Direct Conversion of CO2 into Dimethyl Ether over Al2O3/Cu/ZnO Catalysts Prepared by Sequential Precipitation
Abstract
:1. Introduction
2. Results and Discussion
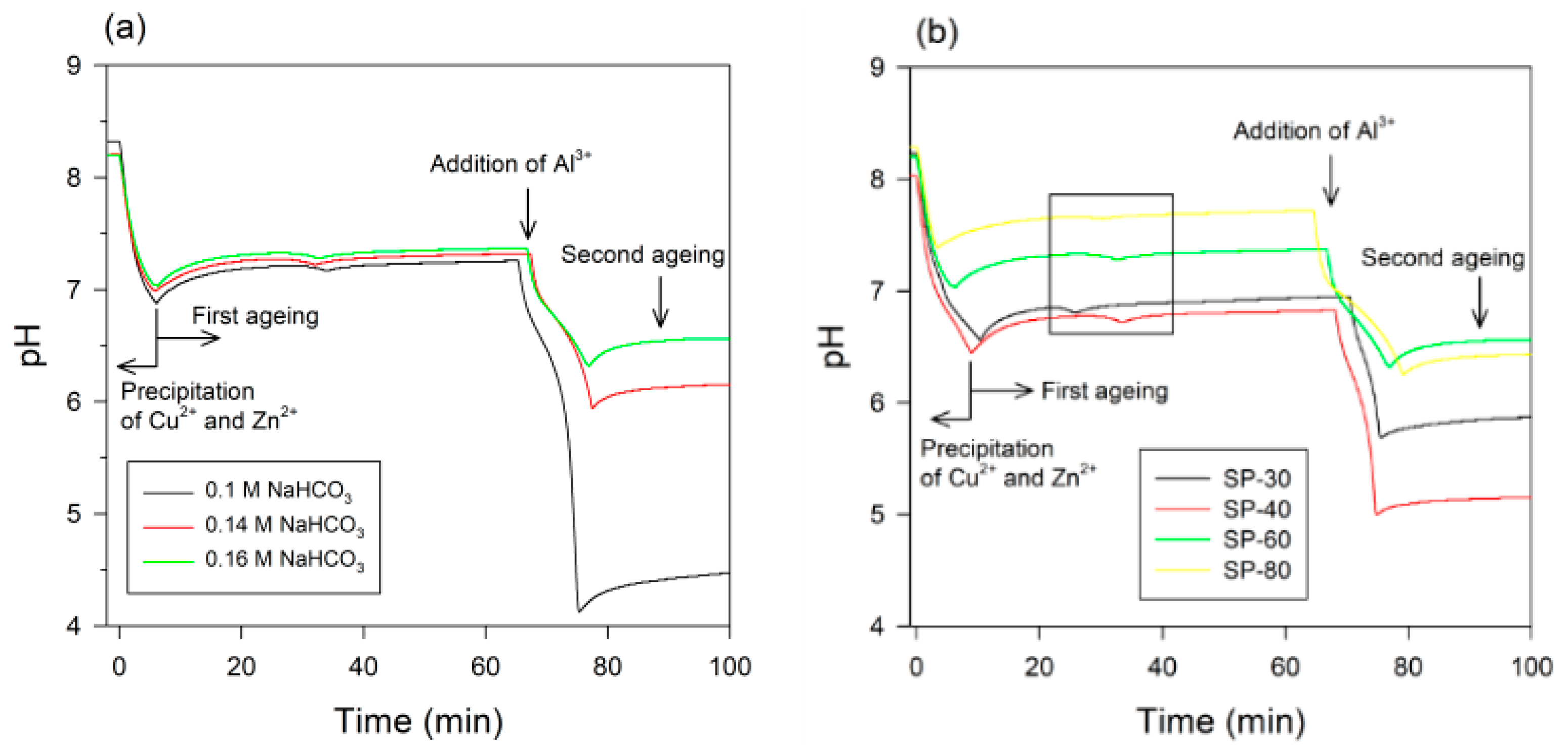
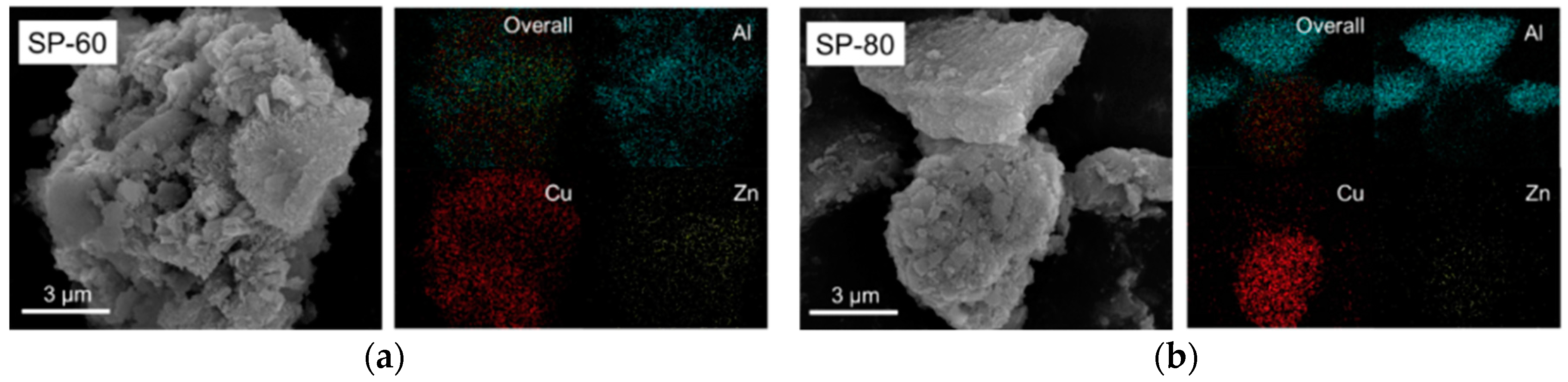
2.1. Preparation of Al2O3/Cu/ZnO Catalyst
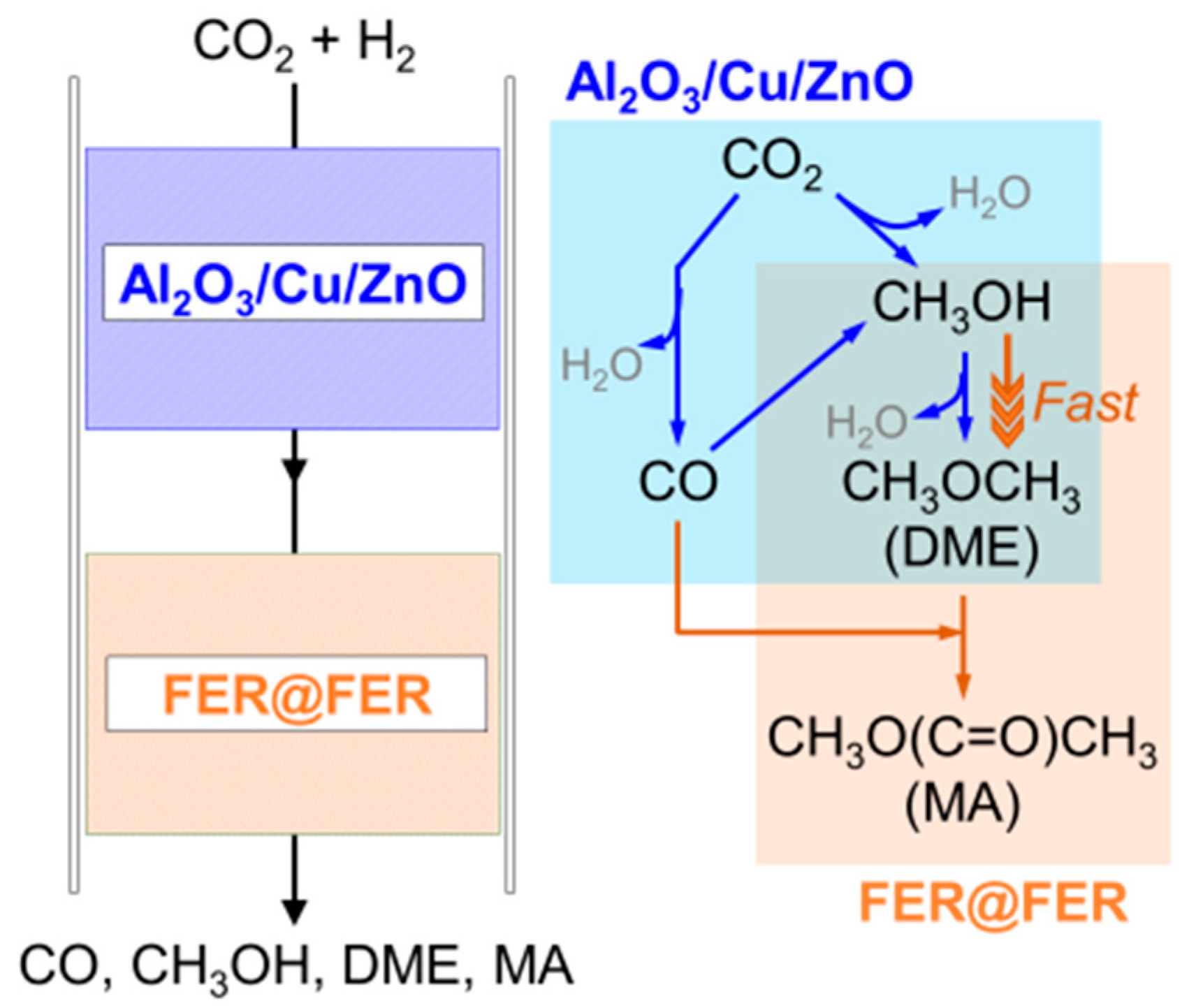
2.2. Direct Conversion of CO2 to DME over Al2O3/Cu/ZnO Catalysts
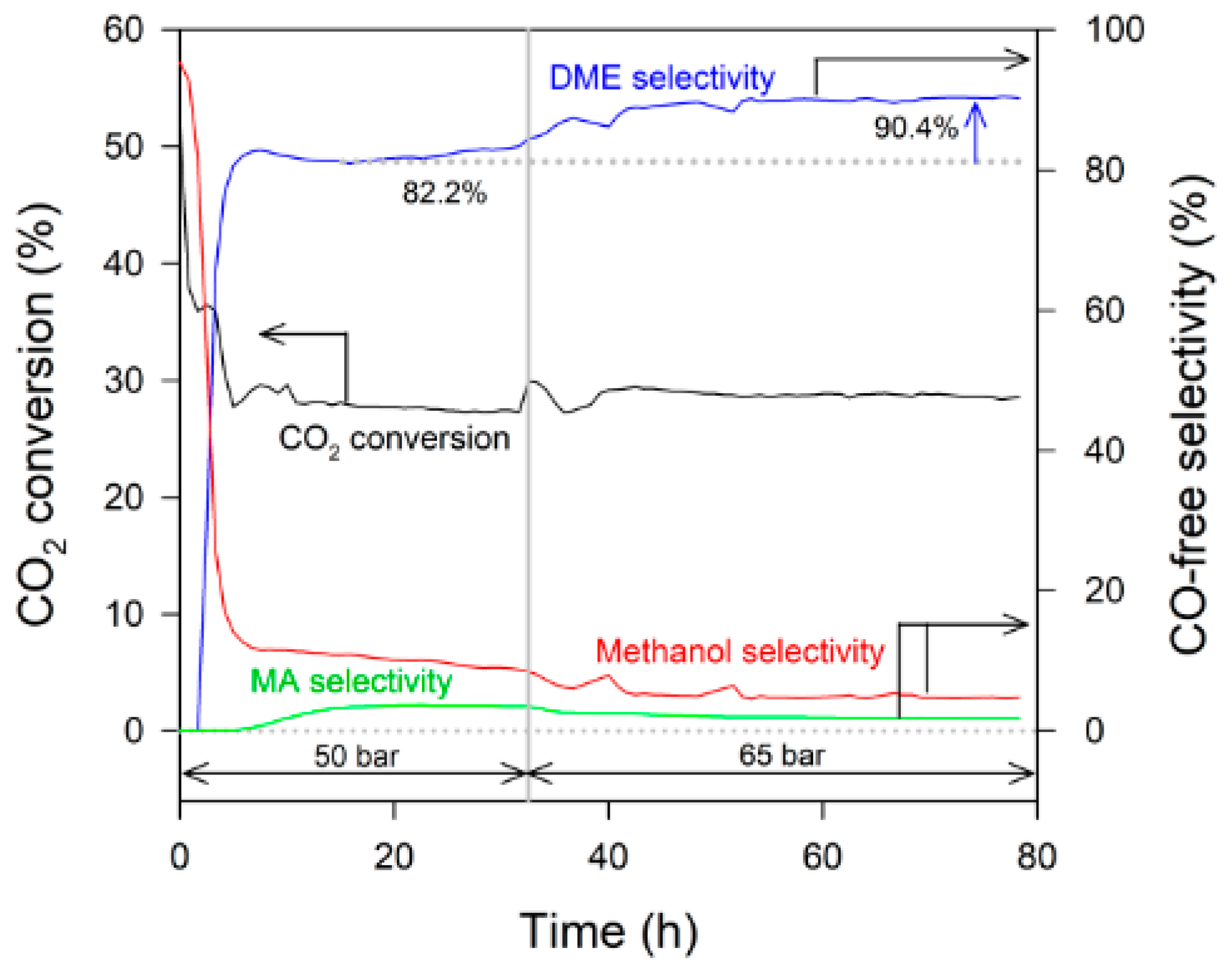
2.3. Attempts to Improve the Selectivity to DME
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.3. Activity Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marlin, D.S.; Sarron, E.; Sigurbjörnsson, Ó. Process advantages of direct CO2 to methanol synthesis. Front. Chem. 2018, 6, 446. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Catalysis chemistry of dimethyl ether synthesis. ACS Catal. 2014, 4, 3346–3356. [Google Scholar] [CrossRef]
- Saravanan, K.; Ham, H.; Tsubaki, N.; Bae, J.W. Recent progress for direct synthesis of dimethyl ether from syngas on the heterogeneous bifunctional hybrid catalysts. Appl. Catal. B 2017, 217, 494–522. [Google Scholar] [CrossRef]
- Catizzone, E.; Bounura, G.; Migliori, M.; Frusteri, F.; Giordano, G. CO2 recycling to dimethyl ether: State-of-the-art and perspectives. Molecules 2018, 23, 31. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Tsubaki, N.; Shamoto, J.; Yoneyama, Y.; Zhang, Y. Confinement effect and synergistic function of H-ZSM-5/Cu-ZnO-Al2O3 capsule catalyst for one-step controlled synthesis. J. Am. Chem. Soc. 2010, 132, 8129–8136. [Google Scholar] [CrossRef]
- Jeong, C.; Ham, H.; Bae, J.W.; Kang, D.-C.; Shin, C.-H.; Baik, J.H.; Suh, Y.-W. Facile structure tuning of a methanol-synthesis catalyst towards the direct synthesis of dimethyl ether from syngas. ChemCatChem 2017, 9, 4484–4489. [Google Scholar] [CrossRef]
- Gherardi, P.; Ruggeri, O.; Trifiro, F.; Vaccari, A.; Piero, G.D.; Manara, G.; Notari, B. Preparation of Cu-Zn-Al mixed hydroxycarbonates precursors of catalysts for the synthesis of methanol at low pressure. Stud. Surf. Sci. Catal. 1983, 16, 723–773. [Google Scholar]
- Kondrat, S.A.; Smith, P.J.; Lu, L.; Bartley, J.K.; Taylor, S.H.; Spencer, M.S.; Kelly, G.J.; Park, C.W.; Kiely, C.J.; Hutchings, G.J. Preparation of a highly active ternary Cu-Zn-Al oxide methanol synthesis catalyst by supercritical CO2 anti-solvent precipitation. Catal. Today 2018, 317, 12–20. [Google Scholar] [CrossRef]
- Behrens, M.; Zander, S.; Kurr, P.; Jacobsen, N.; Senker, J.; Koch, G.; Ressler, T.; Fischer, R.W.; Schlögl, R. Performance improvement of nanocatalysts by promoter-induced defects in the support material: Methanol synthesis over Cu/ZnO:Al. J. Am. Chem. Soc. 2013, 135, 6061–6068. [Google Scholar] [CrossRef]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.-L.; et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 2012, 336, 893–897. [Google Scholar] [CrossRef]
- Silva, R.J.; Pimentel, A.F.; Monteiro, R.S.; Mota, C.J.A. Synthesis of methanol and dimethyl ether from the CO2 hydrogenation over Cu-ZnO supported on Al2O3 and Nb2O5. J. CO2 Util. 2016, 15, 83–88. [Google Scholar] [CrossRef]
- Naik, S.P.; Ryu, T.; Bui, V.; Miller, J.D.; Drinnan, N.B.; Zmierczak, W. Synthesis of DME from CO2/H2 gas mixture. Chem. Eng. J. 2011, 167, 362–368. [Google Scholar] [CrossRef]
- Jia, G.; Tan, Y.; Han, Y. A Comparative study on the thermodynamics of dimethyl ether synthesis from CO hydrogenation and CO2 hydrogenation. Ind. Eng. Chem. Res. 2006, 45, 1152–1159. [Google Scholar] [CrossRef]
- Jeong, C.; Park, J.; Kim, J.; Baik, J.H.; Suh, Y.-W. Effects of Al3+ precipitation onto primitive amorphous Cu-Zn precipitate on methanol synthesis over Cu/ZnO/Al2O3 catalyst. Korean J. Chem. Eng. 2019, 36, 191–196. [Google Scholar] [CrossRef]
- Behrens, M.; Brennecke, D.; Girgsdies, F.; Kißner, S.; Trunschke, A.; Nasrudin, N.; Zakaria, S.; Idris, N.F.; Hamid, S.B.A.; Kniep, B.; et al. Understanding the complexity of a catalyst synthesis: Co-precipitation of mixed Cu,Zn,Al hydroxycarbonate precursors for Cu/ZnO/Al2O3 catalysts investigated by titration experiments. Appl. Catal. A 2011, 392, 93–102. [Google Scholar] [CrossRef]
- Behrens, M.; Kasatkin, I.; Kühl, S.; Weinberg, G. Phase-pure Cu,Zn,Al hydrotalcite-like materials as precursors for copper rich Cu/ZnO/Al2O3 catalysts. Chem. Mater. 2010, 22, 386–397. [Google Scholar] [CrossRef]
- Zander, S.; Seidlhofer, B.; Behrens, M. In Situ EDXRD study of the chemistry of aging of co-precipitated mixed Cu,Zn hydroxycarbonates—Consequences for the preparation of Cu/ZnO catalysts. Dalton Trans. 2012, 41, 13416–13422. [Google Scholar] [CrossRef]
- Cheung, P.; Bhan, A.; Sunley, G.J.; Law, D.J.; Iglesia, E. Site requirements and elementary steps in dimethyl ether carbonylation catalyzed by acidic zeolites. J. Catal. 2007, 245, 110–123. [Google Scholar] [CrossRef]
- Wang, S.; Yin, S.; Guo, W.; Liu, Y.; Zhu, L.; Wang, X. Influence of inlet gas composition on dimethyl ether carbonylation and the subsequent hydrogenation of methyl acetate in two-stage ethanol synthesis. New J. Chem. 2016, 40, 6460–6466. [Google Scholar] [CrossRef]
- Cheung, P.; Bhan, A.; Sunley, G.J.; Iglesia, E. Selective carbonylation of dimethyl ether to methyl acetate catalyzed by acidic zeolites. Angew. Chem. Int. Ed. 2006, 45, 1617–1620. [Google Scholar] [CrossRef]
- Rasmussen, D.B.; Christensen, J.M.; Temel, B.; Studt, F.; Moses, P.G.; Rossmeisl, J.; Riisager, A.; Jensen, A.D. Reaction mechanism of dimethyl ether carbonylation to methyl acetate over mordenite—A combined DFT/experimental study. Catal. Sci. Technol. 2017, 7, 1141–1152. [Google Scholar] [CrossRef]
- Ham, H.; Kim, J.; Lim, J.H.; Sung, W.C.; Lee, D.H.; Bae, J.W. Selective ethanol synthesis via multi-step reactions from syngas: Ferrierite-based catalysts and fluidized-bed reactor application. Catal. Today 2018, 303, 93–99. [Google Scholar] [CrossRef]
- Bansode, A.; Urakawa, A. Towards full one-pass conversion of carbon dioxide to methanol and methanol-derived products. J. Catal. 2014, 309, 66–70. [Google Scholar] [CrossRef]
- Fichtl, M.B.; Schumann, J.; Kasatkin, I.; Jacobsen, N.; Behrens, M.; Schlögl, R.; Muhler, M.; Hinrichsen, O. Counting of oxygen defects versus metal surface sites in methanol synthesis catalysts by different probe molecules. Angew. Chem. Int. Ed. 2014, 53, 7043–7047. [Google Scholar] [CrossRef] [PubMed]
- Kuld, S.; Conradsen, C.; Moses, P.G.; Chorkendorff, I.; Sehested, J. Quantification of zinc atoms in a surface alloy on copper in an industrial-type methanol synthesis catalyst. Angew. Chem. Int. Ed. 2014, 53, 5941–5945. [Google Scholar] [CrossRef] [PubMed]



| Calcined Samples | Reduced Samples | ||||||
|---|---|---|---|---|---|---|---|
| Atomic percentage (%) 1 | SBET (m2 g−1) | SCu (m2 g−1) | DCu (%) 2 | ANH3-TPD3 | |||
| Cu | Zn | Al | |||||
| SP-30 | 48.4 | 21.0 | 30.5 | 131 | 21.9 ± 2.8 | 7.9 ± 1.0 | 1.00 |
| SP-40 | 46.4 | 19.1 | 34.4 | 127 | 21.4 ± 2.9 | 7.9 ± 1.1 | 1.37 |
| SP-60 | 29.4 | 12.4 | 58.2 | 248 | 12.5 ± 2.0 | 6.6 ± 1.0 | 1.45 |
| SP-80 | 14.2 | 6.2 | 79.7 | 309 | 7.5 ± 1.1 | 7.4 ± 1.1 | 1.63 |
| T (K) | XCO2 (%) | YCO (%) | CO-Free Selectivity (%) | ||
|---|---|---|---|---|---|
| CH4 | DME | CH3OH | |||
| 503 | 15.8 | 11.2 | 0.0 | 1.6 | 98.4 |
| 523 | 20.4 | 13.6 | 0.0 | 3.7 | 96.3 |
| 548 | 23.9 | 15.2 | 1.8 | 17.8 | 80.4 |
| 573 | 24.8 | 14.8 | 5.4 | 53.4 | 41.2 |
| 598 | 25.4 | 15.6 | 10.2 | 50.6 | 39.2 |
| Catalyst | XCO2 (%) | YCO (%) | CO-Free Selectivity (%) | ||
|---|---|---|---|---|---|
| CH4 | DME | CH3OH | |||
| SP-30 | 25.2 | 18.5 | 4.2 | 16.9 | 78.9 |
| SP-40 | 25.0 | 19.0 | 5.2 | 36.9 | 57.9 |
| SP-60 | 24.8 | 14.8 | 5.4 | 53.4 | 41.2 |
| SP-80 | 25.2 | 12.6 | 0.5 | 75.1 | 24.4 |
| PM-80 2 | 21.3 | 10.1 | 8.9 | 75.9 | 15.2 |
| Entry | Catalyst | Pressure (Bar) | GHSV (L kgcat−1 h−1) | XCO2 (%) | YCO (%) | CO-Free Selectivity (%) | ||
|---|---|---|---|---|---|---|---|---|
| CH4 | DME | CH3OH | ||||||
| 1 | SP-60 (0.6 g) | 65 | 1450 | 26.9 | 15.0 | 10.9 | 62.3 | 26.8 |
| 2 | SP-60 (2.6 g) | 50 | 335 | 24.7 | 5.6 | 4.4 | 78.0 | 17.6 |
| 3 | SP-60 + γ-Al2O3 1 | 50 | 335 | 24.6 | 12.0 | 1.6 | 84.1 | 14.3 |
| Catalyst | P (Bar) | XCO2 (%) | YCO (%) | CO-Free Selectivity (%) | |||
|---|---|---|---|---|---|---|---|
| CH4 | DME | CH3OH | MA | ||||
| SP-60 and ferrierite | 50 | 24.8 | 11.5 | 3.7 | 82.2 | 10.5 | 3.6 |
| 65 | 28.8 | 9.8 | 2.8 | 90.4 | 4.9 | 1.9 | |
| SP-80 and ferrierite | 50 | 26.6 | 12.5 | 5.4 | 83.0 | 7.3 | 4.3 |
| 65 | 27.9 | 10.7 | 2.3 | 90.9 | 4.6 | 2.2 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, C.; Kim, J.; Kim, J.-H.; Lee, S.; Bae, J.W.; Suh, Y.-W. Direct Conversion of CO2 into Dimethyl Ether over Al2O3/Cu/ZnO Catalysts Prepared by Sequential Precipitation. Catalysts 2019, 9, 524. https://doi.org/10.3390/catal9060524
Jeong C, Kim J, Kim J-H, Lee S, Bae JW, Suh Y-W. Direct Conversion of CO2 into Dimethyl Ether over Al2O3/Cu/ZnO Catalysts Prepared by Sequential Precipitation. Catalysts. 2019; 9(6):524. https://doi.org/10.3390/catal9060524
Chicago/Turabian StyleJeong, Cheonwoo, Jinsung Kim, Ji-Hyeon Kim, Sunghoon Lee, Jong Wook Bae, and Young-Woong Suh. 2019. "Direct Conversion of CO2 into Dimethyl Ether over Al2O3/Cu/ZnO Catalysts Prepared by Sequential Precipitation" Catalysts 9, no. 6: 524. https://doi.org/10.3390/catal9060524
APA StyleJeong, C., Kim, J., Kim, J.-H., Lee, S., Bae, J. W., & Suh, Y.-W. (2019). Direct Conversion of CO2 into Dimethyl Ether over Al2O3/Cu/ZnO Catalysts Prepared by Sequential Precipitation. Catalysts, 9(6), 524. https://doi.org/10.3390/catal9060524







