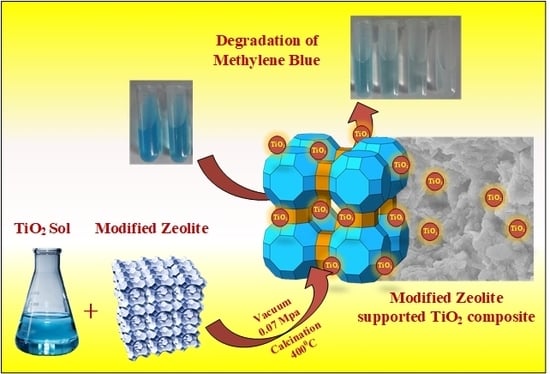
Investigation of Microstructure and Photocatalytic Performance of a Modified Zeolite Supported Nanocrystal TiO2 Composite
Abstract
1. Introduction
2. Results and Discussion
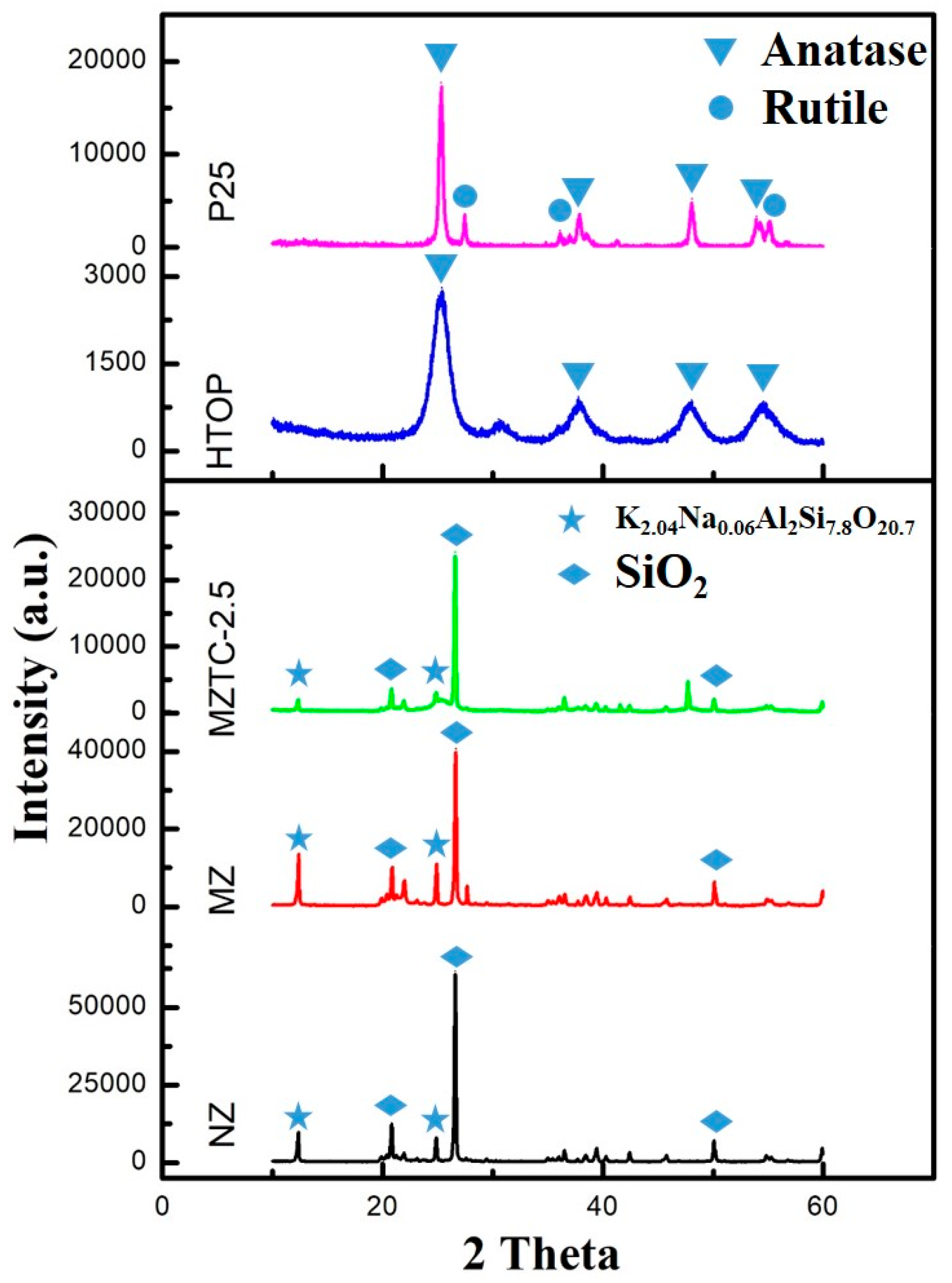
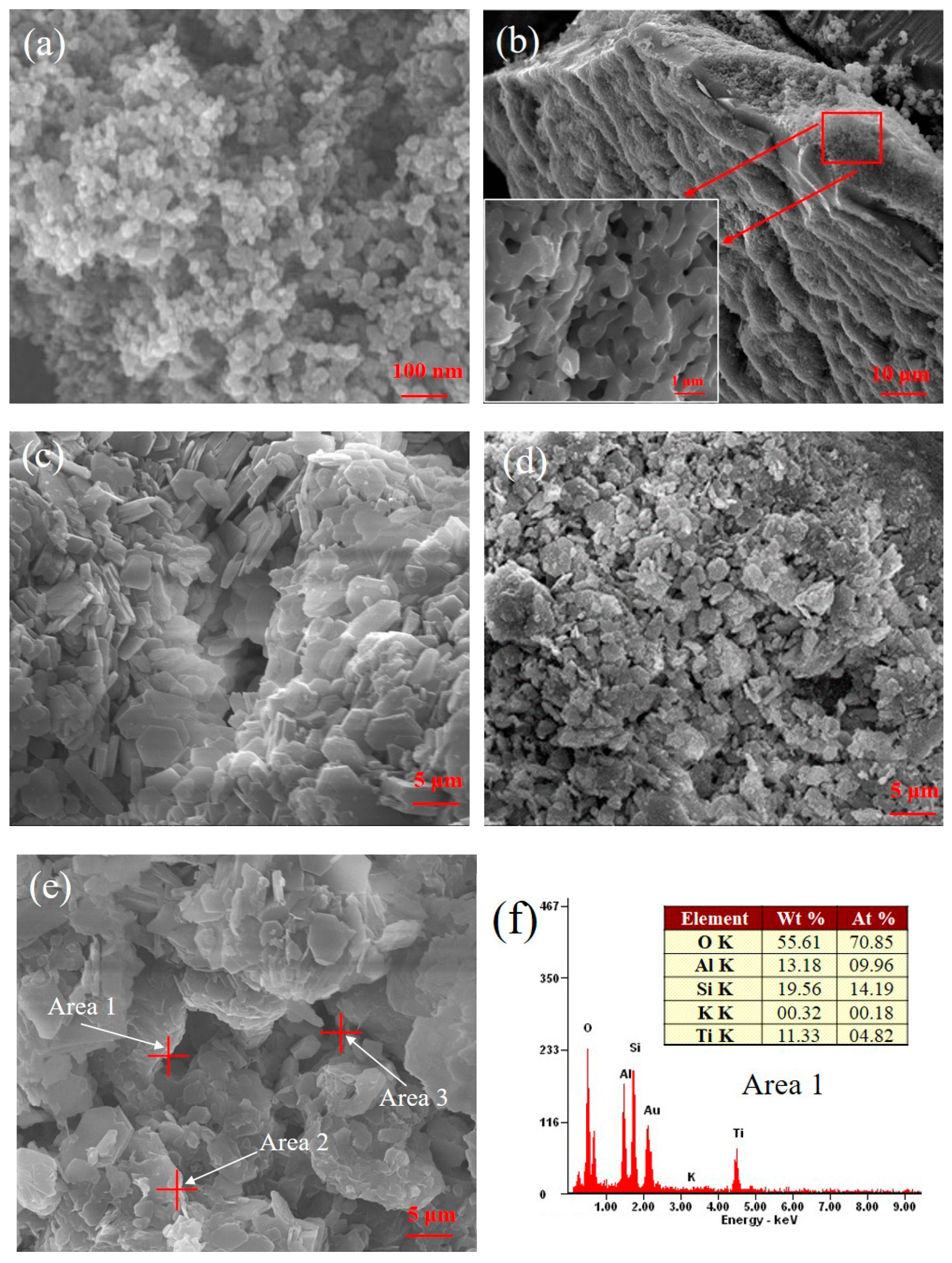
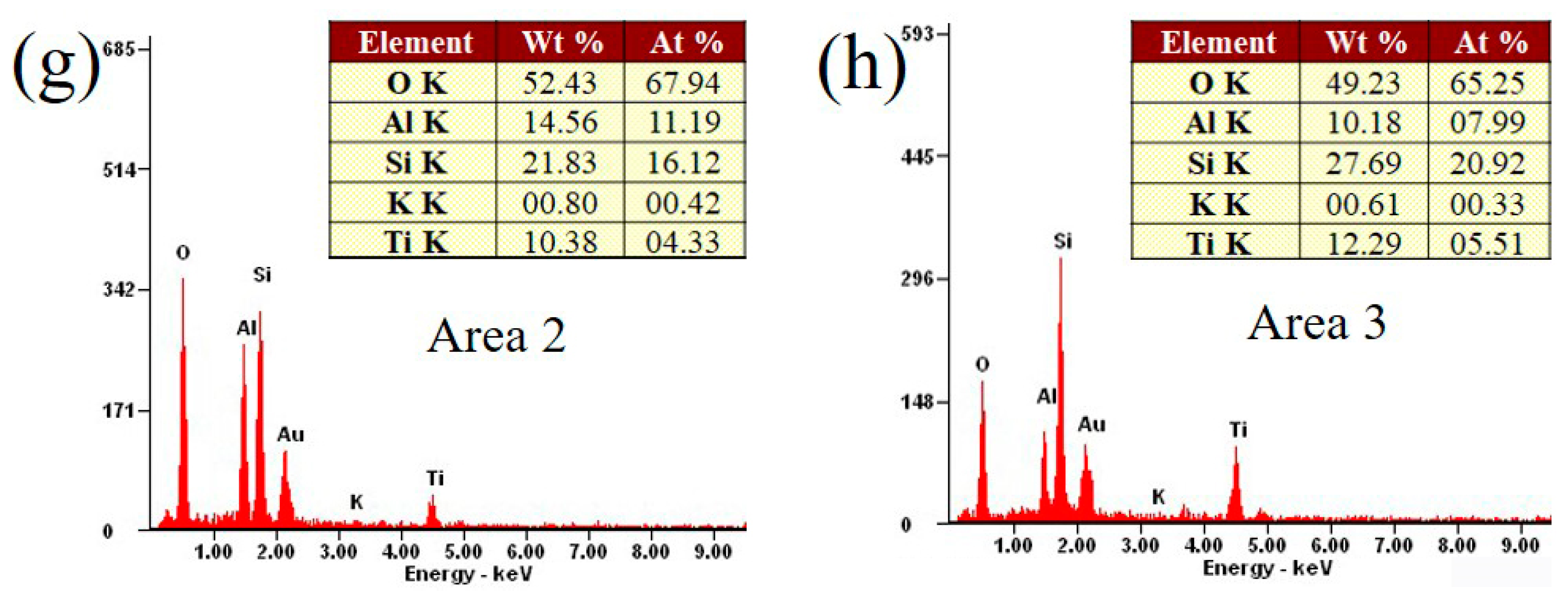
2.1. Physicochemical Properties
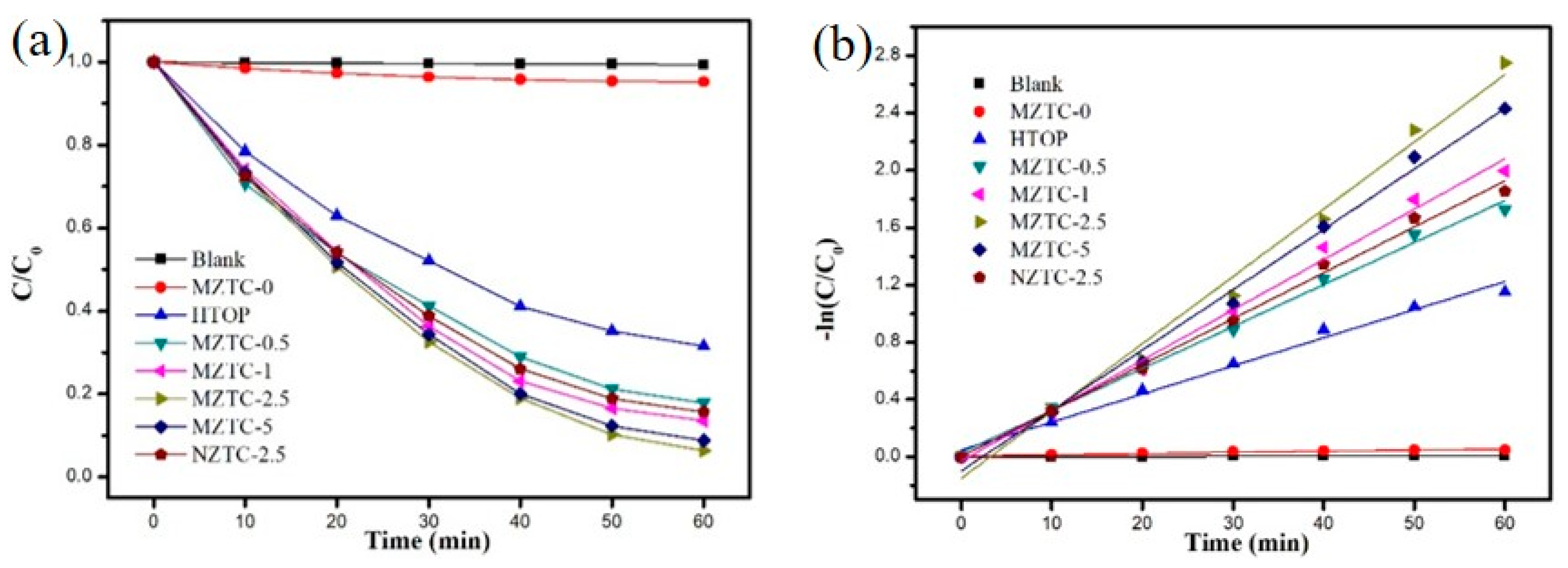
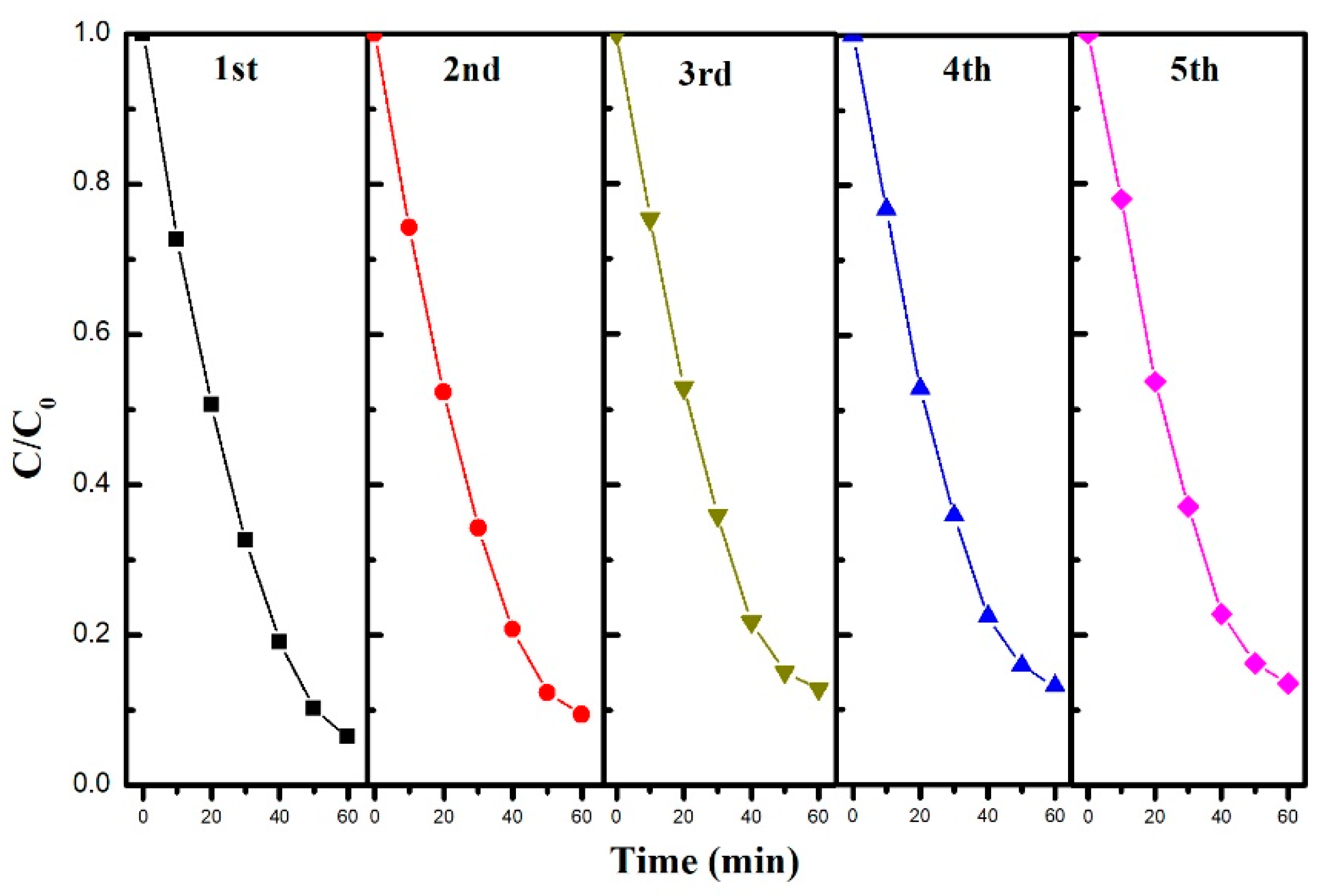
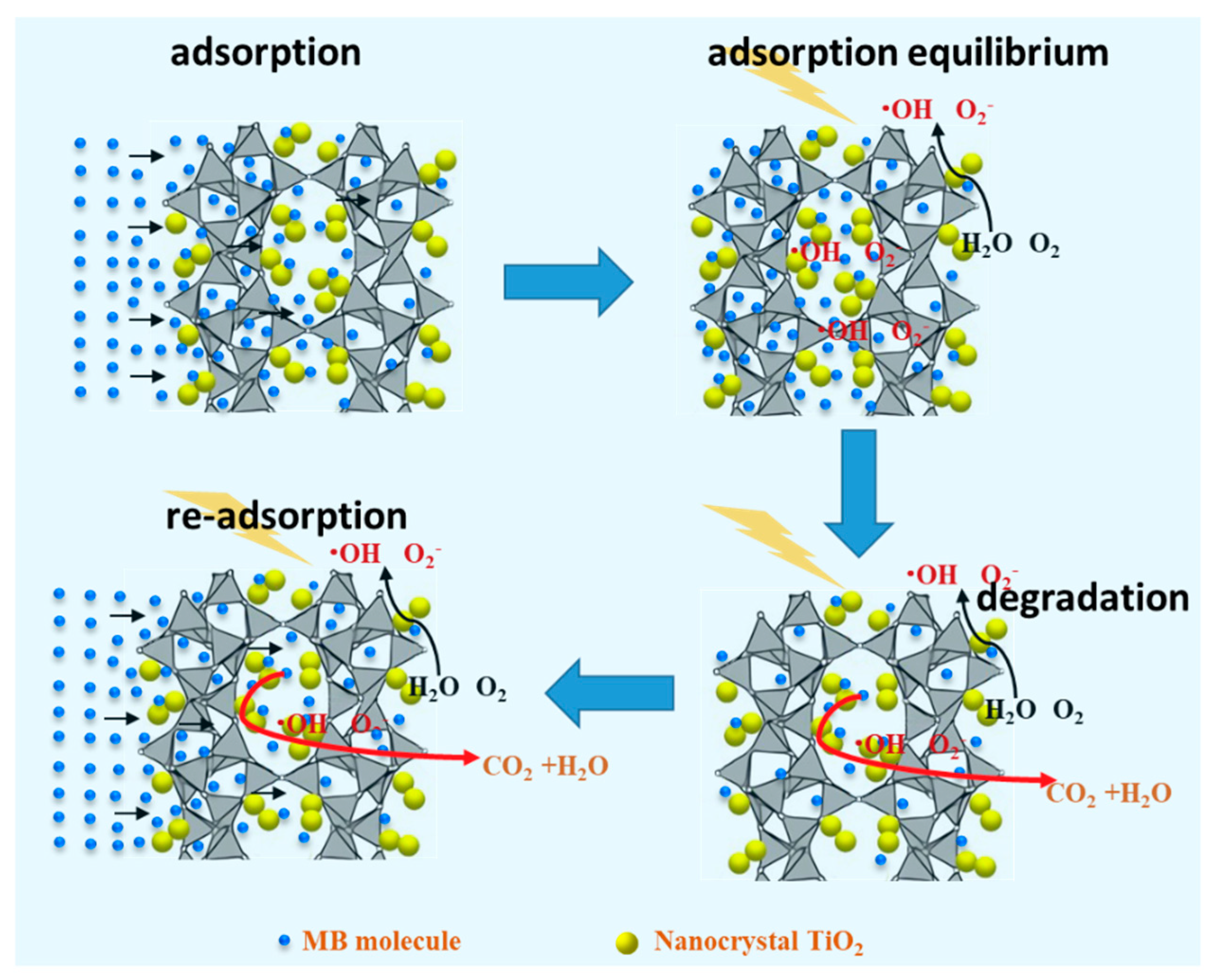
2.2. Evaluation of the Photocatalytic Efficiency
3. Experimental Procedure
3.1. Preparation of MZTC
3.2. Characterization
3.3. Evaluation of Photocatalytic Degradation Efficiency
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef]
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, H.; Zinatizadeh, A.A.L.; Habibi, M.; Akia, M.; Isa, M.H. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination 2010, 261, 3–18. [Google Scholar] [CrossRef]
- Huang, C.C.; Chang, H.T. Parameters for selective colorimetric sensing of mercury(II) in aqueous solutions using mercaptopropionic acid-modified gold nanoparticles. Chem. Commun. 2007, 12, 1215–1217. [Google Scholar] [CrossRef] [PubMed]
- Noemi, R.; Moshe, A.; Gideon, O. A pilot study of constructed wetlands using duckweed (Lemna gibba L.) for treatment of domestic primary effluent in Israel. Water Res. 2004, 38, 2241–2248. [Google Scholar]
- Kuriechen, S.K.; Murugesan, S. Carbon-Doped Titanium Dioxide Nanoparticles Mediated Photocatalytic Degradation of Azo Dyes Under Visible Light. Water Air Soil Pollut. 2013, 224, 1671. [Google Scholar] [CrossRef]
- Kamegawa, T.; Kido, R.; Yamahana, D.; Yamashita, H. Design of TiO2-zeolite composites with enhanced photocatalytic performances under irradiation of UV and visible light. Microporous Mesoporous Mater. 2013, 165, 142–147. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Sharma, A.; Ahmad, J.; Flora, S.J.S. Application of advanced oxidation processes and toxicity assessment of transformation products. Environ. Res. 2018, 167, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, H.; Torimoto, T. Titanium dioxide/adsorbent hybrid photocatalysts for photodestruction of organic substances of dilute concentrations. Catal. Today 2000, 58, 133–140. [Google Scholar] [CrossRef]
- Karuppuchamy, S.; Iwasaki, M.; Minoura, H. Physico-chemical, photoelectrochemical and photocatalytic properties of electrodeposited nanocrystalline titanium dioxide thin films. Vacuum 2007, 81, 708–712. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Fukura, T.; Imai, Y.; Yamaura, H.; Yahiro, H. Photocatalytic activities for partial oxidation of α-methylstyrene over zeolite-supported titanium dioxide and the influence of water addition to reaction solvent. Electrochim. Acta 2010, 55, 7745–7750. [Google Scholar] [CrossRef]
- Fuchs, V.; Méndez, L.; Blanco, M.; Pizzio, L. Mesoporous titania directly modified with tungstophosphoric acid: Synthesis, characterization and catalytic evaluation. Appl. Catal. A Gen. 2009, 358, 73–78. [Google Scholar] [CrossRef]
- Kamegawa, T.; Sonoda, J.; Sugimura, K.; Mori, K.; Yamashita, H. Degradation of isobutanol diluted in water over visible light sensitive vanadium doped TiO2 photocatalyst. J. Alloys Compd. 2009, 486, 685–688. [Google Scholar] [CrossRef]
- Park, H.G.; Kim, J.I.; Kang, M.; Yeo, M.K. The effect of metal-doped TiO2 nanoparticles on zebrafish embryogenesis. Mol. Cell. Toxicol. 2014, 10, 293–301. [Google Scholar] [CrossRef]
- Ghosh, M.; Jana, S.C. Bi-component inorganic oxide nanofibers from gas jet fiber spinning process. RSC Adv. 2015, 5, 105313–105318. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sun, T.Y.; Nowack, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef]
- Farré, M.; Pérez, S.; Gajda-Schrantz, K.; Osorio, V.; Kantiani, L.; Ginebreda, A.; Barceló, D. First determination of C60 and C70 fullerenes and N-methylfulleropyrrolidine C60 on the suspended material of wastewater effluents by liquid chromatography hybrid quadrupole linear ion trap tandem mass spectrometry. J. Hydrol. 2010, 383, 44–51. [Google Scholar] [CrossRef]
- Domoroshchina, E.N.; Chernyshev, V.V.; Kuz’micheva, G.M.; Dorokhov, A.V.; Pirutko, L.V.; Kravchenko, G.V.; Chumakov, R.B. Changing the characteristics and properties of zeolite Y and nano-anatase in the formation of a nano-anatase/Y composite with improved photocatalytic and adsorption properties. Appl. Nanosci. 2018, 8, 19–31. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Maki, K.; Matsumura, Y.; Kamegawa, T.; Mori, K.; Yamashita, H. Hydrophobic Modification of a Mesoporous Silica Surface Using a Fluorine-Containing Silylation Agent and Its Application as an Advantageous Host Material for the TiO2 Photocatalyst. J. Phys. Chem. C 2009, 113, 1552–1559. [Google Scholar] [CrossRef]
- Jiang, G.; Zheng, X.; Wang, Y.; Li, T.; Sun, X. Photo-degradation of methylene blue by multi-walled carbon nanotubes/TiO2 composites. Powder Technol. 2011, 207, 465–469. [Google Scholar] [CrossRef]
- Torimoto, T.; Okawa, Y.; Takeda, N.; Yoneyama, H. Effect of activated carbon content in TiO2-loaded activated carbon on photodegradation behaviors of dichloromethane. J. Photochem. Photobiol. A Chem. 1997, 103, 153–157. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Aoyama, J.; Miyakubo, K.; Eguchi, T.; Kamegawa, T.; Mori, K.; Yamashita, H. TiO2 photocatalyst for degradation of organic compounds in water and air supported on highly hydrophobic FAU zeolite: Structural, sorptive, and photocatalytic studies. J. Catal. 2012, 285, 223–234. [Google Scholar] [CrossRef]
- Kamegawa, T.; Yamahana, D.; Yamashita, H. Graphene Coating of TiO2 Nanoparticles Loaded on Mesoporous Silica for Enhancement of Photocatalytic Activity. J. Phys. Chem. C 2010, 114, 15049–15053. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous Titanium Dioxide Nanofibers with a Significantly Enhanced Photocatalytic Activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Najafabadi, A.T.; Taghipour, F. Physicochemical impact of zeolites as the support for photocatalytic hydrogen production using solar-activated TiO2-based nanoparticles. ENERGY Convers. Manag. 2014, 82, 106–113. [Google Scholar] [CrossRef]
- Reddy, E.P.; Davydov, L.; Smirniotis, P. TiO2-loaded zeolites and mesoporous materials in the sonophotocatalytic decomposition of aqueous organic pollutants: The role of the support. Appl. Catal. B-Environ. 2003, 42, 1–11. [Google Scholar] [CrossRef]
- Al-Harbi, L.M.; Kosa, S.A.; el Maksod, I.H.A.; Hegazy, E.Z. The photocatalytic activity of TiO2-zeolite composite for degradation of dye using synthetic UV and Jeddah sunlight. J. Nanomater. 2015, 16, 46. [Google Scholar] [CrossRef]
- Guesh, K.; Mayoral, Á.; Márquez-Álvarez, C.; Chebude, Y.; Díaz, I. Enhanced photocatalytic activity of TiO2 supported on zeolites tested in real wastewaters from the textile industry of Ethiopia. Microporous Mesoporous Mater. 2016, 225, 88–97. [Google Scholar] [CrossRef]
- Li, Y.J.; Wei, C. Photocatalytic degradation of Rhodamine B using nanocrystalline TiO2-zeolite surface composite catalysts: Effects of photocatalytic condition on degradation efficiency. Catal. Sci. Technol. 2011, 1, 802–809. [Google Scholar]
- Liu, S.; Lim, M.; Amal, R. TiO2-coated natural zeolite: Rapid humic acid adsorption and effective photocatalytic regeneration. Chem. Eng. Sci. 2014, 105, 46–52. [Google Scholar] [CrossRef]
- Lafjah, M.; Djafri, F.; Bengueddach, A. Beta zeolite supported sol-gel TiO2 materials for gas phase photocatalytic applications. J. Hazard. Mater. 2011, 186, 1218–1225. [Google Scholar] [CrossRef]
- Domoroshchina, E.; Kravchenko, G.; Kuz’micheva, G. Nanocomposites of zeolite-titanium(IV) oxides: Preparation, characterization, adsorption, photocatalytic and bactericidal properties. J. Cryst. Growth 2017, 468, 199–203. [Google Scholar] [CrossRef]
- Kravchenko, G.V.; Domoroshchina, E.N.; Kuz’micheva, G.M.; Gaynanova, A.A.; Amarantov, S.V.; Pirutko, L.V.; Tsybinsky, A.M.; Sadovskaya, N.V.; Kopylova, E.V. Zeolite-titanium dioxide nanocomposites: Preparation, characterization, and adsorption properties. Nanotechnol. Russ. 2016, 11, 579–592. [Google Scholar] [CrossRef]
- Zendehdel, M.; Kalateh, Z.; Mortezaii, Z. Photocatalytic activity of the nano-sized TiO2/NaY zeolite for removal of methylene blue. J. Nov. Appl. 2014, 3, 135–141. [Google Scholar]
- Maraschi, F.; Sturini, M.; Speltini, A.; Pretali, L.; Profumo, A.; Pastorello, A.; Kumar, V.; Ferretti, M.; Caratto, V. TiO2-modified zeolites for fluoroquinolones removal from wastewaters and reuse after solar light regeneration. J. Environ. Chem. Eng. 2014, 2, 2170–2176. [Google Scholar] [CrossRef]
- Easwaramoorthi, S.; Natarajan, P. Characterisation and spectral properties of surface adsorbed phenosafranine dye in zeolite-Y and ZSM-5: Photosensitisation of embedded nanoparticles of titanium dioxide. Microporous Mesoporous Mater. 2009, 117, 541–550. [Google Scholar] [CrossRef]
- Wang, J.-J.; Jing, Y.-H.; Ouyang, T.; Chang, C.-T. Preparation of 13X from Waste Quartz and Photocatalytic Reaction of Methyl Orange on TiO2/ZSM-5, 13X and Y-Zeolite. J. Nanosci. Nanotechnol. 2015, 15, 6141–6149. [Google Scholar] [CrossRef]
- Ito, M.; Fukahori, S.; Fujiwara, T. Adsorptive removal and photocatalytic decomposition of sulfamethazine in secondary effluent using TiO2–zeolite composites. Environ. Sci. Pollut. Res. 2014, 21, 834–842. [Google Scholar] [CrossRef]
- Jansson, I.; Suárez, S.; Garcia-Garcia, F.J.; Sánchez, B. Zeolite-TiO2 hybrid composites for pollutant degradation in gas phase. Appl. Catal. B Environ. 2015, 178, 100–107. [Google Scholar] [CrossRef]
- Gomez, S.; Leal, C.; Pizzio, L.; Pierella, L. Preparation and characterization of TiO2/HZSM-11 zeolite for photodegradation of dichlorvos in aqueous solution. J. Hazard. Mater. 2013, 258, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kruk, M.; Jaroniec, M. Gas Adsorption Characterization of Ordered Organic−Inorganic Nanocomposite Materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Sing, K.S.; Everett, D.H.; Haul, R.A.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Mozgawa, W. The influence of some heavy metals cations on the FTIR spectra of zeolites. J. Mol. Struct. 2000, 555, 299–304. [Google Scholar] [CrossRef]
- Pechar, F.; Rykl, D. Infrared spectra of natural zeolites of the stilbite group. Chem. Zvesti. 1981, 35, 189–202. [Google Scholar]
- Weckhuysen, B.M.; Yu, J. Recent advances in zeolite chemistry and catalysis. Chem. Soc. Rev. 2015, 44, 7022–7024. [Google Scholar] [CrossRef] [PubMed]
- Mozgawa, W.; Król, M.; Barczyk, K.; Science, M. FT-IR studies of zeolites from different structural groups. Chemik 2011, 65, 671–674. [Google Scholar]
- Perego, G.; Bellussi, G.; Corno, C.; Taramasso, M.; Buonomo, F.; Esposito, A. New developments in zeolite science and technology. Stud. Surf. Sci. Catal. 1986, 28, 129–136. [Google Scholar]
- De Man, A.J.M.; Sauer, J. Coordination, Structure, and Vibrational Spectra of Titanium in Silicates and Zeolites in Comparison with Related Molecules. An ab Initio Study. J. Phys. Chem. 1996, 100, 551–559. [Google Scholar] [CrossRef]
- Zhang, G.; Song, A.; Duan, Y.; Zheng, S. Enhanced photocatalytic activity of TiO2/zeolite composite for abatement of pollutants. Microporous Mesoporous Mater. 2017, 255, 61–68. [Google Scholar] [CrossRef]
- Setthaya, N.; Chindaprasirt, P.; Yin, S.; Pimraksa, K. TiO2-zeolite photocatalysts made of metakaolin and rice husk ash for removal of methylene blue dye. Powder Technol. 2017, 313, 417–426. [Google Scholar] [CrossRef]
- Soltani, N.; Saion, E.; Hussein, M.Z.; Erfani, M.; Abedini, A. Visible Light-Induced Degradation of Methylene Blue in the Presence of Photocatalytic ZnS and CdS Nanoparticles. Int. J. Mol. Sci. 2012, 13, 12242–12258. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Zhang, Y.; Xu, Q.; Lamson, J.J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L.; Cheng, B.; Su, Y. Hydrothermal preparation and photocatalytic activity of hierarchically sponge-like macro-/mesoporous Titania. J. Phys. Chem. C 2007, 111, 10582–10589. [Google Scholar] [CrossRef]
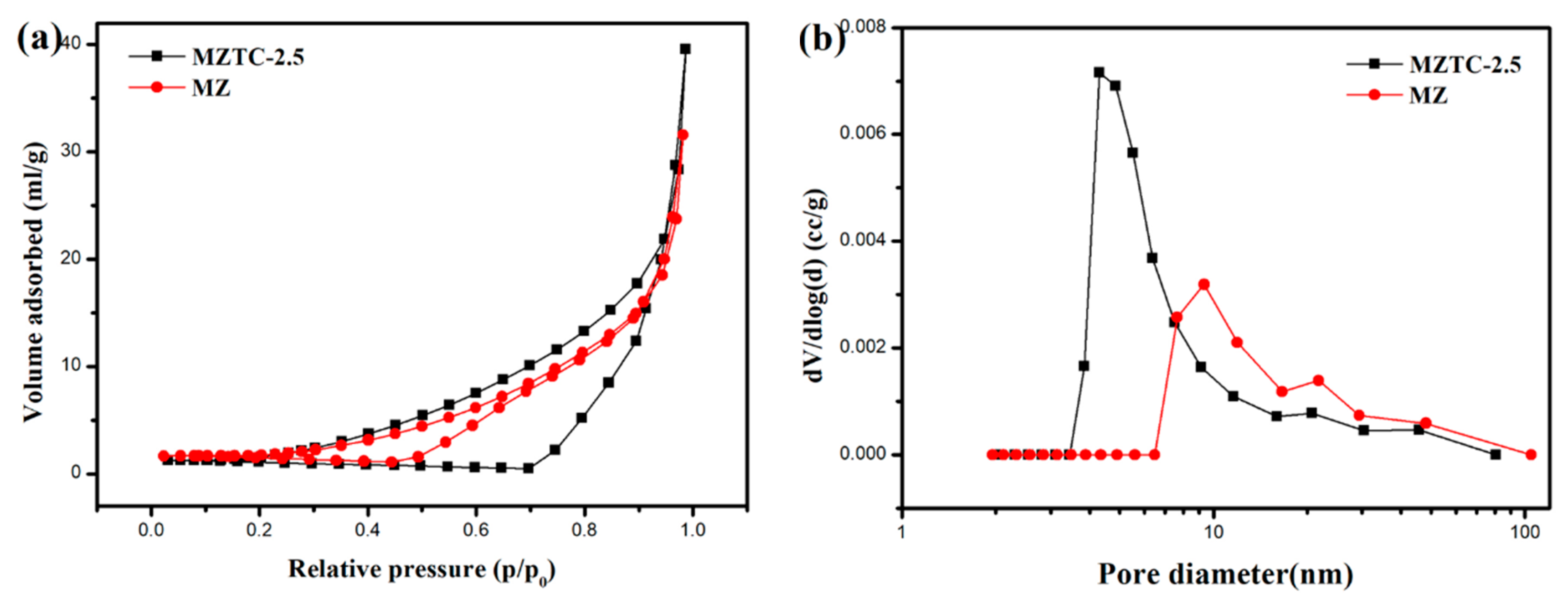
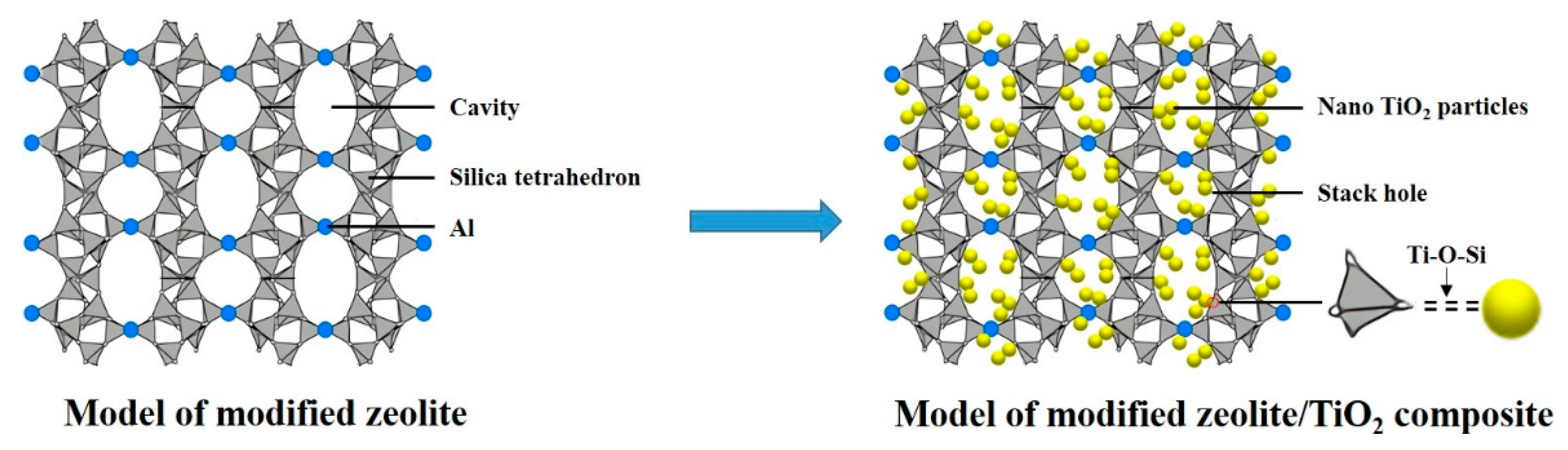
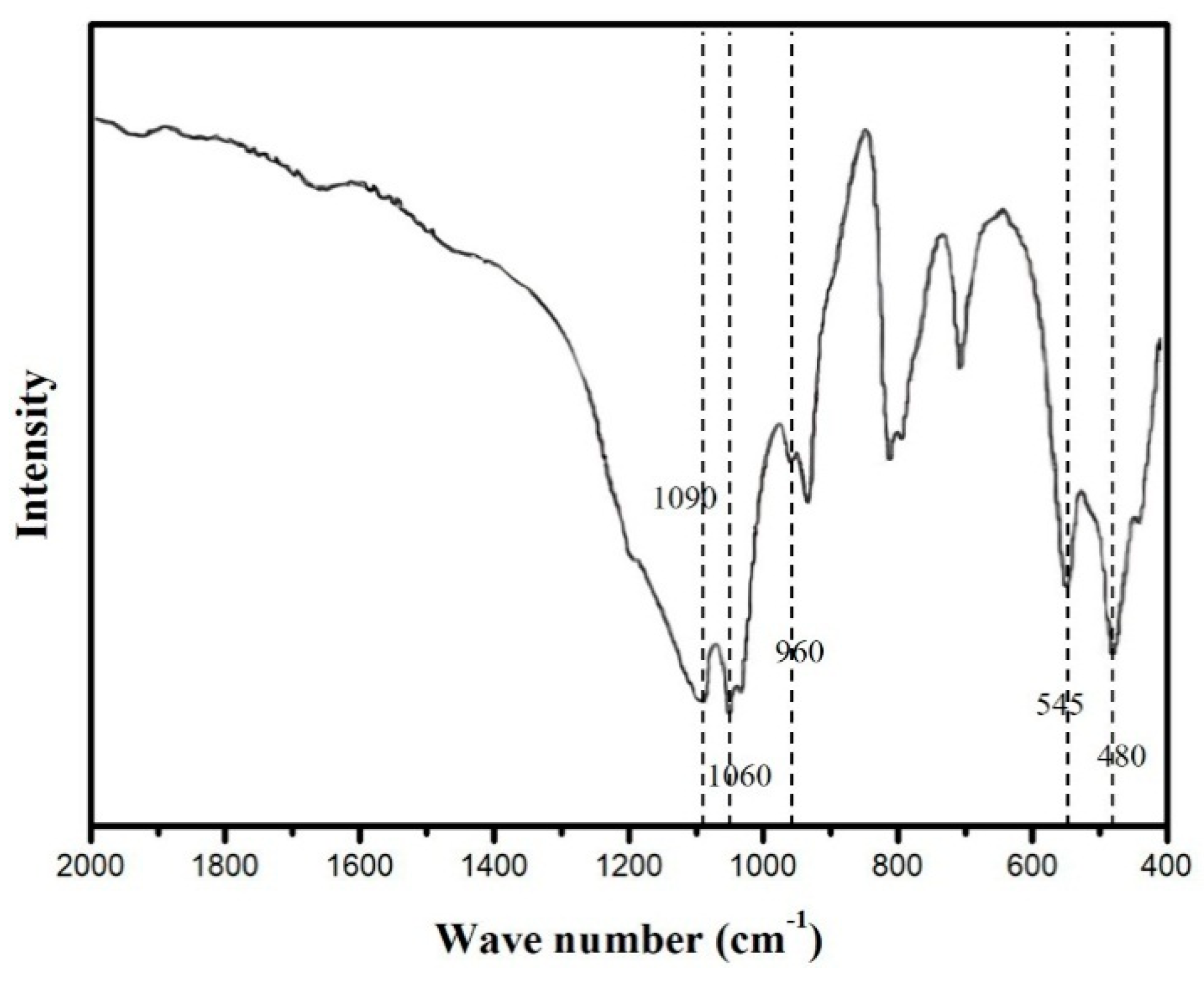
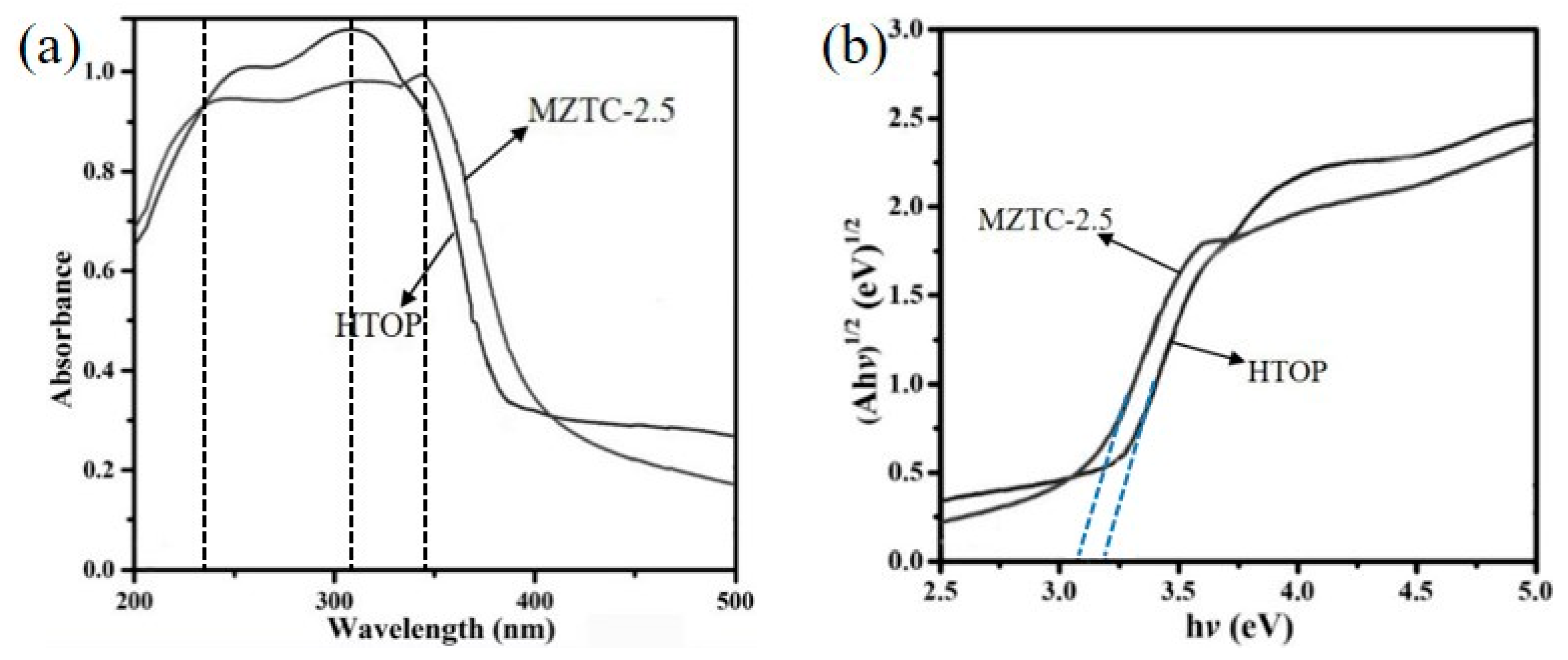
| Sample | S (m2g−1) | V (cm3g−1) | D (nm) |
|---|---|---|---|
| MZ | 392 | 0.29 | 12.02 |
| MZTC-2.5 | 293 | 0.21 | 9.27 |
| Specimen | k (min−1) | R2 |
|---|---|---|
| Blank | 9.74 × 10−4 | 0.98574 |
| MZTC-0 | 8.05 × 10−4 | 0.98691 |
| HTOP | 0.01959 | 0.98693 |
| NZTC-2.5 | 0.03204 | 0.99422 |
| MZTC-0.5 | 0.02930 | 0.99492 |
| MZTC-1 | 0.03512 | 0.99140 |
| MZTC-2.5 | 0.04694 | 0.98633 |
| MZTC-5 | 0.04214 | 0.99194 |
| SiO2 | Al2O3 | Na2O | CaO | K2O | MgO | Fe2O3 | FeO | TiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| 60~70 | 17.8 | 4.2 | 2.6 | 3.2 | 0.8 | 1.6 | 1.2 | 0.6 | 0.26 |
| Specimen | NZ Content/wt% | MZ Content/wt% | TiO2 Content/wt% |
|---|---|---|---|
| HTOP | - | - | 100 |
| NZTC-2.5 | 93.92 | - | 6.08 |
| MZTC-0 | - | 100 | 0 |
| MZTC-0.5 | - | 98.72 | 1.28 |
| MZTC-1 | - | 97.48 | 2.52 |
| MZTC-2.5 | - | 93.92 | 6.08 |
| MZTC-5 | - | 88.53 | 11.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, G.; He, W.; He, Y. Investigation of Microstructure and Photocatalytic Performance of a Modified Zeolite Supported Nanocrystal TiO2 Composite. Catalysts 2019, 9, 502. https://doi.org/10.3390/catal9060502
Liao G, He W, He Y. Investigation of Microstructure and Photocatalytic Performance of a Modified Zeolite Supported Nanocrystal TiO2 Composite. Catalysts. 2019; 9(6):502. https://doi.org/10.3390/catal9060502
Chicago/Turabian StyleLiao, Gang, Wei He, and Yuming He. 2019. "Investigation of Microstructure and Photocatalytic Performance of a Modified Zeolite Supported Nanocrystal TiO2 Composite" Catalysts 9, no. 6: 502. https://doi.org/10.3390/catal9060502
APA StyleLiao, G., He, W., & He, Y. (2019). Investigation of Microstructure and Photocatalytic Performance of a Modified Zeolite Supported Nanocrystal TiO2 Composite. Catalysts, 9(6), 502. https://doi.org/10.3390/catal9060502