Information Center. Available online: http://www.znoxide.org/index.html (accessed on 1 April 1997).
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185, 1–22. [Google Scholar] [CrossRef]
- Das, A.; Wang, D.-Y.; Leuteritz, A.; Subramaniam, K.; Greenwell, H.C.; Wagenknecht, U.; Heinrich, G. Preparation of zinc oxide free, transparent rubber nanocomposites using a layered double hydroxide filler. J. Mater. Chem. 2011, 21, 7194. [Google Scholar] [CrossRef]
- Cai, G.-R. Redefining Vulcanization. Technol. Cult. 2010, 51, 357–387. [Google Scholar]
- Coleman, M.M.; Shelton, J.R.; Koenig, J.L. Sulfur Vulcanization of Hydrocarbon Diene Elastomers. Ind. Eng. Chem. Prod. Res. Dev. 1974, 13, 154–166. [Google Scholar] [CrossRef]
- Ducháček, V.; Kuta, A.; Pribyl, P. Efficiency of metal activators of accelerated sulfur vulcanization. J. Appl. Polym. Sci. 1993, 47, 743–746. [Google Scholar] [CrossRef]
- Heideman, G. Reduced Zinc Oxide Levels in Sulphur Vulcanisation of Rubber Compounds. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2004. [Google Scholar]
- Krejsa, M.R.; Koenig, J.L. A Review of Sulfur Crosslinking Fundamentals for Accelerated and Unaccelerated Vulcanization. Rubber Chem. Technol. 1993, 66, 376–410. [Google Scholar] [CrossRef]
- Morrison, N.J.; Porter, M. Temperature effects on the stability of intermediates and crosslinks in sulfur vulcanization. Rubber Chem. Technol. 1984, 57, 63–85. [Google Scholar] [CrossRef]
- Councell, T.B.; Duckenfield, K.U.; Landa, E.R.; Callender, E. Tire-wear particles as a source of zinc to the environment. Environ. Sci. Technol. 2004, 38, 4206–4214. [Google Scholar] [CrossRef]
- Callender, E.; Rice, K.C. The urban environmental gradient: Anthropogenic influences on the spatial and temporal distributions of lead and zinc in sediments. Environ. Sci. Technol. 2000, 34, 232–238. [Google Scholar] [CrossRef]
- Santa Clara Valley Nonpoint Source Control Program. Santa Clara Valley Nonpoint Source Control Program, Source Identification and Control Report; Woodward-Clyde Consultants: Santa Clara, CA, USA, 1992. [Google Scholar]
- Wolfenden, L. Tyres Report; British Energy Agency: London, UK, 2001. [Google Scholar]
- Lander, L.; Lindström, L. Zinc in Society and the Environment: An Account of the Facts on Fluxes, Amounts and Effects of Zinc in Sweden; Swedish Environmental Research Group: Fryksta, Sweden, 1998. [Google Scholar]
- EPA. Environmental Protection Agency National Recommended Water Qaulity Criteria; EPA: Washington, DC, USA, 1999.
- EPA. Environmental Protection Agency National Secondary Drinking Water Regulations; EPA: Washington, DC, USA, 1984.
- Ramos, G.; Alguacil, F.J.; López, F.A. The recycling of end-of-life tyres. Technological review. Rev. Metal. 2011, 47, 273–284. [Google Scholar]
- Eldin, N.N.; Senouci, A.B. Rubber-tire particles as concrete aggregate. J. Mater. Civ. Eng. 1993, 5, 478–496. [Google Scholar] [CrossRef]
- Heideman, G.; Noordermeer, J.W.M.; Datta, R.N.; Van Baarle, B. Effect of metal oxides as activator for sulphur vulcanisation in various rubbers. KGK Kautsch. Gummi Kunstst. 2005, 58, 30–42. [Google Scholar]
- Lautenschlaeger, F.K.; Edwards, K. Model compound vulcanization—Part V. The effect of chemical additives and fillers. Rubber Chem. Technol. 1980, 53, 27–47. [Google Scholar] [CrossRef]
- Guzmán, M.; Vega, B.; Agulló, N.; Giese, U.; Borrós, S. Zinc oxide versus magnesium oxide revisited. Part 1. Rubber Chem. Technol. 2012, 85, 38–55. [Google Scholar] [CrossRef]
- Scotti, R.; D’Arienzo, M.; Di Credico, B.; Giannini, L.; Morazzoni, F. Silica–Polymer Interface and Mechanical Reinforcement in Rubber Nanocomposites. In Hybrid Organic-Inorganic Interfaces: Towards Advanced Functional Materials; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Barlow, C.; Jayasuriya, S.; Suan Tan, C. The World Rubber Industry; Routledge Revivals: Abingdon, UK, 1994. [Google Scholar]
- Garcés, J.M.; Moll, D.J.; Bicerano, J.; Fibiger, R.; McLeod, D.G. Polymeric nanocomposites for automotive applications. Adv. Mater. 2000, 12, 1835–1839. [Google Scholar] [CrossRef]
- Donnet, J.B. Nano and microcomposites of polymers elastomers and their reinforcement. Compos. Sci. Technol. 2003, 63, 1085–1088. [Google Scholar] [CrossRef]
- Di Credico, B.; Cobani, E.; Callone, E.; Conzatti, L.; Cristofori, D.; D’Arienzo, M.; Dirè, S.; Giannini, L.; Hanel, T.; Scotti, R.; et al. Size-controlled self-assembly of anisotropic sepiolite fibers in rubber nanocomposites. Appl. Clay Sci. 2018, 152, 51–64. [Google Scholar] [CrossRef]
- Di Credico, B.; Tagliaro, I.; Cobani, E.; Conzatti, L.; D’Arienzo, M.; Giannini, L.; Mascotto, S.; Scotti, R.; Stagnaro, P.; Tadiello, L. A Green Approach for Preparing High-Loaded Sepiolite/Polymer Biocomposites. Nanomaterials 2018, 9, 46. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Diré, S.; Redaelli, M.; Borovin, E.; Callone, E.; Di Credico, B.; Morazzoni, F.; Pegoretti, A.; Scotti, R. Unveiling the hybrid interface in polymer nanocomposites enclosing silsesquioxanes with tunable molecular structure: Spectroscopic, thermal and mechanical properties. J. Colloid Interface Sci. 2018, 512, 609–617. [Google Scholar] [CrossRef]
- Redaelli, M.; D’Arienzo, M.; Brus, J.; Di Credico, B.; Geppi, M.; Giannini, L.; Matejka, L.; Martini, F.; Panattoni, F.; Spirkova, M.; et al. On the key role of SiO2@POSS hybrid filler in tailoring networking and interfaces in rubber nanocomposites. Polym. Test. 2018, 65, 429–439. [Google Scholar] [CrossRef]
- Spence, D.; Ferry, J.D. Enhanced Polymerization and Depolymerization of Natural Rubber by Quinones and Related Compounds. Rubber Chem. Technol. 2011, 11, 47–56. [Google Scholar] [CrossRef]
- Scott, K.W.; Lorenz, O.; Parks, C.R. Network degradation accompanying the vulcanization of natural rubber with a sulfur–diphenylguanidine system. J. Appl. Polym. Sci. 1964, 8, 2909–2922. [Google Scholar] [CrossRef]
- Ghosh, P.; Katare, S.R.; Patkar, P.R.; Caruthers, J.M.; Venkatasubramanian, V.; Walker, K.A. Sulfur Vulcanization of Natural Rubber for Benzothiazole Accelerated Formulations: From reaction mechanisms to a rational kinetic model. Rubber Chem. Technol. 2003, 76, 592–693. [Google Scholar] [CrossRef]
- Reddy, C.M.; Quinn, J.G. Environmental chemistry of benzothiazeles derived from rubber. Environ. Sci. Technol. 1997, 31, 2847–2853. [Google Scholar] [CrossRef]
- Aprem, A.S.; Joseph, K.; Mathew, T.; Altstaedt, V.; Thomas, S. Studies on accelerated sulphur vulcanization of natural rubber using 1-phenyl-2, 4-dithiobiuret/tertiary butyl benzothiazole sulphenamide. Eur. Polym. J. 2003, 39, 1451–1460. [Google Scholar] [CrossRef]
- Nieuwenhuizen, P.J.; Reedijk, J.; van Duin, M.; McGill, W.J. Thiuram and Dithiocarbamate-Accelerated Sulfur Vulcanization from the Chemist’s Perspective; Methods, Materials and Mechanisms Reviewed. Rubber Chem. Technol. 2011, 70, 368–429. [Google Scholar] [CrossRef]
- Hernández, M.; Ezquerra, T.A.; Verdejo, R.; López-Manchado, M.A. Role of vulcanizing additives on the segmental dynamics of natural rubber. Macromolecules 2012, 45, 1070–1075. [Google Scholar] [CrossRef]
- Wolfe, J.R.; Pugh, T.L.; Killian, A.S. The Chemistry of Sulfur Curing. III. Effects of Zinc Oxide on the Mechanism of the Reaction of Cyclohexene with Sulfur. Rubber Chem. Technol. 2011, 41, 1329–1338. [Google Scholar] [CrossRef]
- Isayev, A.I.; Deng, J.S. Nonisothermal vulcanization of rubber compounds. Rubber Chem. Technol. 1987, 61, 340–361. [Google Scholar] [CrossRef]
- Ding, R.; Lenov, A.I.; Coran, A.Y. study of the vulcanization kinetics of an accelerated-sulfur SBR compound. Rubber Chem. Technol. 1995, 69, 81–91. [Google Scholar] [CrossRef]
- Milani, G.; Leroy, E.; Milani, F.; Deterre, R. Mechanistic modeling of reversion phenomenon in sulphur cured natural rubber vulcanization kinetics. Polym. Test. 2013, 32, 1052–1063. [Google Scholar] [CrossRef]
- Milani, G.; Milani, F. Closed form numerical approach for a kinetic interpretation of high-cis polybutadiene rubber vulcanization with sulphur. J. Math. Chem. 2017, 55, 552–583. [Google Scholar] [CrossRef]
- Mathew, A.P.; Packirisamy, S.; Thomas, S. Studies on the thermal stability of natural rubber/polystyrene interpenetrating polymer networks: Thermogravimetric analysis. Polym. Degrad. Stab. 2001, 72, 423–439. [Google Scholar] [CrossRef]
- Tiwari, M.; Noordermeer, J.W.M.; Dierkes, W.K.; van Ooij, W.J. Effect of Plasma Polymerization on the Performance of Silica in NBR, EPDM and NBR/EPDM Blends. Rubber Chem. Technol. 2011, 81, 276–296. [Google Scholar] [CrossRef]
- Coran, A.Y. Vulcanization. Part V. The formation of crosslinks in the system: Natural rubber-sulfur-MBT-zinc ion. Rubber Chem. Technol. 1964, 37, 679–688. [Google Scholar] [CrossRef]
- Norvez, S.; Ikeda, Y.; Higashitani, N.; Hijikata, K.; Kokubo, Y.; Morita, Y.; Shibayama, M.; Osaka, N.; Suzuki, T.; Endo, H.; et al. Vulcanization: New focus on a traditional technology by small-angle neutron scattering. Macromolecules 2009, 42, 2741–2748. [Google Scholar]
- Van den Berg, J.H.M.; Beulen, J.W.; Duynstee, E.F.J.; Nelissen, H.L. Model Vulcanization of EPDM Compounds—Part I: Structure Determination of Vulcanization Products from Ethylidene Norbornane. Rubber Chem. Technol. 1984, 57, 265–274. [Google Scholar] [CrossRef]
- Fujimoto, K.; Wataya, K. The study of polymers by high-temperature ATR spectroscopy. J. Appl. Polym. Sci. 1969, 13, 2513–2526. [Google Scholar] [CrossRef]
- Armstrong, R.T.; Little, J.R.; Doak, K.W. Chemistry of Sulfur-Olefin Reactions. Ind. Eng. Chem. 1944, 36, 628–633. [Google Scholar] [CrossRef]
- Choi, W. The Main Mechanism and Cross-Linking Structure for Accelerated Sulfur Vulcanization. e-J. Soft Mater. 2006, 2, 47–55. [Google Scholar] [CrossRef]
- Nieuwenhuizen, P.J.; Sandjaj, T.; Van Veen, J.M. Homogeneous zinc (II) catalysis in accelerated vulcanization I. Reaction-stage modeling and cross-link formation. Rubber Chem. Technol. 1998, 71, 750–765. [Google Scholar] [CrossRef]
- Damen, R.; Nieuwenhuizen, P.J.; Haasnoot, J.G.; Reedijk, J.; Couchman, S.M.; Jeffery, J.; McCleverty, J.A. Homogeneous zinc (II) catalysis in accelerated vulcanization: IV. The prevailing mechanism of crosslink formation in mercaptobenzothiazole systems. Rubber Chem. Technol. 2003, 76, 82–100. [Google Scholar] [CrossRef]
- Hahn, J.; Palloch, P.; Thelen, N.; Weidenhaupt, H.-J. Reversion Stable Networks with Polysulfide Polymers as Vulcanization Agents. Rubber Chem. Technol. 2011, 74, 28–43. [Google Scholar] [CrossRef]
- Heideman, G.; Datta, R.N.; Noordermeer, J.W.M.; Van Baarle, B. Influence of zinc oxide during different stages of sulfur vulcanization. elucidated by model compound studies. J. Appl. Polym. Sci. 2005, 95, 1388–1404. [Google Scholar] [CrossRef]
- Heideman, G.; Datta, R.N.; Noordermeer, J.W.M.; Van Baarle, B. Activators in Accelerated Sulfur Vulcanization. Rubber Chem. Technol. 2004, 77, 512–541. [Google Scholar] [CrossRef]
- Ignatz-Hoover, F. Review of vulcanization chemistry. Rubber World 1999, 220. [Google Scholar]
- Chapman, A.V. The influence of excess zinc stearate on the chemistry of sulphur vulcanization of natural rubber. Phosphorus Sulfur Silicon Relat. Elem. 1991, 59, 271–274. [Google Scholar] [CrossRef]
- Heideman, G.; Noordermeer, J.W.M.; Datta, R.N.; van Baarle, B. Multifunctional Additives as Zinc-Free Curatives for Sulfur Vulcanization. Rubber Chem. Technol. 2006, 79, 561–588. [Google Scholar] [CrossRef]
- Versloot, P.; Haasnoot, J.G.; Nieuwenhuizen, P.J.; Reedijk, J.; van Duin, M.; Put, J. Sulfur Vulcanization of Simple Model Olefins, Part V: Double Bond Isomerization during Accelerated Sulfur Vulcanization as Studied by Model Olefins. Rubber Chem. Technol. 2011, 70, 106–119. [Google Scholar] [CrossRef]
- Mcgill, W.J.; Shelver, S.R. Effect of carboxylic acids on 2-bisbenzothiazole-2,2′-disulfide- and tetramethylthiuram disulfide-accelerated sulfur vulcanization. II. Vulcanization of polyisoprene in the absence of ZnO. J. Appl. Polym. Sci. 1999, 72, 1007–1012. [Google Scholar] [CrossRef]
- Ikeda, Y.; Yasuda, Y.; Ohashi, T.; Yokohama, H.; Minoda, S.; Kobayashi, H.; Honma, T. Dinuclear bridging bidentate zinc/stearate complex in sulfur cross-linking of rubber. Macromolecules 2015, 48, 462–475. [Google Scholar] [CrossRef]
- Coran, A.Y. Chemistry of the vulcanization and protection of elastomers: A review of the achievements. J. Appl. Polym. Sci. 2003, 87, 24–30. [Google Scholar] [CrossRef]
- Morgan, B.; Mcgill, W.J. 2-Mercaptobenzothiazole as Accelerator for 2, 3-Dimethyl-2-butene. J. Appl. Polym. Sci. 2000, 76, 1377–1385. [Google Scholar] [CrossRef]
- Gradwell, M.H.S.; Van Der Merwe, M.J. 2- t butylbenzothiazole sulfenamide accelerated sulfur vulcanization of polyisoprene. Rubber Chem. Technol. 1999, 72, 55–64. [Google Scholar] [CrossRef]
- Farmer, E.H.; Shipley, F.W. The Reaction of Sulphur and Sulphur Compounds with Olefinic Substances. Part I. The reaction of Sulphur with mono-olefins and with 1:5-diolefins. J. Chem. Soc. 1947, 298, 1519–1532. [Google Scholar] [CrossRef]
- Bateman, L.; Moore, C.G.; Porter, M. The Reaction of Sulphur and Sulphur Compounds with Olefinic substances. Part XI. The Mechanim of Interaction of Sulphur with Mono-olefins and 1: 5-Dienes. J. Chem. Soc. 1958, 2866–2879. [Google Scholar] [CrossRef]
- Sadequl, A.M.; Ishiaku, U.S.; Ismail, H.; Poh, B.T. The effect of accelerator/sulphur ratio on the scorch time of epoxidized natural rubber. Eur. Polym. J. 1998, 34, 51–57. [Google Scholar] [CrossRef]
- Poh, B.T.; Tang, W.L. Concentration effect of stearic acid on scorch behavior of epoxidized natural rubber. J. Appl. Polym. Sci. 1995, 55, 537–542. [Google Scholar] [CrossRef]
- Susanna, A.; D’Arienzo, M.; Di Credico, B.; Giannini, L.; Hanel, T.; Grandori, R.; Morazzoni, F.; Mostoni, S.; Santambrogio, C.; Scotti, R. Catalytic effect of ZnO anchored silica nanoparticles on rubber vulcanization and cross-link formation. Eur. Polym. J. 2017, 93, 63–74. [Google Scholar] [CrossRef]
- Roy, K.; Alam, M.N.; Mandal, S.K.; Debnath, S.C. Preparation of zinc-oxide-free natural rubber nanocomposites using nanostructured magnesium oxide as cure activator. J. Appl. Polym. Sci. 2015, 132, 1–7. [Google Scholar] [CrossRef]
- Guzmán, M.; Vega, B.; Agulló, N.; Borrós, S. Zinc Oxide Versus Magnesium Oxide Revisited. Part 2. Rubber Chem. Technol. 2012, 85, 56–67. [Google Scholar] [CrossRef]
- Sahoo, S.; Maiti, M.; Ganguly, A.; George, J.J.; Bhowmick, A.K. Effect of zinc oxide nanoparticles as cure activator on the properties of natural rubber and nitrile rubber. J. Appl. Polym. Sci. 2007, 105, 2407–2415. [Google Scholar] [CrossRef]
- Przybyszewska, M.; Zaborski, M. The effect of zinc oxide nanoparticle morphology on activity in crosslinking of carboxylated nitrile elastomer. Express Polym. Lett. 2009, 3, 542–552. [Google Scholar] [CrossRef]
- Panampilly, B.; Thomas, S. Nano ZnO as Cure activator and reinforcing filler in natural rubber. Polym. Eng. Sci. 2013, 53, 1337–1346. [Google Scholar] [CrossRef]
- Tan, E.-H.; Wolff, S.; Haddeman, M.; Grewatta, H.P.; Wang, M.-J. Filler-Elastomer interactions. Part IX. Performance of silicas in polar elastomers. Rubber Chem. Technol. 1993, 66, 594–604. [Google Scholar] [CrossRef]
- Roy, K.; Alam, M.N.; Mandal, S.K.; Debnath, S.C. Sol-gel derived nano zinc oxide for the reduction of zinc oxide level in natural rubber compounds. J. Sol Gel Sci. Technol. 2014, 70, 378–384. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L.; Wu, W.; Cheng, Z.; Sun, Y.; Jiang, H.; Li, C. Zinc oxide with dominant (1 0 0) facets boosts vulcanization activity. Eur. Polym. J. 2019, 113, 148–154. [Google Scholar] [CrossRef]
- Chen, Y.; Kim, M.; Lian, G.; Johnson, M.B.; Peng, X. Side reactions in controlling the quality, yield, and stability of high quality colloidal nanocrystals. J. Am. Chem. Soc. 2005, 127, 13331–13337. [Google Scholar] [CrossRef]
- Heideman, G.; Noordermeer, J.W.M.; Datta, R.N.; van Baarle, B. Effect of Zinc Complexes as Activator for Sulfur Vulcanization in Various Rubbers. Rubber Chem. Technol. 2005, 78, 245–257. [Google Scholar] [CrossRef]
- Heideman, G.; Noordermeer, J.W.M.; Datta, R.N.; Van Baarle, B. Various ways to reduce zinc oxide levels in S-SBR rubber compounds. Macromol. Symp. 2006, 245, 657–667. [Google Scholar] [CrossRef]
- Steudel, R.; Steudel, Y.; Wong, M.W. Complexation of the vulcanization accelerator tetramethylthiuram disulfide and related molecules with zinc compounds including zinc oxide clusters (Zn4O4). Chem. A Eur. J. 2008, 14, 919–932. [Google Scholar] [CrossRef]
- Przybyszewska, M.; Zaborski, M.; Jakubowski, B.; Zawadiak, J. Zinc chelates as new activators for sulphur vulcanization of acrylonitrile-butadiene elastomer. Express Polym. Lett. 2009, 3, 256–266. [Google Scholar] [CrossRef]
- Alam, M.N.; Mandal, S.K.; Debnath, S.C. Effect of Zinc Dithiocarbamates and Thiazole-Based Accelerators on the Vulcanization of Natural Rubber. Rubber Chem. Technol. 2012, 85, 120–131. [Google Scholar] [CrossRef]
- Henning, S.K. Reduced Zinc Loading: Using Zinc Monomethacrylate to Activate Accelerated Sulfur Vulcanization. In Proceedings of the Fall 172nd Technical Meeting of the Rubber Division, American Chemical Society, Cray Valley USA, LLC Exton, Pennsylvania USA, Cleveland, OH, USA, 16 October 2007. [Google Scholar]
- Moresco, S.; Giovanela, M.; Carli, L.N.; Crespo, J.S. Development of passenger tire treads: Reduction in zinc content and utilization of a bio-based lubricant. J. Clean. Prod. 2016, 117, 199–206. [Google Scholar] [CrossRef]
- Zanchet, A.; De Sousa, F.D.B.; Crespo, J.S.; Scuracchio, C.H. Activator from sugar cane as a green alternative to conventional vulcanization additives. J. Clean. Prod. 2018, 174, 437–446. [Google Scholar] [CrossRef]
- Zanchet, A.; Demori, R.; de Sousa, F.D.B.; Ornaghi, H.L.; Schiavo, L.S.A.; Scuracchio, C.H. Sugar cane as an alternative green activator to conventional vulcanization additives in natural rubber compounds: Thermal degradation study. J. Clean. Prod. 2019, 207, 248–260. [Google Scholar] [CrossRef]
- Gardiner, B.J. Curative diffusion between dissimilar elastomers and its influence on adhesion. Rubber Chem. Technol. 1968, 41, 1312–1328. [Google Scholar] [CrossRef]
- Chen, L.; Guo, X.; Luo, Y.; Jia, Z.; Chen, Y.; Jia, D. Inorganic and Organic Hybrid Nanoparticles as Multifunctional Crosslinkers for Rubber Vulcanization with High-Filler Rubber Interaction. Polymers 2018, 10, 1138. [Google Scholar] [CrossRef]
- Chen, L.; Guo, X.; Luo, Y.; Jia, Z.; Bai, J.; Chen, Y.; Jia, D. Effect of novel supported vulcanizing agent on the interfacial interaction and strain-induced crystallization properties of natural rubber nanocomposites. Polymer 2018, 148, 390–399. [Google Scholar] [CrossRef]
- Mao, Y.; Tian, Q.; Zhang, C.; Tang, Y.; Wang, Y.; Li, X.; Ding, T. Vulcanization accelerator functionalized nanosilica: Effect on the reinforcement behavior of SSBR/BR. Polym. Eng. Sci. 2019, 59, 1270–1278. [Google Scholar] [CrossRef]
- Zhong, B.; Jia, Z.; Luo, Y.; Jia, D. Surface modification of silica with N-cyclohexyl-2-benzothiazole sulfenamide for styrene-butadiene rubber composites with dramatically improved mechanical property. Mater. Lett. 2015, 145, 41–43. [Google Scholar] [CrossRef]
- Zhong, B.; Jia, Z.; Luo, Y.; Jia, D. A method to improve the mechanical performance of styrene-butadiene rubber via vulcanization accelerator modified silica. Compos. Sci. Technol. 2015, 117, 46–53. [Google Scholar] [CrossRef]
- Heideman, G.; Noordermeer, J.W.M.; Datta, R.N.; Baarle, B. Van Modified Clays as Activator in Sulphur Vulcanisation. Kautsch. Gummi Kunstst. 2003, 56, 650–656. [Google Scholar]
- Heideman, G.; Noordermeer, J.W.M.; Datta, R.N. Zinc Loaded Clay as Activator in Sulfur Vulcanization: A new route for zinc oxide reduction in rubber compounds. Rubber Chem. Technol. 2004, 77, 336–355. [Google Scholar] [CrossRef]
- Eshwaran, S.B.; Basu, D.; Kutlu, B.; Leuteritz, A.; Wagenknecht, U.; Stöckelhuber, K.W.; Naskar, K.; Das, A.; Heinrich, G. Stearate Modified Zinc-Aluminum Layered Double Hydroxides and Acrylonitrile Butadiene Rubber Nanocomposites. Polym. Plast. Technol. Eng. 2014, 53, 65–73. [Google Scholar] [CrossRef]
- Qi, J.Y.; Wu, L.X.; Zhuo, D.X. Preparation and Properties of BR/SBR Blends Using Surface-Modified Nano Zinc Oxide. Adv. Mater. Res. 2014, 910, 101–104. [Google Scholar] [CrossRef]
- Gujel, A.A.; Bandeira, M.; Menti, C.; Perondi, D.; Guégan, R.; Roesch-Ely, M.; Giovanela, M.; Crespo, J.S. Evaluation of vulcanization nanoactivators with low zinc content: Characterization of zinc oxides, cure, physico-mechanical properties, Zn2+ release in water and cytotoxic effect of EPDM compositions. Polym. Eng. Sci. 2018, 58, 1800–1809. [Google Scholar] [CrossRef]
- Niemantsverdriet, J.W.; Chorkendorff, I. Concept of Modern Catalysis and Kinetics; WILEY-VCH Verlag GmbH & Co.: Weinheim, Germany, 2003. [Google Scholar]
- Murata, K.; Mahara, Y.; Ohyama, J.; Yamamoto, Y.; Arai, S.; Satsuma, A. The Metal-Support Interaction Concerning the Particle Size Effect of Pd/Al2O3 on Methane Combustion. Angew. Chem. 2017, 129, 16209–16213. [Google Scholar] [CrossRef]
- Kuo, T.J.; Lin, C.N.; Kuo, C.L.; Huang, M.H. Growth of ultralong ZnO nanowires on silicon substrates by vapor transport and their use as recyclable photocatalysts. Chem. Mater. 2007, 19, 5143–5147. [Google Scholar] [CrossRef]
- Mihai, G.D.; Meynen, V.; Mertens, M.; Bilba, N.; Cool, P.; Vansant, E.F. ZnO nanoparticles supported on mesoporous MCM-41 and SBA-15: A comparative physicochemical and photocatalytic study. J. Mater. Sci. 2010, 45, 5786–5794. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, F.; Hua, W.; Yue, Y.; Gao, Z. ZnO supported on high silica HZSM-5 as new catalysts for dehydrogenation of propane to propene in the presence of CO2. Catal. Today 2009, 148, 316–322. [Google Scholar] [CrossRef]
- Susanna, A.; Armelao, L.; Callone, E.; Dirè, S.; D’Arienzo, M.; Di Credico, B.; Giannini, L.; Hanel, T.; Morazzoni, F.; Scotti, R. ZnO nanoparticles anchored to silica filler. A curing accelerator for isoprene rubber composites. Chem. Eng. J. 2015, 275, 245–252. [Google Scholar] [CrossRef]
- Acikgoz, C.; Sahbaz, D.A.; Kockar, O.M. ZnO nanoparticles bonded to SiO2 filler as a curing accelerator in cold vulcanizing adhesives. Acta Phys. Pol. A 2017, 131, 200–203. [Google Scholar] [CrossRef]
- Alam, M.N.; Potiyaraj, P. Precipitated Nano Zinc Hydroxide on the Silica Surface as an Alternative Cure Activator in the Vulcanization of Natural Rubber. Rubber Chem. Technol. 2017, 90, 714–727. [Google Scholar] [CrossRef]
- Lin, Y.; Zeng, Z.; Zhu, J.; Chen, S.; Yuan, X.; Liu, L. Graphene nanosheets decorated with ZnO nanoparticles: Facile synthesis and promising application for enhancing the mechanical and gas barrier properties of rubber nanocomposites. RSC Adv. 2015, 5, 57771–57780. [Google Scholar] [CrossRef]
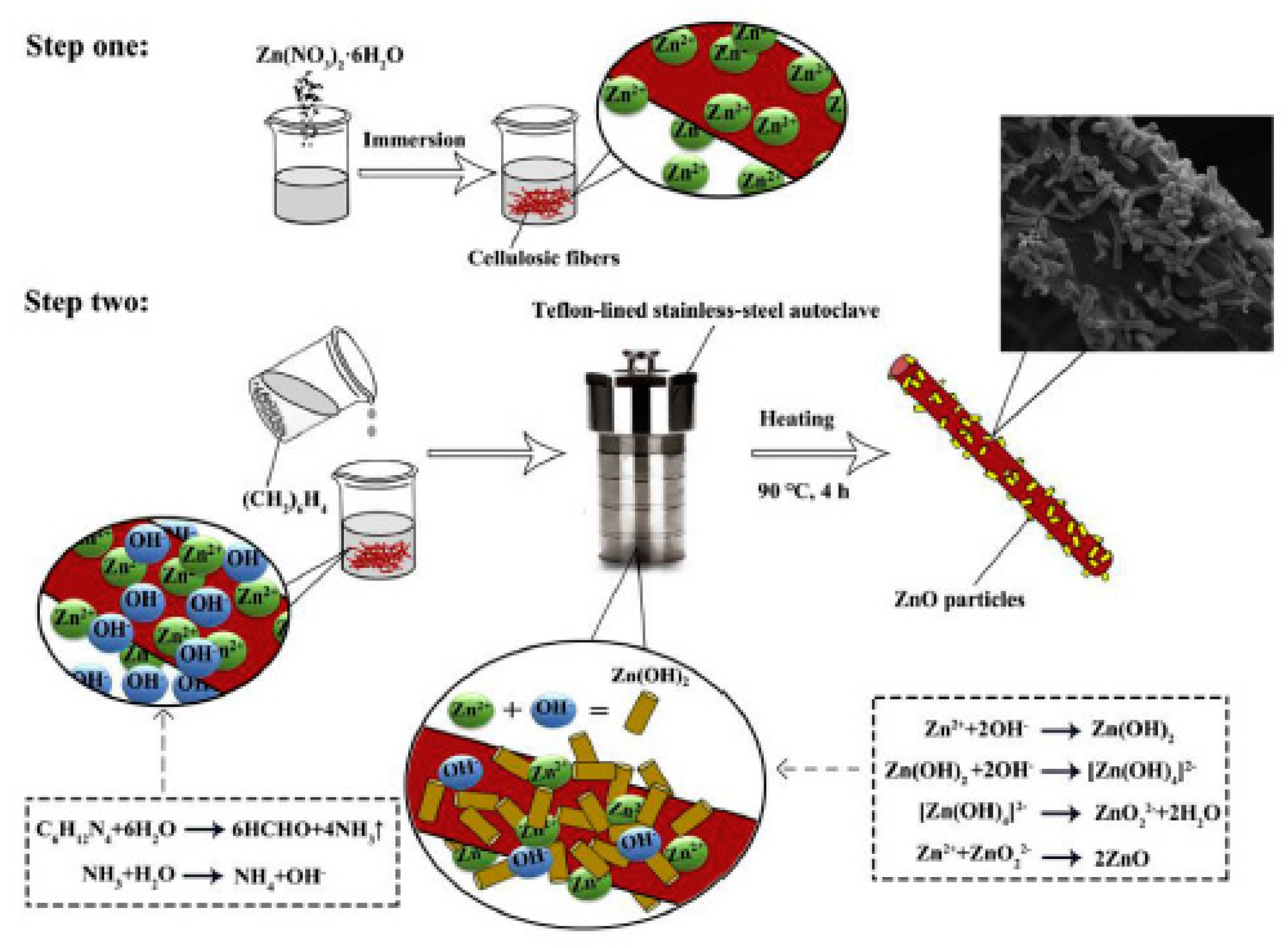
- Li, Y.; Sun, H.; Zhang, Y.; Xu, M.; Shi, S.Q. The three-dimensional heterostructure synthesis of ZnO/cellulosic fibers and its application for rubber composites. Compos. Sci. Technol. 2019, 177, 10–17. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostoni, S.; Milana, P.; Di Credico, B.; D’Arienzo, M.; Scotti, R. Zinc-Based Curing Activators: New Trends for Reducing Zinc Content in Rubber Vulcanization Process. Catalysts 2019, 9, 664. https://doi.org/10.3390/catal9080664
Mostoni S, Milana P, Di Credico B, D’Arienzo M, Scotti R. Zinc-Based Curing Activators: New Trends for Reducing Zinc Content in Rubber Vulcanization Process. Catalysts. 2019; 9(8):664. https://doi.org/10.3390/catal9080664
Chicago/Turabian StyleMostoni, Silvia, Paola Milana, Barbara Di Credico, Massimiliano D’Arienzo, and Roberto Scotti. 2019. "Zinc-Based Curing Activators: New Trends for Reducing Zinc Content in Rubber Vulcanization Process" Catalysts 9, no. 8: 664. https://doi.org/10.3390/catal9080664
APA StyleMostoni, S., Milana, P., Di Credico, B., D’Arienzo, M., & Scotti, R. (2019). Zinc-Based Curing Activators: New Trends for Reducing Zinc Content in Rubber Vulcanization Process. Catalysts, 9(8), 664. https://doi.org/10.3390/catal9080664





