Reactive Grinding Synthesis of LaBO3 (B: Mn, Fe) Perovskite; Properties for Toluene Total Oxidation
Abstract
:1. Introduction
2. Results
2.1. Physico-Chemical Characterization
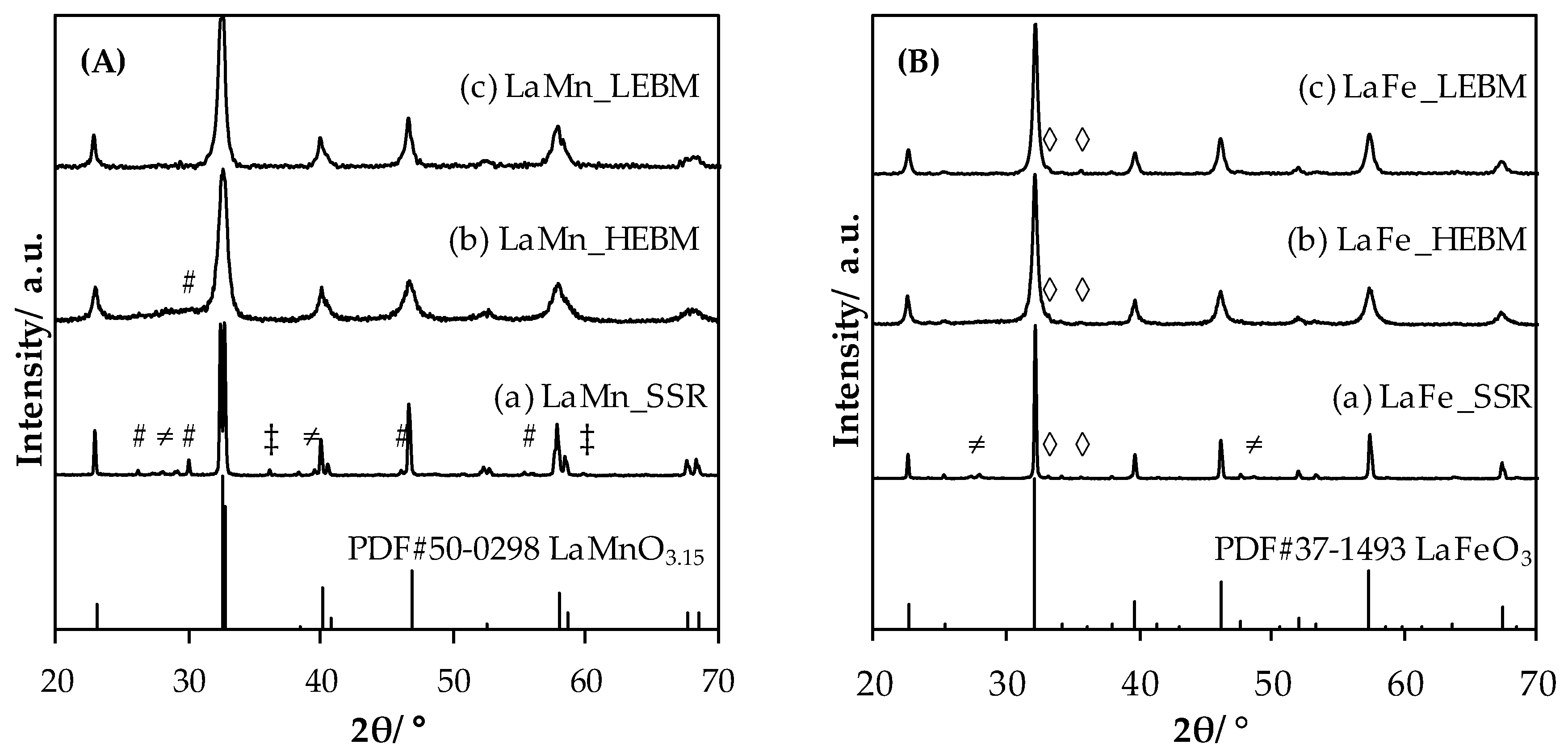
2.1.1. Structural Properties Evolution upon Grinding
2.1.2. Textural Properties Evolution upon Grinding
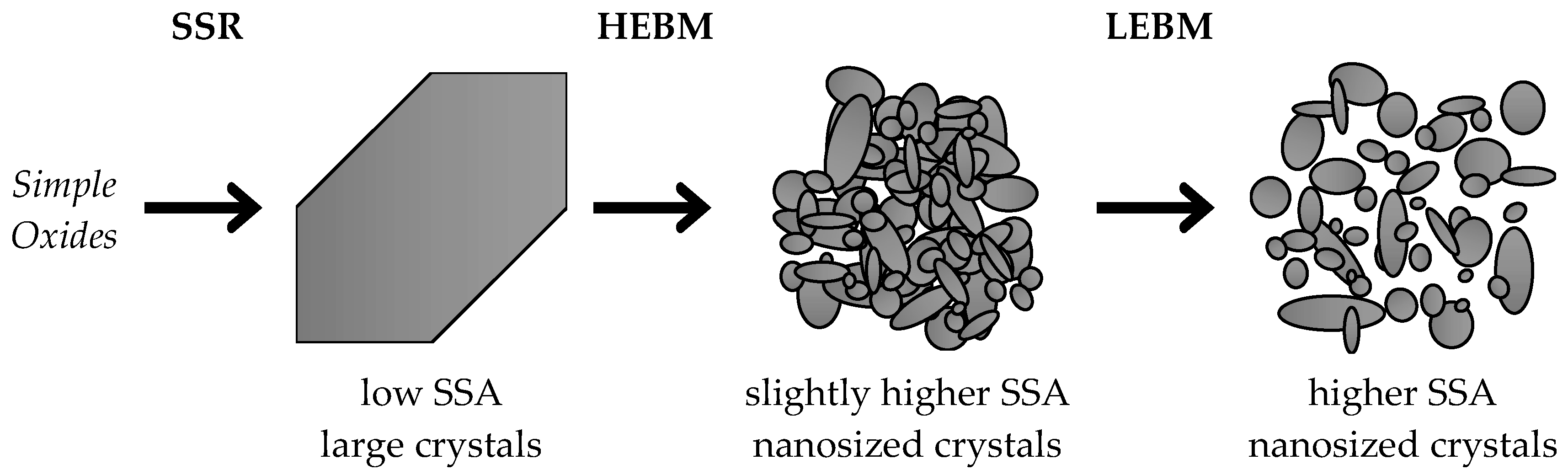
2.1.3. Morphology Evolution upon Grinding
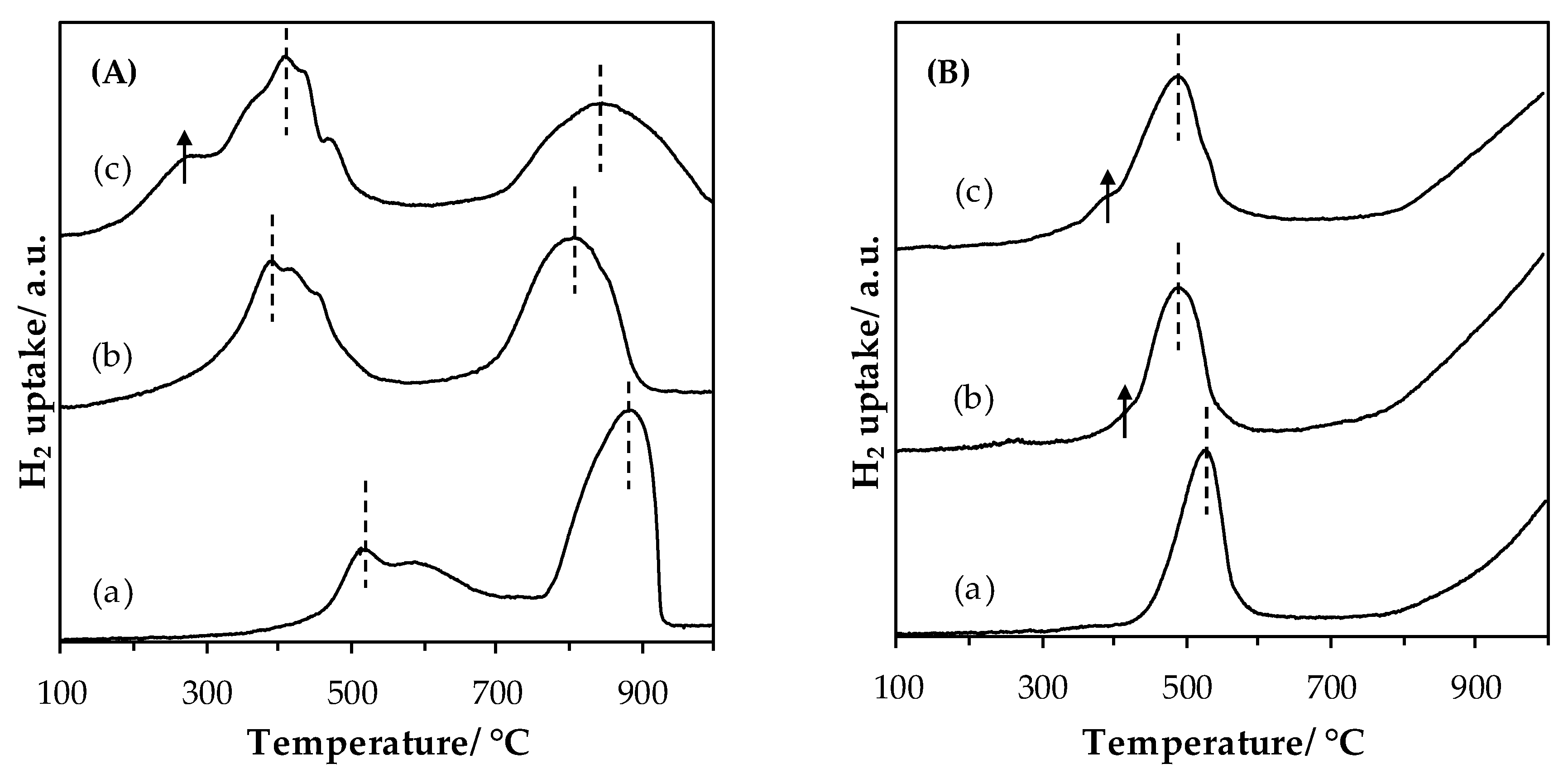
2.1.4. Evolution of Transition Metal Reducibility upon Grinding
- -
- Complete reduction of Fe(+III) to Fe(+II), with a possible additional hydrogen consumption of surface Fe(+II) reduction,
- -
- Reduction of remaining Fe(+II) to Fe(0), and obtaining of La2O3 + Fe(0) individual phases.
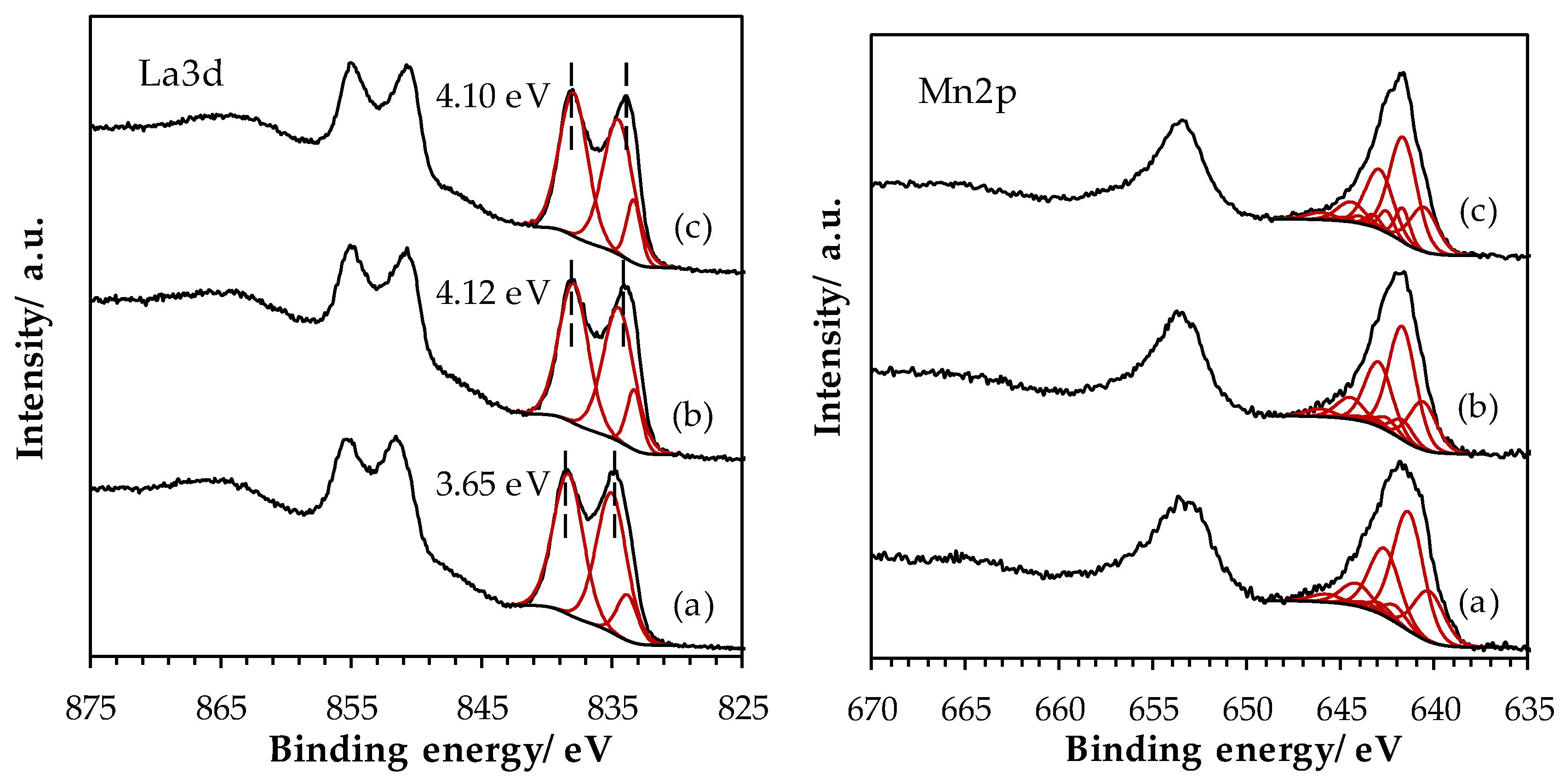
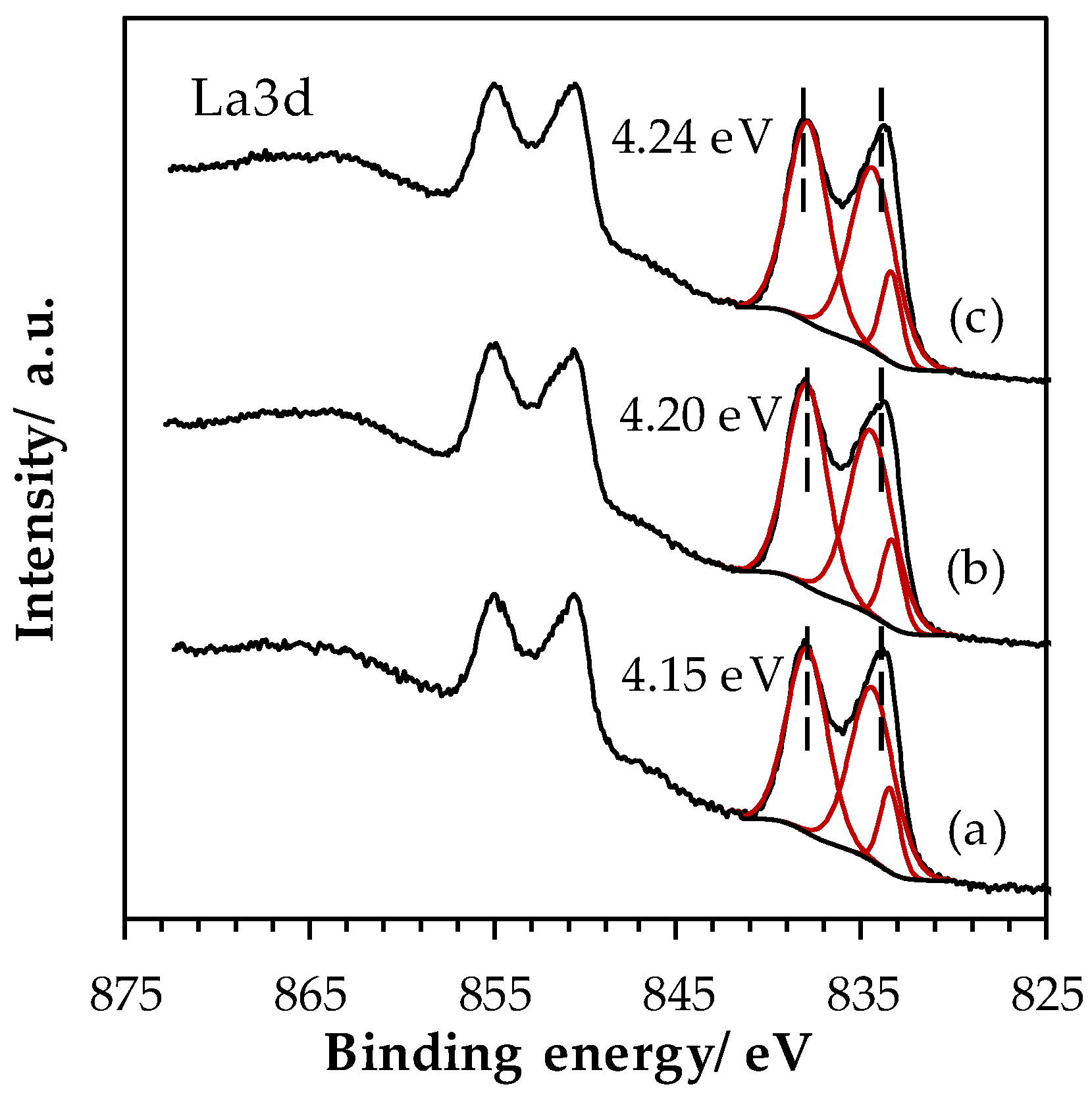
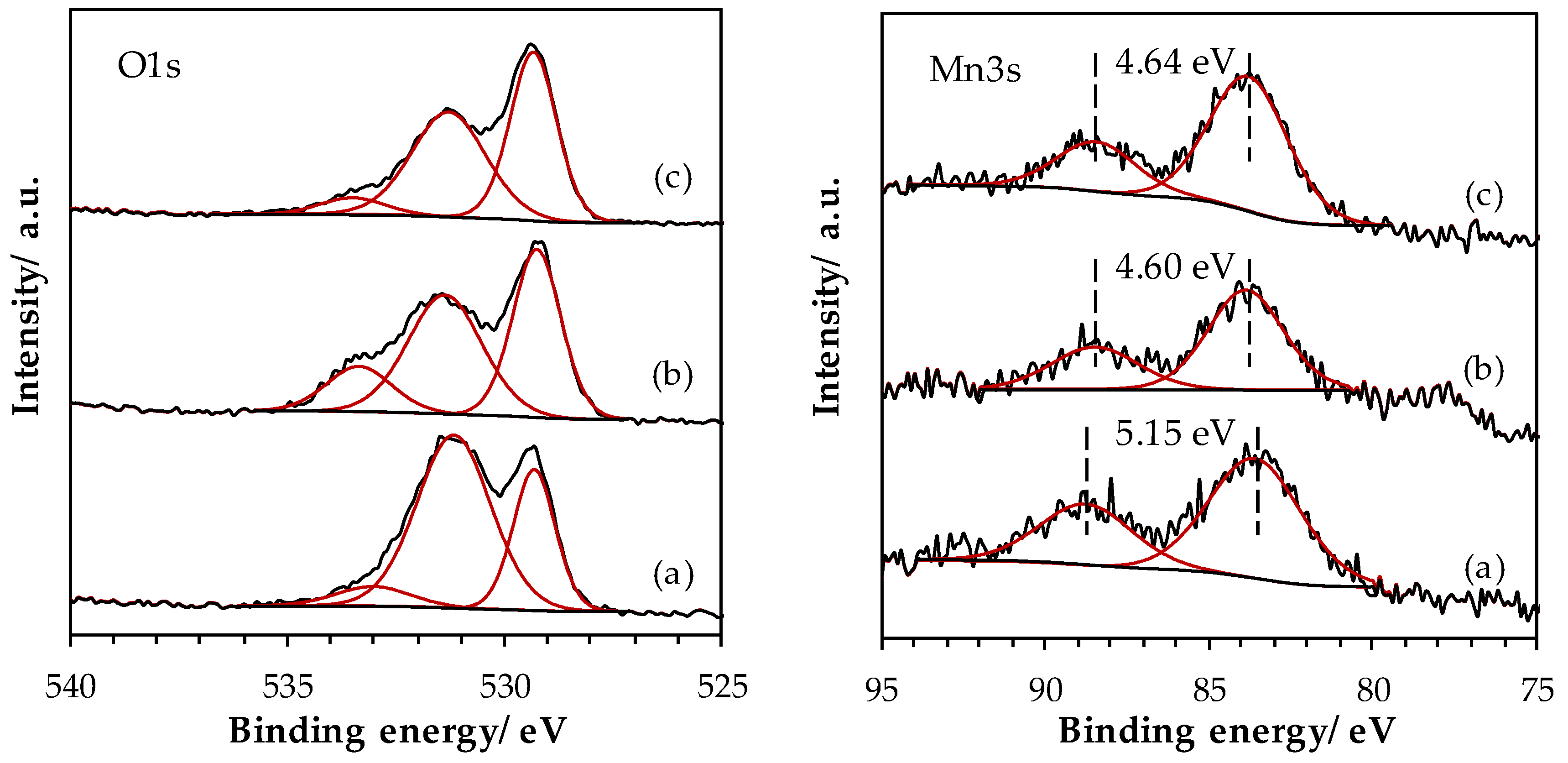
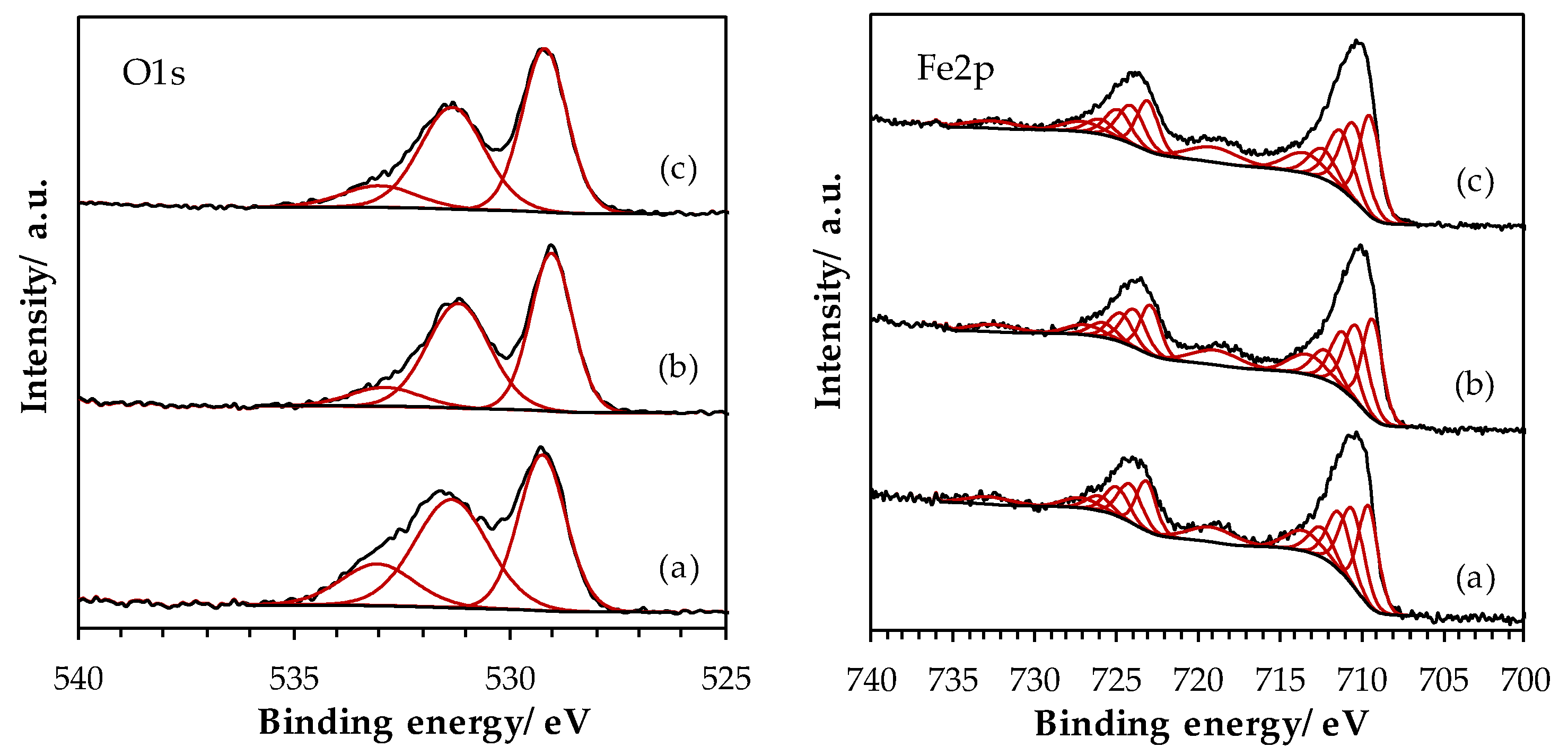
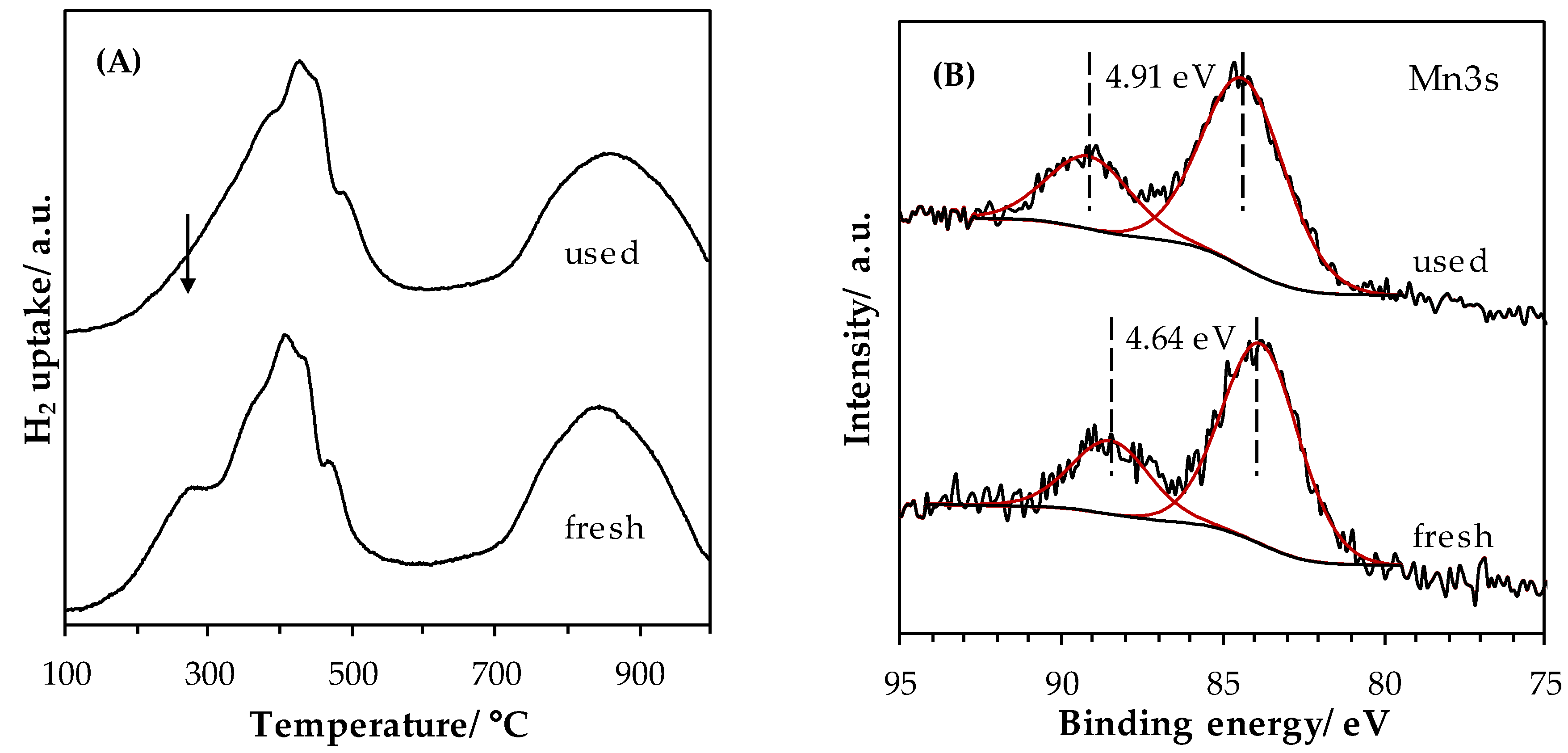
2.1.5. Surface Properties of Materials
2.2. Catalytic Performances
2.2.1. Catalysts Activity
2.2.2. Stability Tests
3. Materials and Methods
3.1. Synthesis
- -
- STEP 1, Solid state reaction—SSR: oxide precursors (La2O3 and Mn2O3 or Fe3O4) were homogeneously mixed in such a way as the molar La:B be equal to 1.0 and calcined for 4 h at 1100 °C under static air atmosphere to obtain the perovskite phase (confirmed by XRD analysis). Materials produced at this step were LaMn_SSR and LaFe_SSR.
- -
- STEP 2, High-energy ball milling—HEBM: a first high energy milling step was performed for 90 min (SPEX 8000D grinder with stainless steel equipment and balls (ø 1 × 11 mm, 2 × 12.5 mm), under static air atmosphere) at a revolution frequency of 17.5 Hz (1060 cycle·min−1).
- -
- STEP 3, Low-energy ball milling—LEBM: a second low energy ball milling was performed for 120 min (Union Process’ Svegvari attritor with stainless steel equipment and balls (ø 5 mm), with addition of a small amount of water (0.4 mL per g of material)) at a rotation speed of 450 rpm. The muddy sample was recovered using 500 mL of water and dried at 150 °C overnight.
3.2. Physico-Chemical Characterizations
3.3. Catalytic Activity Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Sample | BE (eV) | |||
|---|---|---|---|---|
| La3d5/2 | Mn2p3/2 | Fe2p3/2 | O1s 1 | |
| LaMn_SSR | 834.7 | 641.6 | 529.3/531.2/533.0 | |
| LaMn_HEBM | 834.1 | 641.6 | 529.2/531.3/533.3 | |
| LaMn_LEBM | 833.9 | 641.7 | 529.4/531.3/533.5 | |
| LaFe_SSR | 833.9 | 710.8 | 529.3/531.4/533.1 | |
| LaFe_HEBM | 833.7 | 710.6 | 529.0/531.2/532.9 | |
| LaFe_LEBM | 833.6 | 709.6 | 529.2/531.3/533.0 | |
References
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Publications office of the European Union website. European Union Emission Inventory Report 1990–2015 under the UNECE Convention on Long-Range Transboundary Air Pollution (LRTAP). Available online: https://publications.europa.eu/s/kInt (accessed on 6 March 2019).
- Donald, J.M.; Hooper, K.; Hopenhayn-Rich, C. Reproductive and developmental toxicity of toluene: A review. Environ. Health Perspect. 1991, 94, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.F.; Webb, P.J.; Lewis, A.C.; Reviejo, M.M. Quantifying small molecules in secondary organic aerosol formed during the photo-oxidation of toluene with hydroxyl radicals. Atmos. Environ. 2005, 39, 7263–7275. [Google Scholar] [CrossRef]
- Eur-LEX European Union Law website. Official Journal of the European Union, Commission Directive 2006/15/EC. Available online: https://eur-lex.europa.eu/eli/dir/2006/15/oj (accessed on 6 March 2019).
- Amann, M.; Lutz, M. The revision of the air quality legislation in the European Union related to ground-level ozone. J. Hazard. Mater. 2000, 78, 41–62. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Spivey, J.J.; Butt, J.B. Literature review: Deactivation of catalysts in the oxidation of volatile organic compounds. Catal. Today 1992, 11, 465–500. [Google Scholar] [CrossRef]
- Ihm, S.-K.; Jun, Y.-D.; Kim, D.-C.; Jeong, K.-E. Low-temperature deactivation and oxidation state of Pd/γ-Al2O3 catalysts for total oxidation of n-hexane. Catal. Today 2004, 93–95, 149–154. [Google Scholar] [CrossRef]
- Johnson Matthey website. PGM Market Report. Available online: http://www.platinum.matthey.com/ (accessed on 6 March 2019).
- Royer, S.; Duprez, D. Catalytic Oxidation of Carbon Monoxide over Transition Metal Oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Quiroz Torres, J.; Royer, S.; Bellat, J.-P.; Giraudon, J.-M.; Lamonier, J.-F. Formaldehyde: Catalytic Oxidation as a Promising Soft Way of Elimination. ChemSusChem 2013, 6, 578–592. [Google Scholar] [CrossRef]
- Twu, J.; Gallagher, P.K. Chapter 1: Preparation of Bulk and Supported Perovskites. In Properties and Applications of Perovskite-Type Oxides, 1st ed.; Tejuca, L.G., Fierro, J.L.G., Eds.; CRC Press: Boca Raton, FL, USA, 1992; pp. 1–23. [Google Scholar]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as substitutes of noble metals for heterogeneous catalysis: Dream or reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef] [PubMed]
- Wachowski, L. Influence of the method of preparation on the porous structure of perovskite oxides. Surf. Coat. Technol. 1986, 29, 303–311. [Google Scholar] [CrossRef]
- Royer, S.; Bérubé, F.; Kaliaguine, S. Effect of the synthesis conditions on the redox and catalytic properties in oxidation reactions of LaCo1−xFexO3. Appl. Catal. A Gen. 2005, 282, 273–284. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Y.; Guo, Y.; Lu, G.; Boreave, A.; Retailleau, L.; Baylet, A.; Giroir-Fendler, A. LaMnO3 perovskite oxides prepared by different methods for catalytic oxidation of toluene. Appl. Catal. B Environ. 2014, 148–149, 490–498. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Manuel Criado, J.; Delogu, F.; Dutková, E.; Gaffet, E.; José Gotor, F.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Alamdari, H.; Royer, S. Chapter 2: Mechanochemistry. In Perovskites and Related Mixed Oxides; Granger, P., Parvulescu, V.I., Prellier, W., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 25–46. [Google Scholar]
- Ciotonea, C.; Averlant, R.; Rochard, G.; Mamede, A.S.; Giraudon, J.M.; Alamdari, H.; Lamonier, J.-F.; Royer, S. A Simple and Green Procedure to Prepare Efficient Manganese Oxide Nanopowder for the Low Temperature Removal of Formaldehyde. ChemCatChem 2017, 9, 2366–2376. [Google Scholar] [CrossRef]
- Laassiri, S.; Bion, N.; Duprez, D.; Royer, S.; Alamdari, H. Clear microstructure–performance relationships in Mn-containing perovskite and hexaaluminate compounds prepared by activated reactive synthesis. Phys. Chem. Chem. Phys. 2014, 16, 4050–4060. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, B.; Kaliaguine, S. Methanol oxidation on LaBO3 (B = Co, Mn, Fe) perovskite-type catalysts prepared by reactive grinding. Appl. Catal. A Gen. 2008, 343, 29–38. [Google Scholar] [CrossRef]
- Kaliaguine, S.; Van Neste, A. Process for Synthesizing Metal Oxides and Metal Oxides Having A Perovskite or Perovskite-Like Crystal Structure. U.S. Patent 6,770,256, 3 August 2004. [Google Scholar]
- Ghasdi, M.; Alamdari, H.; Royer, S.; Adnot, A. Electrical and CO gas sensing properties of nanostructured La1−xCexCoO3 perovskite prepared by activated reactive synthesis. Sens. Actuators B Chem. 2011, 156, 147–155. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Laassiri, S.; Duprez, D.; Royer, S.; Alamdari, H. Solvent free synthesis of nanocrystalline hexaaluminate-type mixed oxides with high specific surface areas for CO oxidation reaction. Catal. Sci. Technol. 2011, 1, 1124–1127. [Google Scholar] [CrossRef]
- Kaliaguine, S.; Van Neste, A.; Szabo, V.; Gallot, J.E.; Bassir, M.; Muzychuk, R. Perovskite-type oxides synthesized by reactive grinding: Part I. Preparation and characterization. Appl. Catal. A. 2001, 209, 345–358. [Google Scholar] [CrossRef]
- Vogel, E.M.; Johnson, D.W., Jr.; Gallagher, P.K. Oxygen Stoichiometry in LaMn1-xCuxO3+y by Thermogravimetry. J. Am. Ceram. Soc. 1977, 60, 31–33. [Google Scholar] [CrossRef]
- Irusta, S.; Pina, M.P.; Menéndez, M.; Santamaría, J. Catalytic Combustion of Volatile Organic Compounds over La-Based Perovskites. J. Catal. 1998, 179, 400–412. [Google Scholar] [CrossRef]
- Faye, J.; Baylet, A.; Trentesaux, M.; Royer, S.; Dumeignil, F.; Duprez, D.; Valange, S.; Tatibouët, J.M. Influence of lanthanum stoichiometry in La1-xFeO3-δ perovskites on their structure and catalytic performance in CH4 total oxidation. Appl. Catal. B Environ. 2012, 126, 134–143. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Zhan, W.; Guo, Y.; Guo, Y.; Lu, G.; Baylet, A.; Giroir-Fendler, A. Catalytic oxidation of vinyl chloride emission over LaMnO3 and LaB0.2Mn0.8O3 (B = Co, Ni, Fe) catalysts. Appl. Catal. B Environ. 2013, 129, 509–516. [Google Scholar] [CrossRef]
- Fierro, J.L.G.; Gonzalez Tejuca, L. Non-stoichiometric surface behaviour of LaMO3 oxides as evidenced by XPS. Appl. Surf. Sci. 1987, 27, 453–457. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, L.; Dai, H.; He, H.; Au, C.T. Strontium-Doped Lanthanum Cobaltite and Manganite: Highly Active Catalysts for Toluene Complete Oxidation. Ind. Eng. Chem. Res. 2008, 47, 8175–8183. [Google Scholar] [CrossRef]
- Fierro, J.L.G. Structure and composition of perovskite surface in relation to adsorption and catalytic properties. Catal. Today 1990, 8, 153–174. [Google Scholar] [CrossRef]
- Sunding, M.F.; Hadidi, K.; Diplas, S.; Løvvik, O.M.; Norby, T.E.; Gunnæs, A.E. XPS characterisation of in situ treated lanthanum oxide and hydroxide using tailored charge referencing and peak fitting procedures. J. Electron. Spectros. Relat. Phenom. 2011, 184, 399–409. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Bagus, P.S.; Broer, R.; Ilton, E.S. A new near degeneracy effect for photoemission in transition metals. Chem. Phys. Lett. 2004, 94, 150–154. [Google Scholar] [CrossRef]
- Galakhov, V.R.; Demeter, M.; Bartkowski, S.; Neumann, M.; Ovechkina, N.A.; Kurmaev, E.Z.; Lobachevskaya, N.I.; Mukovskii, Y.M.; Mitchell, J.; Ederer, D.L. Mn exchange splitting in mixed-valence manganites. Phys. Rev. B 2002, 65, 113102. [Google Scholar] [CrossRef]
- McCarty, J.G.; Wise, H. Perovskite catalysts for methane combustion. Catal. Today 1990, 8, 231–248. [Google Scholar] [CrossRef]
- Pecchi, G.; Jiliberto, M.G.; Delgado, E.J.; Cadús, L.E.; Fierro, J.L.G. Effect of B-site cation on the catalytic activity of La1−xCaxBO3 (B = Fe, Ni) perovskite-type oxides for toluene combustion. J. Chem. Technol. Biotechnol. 2011, 86, 1067–1073. [Google Scholar] [CrossRef]
- Behar, S.; Gómez-Mendoza, N.A.; Gómez-García, M.Á.; Świerczyński, D.; Quignard, F.; Tanchoux, N. Study and modelling of kinetics of the oxidation of VOC catalyzed by nanosized Cu–Mn spinels prepared via an alginate route. Appl. Catal. A Gen. 2015, 504, 203–210. [Google Scholar] [CrossRef]





| Sample | XRD Phase | Dcryst (nm) | SSA 2 (m2·g−1) | Fe cont. 3 (at.%) |
|---|---|---|---|---|
| LaMn_SSR | P, La2O3, La(OH)3, Mn3O4 | >500 1 | 1.0 | 0.0 |
| LaMn_HEBM | P, La2O3 | 14 | 3.5 | 0.7 |
| LaMn_LEBM | P | 16 | 9.9 | 2.9 |
| LaFe_SSR | P, La(OH)3, Fe2O3 | >500 1 | 1.6 | - |
| LaFe_HEBM | P, Fe2O3 | 17 | 3.4 | - |
| LaFe_LEBM | P, Fe2O3 | 22 | 18.8 | - |
| Sample | T1,max (°C) | T2,max (°C) | H2 Uptake 1 (mmol(H2)·g−1) | TM AOS 2 |
|---|---|---|---|---|
| LaMn_SSR | 512 | 882 | 1.04 (2.63) | 3.3 |
| LaMn_HEBM | 390 | 806 | 1.54 (3.02) | 3.4 |
| LaMn_LEBM | 404 | 845 | 2.09 (3.14) | 3.4 |
| LaFe_SSR | 523 | - | 0.89 | 2.4 |
| LaFe_HEBM | 490 | - | 0.92 | 2.5 |
| LaFe_LEBM | 489 | - | 1.18 | 2.6 |
| Sample | La (at.%) | Mn (at.%) | Fe (at.%) | La/(Fe+Mn) (-) | Ototal (at.%) | OI (at.%) | OII (at.%) | OIII (at.%) | AOS 1 |
|---|---|---|---|---|---|---|---|---|---|
| LaMn_SSR | 19.2 | 9.9 | - | 2.01 | 70.9 | 20.9 | 44.9 | 5.2 | 3.2 |
| LaMn_HEBM | 16.4 | 11.3 | n.q. | 1.45 | 72.3 | 29.4 | 32.3 | 10.6 | 3.8 |
| LaMn_LEBM | 16.3 | 11.5 | 1.4 | 1.26 | 70.7 | 33.0 | 32.8 | 4.9 | 3.7 |
| LaFe_SSR | 14.4 | - | 9.1 | 1.58 | 76.5 | 30.7 | 33.2 | 12.7 | - |
| LaFe_HEBM | 19.9 | - | 10.0 | 1.99 | 70.1 | 32.0 | 31.7 | 6.4 | - |
| LaFe_LEBM | 19.0 | - | 11.1 | 1.71 | 69.9 | 33.2 | 29.5 | 7.2 | - |
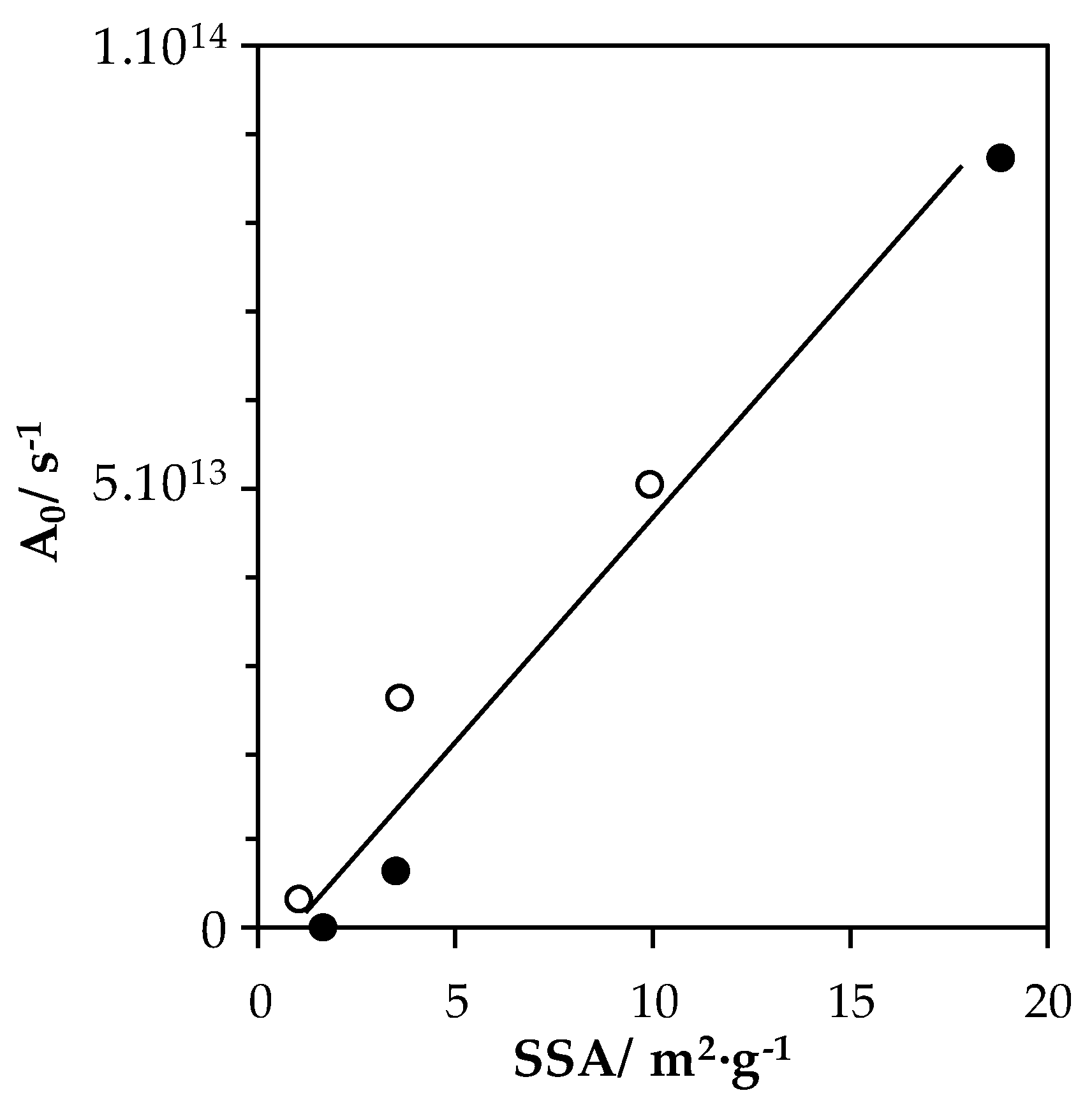
| Sample | T10 (°C) | T50 (°C) | T90 (°C) | Activity 1 (mmol·s−1·m−2) | Ea 2 (kJ·mol−1) | A0 3 (s−1) |
|---|---|---|---|---|---|---|
| LaMn_SSR | 290 | / | / | / | 126 | 3.2 × 1012 |
| LaMn_HEBM | 251 | 283 | 317 | 8.68 × 10−6 | 146 | 2.6 × 1013 |
| LaMn_LEBM | 242 | 271 | 295 | 6.41 × 10−6 | 140 | 5.0 × 1013 |
| LaFe_SSR | 325 | / | / | / | 164 | 3.6 × 1011 |
| LaFe_HEBM | 273 | 301 | 323 | 2.44 × 10−6 | 155 | 6.5 × 1012 |
| LaFe_LEBM | 234 | 261 | 283 | 5.39 × 10−6 | 137 | 8.7 × 1013 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidinger, B.; Royer, S.; Alamdari, H.; Giraudon, J.-M.; Lamonier, J.-F. Reactive Grinding Synthesis of LaBO3 (B: Mn, Fe) Perovskite; Properties for Toluene Total Oxidation. Catalysts 2019, 9, 633. https://doi.org/10.3390/catal9080633
Heidinger B, Royer S, Alamdari H, Giraudon J-M, Lamonier J-F. Reactive Grinding Synthesis of LaBO3 (B: Mn, Fe) Perovskite; Properties for Toluene Total Oxidation. Catalysts. 2019; 9(8):633. https://doi.org/10.3390/catal9080633
Chicago/Turabian StyleHeidinger, Bertrand, Sébastien Royer, Houshang Alamdari, Jean-Marc Giraudon, and Jean-François Lamonier. 2019. "Reactive Grinding Synthesis of LaBO3 (B: Mn, Fe) Perovskite; Properties for Toluene Total Oxidation" Catalysts 9, no. 8: 633. https://doi.org/10.3390/catal9080633
APA StyleHeidinger, B., Royer, S., Alamdari, H., Giraudon, J.-M., & Lamonier, J.-F. (2019). Reactive Grinding Synthesis of LaBO3 (B: Mn, Fe) Perovskite; Properties for Toluene Total Oxidation. Catalysts, 9(8), 633. https://doi.org/10.3390/catal9080633







