Insights over Titanium Modified FeMgOx Catalysts for Selective Catalytic Reduction of NOx with NH3: Influence of Precursors and Crystalline Structures
Abstract
1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Activity Test
2.3. Catalyst Characterization
3. Results and Discussion
3.1. Effect of Different Precursors on Catalytic Performance Over Titanium Modified FeMgOx Catalysts
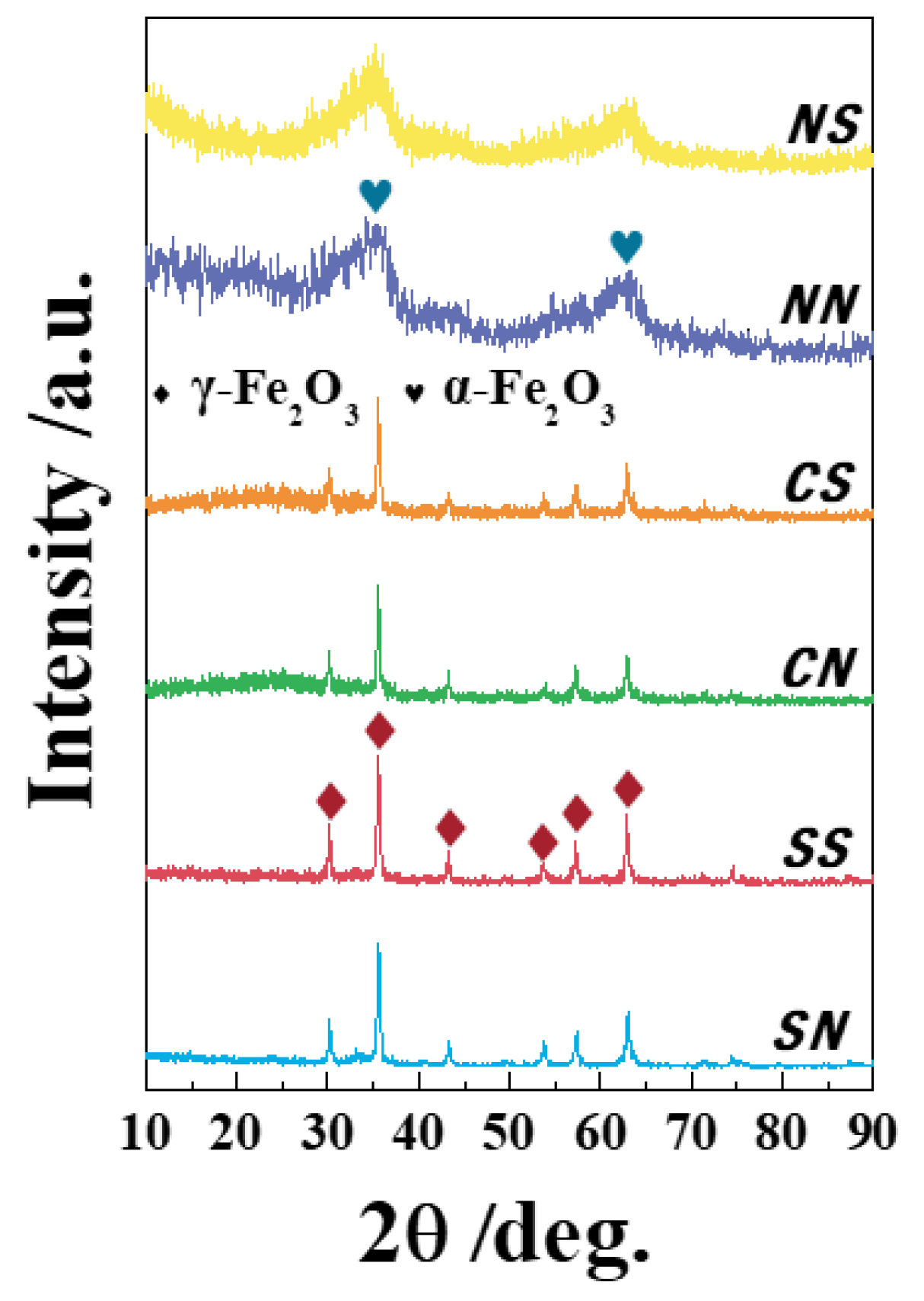
3.2. X-ray Diffraction (XRD) Patterns
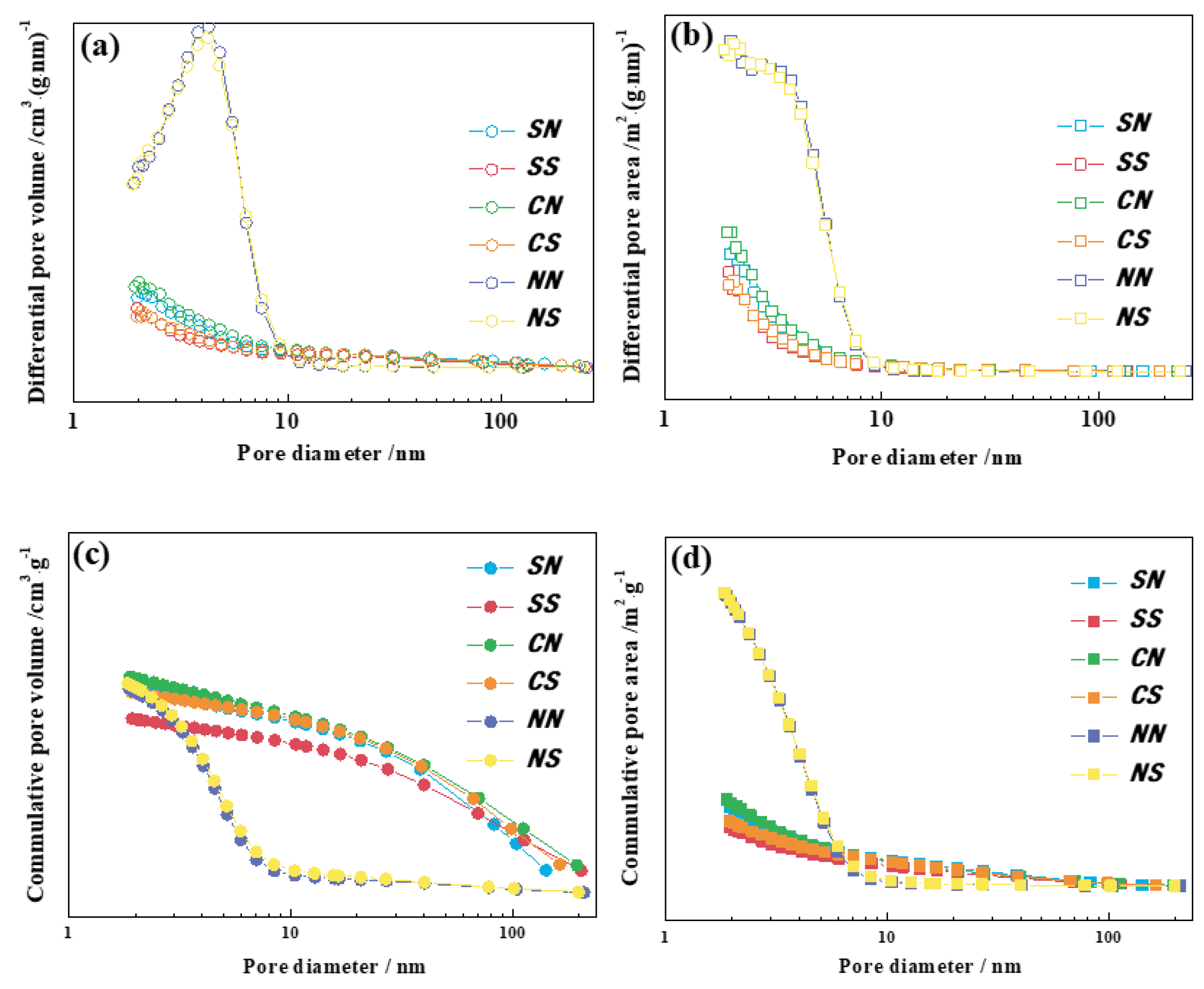
3.3. N2-Adsorption-Desorption
3.4. SEM and Energy Dispersive Spectrometer (EDS)
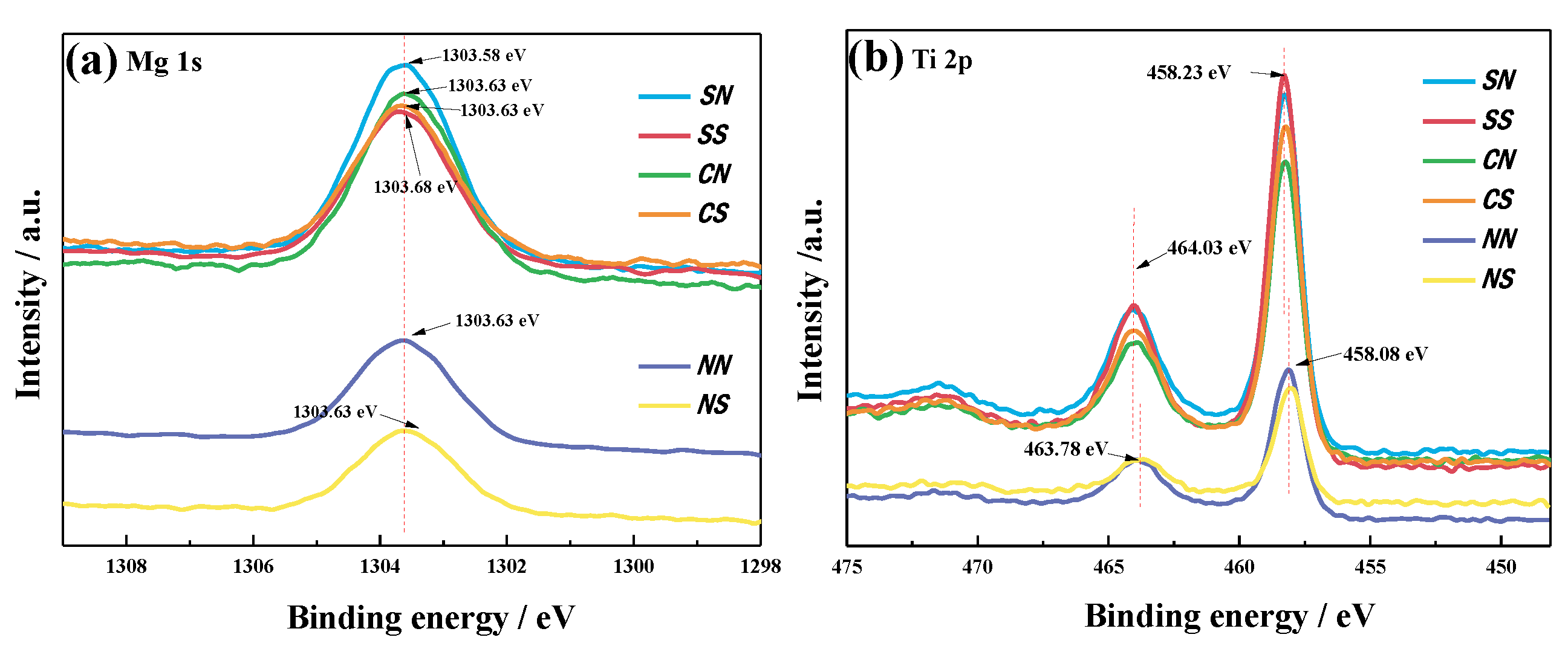
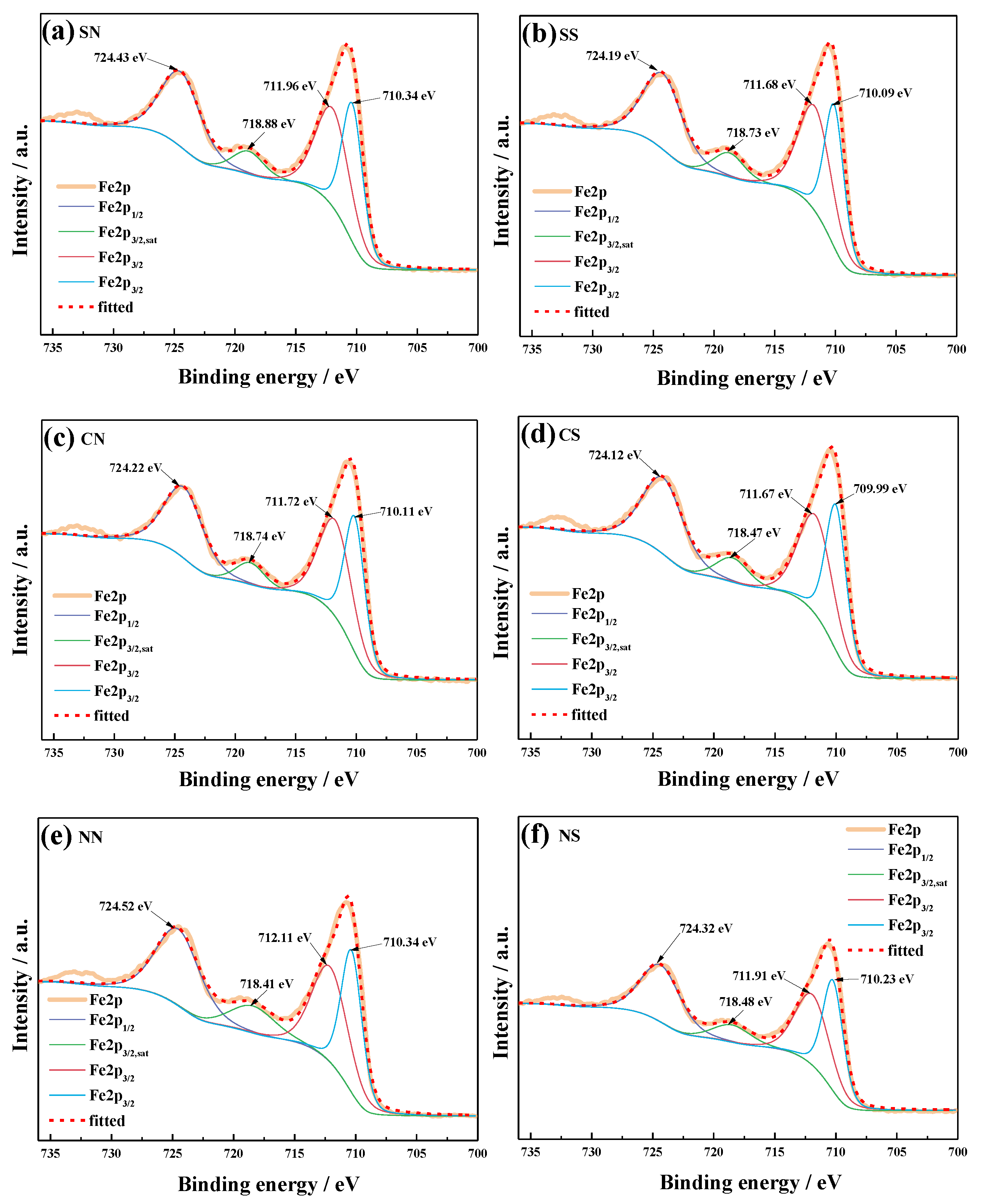
3.5. X-ray Photoelectron Spectroscopy (XPS)
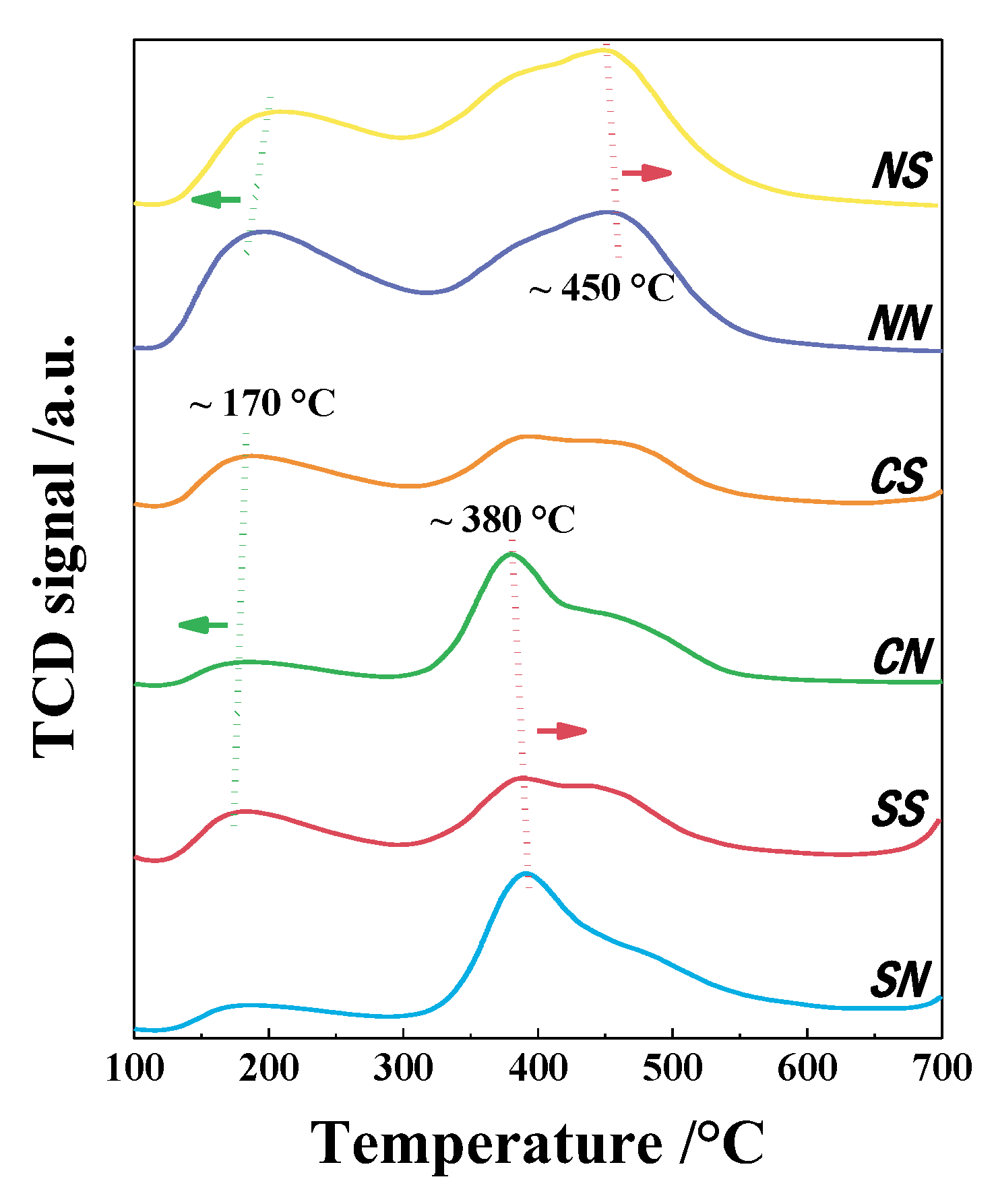
3.6. NH3-TPD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Baek, J.; Lee, S.; Park, J.; Jeong, J.; Hwang, R.; Ko, C.; Jeon, S.; Choi, T.; Yi, K. Effects of steam introduction on deactivation of Fe-BEA catalyst in NH3-SCR of N2O and NO. J. Ind. Eng. Chem. 2017, 48, 194–201. [Google Scholar] [CrossRef]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Wang, D.; Luo, J.; Yang, Q.; Yan, J.; Zhang, K.; Zhang, W.; Peng, Y.; Li, J.; Crittenden, J. Deactivation mechanism of multipoisons in cement furnace flue gas on selective catalytic reduction catalysts. Environ. Sci. Technol. 2019, 53, 6937–6944. [Google Scholar] [CrossRef] [PubMed]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.; Peng, Y.; Si, W.; Li, X.; Li, B.; Li, J. Dechlorination of chlorobenzene on vanadium-based catalysts for low-temperature SCR. Chem. Commun. 2018, 54, 2032–2035. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Yang, R.T. Performance and kinetics study for low-temperature SCR of NO with NH3 over MnOx–CeO2 catalyst. J. Catal. 2003, 217, 434–441. [Google Scholar] [CrossRef]
- France, L.J.; Yang, Q.; Li, W.; Chen, Z.; Guang, J.; Guo, D.; Wang, L.; Li, X. Ceria modified FeMnOx—Enhanced performance and sulphur resistance for low-temperature SCR of NOx. Appl. Catal. B Environ. 2017, 206, 203–215. [Google Scholar] [CrossRef]
- Shelef, M. Selective catalytic reduction of NOx with N-free reductants. Chem. Rev. 1995, 95, 209–225. [Google Scholar] [CrossRef]
- Wang, D.; Peng, Y.; Yang, Q.; Xiong, S.; Li, J.; Crittenden, J. Performance of Modified LaxSr1–xMnO3 Perovskite Catalysts for NH3 Oxidation: TPD, DFT, and Kinetic Studies. Environ. Sci. Technol. 2018, 52, 7443–7449. [Google Scholar] [CrossRef]
- Shi, X.; Liu, F.; Xie, L.; Shan, W.; He, H. NH3-SCR performance of fresh and hydrothermally aged Fe-ZSM-5 in standard and fast selective catalytic reduction reactions. Environ. Sci. Technol. 2013, 47, 3293–3298. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Park, R.; Jeon, J.; Kim, D.; Jung, S.; Kim, S.; Park, Y. Effect of surfactant, HCl and NH3 treatments on the regeneration of waste activated carbon used in selective catalytic reduction unit. J. Ind. Eng. Chem. 2015, 32, 109–112. [Google Scholar] [CrossRef]
- Fan, Y.; Ling, W.; Huang, B.; Dong, L.; Yu, C.; Xi, H. The synergistic effects of cerium presence in the framework and the surface resistance to SO2 and H2O in NH3-SCR. J. Ind. Eng. Chem. 2017, 56, 108–119. [Google Scholar] [CrossRef]
- Boningari, T.; Ettireddy, P.R.; Somogyvari, A.; Liu, Y.; Vorontsov, A.; McDonald, C.A.; Smirniotis, P.G. Influence of elevated surface texture hydrated titania on Ce-doped Mn/TiO2 catalysts for the low-temperature SCR of NOx under oxygen-rich conditions. J. Catal. 2016, 325, 145–155. [Google Scholar] [CrossRef]
- Aguilar-Romero, M.; Camposeco, R.; Castillo, S.; Marín, J.; Rodríguez-González, V.; García-Serrano, L.; Mejía-Centeno, I. Acidity, surface species, and catalytic activity study on V2O5-WO3/TiO2 nanotube catalysts for selective NO reduction by NH3. Fuel 2017, 198, 123–133. [Google Scholar] [CrossRef]
- Pappas, D.K.; Boningari, T.; Boolchand, P.; Smirniotis, P.G. Novel manganese oxide confined interweaved titania nanotubes for the low-temperature Selective Catalytic Reduction (SCR) of NOx by NH3. J. Catal. 2016, 334, 1–13. [Google Scholar] [CrossRef]
- Song, I.; Youn, S.; Lee, H.; Lee, S.G.; Cho, S.; Kim, D. Effects of microporous TiO2 support on the catalytic and structural properties of V2O5/microporous TiO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2017, 210, 421–431. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations. J. Catal. 2012, 288, 74–83. [Google Scholar] [CrossRef]
- He, Y.; Ford, M.E.; Zhu, M.; Liu, Q.; Tumuluri, U.; Wu, Z.; Wachs, I.E. Influence of catalyst synthesis method on selective catalytic reduction (SCR) of NO by NH3 with V2O5-WO3/TiO2 catalysts. Appl. Catal. B 2016, 193, 141–150. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Xu, B.; Cao, Y.; Feng, X.; Sun, M.; Gong, M.; Chen, Y. Effectively promote catalytic performance by adjusting W/Fe molar ratio of FeWx/Ce0. 68Zr0. 32O2 monolithic catalyst for NH3-SCR. J. Ind. Eng. Chem. 2016, 36, 334–345. [Google Scholar] [CrossRef]
- Wang, D.; Peng, Y.; Xiong, S.; Li, B.; Gan, L.; Lu, C.; Chen, J.; Ma, Y.; Li, J. De-reducibility mechanism of titanium on maghemite catalysts for the SCR reaction: An in situ DRIFTS and quantitative kinetics study. Appl. Catal. B 2018, 221, 556–564. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Zhang, C.; Feng, Z.; Zheng, L.; Xie, Y.; Hu, T. Selective catalytic reduction of NO with NH3 over iron titanate catalyst: Catalytic performance and characterization. Appl. Catal. B Environ. 2010, 96, 408–420. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Wang, C.; Chen, J.; Ma, L.; Chang, H.; Chen, L.; Peng, Y.; Yan, N. Fe–Ti spinel for the selective catalytic reduction of NO with NH3: Mechanism and structure–activity relationship. Appl. Catal. B Environ. 2012, 117, 73–80. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Chen, B.; Zhu, T.; Ma, L. Novel Fe–Ce–Ti catalyst with remarkable performance for the selective catalytic reduction of NOx by NH3. Catal. Sci. Technol. 2016, 6, 6688–6696. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Chen, Y.; Gao, X.; Zhang, L. Supported metal sulfates on Ce–TiOx as catalysts for NH3–SCR of NO: High resistances to SO2 and potassium. J. Ind. Eng. Chem. 2016, 36, 271–278. [Google Scholar] [CrossRef]
- Xiong, Z.; Peng, B.; Zhou, F.; Wu, C.; Lu, W.; Jin, J.; Ding, S. Magnetic iron-cerium-tungsten mixed oxide pellets prepared through critic acid sol-gel process assisted by microwave irradiation for selective catalytic reduction of NOx with NH3. Powder Technol. 2017, 319, 19–25. [Google Scholar] [CrossRef]
- Xiong, Z.; Liu, J.; Zhou, F.; Liu, D.; Lu, W.; Jin, J.; Ding, S. Selective catalytic reduction of NOx with NH3 over iron-cerium-tungsten mixed oxide catalyst prepared by different methods. Appl. Surf. Sci. 2017, 406, 218–225. [Google Scholar] [CrossRef]
- Xu, H.; Xie, J.; Ma, Y.; Qu, Z.; Zhao, S.; Chen, W.; Huang, W.; Yan, N. The cooperation of FeSn in a MnOx complex sorbent used for capturing elemental mercury. Fuel 2015, 140, 803–809. [Google Scholar] [CrossRef]
- Shu, Y.; Aikebaier, T.; Quan, X.; Chen, S.; Yu, H. Selective catalytic reaction of NOx with NH3 over Ce–Fe/TiO2-loaded wire-mesh honeycomb: Resistance to SO2 poisoning. Appl. Catal. B Environ. 2014, 150, 630–635. [Google Scholar] [CrossRef]
- Liu, Z.; Su, H.; Chen, B.; Li, J.; Woo, S. Activity enhancement of WO3 modified Fe2O3 catalyst for the selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2016, 299, 255–262. [Google Scholar] [CrossRef]
- Foo, R.; Vazhnova, T.; Lukyanov, D.B.; Millington, P.; Collier, J.; Rajaram, R.; Golunski, S. Formation of reactive Lewis acid sites on Fe/WO3–ZrO2 catalysts for higher temperature SCR applications. Appl. Catal. B Environ. 2015, 162, 174–179. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Jensen, A.D.; Siret, B.; Tabaries, F.; Fehrmann, R. Mn/TiO2 and Mn–Fe/TiO2 catalysts synthesized by deposition precipitation—Promising for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2015, 165, 628–635. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, L.; Wang, X.; Zou, W.; Cao, Y.; Sun, J.; Tang, C.; Gao, F.; Deng, Y.; Dong, L. Synthesis, characterization and catalytic performance of FeMnTiOx mixed oxides catalyst prepared by a CTAB-assisted process for mid-low temperature NH3-SCR. Appl. Catal. A Gen. 2015, 505, 235–242. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Xiong, Z.; Wu, C.; Hu, Q.; Wang, Y.; Jin, J.; Lu, C.; Guo, D. Promotional effect of microwave hydrothermal treatment on the low-temperature NH3-SCR activity over iron-based catalyst. Chem. Eng. J. 2016, 286, 459–466. [Google Scholar] [CrossRef]
- Cao, F.; Su, S.; Xiang, J.; Wang, P.; Hu, S.; Sun, L.; Zhang, A. The activity and mechanism study of Fe–Mn–Ce/γ-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3. Fuel 2015, 139, 232–239. [Google Scholar] [CrossRef]
- Casanova, M.; Llorca, J.; Sagar, A.; Schermanz, K.; Trovarelli, A. Mixed iron–erbium vanadate NH3-SCR catalysts. Catal. Today 2015, 241, 159–168. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, Z.; Yang, H.; Wang, C. Comparison study of Cu-Fe-Ti and Co-Fe-Ti oxide catalysts for selective catalytic reduction of NO with NH3 at low temperature. J. Collid Interface Sci. 2016, 478, 11–21. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Zhang, C.; Shan, W.; Shi, X. Mechanism of the selective catalytic reduction of NOx with NH3 over environmental-friendly iron titanate catalyst. Catal. Today 2011, 175, 18–25. [Google Scholar] [CrossRef]
- Liu, F.; He, H. Structure−Activity Relationship of Iron Titanate Catalysts in the Selective Catalytic Reduction of NOx with NH3. J. Phys. Chem. C 2010, 114, 16929–16936. [Google Scholar] [CrossRef]
- Ma, L.; Li, J.; Ke, R.; Fu, L. Catalytic Performance, Characterization, and Mechanism Study of Fe2(SO4)3/TiO2 Catalyst for Selective Catalytic Reduction of NOx by Ammonia. J. Phys. Chem. C 2011, 115, 7603–7612. [Google Scholar] [CrossRef]
- Xu, L.; Niu, S.; Lu, C.; Wang, D.; Zhang, K.; Li, J. NH3-SCR performance and characterization over magnetic iron-magnesium mixed oxide catalysts. Korean J. Chem. Eng. 2017, 34, 1576–1583. [Google Scholar] [CrossRef]
- Xu, L.; Niu, S.; Wang, D.; Lu, C.; Zhang, Q.; Zhang, K.; Li, J. Selective catalytic reduction of NOx with NH3 over titanium modified FexMgyOz catalysts: Performance and characterization. J. Ind. Eng. Chem. 2018, 63, 391–404. [Google Scholar] [CrossRef]
- Xu, L.; Niu, S.; Lu, C.; Zhang, Q.; Li, J. Influence of calcination temperature on Fe0.8Mg0.2Oz catalyst for selective catalytic reduction of NOx with NH3. Fuel 2018, 219, 248–258. [Google Scholar] [CrossRef]
- Wang, D.; Peng, Y.; Yang, Q.; Hu, F.; Li, J.; Crittenden, J. NH3-SCR performance of WO3 blanketed CeO2 with different morphology: Balance of surface reducibility and acidity. Catal. Today 2019, 332, 42–48. [Google Scholar] [CrossRef]




| Label | Catalyst | Precursor |
|---|---|---|
| SN | Ti modified FeMgOx | FeSO4·7H2O, Mg(NO3)2·6H2O |
| SS | FeSO4·7H2O, MgSO4·7H2O | |
| CN | FeCl2·7H2O, Mg(NO3)2·6H2O | |
| CS | FeCl2·7H2O, MgSO4·7H2O | |
| NN | Fe(NO3)3·9H2O, Mg(NO3)2·6H2O | |
| NS | Fe(NO3)3·9H2O, MgSO4·7H2O |
| Catalysts | SBET/m2·g−1 | VBJH/cm3·g−1 | Average Pore Diameter/nm |
|---|---|---|---|
| SN | 55.1245 | 0.2291 | 16.2670 |
| SS | 46.6441 | 0.1951 | 18.6213 |
| CN | 58.6066 | 0.2413 | 15.5461 |
| CS | 51.8948 | 0.2243 | 19.2990 |
| NN | 181.3934 | 0.2279 | 4.3710 |
| NS | 180.4130 | 0.2340 | 4.4512 |
| Samples | t-Plot Microporous Area/m2·g−1 | t-Plot External Surface Area/m2·g−1 | t-Plot Microporous Volume/cm3·g−1 |
|---|---|---|---|
| SN | 0.5467 | 54.5777 | 6.5000 × 10−5 |
| SS | 5.1114 | 41.5327 | 2.5290 × 10−3 |
| CN | 4.3249 | 54.2817 | 2.0670 × 10−3 |
| CS | 6.5865 | 45.3083 | 3.3030 × 10−3 |
| NN | 2.7968 | 178.5966 | 3.5550 × 10−4 |
| NS | 3.1251 | 177.2879 | 2.54 × 10−3 |
| Catalyst | Percentage by Weight/wt % | Percentage by Atomicity/at % | ||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Mg | Ti | O | Fe | Mg | Ti | O | |
| SN | 59.06 | 1.68 | 4.79 | 34.47 | 30.82 | 2.85 | 3.21 | 63.12 |
| SS | 59.25 | 3.10 | 4.73 | 32.92 | 31.72 | 3.81 | 2.95 | 61.52 |
| CN | 60.66 | 3.64 | 4.60 | 31.10 | 33.16 | 4.57 | 2.93 | 59.34 |
| CS | 59.33 | 3.18 | 4.40 | 33.09 | 31.68 | 3.90 | 2.74 | 61.68 |
| NN | 68.39 | 2.90 | 5.30 | 23.41 | 41.97 | 4.09 | 3.79 | 50.16 |
| NS | 67.93 | 3.47 | 4.88 | 23.72 | 41.53 | 4.45 | 3.17 | 50.85 |
| Catalyst | Fe 2p/% | Mg 1s/% | O 1s/% | Ti 2p/% |
|---|---|---|---|---|
| SN | 38.88 | 6.92 | 46.85 | 7.35 |
| SS | 46.35 | 7.89 | 37.20 | 8.57 |
| CN | 45.75 | 9.54 | 37.75 | 6.95 |
| CS | 47.49 | 7.47 | 37.48 | 7.55 |
| NN | 56.74 | 6.43 | 32.92 | 3.91 |
| NS | 56.65 | 6.20 | 32.86 | 4.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Yang, Q.; Hu, L.; Wang, D.; Peng, Y.; Shao, Z.; Lu, C.; Li, J. Insights over Titanium Modified FeMgOx Catalysts for Selective Catalytic Reduction of NOx with NH3: Influence of Precursors and Crystalline Structures. Catalysts 2019, 9, 560. https://doi.org/10.3390/catal9060560
Xu L, Yang Q, Hu L, Wang D, Peng Y, Shao Z, Lu C, Li J. Insights over Titanium Modified FeMgOx Catalysts for Selective Catalytic Reduction of NOx with NH3: Influence of Precursors and Crystalline Structures. Catalysts. 2019; 9(6):560. https://doi.org/10.3390/catal9060560
Chicago/Turabian StyleXu, Liting, Qilei Yang, Lihua Hu, Dong Wang, Yue Peng, Zheru Shao, Chunmei Lu, and Junhua Li. 2019. "Insights over Titanium Modified FeMgOx Catalysts for Selective Catalytic Reduction of NOx with NH3: Influence of Precursors and Crystalline Structures" Catalysts 9, no. 6: 560. https://doi.org/10.3390/catal9060560
APA StyleXu, L., Yang, Q., Hu, L., Wang, D., Peng, Y., Shao, Z., Lu, C., & Li, J. (2019). Insights over Titanium Modified FeMgOx Catalysts for Selective Catalytic Reduction of NOx with NH3: Influence of Precursors and Crystalline Structures. Catalysts, 9(6), 560. https://doi.org/10.3390/catal9060560




