In Situ DRIFTS Investigation on CeOx Catalyst Supported by Fly-Ash-Made Porous Cordierite Ceramics for Low-Temperature NH3-SCR of NOX
Abstract
:1. Introduction
2. Results and Discussion
2.1. The NH3-SCR Activity
2.2. Materials Characterization
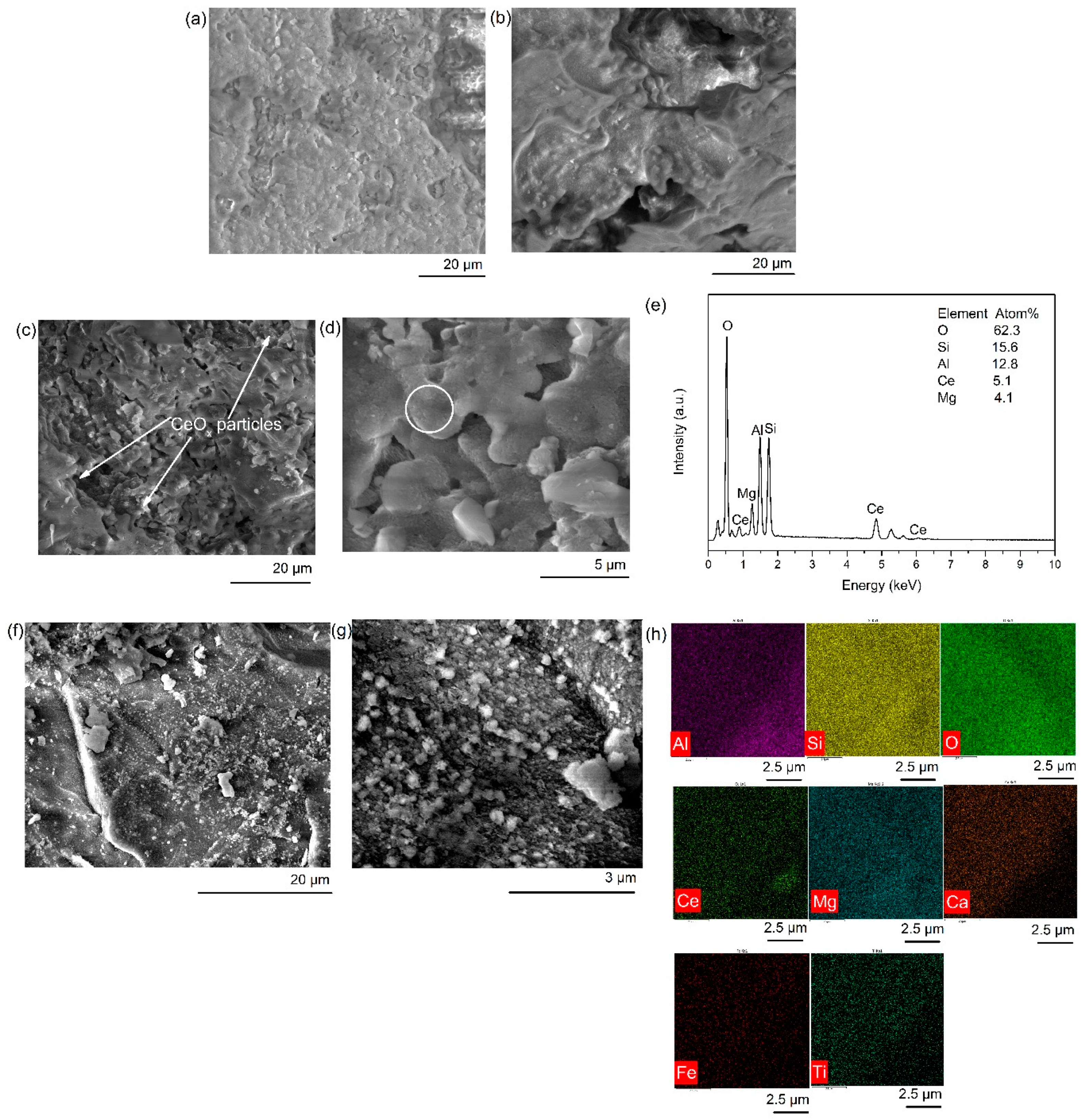
2.2.1. SEM Analysis
2.2.2. Reducibility (H2-TPR)
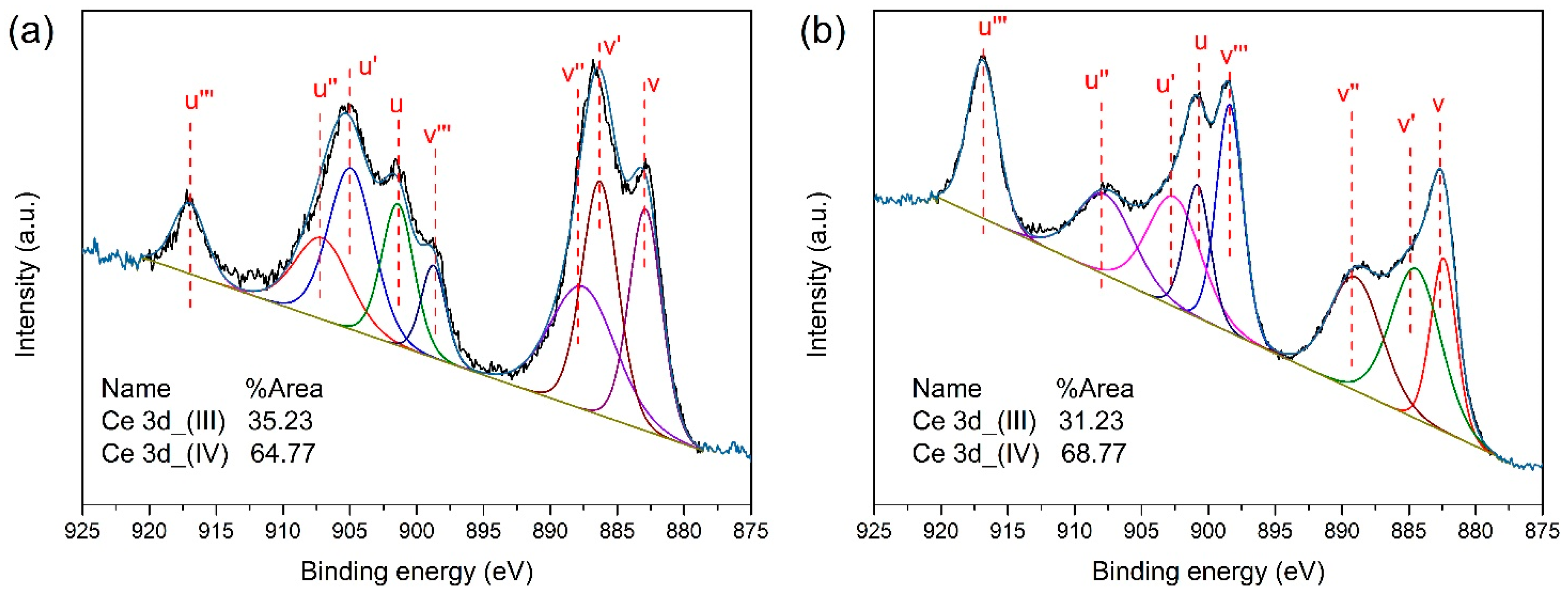
2.2.3. XPS Analysis
2.3. In Situ DRIFTS Studies
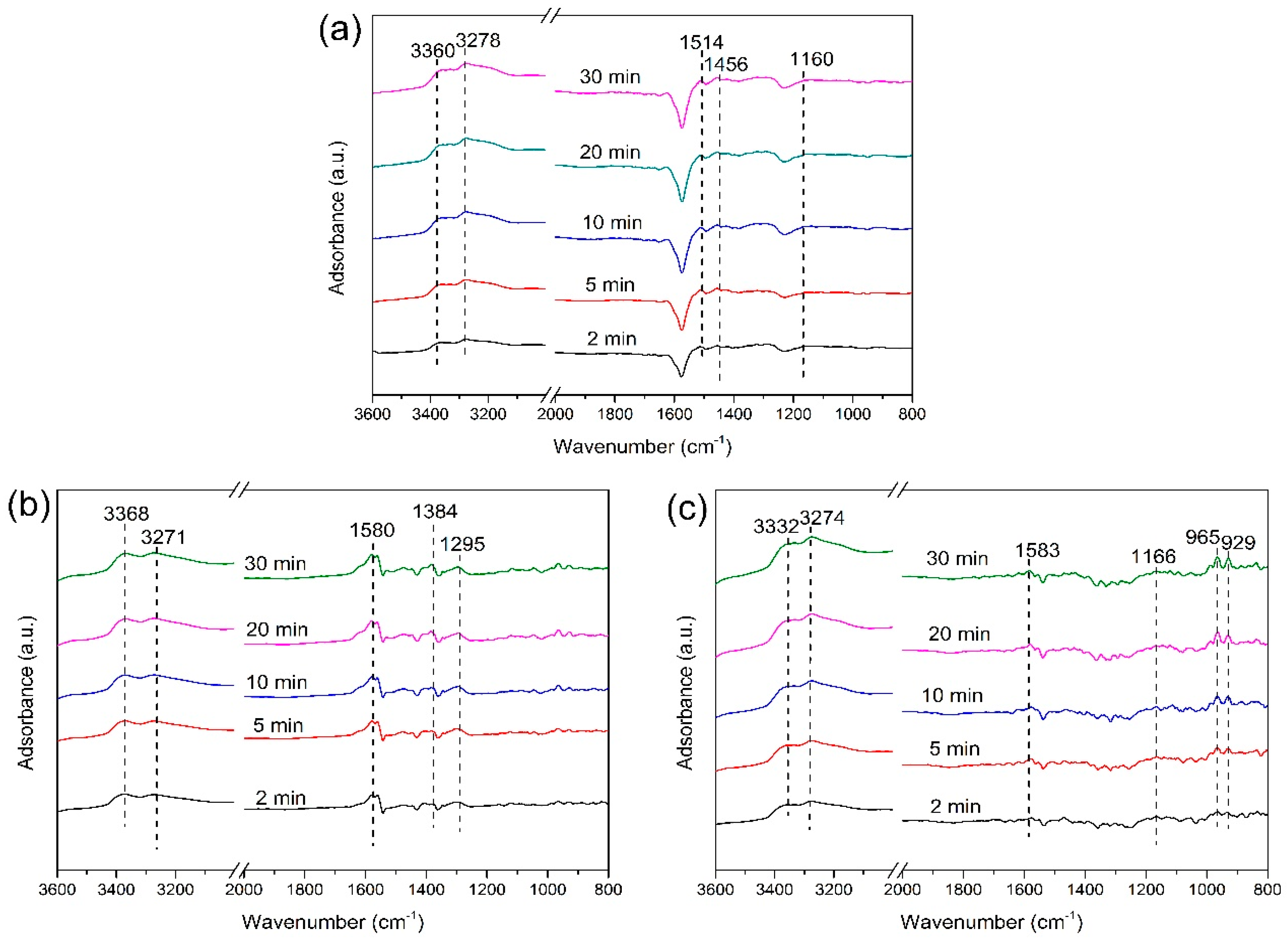
2.3.1. Adsorption of NH3
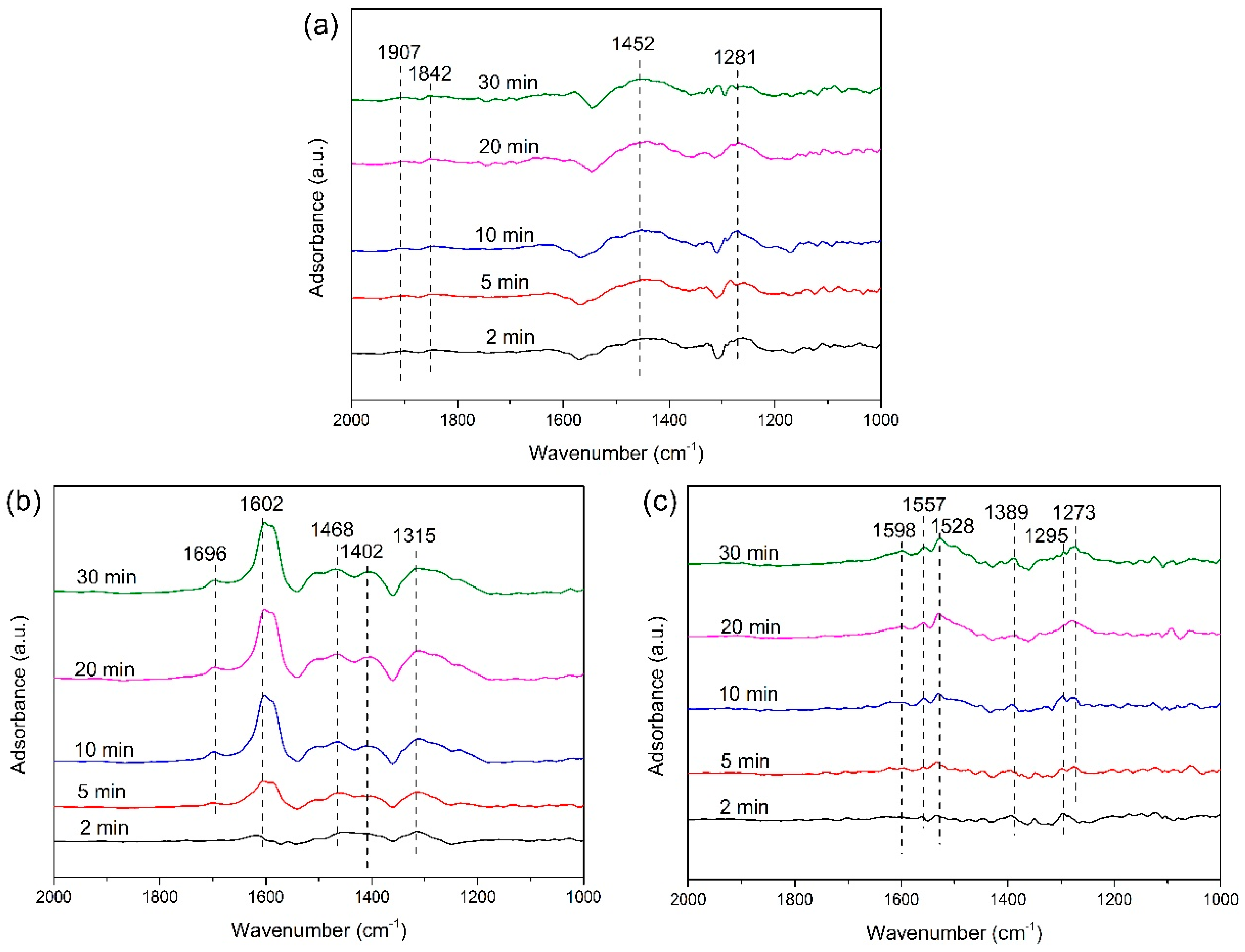
2.3.2. Reaction between Nitrogen Oxides and ad-NH3 Species
2.3.3. Co-Adsorption of NO + O2
2.3.4. Reaction between NH3 and Adsorbed Nitrogen Oxides
2.4. Denitration Mechanism of the 0.6Ce/SPCC Catalyst
- E-R mechanism:
- L-H mechanism:(*: active sites on the catalyst surface)
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. SCR Activity Measurements
3.5. In Situ DRIFTS Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, B.; Wang, S.; Liu, H.; Xu, J.; Fu, K.; Klimont, Z.; Hao, J.; He, K.; Cofala, J.; Amann, M. NOx emissions in China: historical trends and future perspectives. Atmos. Chem. Phys. 2013, 13, 9869–9897. [Google Scholar] [CrossRef]
- Xie, Z.; Zhou, X.; Wu, H.; Chen, L.; Zhao, H.; Liu, Y.; Pan, L.; Chen, H. One-pot hydrothermal synthesis of CuBi co-doped mesoporous zeolite Beta for the removal of NOx by selective catalytic reduction with ammonia. Sci. Rep. 2016, 6, 30132. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gong, Z.; Lu, C.; Niu, S.; Ding, K.; Xu, L.; Zhang, K. Preparation and Performance of Modified Red Mud-Based Catalysts for Selective Catalytic Reduction of NOx with NH3. Catalysts 2018, 8, 35. [Google Scholar] [CrossRef]
- Paolucci, C.; Khurana, I.; Parekh, A.A.; Li, S.; Shih, A.J.; Li, H.; Di Iorio, J.R.; Albarracin-Caballero, J.D.; Yezerets, A.; Miller, J.T. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 2017, 357, 898–903. [Google Scholar] [CrossRef]
- Topsøe, N.-Y. Mechanism of the selective catalytic reduction of nitric oxide by ammonia elucidated by in situ on-line Fourier transform infrared spectroscopy. Science 1994, 265, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yang, K. The role of Fe2O3 species in depressing the formation of N2O in the selective reduction of NO by NH3 over V2O5/TiO2-based catalysts. Catalysts 2018, 8, 134. [Google Scholar]
- Wu, R.; Zhang, N.; Li, L.; He, H.; Song, L.; Qiu, W. DRIFT Study on Promotion Effect of the Keggin Structure over V2O5-MoO3/TiO2 Catalysts for Low Temperature NH3-SCR Reaction. Catalysts 2018, 8, 143. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.-S.; Crocker, M.; Zhang, Z.-S.; Zhu, A.-M.; Shi, C. A study of the mechanism of low-temperature SCR of NO with NH3 on MnOx/CeO2. J. Mol. Catal. A-Chem. 2013, 378, 82–90. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Z.; Liu, L.; Zhang, Y.; Zhang, Z.; Wang, X. Low-temperature SCR of NO with NH3 over activated semi-coke composite-supported rare earth oxides. Appl. Surf. Sci. 2014, 309, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Liu, L.; Mi, L.; Wang, X. In situ DRIFTS studies on MnOx nanowires supported by activated semi-coke for low temperature selective catalytic reduction of NOx with NH3. Appl. Surf. Sci. 2016, 366, 139–147. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Z.; Wang, Y.; Fan, R.; Zhang, C.; Wang, Y.; Guo, X.; Wang, R.; Zhang, S. Ce and Zr Modified WO3-TiO2 Catalysts for Selective Catalytic Reduction of NOx by NH3. Catalysts 2018, 8, 375. [Google Scholar] [CrossRef]
- Shan, W.; Liu, F.; He, H.; Shi, X.; Zhang, C. Novel cerium–tungsten mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Chem. Commun. 2011, 47, 8046–8048. [Google Scholar] [CrossRef]
- Zhou, G.; Zhong, B.; Wang, W.; Guan, X.; Huang, B.; Ye, D.; Wu, H. In situ DRIFTS study of NO reduction by NH3 over Fe–Ce–Mn/ZSM-5 catalysts. Catal. Today. 2011, 175, 157–163. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Li, J.; Ma, L. Promoting effect of MoO3 on the NOx reduction by NH3 over CeO2/TiO2 catalyst studied with in situ DRIFTS. Appl. Catal. B-Environ. 2014, 144, 90–95. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, S.; Zeng, Y.; Wang, P.; Zhong, Q. Active site of O2 and its improvement mechanism over Ce-Ti catalyst for NH3-SCR reaction. Catalysts 2018, 8, 336. [Google Scholar] [CrossRef]
- Cao, L.; Chen, L.; Wu, X.; Ran, R.; Xu, T.; Chen, Z.; Weng, D. TRA and DRIFTS studies of the fast SCR reaction over CeO2/TiO2 catalyst at low temperatures. Appl. Catal. A- Gen. 2018, 557, 46–54. [Google Scholar] [CrossRef]
- Qu, L.; Li, C.; Zeng, G.; Zhang, M.; Fu, M.; Ma, J.; Zhan, F.; Luo, D. Support modification for improving the performance of MnOx–CeOy/γ-Al2O3 in selective catalytic reduction of NO by NH3. Chem. Eng. J. 2014, 242, 76–85. [Google Scholar] [CrossRef]
- Li, P.; Xin, Y.; Li, Q.; Wang, Z.; Zhang, Z.; Zheng, L. Ce–Ti amorphous oxides for selective catalytic reduction of NO with NH3: confirmation of Ce–O–Ti active sites. Environ. Sci. Technol. 2012, 46, 9600–9605. [Google Scholar] [CrossRef]
- Palma, V.; Barba, D.; Vaiano, V.; Colozzi, M.; Palo, E.; Barbato, L.; Cortese, S.; Miccio, M. Honeycomb Structured Catalysts for H2 Production via H2S Oxidative Decomposition. Catalysts 2018, 8, 488. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, B.; Du, J.; Tang, Q.; Liu, Z.; Liu, R.; Tao, C. The monolithic cordierite supported V2O5–MoO3/TiO2 catalyst for NH3-SCR. Chem. Eng. J. 2016, 294, 264–272. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Su, J. Al2O3-coated cordierite honeycomb supported CuO catalyst for selective catalytic reduction of NO by NH3: surface properties and reaction mechanism. Catal. Today. 2010, 158, 370–376. [Google Scholar] [CrossRef]
- Su, J.; Liu, Q.; Liu, Z.; Huang, Z. Honeycomb CuO/Al2O3/Cordierite Catalyst for Selective Catalytic Reduction of NO by NH3-Effect of Al2O3 Coating. Ind. Eng. Chem. Res. 2008, 47, 4295–4301. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Tan, K.; Zhang, L.; Tang, K.; Shi, X. Fabrication and characterization of porous cordierite ceramics prepared from ferrochromium slag. Ceram. Int. 2016, 42, 734–742. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Chen, Z.; Ji, R.; Liu, L.; Wang, X. Fabrication and characterization of porous cordierite ceramics prepared from fly ash and natural minerals. Ceram. Int. 2019. under review. [Google Scholar]
- Zhan, S.; Zhang, H.; Zhang, Y.; Shi, Q.; Li, Y.; Li, X. Efficient NH3-SCR removal of NOx with highly ordered mesoporous WO3 (χ)-CeO2 at low temperatures. Appl. Catal. B-Environ. 2017, 203, 199–209. [Google Scholar] [CrossRef]
- Yao, X.; Chen, L.; Cao, J.; Yang, F.; Tan, W.; Dong, L. Morphology and Crystal-Plane Effects of CeO2 on TiO2/CeO2 Catalysts during NH3-SCR Reaction. Ind. Eng. Chem. Res. 2018, 57, 12407–12419. [Google Scholar] [CrossRef]
- Sun, X.; Guo, R.-t.; Liu, S.-w.; Liu, J.; Pan, W.-g.; Shi, X.; Qin, H.; Wang, Z.-y.; Qiu, Z.-z.; Liu, X.-y. The promoted performance of CeO2 catalyst for NH3-SCR reaction by NH3 treatment. Appl. Surf. Sci. 2018, 462, 187–193. [Google Scholar] [CrossRef]
- Vorokhta, M.; Khalakhan, I.; Matolínová, I.; Nováková, J.; Haviar, S.; Lančok, J.; Novotný, M.; Yoshikawa, H.; Matolín, V. PLD prepared nanostructured Pt-CeO2 thin films containing ionic platinum. Appl. Surf. Sci. 2017, 396, 278–283. [Google Scholar] [CrossRef]
- Nguyen, T.-S.; Postole, G.; Loridant, S.; Bosselet, F.; Burel, L.; Aouine, M.; Massin, L.; Gélin, P.; Morfin, F.; Piccolo, L. Ultrastable iridium–ceria nanopowders synthesized in one step by solution combustion for catalytic hydrogen production. J. Mater. Chem. A. 2014, 2, 19822–19832. [Google Scholar] [CrossRef]
- Jiang, Y.; Bao, C.; Liu, S.; Liang, G.; Lu, M.; Lai, C.; Shi, W.; Ma, S. Enhanced activity of Nb-modified CeO2/TiO2 catalyst for the selective catalytic reduction of NO with NH3. Aerospl. Air. Qual. Res. 2018, 18, 2121–2130. [Google Scholar] [CrossRef]
- Fan, J.; Ning, P.; Song, Z.; Liu, X.; Wang, L.; Wang, J.; Wang, H.; Long, K.; Zhang, Q. Mechanistic aspects of NH3-SCR reaction over CeO2/TiO2-ZrO2-SO42− catalyst: In situ DRIFTS investigation. Chem. Eng. J. 2018, 334, 855–863. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wu, Z.; Wang, H.; Gu, T. Low-temperature selective catalytic reduction of NO with NH3 over MnCe oxides supported on TiO2 and Al2O3: A comparative study. Chemosphere 2010, 78, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Larrubia, M.A.; Ramis, G.; Busca, G. An FT-IR study of the adsorption and oxidation of N-containing compounds over Fe2O3-TiO2 SCR catalysts. Appl. Catal. B-Environ. 2001, 30, 101–110. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yan, Z.; Liu, L.; Zhang, Z.; Wang, X. Promoting effect of Nd on the reduction of NO with NH3 over CeO2 supported by activated semi-coke: an in situ DRIFTS study. Catal. Sci. Technol. 2015, 5, 2251–2259. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, X.; Huang, T.; Shen, K.; Lu, B. In situ DRIFTS studies of NH3-SCR mechanism over V2O5-CeO2/TiO2-ZrO2 catalysts for selective catalytic reduction of NOx. Materials 2018, 11, 1307. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, J.; Ning, P.; Song, Z.; Liu, X.; Wang, L.; Wang, J.; Wang, H.; Long, K. In situ DRIFTS investigation of NH3-SCR reaction over CeO2/zirconium phosphate catalyst. Appl. Surf. Sci. 2018, 435, 1037–1045. [Google Scholar] [CrossRef]
- Sazama, P.; Čapek, L.; Drobná, H.; Sobalik, Z.; Dědeček, J.; Arve, K.; Wichterlová, B. Enhancement of decane-SCR-NOx over Ag/alumina by hydrogen. Reaction kinetics and in situ FTIR and UV–vis study. J. Catal. 2005, 232, 302–317. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, W.; Luo, N.; Li, P.; Lei, Z.; Chen, B. Low-temperature NH3-SCR of NO by lanthanum manganite perovskites: Effect of A-/B-site substitution and TiO2/CeO2 support. Appl. Catal. B-Environ. 2014, 146, 94–104. [Google Scholar] [CrossRef]
- Chen, L.; Si, Z.; Wu, X.; Weng, D. DRIFT study of CuO–CeO2–TiO2 mixed oxides for NOx reduction with NH3 at Low temperatures. Acs. Appl. Mater. Inter. 2014, 6, 8134–8145. [Google Scholar] [CrossRef]
- Łamacz, A.; Krztoń, A.; Djéga-Mariadassou, G. Study on the selective catalytic reduction of NO with toluene over CuO/CeZrO2. A confirmation for the three-function model of HC-SCR using the temperature programmed methods and in situ DRIFTS. Appl. Catal. B-Environ. 2013, 142, 268–277. [Google Scholar] [CrossRef]
- Wang, W.; McCool, G.; Kapur, N.; Yuan, G.; Shan, B.; Nguyen, M.; Graham, U.M.; Davis, B.H.; Jacobs, G.; Cho, K. Mixed-phase oxide catalyst based on Mn-mullite (Sm, Gd) Mn2O5 for NO oxidation in diesel exhaust. Science 2012, 337, 832–835. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Characterization and FTIR studies of MnOx−CeO2 catalyst for low-temperature selective catalytic reduction of NO with NH3. J. Phys. Chem. B 2004, 108, 15738–15747. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Z.; Liu, L.; Chen, Y.; Zhang, Z.; Wang, X. In situ DRIFTS investigation on the SCR of NO with NH3 over V2O5 catalyst supported by activated semi-coke. Appl. Surf. Sci. 2014, 313, 660–669. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Li, H.; Hu, H.; Hu, X.; Shi, L.; Zhang, D. Promotional effects of zirconium doped CeVO4 for the low-temperature selective catalytic reduction of NOx with NH3. Appl. Catal. B-Environ. 2016, 183, 269–281. [Google Scholar] [CrossRef]
- Jiang, Y.; Bao, C.; Liu, Q.; Liang, G.; Lu, M.; Ma, S. A novel CeO2-MoO3-WO3/TiO2 catalyst for selective catalytic reduction of NO with NH3. Catal. Commun. 2018, 103, 96–100. [Google Scholar] [CrossRef]




| Sample | Ce(NO3)3 Concentration (mol/L) | Catalyst Support | Temperature (°C) | Time (h) |
|---|---|---|---|---|
| 0.05Ce/CPCC | 0.05 | CPCC | 160 | 24 |
| 0.2Ce/CPCC | 0.2 | CPCC | 160 | 24 |
| 0.4Ce/CPCC | 0.4 | CPCC | 160 | 24 |
| 0.6Ce/CPCC | 0.6 | CPCC | 160 | 24 |
| 0.8Ce/CPCC | 0.8 | CPCC | 160 | 24 |
| 0.6Ce/SPCC | 0.6 | SPCC | 160 | 24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Chen, Z.; He, B.; Yan, Z.; Wang, H.; Liu, L.; Wang, X. In Situ DRIFTS Investigation on CeOx Catalyst Supported by Fly-Ash-Made Porous Cordierite Ceramics for Low-Temperature NH3-SCR of NOX. Catalysts 2019, 9, 496. https://doi.org/10.3390/catal9060496
Wang S, Chen Z, He B, Yan Z, Wang H, Liu L, Wang X. In Situ DRIFTS Investigation on CeOx Catalyst Supported by Fly-Ash-Made Porous Cordierite Ceramics for Low-Temperature NH3-SCR of NOX. Catalysts. 2019; 9(6):496. https://doi.org/10.3390/catal9060496
Chicago/Turabian StyleWang, Shaoxin, Ziwei Chen, Beini He, Zheng Yan, Hao Wang, Lili Liu, and Xidong Wang. 2019. "In Situ DRIFTS Investigation on CeOx Catalyst Supported by Fly-Ash-Made Porous Cordierite Ceramics for Low-Temperature NH3-SCR of NOX" Catalysts 9, no. 6: 496. https://doi.org/10.3390/catal9060496
APA StyleWang, S., Chen, Z., He, B., Yan, Z., Wang, H., Liu, L., & Wang, X. (2019). In Situ DRIFTS Investigation on CeOx Catalyst Supported by Fly-Ash-Made Porous Cordierite Ceramics for Low-Temperature NH3-SCR of NOX. Catalysts, 9(6), 496. https://doi.org/10.3390/catal9060496






