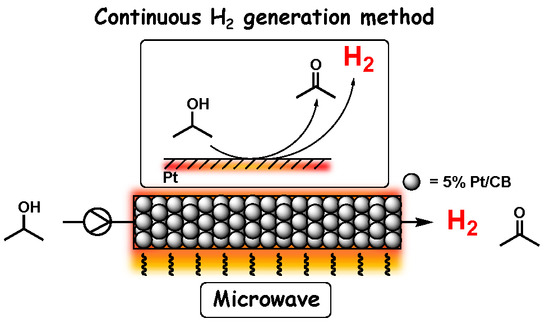
Microwave-Mediated Continuous Hydrogen Abstraction Reaction from 2-PrOH Catalyzed by Platinum on Carbon Bead
Abstract
1. Introduction
2. Results and Discussion
2.1. Microwave Heating Property under Existence of 5% Pt/CB and 2-PrOH
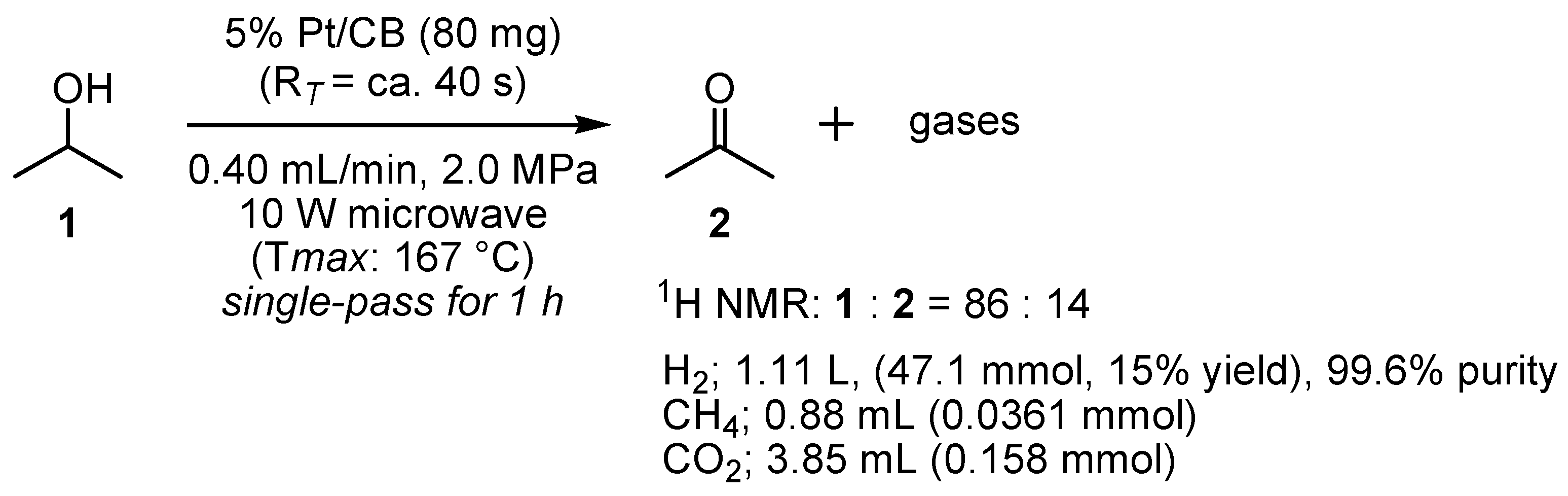
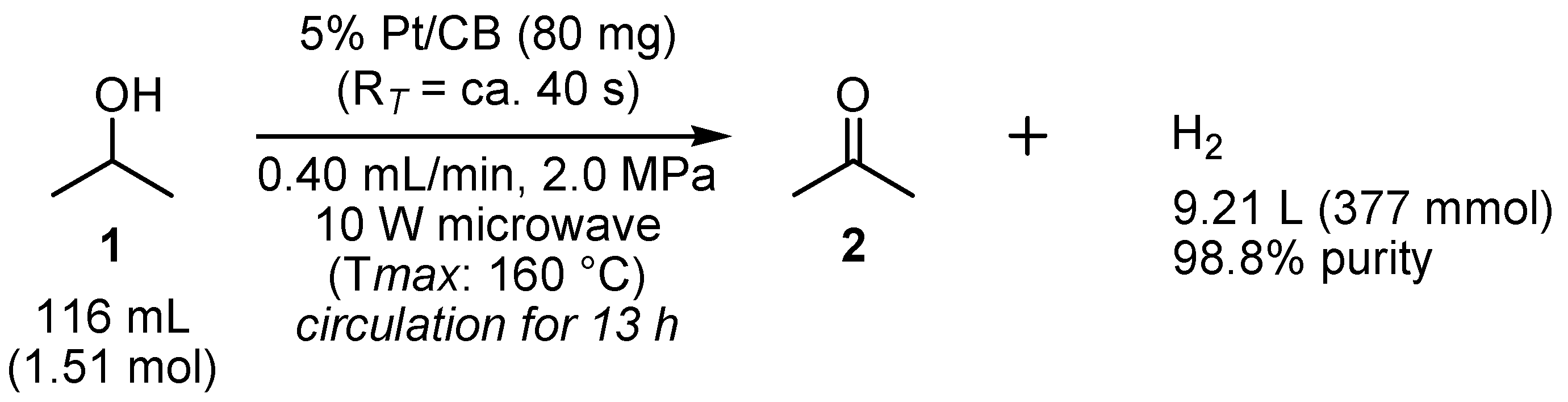
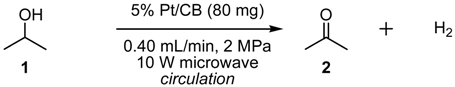
2.2. Dehydrogenation of 2-PrOH
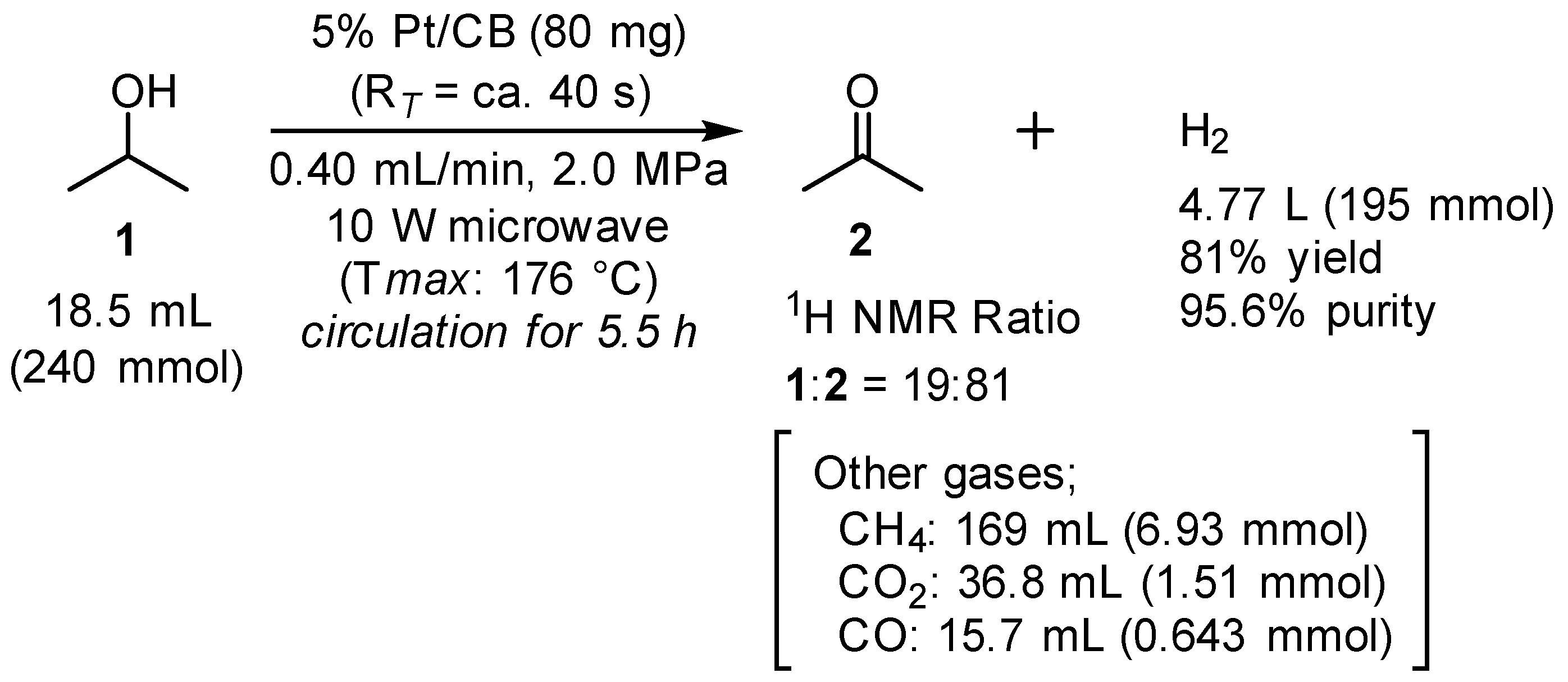
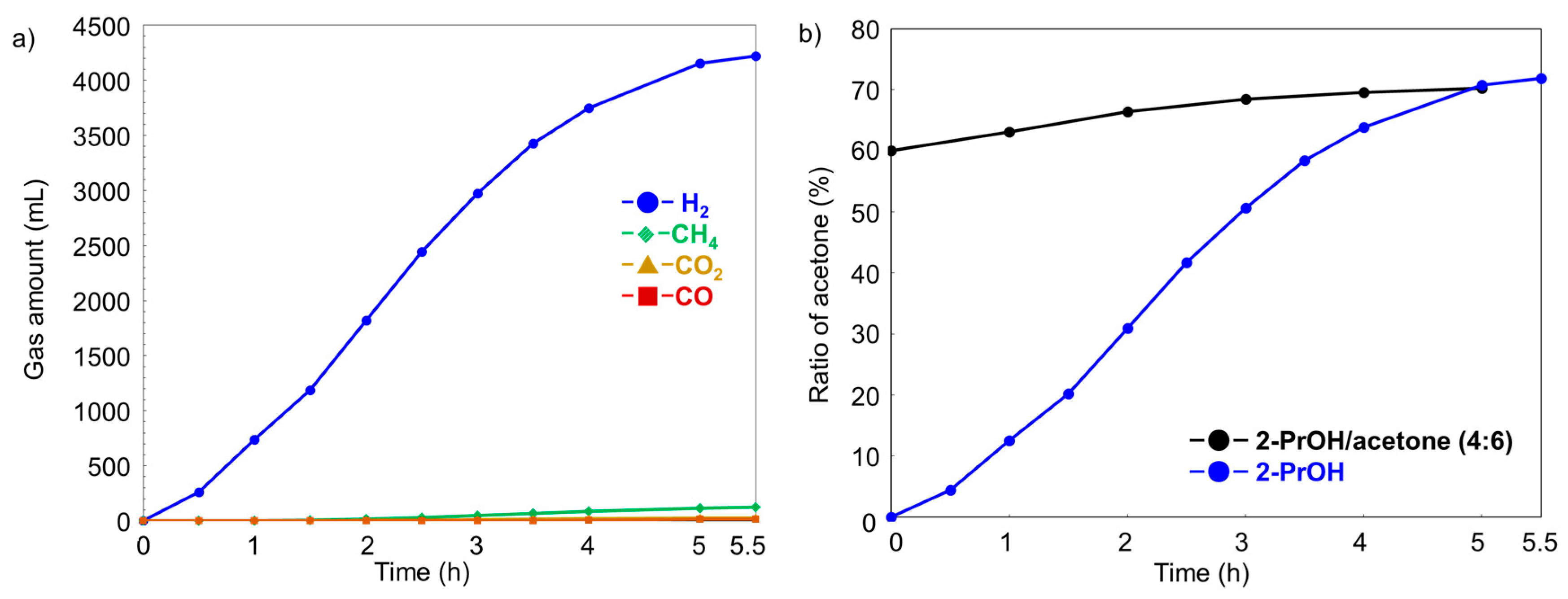
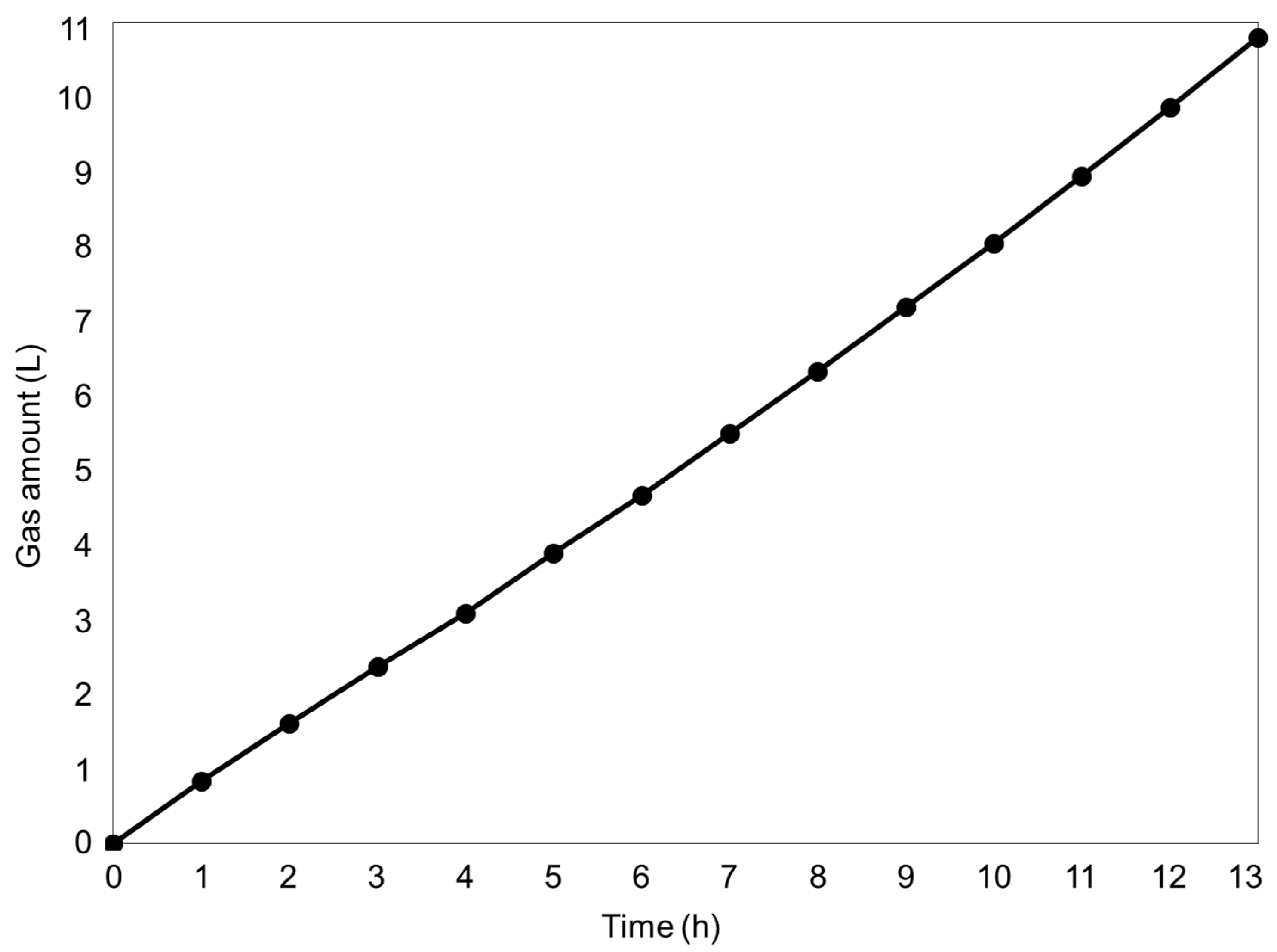
2.3. Long-Term Experiment
2.4. Effects of Reaction Conditions
3. Materials and Methods
3.1. General
3.2. Experimentals
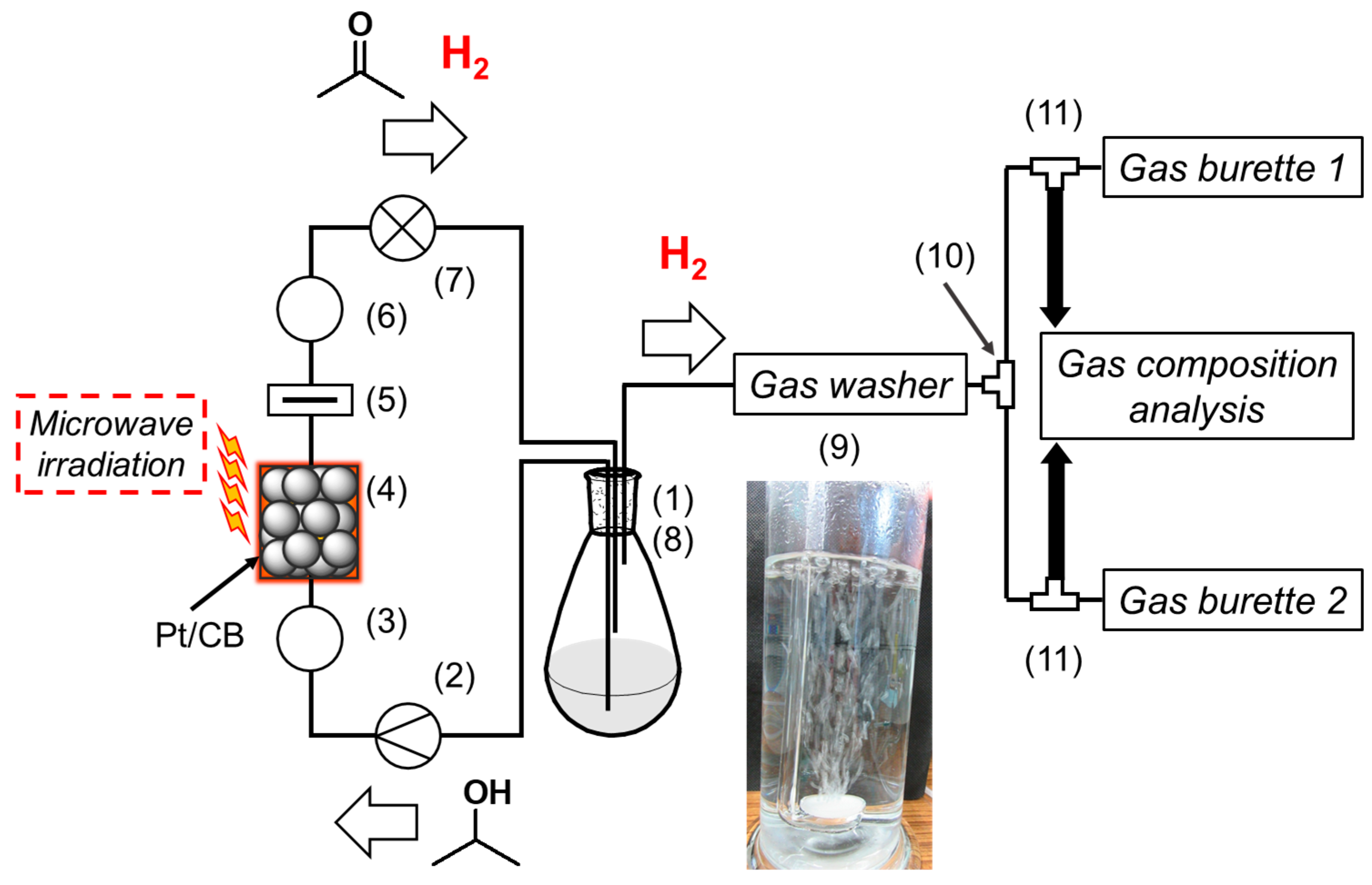
3.2.1. Reaction System
3.2.2. Preparation of Catalyst Cartridge
3.2.3. Hydrogen Generation from 2-PrOH
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming A review. Appl. Catal. B 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P. Liquid Organic Hydrogen Carriers as an efficient vector for the transport and storage of renewable energy. Int. J. Hydrogen Energy 2012, 37, 18118–18132. [Google Scholar] [CrossRef]
- Dalebrook, A.F.; Gan, W.; Grasemann, M.; Moret, S.; Laurenczy, G. Hydrogen storage: beyond conventional methods. Chem. Commun. 2013, 49, 8735–8751. [Google Scholar] [CrossRef] [PubMed]
- Sartbaeva, A.; Kuznetsov, V.L.; Wells, S.A.; Edwards, P.P. Hydrogen nexus in a sustainable energy future. Energy Environ. Sci. 2008, 1, 79–85. [Google Scholar] [CrossRef]
- Horikoshi, S.; Kamata, M.; Sumi, T.; Serpone, N. Selective heating of Pd/AC catalyst in heterogeneous systems for the microwave-assisted continuous hydrogen evolution from organic hydrides: Temperature distribution in the fixed-bed reactor. Int. J. Hydrogen Energy 2016, 41, 12029–12037. [Google Scholar] [CrossRef]
- Murray, L.J.; Dincă, M.; Long, J.R. Hydrogen storage in metal−organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef] [PubMed]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Li, J.; Chai, Y.; Liu, B.; Wu, Y.; Li, X.; Tang, Z.; Liu, Y.; Liu, C. The catalytic performance of Ni2P/Al2O3 catalyst in comparison with Ni/Al2O3 catalyst in dehydrogenation of cyclohexane. Appl. Catal. A 2014, 469, 434–441. [Google Scholar] [CrossRef]
- Suttisawat, Y.; Sakai, H.; Abe, M.; Rangsunvigit, P.; Horikoshi, S. Microwave effect in the dehydrogenation of tetralin and decalin with a fixed-bed reactor. Int. J. Hydrogen Energy 2012, 37, 3242–3250. [Google Scholar] [CrossRef]
- Li, X.; Tuo, Y.; Li, P.; Duan, X.; Jiang, H.; Zhou, X. Effects of carbon support on microwave-assisted catalytic dehydrogenation of decalin. Carbon 2014, 67, 775–783. [Google Scholar] [CrossRef]
- Lázaro, M.P.; García–Bordejé, E.; Sebastián, D.; Moliner, R. In situ hydrogen generation from cycloalkanes using a Pt/CNF catalyst. Catal. Today 2008, 138, 203–209. [Google Scholar] [CrossRef]
- Usman, M.R.; Alotaibi, F.M.; Aslam, R. Dehydrogenation–hydrogenation of methylcyclohexane-toluene system on 1.0 wt % Pt/zeolite beta catalyst. Prog. React. Kinet. Mec. 2015, 40, 353–366. [Google Scholar] [CrossRef]
- Kariya, N.; Fukuoka, A.; Ichikawa, M. Efficient evolution of hydrogen from liquid cycloalkanes over Pt-containing catalysts supported on active carbons under “wet–dry multiphase conditions”. Appl. Catal. A 2002, 233, 91–102. [Google Scholar] [CrossRef]
- Alhumaidan, F.; Tsakiris, D.; Cresswell, D.; Garforth, A. Hydrogen storage in liquid organic hydride: Selectivity of MCH dehydrogenation over monometallic and bimetallic Pt catalysts. Int. J. Hydrogen Energy 2013, 38, 14010–14026. [Google Scholar] [CrossRef]
- Nakano, A.; Manabe, S.; Higo, T.; Seki, H.; Nagatake, S.; Yabe, T.; Ogo, S.; Nagatsuka, T.; Sugiura, Y.; Iki, H.; et al. Effects of Mn addition on dehydrogenation of methylcyclohexane over Pt/Al2O3 catalyst. Appl. Catal. A 2017, 543, 75–81. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Tsuru, T.; Kang, D.-Y. Simulation and design of catalytic membrane reactor for hydrogen production via methylcyclohexane dehydrogenation. Int. J. Hydrogen Energy 2017, 42, 26296–26307. [Google Scholar] [CrossRef]
- Boufaden, N.; Akkari, R.; Pawelec, B.; Fierro, J.L.G.; Said Zina, M.; Ghorbei, A. Dehydrogenation of methylcyclohexane to toluene over partially reduced silica-supported Pt-Mo catalysts. J. Mol. Catal. A 2016, 420, 96–106. [Google Scholar] [CrossRef]
- Yolcular, S. Organic chemical hydride dehydrogenation over nickel catalysts supported with SiO2 for hydrogen recovery. Energy Sources Part A 2016, 38, 2031–2034. [Google Scholar] [CrossRef]
- Mitsui Chemicals, Inc. Method for Producing Isopropyl Alcohol. JP4321838B2, 12 June 2009. [Google Scholar]
- Yamashita, M.; Kawamura, T.; Suzuki, M.; Saito, Y. Characteristics of suspended Ru/carbon catalyst for 2-propanol dehydrogenation applicable to chemical heat pomp. Bull. Chem. Soc. Jpn. 1991, 64, 272–278. [Google Scholar] [CrossRef]
- Yamashita, M.; Dai, F.-Y.; Suzuki, M.; Saito, Y. Mechanism of 2-PrOH dehydrogenation with suspended Nickel fine-particle catalyst. Bull. Chem. Soc. Jpn. 1991, 64, 628–634. [Google Scholar] [CrossRef]
- Mooksuwan, W.; Kumar, S. Study on 2-propanol/acetone/hydrogen chemical heat pump: endothermic dehydrogenation of 2-propanol. Int. J. Energy Res. 2000, 24, 1109–1122. [Google Scholar] [CrossRef]
- Ando, Y.; Yamashita, M.; Saito, Y. Reaction Mechanism of 2-propanol dehydrogenation with a carbon-supported Ru–Pt composite catalyst in the liquid phase. Bull. Chem. Soc. Jpn. 2003, 76, 2045–2049. [Google Scholar] [CrossRef]
- Ukisu, Y.; Miyadera, T. Dehydrogenation of 2-Prpanool with suspended noble metal catalysts: activity enhancement by the addition of sodium hydroxide. React. Kinet. Catal. Lett. 2004, 81, 305–311. [Google Scholar] [CrossRef]
- Behar, D.; Rabani, J. Kinetics of hydrogen production upon reduction of aqueous TiO2 nanoparticles catalyzed by Pd0, Pt0, or Au0 coatings and an unusual hydrogen abstraction; steady state and pulse radiolysis study. J. Phys. Chem. 2006, 110, 8750–8755. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ramirez, Z.; Gonzarez-Calderon, J.A.; Almendarez-Camarillo, A.; Fierro-Gonzalez, J.C. Adsorption and dehydrogenation of 2-propanol on the surface of γ-Al2O3-supported gold. Surf. Sci. 2012, 606, 1167–1172. [Google Scholar] [CrossRef]
- Sakurai, M.; Honda, H.; Kameyama, H. Fundamental study of a non-steady operation for 2-propanol de-hydrogenation. Int. J. Hydrogen Energy 2007, 32, 1303–1308. [Google Scholar] [CrossRef]
- Zieliñska, B.; Sreñscek-Nazzal, J.; Kaleñczuk, R.J. Photocatalytic hydrogen generation over alkali niobates in the presence of organic compounds. Pol. J. Chem. Tech. 2008, 10, 1–3. [Google Scholar] [CrossRef]
- Karakuș, Y.; Aynacı, F.; Kıpҫak, E.; Akgün, M. Hydrogen production from 2-propanol over Pt/Al2O3 and Ru/Al2O3 catalysts in supercritical water. Int. J. Hydrogen Energy 2013, 38, 7298–7306. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Mechtaeva, E.V.; Zvereva, I.A. Photocatalytic Activity of TiO2−MOx Composites in the Reaction of Hydrogen Generation from Aqueous Isopropanol Solution. Russ. J. Gen. Chem. 2014, 84, 611–616. [Google Scholar] [CrossRef]
- Huang, S.; Wu, X.; Chen, W.; Ma, L.; Liu, S.; He, G. Electrocatalytic dehydrogenation of 2-propanol in electrochemicalhydrogen pump reactor. Catal. Today 2016, 276, 128–132. [Google Scholar] [CrossRef]
- Huang, S.; Wu, X.; Chen, W.; Wang, T.; Wu, Y.; He, G. A bilateral electrochemical hydrogen pump reactor for 2-propanol dehydrogenation and phenol hydrogenation. Green Chem. 2016, 18, 2353–2362. [Google Scholar] [CrossRef]
- Chai, Z.; Zeng, T.-T.; Li, Q.; Lu, L.-Q.; Xiao, W.-J.; Xu, D. Efficient Visible Light-Driven Splitting of Alcohols into Hydrogen and Corresponding Carbonyl Compounds over a Ni-Modified CdS Photocatalyst. J. Am. Chem. Soc. 2016, 138, 10128–10131. [Google Scholar] [CrossRef]
- López-Tenlladoa, F.J.; Hidalgo-Carrilloa, J.; Montesa, V.; Marinasa, A.; Urbanoa, F.J.; Marinasa, J.M.; Ilievab, L.; Tabakovab, T.; Reid, F. A comparative study of hydrogen photocatalytic production fromglycerol and propan-2-ol on M/TiO2 systems (M = Au, Pt, Pd). Catal. Today 2017, 280, 58–64. [Google Scholar] [CrossRef]
- Haneishi, N.; Tsubaki, S.; Abe, E.; Maitani, M.M.; Suzuki1, E.; Fujii, S.; Fukushima, J.; Takizawa, H.; Wada, Y. Reactions under Microwave Irradiation by Local Heating at the Vicinal Contact Points of Catalyst Particles. Sci. Rep. 2019, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- López-Tenllado, F.J.; Hidalgo-Carrillo, J.; Montes-Jiménez, V.; Sánchez-López, E.; Urbano, F.J.; Marinas, A. Photocatalytic production of hydrogen from binary mixtures of C-3 alcohols on Pt/TiO2: Influence of alcohol structure. Catal. Today 2019, 328, 2–7. [Google Scholar] [CrossRef]
- Sawama, Y.; Morita, K.; Asai, S.; Kozawa, M.; Tadokoro, S.; Nakajima, J.; Monguchi, Y.; Sajiki, H. Palladium on Carbon-Catalyzed Aqueous Transformation of Primary Alcohols to Carboxylic Acids Based on Dehydrogenation under Mildly Reduced Pressure. Adv. Synth. Catal. 2015, 357, 1205–1210. [Google Scholar] [CrossRef]
- Sawama, Y.; Morita, K.; Yamada, T.; Nagata, S.; Yabe, Y.; Monguchi, Y.; Sajiki, H. Rhodium-on-carbon catalyzed hydrogen scavenger- and oxidant-free dehydrogenation of alcohols in aqueous media. Green Chem. 2014, 16, 3439–3443. [Google Scholar] [CrossRef]
- Ichikawa, T.; Matsuo, T.; Tachikawa, T.; Yamada, T.; Yoshimura, T.; Yoshimura, M.; Takagi, Y.; Sawama, Y.; Sugiyama, J.; Monguchi, Y.; et al. Microwave-Mediated Site-Selective Heating of Spherical-Carbon-Bead-Supported Platinum for the Continuous, Efficient Catalytic Dehydrogenative Aromatization of Saturated Cyclic Hydrocarbons. ACS Sustain. Chem. Eng. 2019, 7, 3052–3061. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, Y.; Zhao, G.; Wang, D.; Liu, L.; He, W.; Li, Y. Porous bimetallic Pt–Fe nanocatalysts for highly efficient hydrogenation of acetone. Nano Res. 2015, 8, 2706–2713. [Google Scholar] [CrossRef]
- Sen, B.; Vannice, M.A. Metal-support effects on acetone hydrogenation over platinum catalysts. J. Catal. 1988, 113, 52–71. [Google Scholar] [CrossRef]
- Chiyoda Corp. Storage-Transport System of Hydrogen. JP2007-269522, 18 October 2007. [Google Scholar]
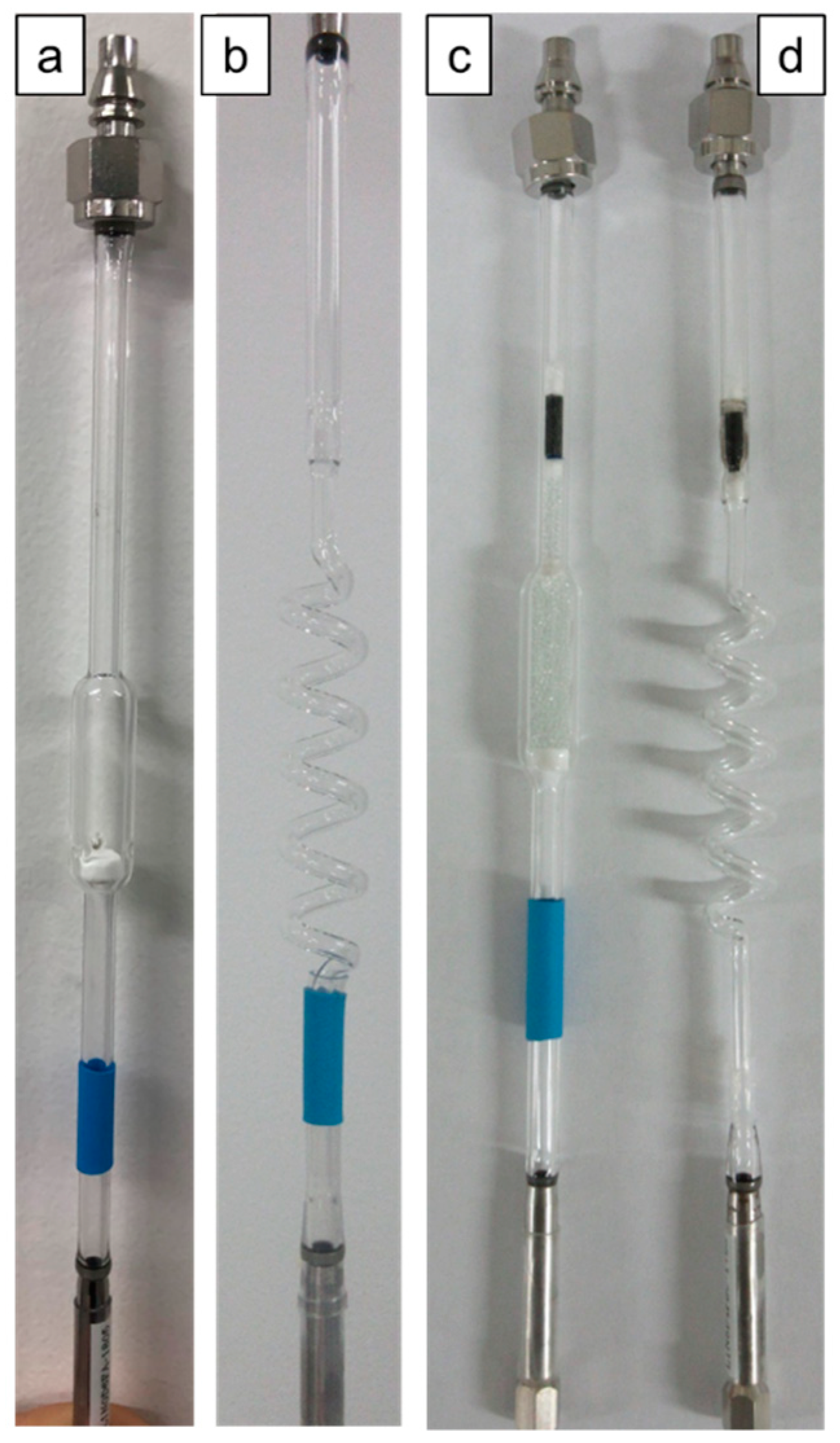
| Entry | Type of Catalyst Cartridge | Adjuster Tube 2 | Resonant Frequency (GHz) |
|---|---|---|---|
| 1 | Straight | None | 2.446 3 |
| 2 | Straight | Glass | Not detected 4 |
| 3 | Straight | PTFE | 2.382 |
| 4 | Helix−Straight | None | 2.566 |
| 5 | Helix−Straight | Glass | 2.412 |
| 6 | Helix−Straight | PTFE | 2.505 |
| Entry | Deviation from Above | t (h) | T (°C) | H2 Gas | Other Gases (mmol) | |||
|---|---|---|---|---|---|---|---|---|
| Total Amount (mmol) | Production Rate (mmol/h) | Yield (%) | Purity (%) | |||||
| 1 | none | 5.5 | 176 | 195 | 35.5 | 81 | 96 | CH4 (6.93), CO2 (1.51), CO (0.643) |
| 2 | 0.8 mL/min | 1 | 157 | 13.0 | 13.0 | 5 | 98 | CH4 (0.121), CO2 (0.206), |
| 3 | 1 MPa | 6 | 142 | 181 | 30.2 | 75 | 97 | CH4 (3.98), CO2 (1.16) |
| 4 | w/H2O (20 mol %) 2 | 5.5 | 176 | 166 | 30.2 | 72 | 97 | CH4 (4.05), CO2 (1.75) |
| 5 | w/H2O (5 mol %) 3 | 5 | 168 | 167 | 33.4 | 71 | 93 | CH4 (9.59), CO2 (2.80) |
| 6 | 5% Pt/CB (60 mg) 4 | 5 | 171 | 68.7 | 13.7 | 98 | 89 | CH4 (7.10) CO2 (1.57) |
| 7 | w/H2O (20 mol %) 2, 5% Pt/CB (60 mg) | 3.5 | 154 | 220 | 62.9 | 95 | 89 | CH4 (18.9), CO2 (7.31) |
| 8 | w/H2O (5 mol %) 3, 5% Pt/CB (60 mg) | 4.8 | 150 | 209 | 43.5 | 90 | 87 | CH4 (18.3), CO2 (8.21), CO (4.96), C2H6 (0.283) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichikawa, T.; Matsuo, T.; Tachikawa, T.; Teranishi, W.; Yamada, T.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Microwave-Mediated Continuous Hydrogen Abstraction Reaction from 2-PrOH Catalyzed by Platinum on Carbon Bead. Catalysts 2019, 9, 655. https://doi.org/10.3390/catal9080655
Ichikawa T, Matsuo T, Tachikawa T, Teranishi W, Yamada T, Sawama Y, Monguchi Y, Sajiki H. Microwave-Mediated Continuous Hydrogen Abstraction Reaction from 2-PrOH Catalyzed by Platinum on Carbon Bead. Catalysts. 2019; 9(8):655. https://doi.org/10.3390/catal9080655
Chicago/Turabian StyleIchikawa, Tomohiro, Tomohiro Matsuo, Takumu Tachikawa, Wataru Teranishi, Tsuyoshi Yamada, Yoshinari Sawama, Yasunari Monguchi, and Hironao Sajiki. 2019. "Microwave-Mediated Continuous Hydrogen Abstraction Reaction from 2-PrOH Catalyzed by Platinum on Carbon Bead" Catalysts 9, no. 8: 655. https://doi.org/10.3390/catal9080655
APA StyleIchikawa, T., Matsuo, T., Tachikawa, T., Teranishi, W., Yamada, T., Sawama, Y., Monguchi, Y., & Sajiki, H. (2019). Microwave-Mediated Continuous Hydrogen Abstraction Reaction from 2-PrOH Catalyzed by Platinum on Carbon Bead. Catalysts, 9(8), 655. https://doi.org/10.3390/catal9080655