Sulfur Poisoning Effects on Modern Lean NOx Trap Catalysts Components
Abstract
1. Introduction
2. Results
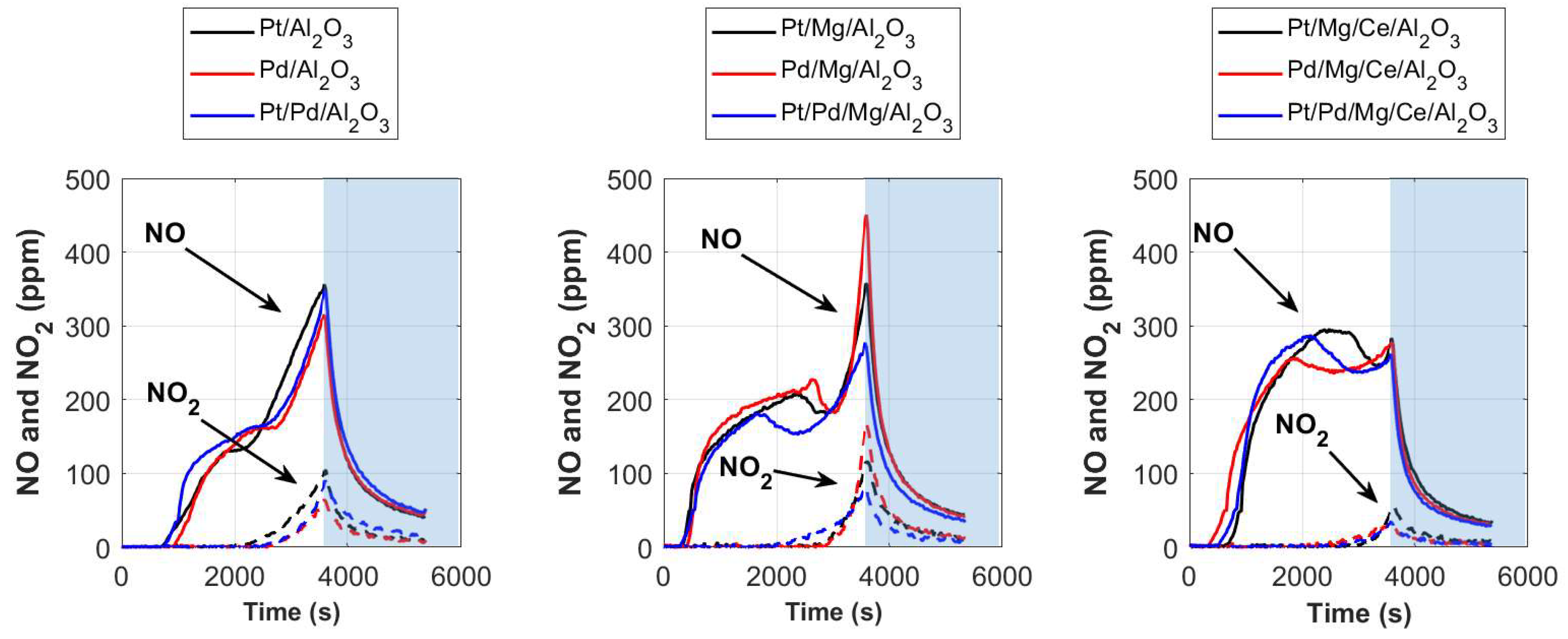
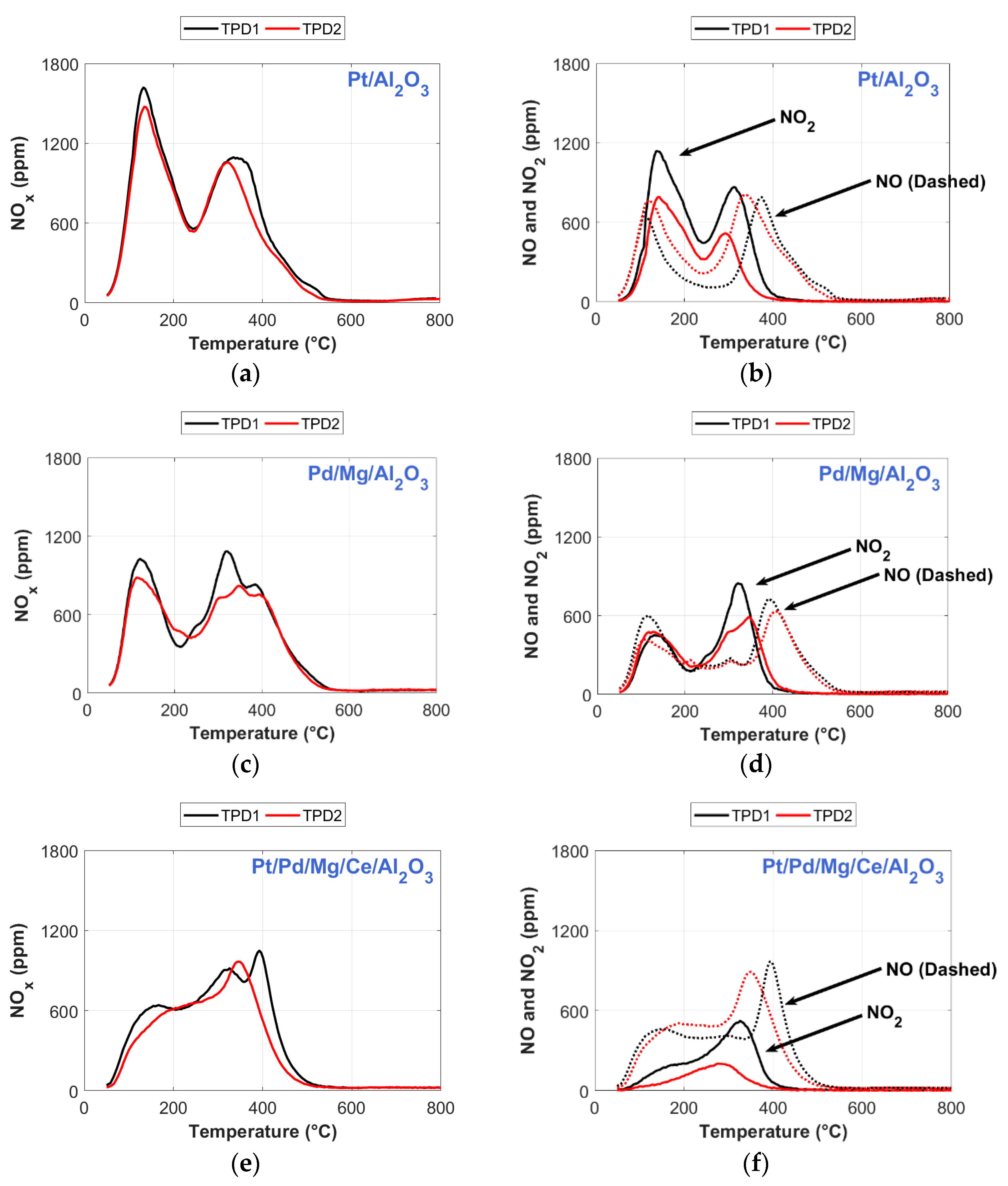
2.1. NO2-TPD Experiments
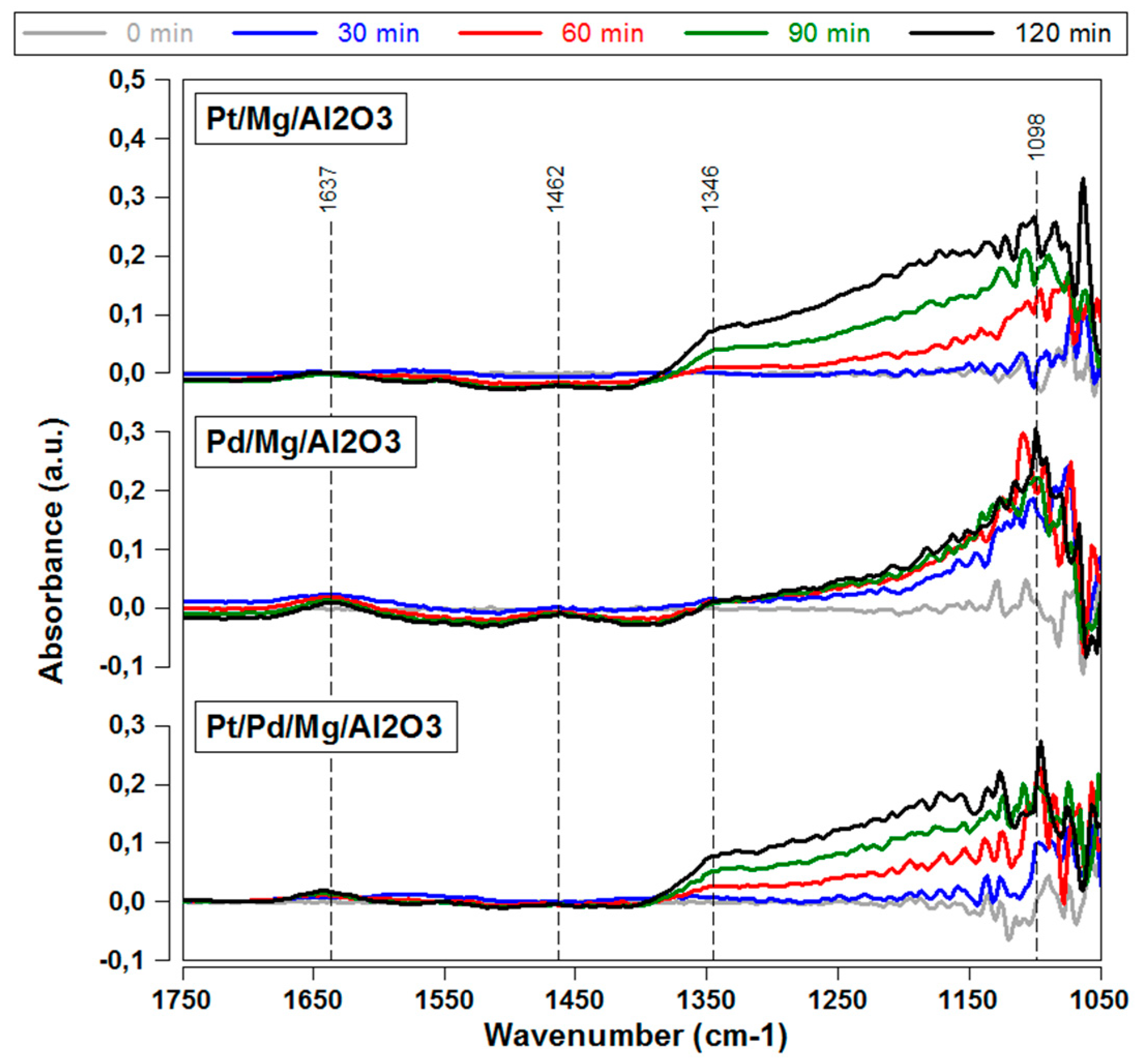
2.2. Sulfur Adsorption Characteristics—DRIFTS Experiments
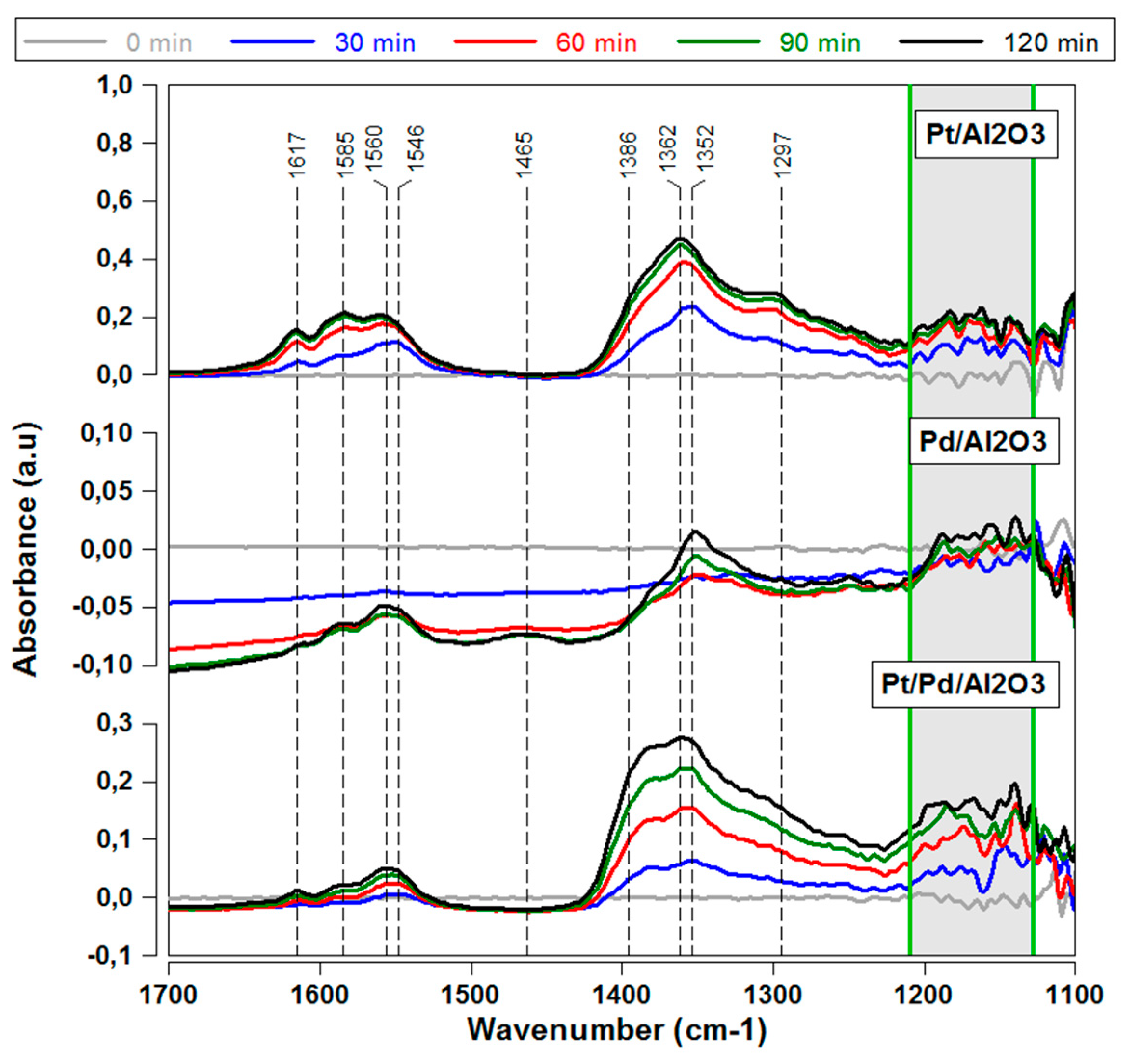
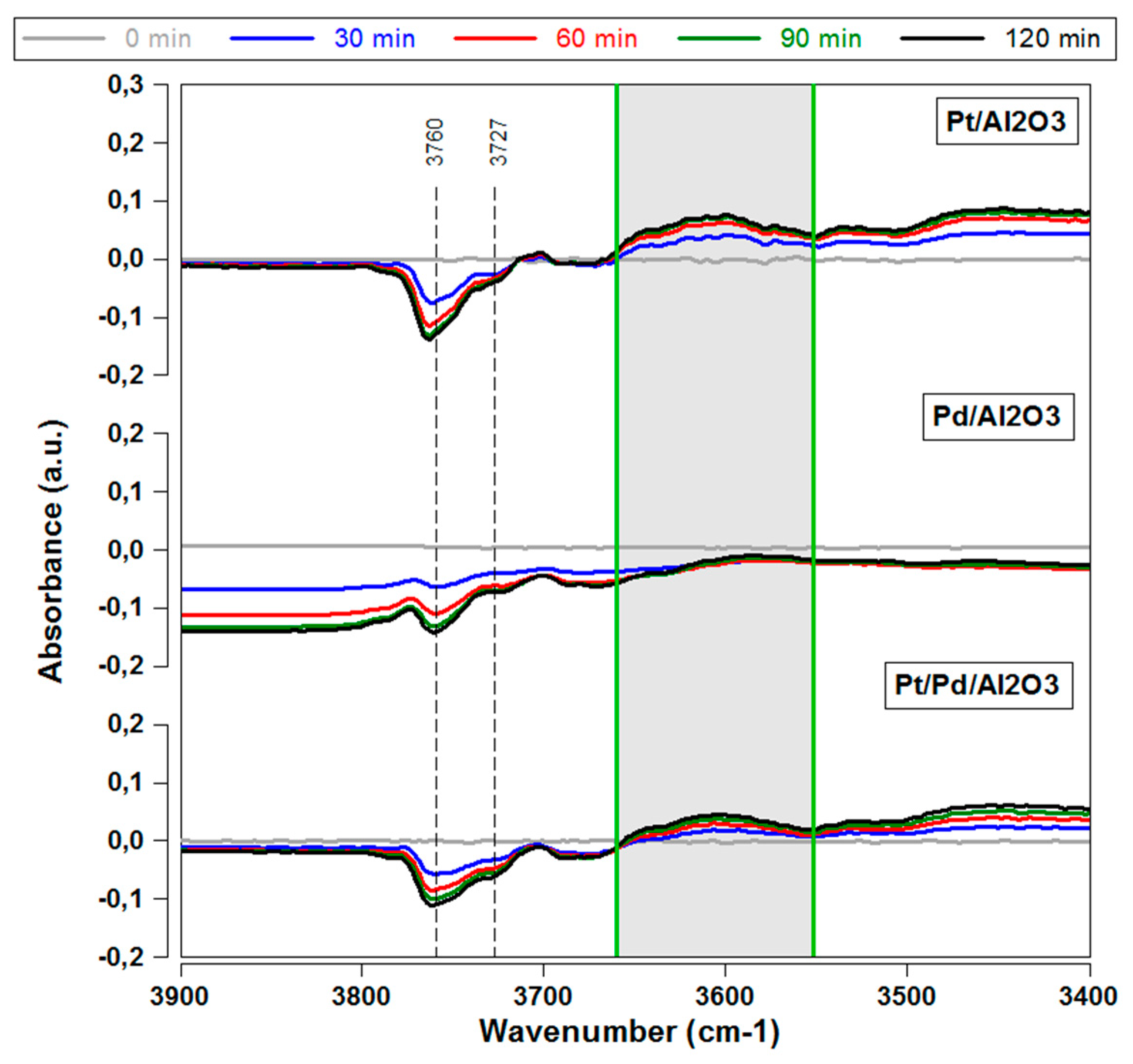
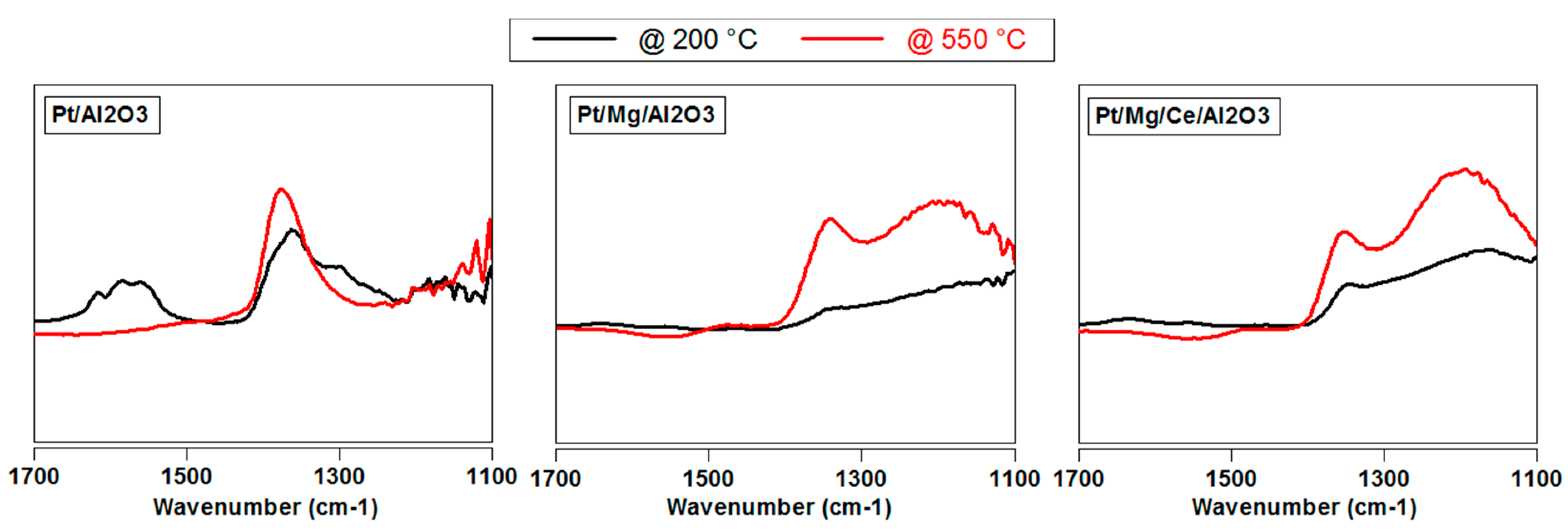
2.2.1. Influence of Sulfur on Different Precious Metal Species
2.2.2. Influence of Sulfur on Magnesium Compound
2.2.3. Influence of Sulfur on Ceria Compound
2.2.4. Influence of Catalyst Temperature on SO2 Poisoning
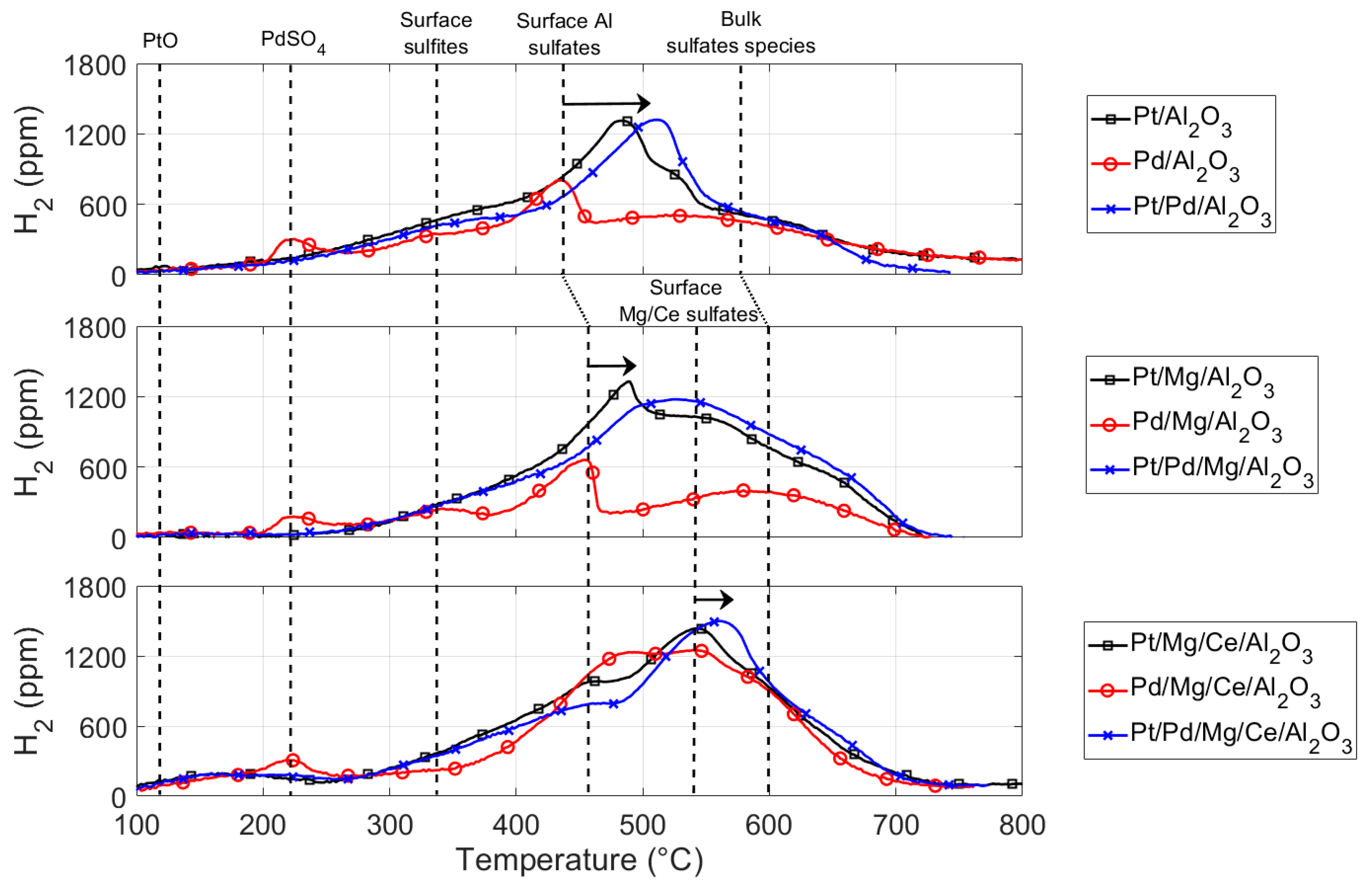
2.3. Sulfur Regeneration
2.4. Temperature Programmed Desorption (TPD) after Sulfur Regeneration
2.5. Interpretation of the Sulfur Poisoning and Regeneration Results
3. Experimental
3.1. Catalyst Preparation
3.2. Catalytic Evaluation Experiments
3.2.1. NO2-TPD Experiments
3.2.2. SO2 Poisoning and H2-TPR Experiments
3.3. DRIFT Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| [47] | (A1) | |
| [8] | (A2) | |
| [47] | (A3) | |
| [47] | (A4) | |
| [8] | (A5) |
| [5] | (A6) |
References
- Ottinger, N.A.; Toops, T.J.; Pihl, J.A.; Roop, J.T.; Choi, J.-S.; Partridge, W.P. Sulfate storage and stability on representative commercial lean NOx trap components. Appl. Catal. B Environ. 2012, 117–118, 167–176. [Google Scholar] [CrossRef]
- De Abreu Goes, J.E.; Olsson, L.; Berggrund, M.; Kristoffersson, A.; Gustafson, L.; Hicks, M. Performance Studies and Correlation between Vehicle- and Rapid-Aged Commercial Lean NOx Trap Catalysts. SAE Int. J. Engines 2017, 10, 1613–1626. [Google Scholar] [CrossRef]
- Johnson, T.; Joshi, A. Review of Vehicle Engine Efficiency and Emissions. SAE Int. J. Engines 2018, 11, 1307–1330. [Google Scholar] [CrossRef]
- Choi, J.-S.; Partridge, W.P.; Pihl, J.A.; Daw, C.S. Sulfur and temperature effects on the spatial distribution of reactions inside a lean NOx trap and resulting changes in global performance. Catal. Today 2008, 136, 173–182. [Google Scholar] [CrossRef]
- Dawody, J.; Skoglundh, M.; Olsson, L.; Fridell, E. Kinetic modelling of sulfur deactivation of Pt/BaO/Al2O3 and BaO/Al2O3 NOx storage catalysts. Appl. Catal. B Environ. 2007, 70, 179–188. [Google Scholar] [CrossRef]
- Liu, Y.; He, H.; Xu, W.; Yu, Y. Mechanism of Heterogeneous Reaction of Carbonyl Sulfide on Magnesium Oxide. The J. Phys. Chem. A 2007, 111, 4333–4339. [Google Scholar] [CrossRef] [PubMed]
- Rohr, F.; Göbel, U.; Kattwinkel, P.; Kreuzer, T.; Müller, W.; Philipp, S.; Gélin, P. New insight into the interaction of sulfur with diesel NOx storage catalysts. Appl. Catal. B Environ. 2007, 70, 189–197. [Google Scholar] [CrossRef]
- Lafossas, F.-A.; Manetas, C.; Mohammadi, A.; Kalogirou, M.; Koltsakis, G.; Samaras, Z.; Iida, M.; Inoue, M. Sulfation and Lean/Rich Desulfation of a NOx storage Reduction Catalyst: Experimental and Simulation Study. Int. J. Engine Res. 2015, 16, 197–212. [Google Scholar] [CrossRef]
- Hadl, K.; Ratzberger, R.; Eichlseder, H.; Schuessler, M.; Linares, W.; Pucher, H. Sulfur Poisoning of a NOx Storage Catalyst—A Comprehensive Modelling Approach. SAE Int. J. Engines 2016. [Google Scholar] [CrossRef]
- Olsson, L.; Blint, R.J.; Fridell, E. Global Kinetic Model for Lean NOx Traps. Ind. Eng. Chem. Res. 2005, 44, 3021–3032. [Google Scholar] [CrossRef]
- AL-Harbi, M.; Epling, W.S. Investigating the Effect of NO Versus NO2 on the Performance of a Model NOx Storage/Reduction Catalyst. Catal. Lett. 2009, 130, 121–129. [Google Scholar] [CrossRef]
- Benramdhane, S.; Millet, C.-N.; Jeudy, E.; Lavy, J.; Blasin-Aubé, V.; Daturi, M. Diesel Lean NOx-Trap Thermal Aging and Performance Evolution Characterization. Oil Gas Sci. Technol. Rev. IFP Energies nouvelles 2011, 66, 845–853. [Google Scholar] [CrossRef]
- Rohr, F.; Peter, S.D.; Lox, E.; Kögel, M.; Sassi, A.; Juste, L.; Rigaudeau, C.; Belot, G.; Gélin, P.; Primet, M. On the Mechanism of Sulphur Poisoning and Regeneration of a Commercial Gasoline NOx-Storage Catalyst. Appl. Catal. B Environ. 2005, 56, 201–212. [Google Scholar] [CrossRef]
- Ji, Y.; Toops, T.J.; Crocker, M. Effect of Ceria on the Sulfation and Desulfation Characteristics of a Model Lean NO x Trap Catalyst. Catal. Lett. 2009, 127, 55–62. [Google Scholar] [CrossRef]
- Easterling, V.; Ji, Y.; Crocker, M.; Ura, J.; Theis, J.R.; McCabe, R.W. Effect of ceria on the desulfation characteristics of model lean NOx trap catalysts. Catal. Today 2010, 151, 338–346. [Google Scholar] [CrossRef]
- Choi, J.-S.; Partridge, W.P.; Lance, M.J.; Walker, L.R.; Pihl, J.A.; Toops, T.J.; Finney, C.E.A.; Daw, C.S. Nature and Spatial Distribution of Sulfur Species in a Sulfated Barium-Based Commercial Lean NOx Trap Catalyst. Catal. Today 2010, 151, 354–361. [Google Scholar] [CrossRef]
- Basile, F.; Fornasari, G.; Gambatesa, A.; Livi, M.; Vaccari, A. Reactivity of Mg-Ba catalyst in NOx storage/reduction. Catal. Today 2007, 119, 59–63. [Google Scholar] [CrossRef]
- Jeong, S.; Youn, S.; Kim, D.H. Effect of Mg/Al ratios on the NOx storage activity over Pt-BaO/Mg–Al mixed oxides. Catal. Today 2014, 231, 155–163. [Google Scholar] [CrossRef]
- Luo, J.; Gao, F.; Karim, A.M.; Xu, P.; Browning, N.D.; Peden, C.H.F. Advantages of MgAlOx over γ-Al2O3 as a Support Material for Potassium-Based High-Temperature Lean NOx Traps. ACS Catal. 2015, 5, 4680–4689. [Google Scholar] [CrossRef]
- Nova, I.; Castoldi, L.; Prinetto, F.; Dal Santo, V.; Lietti, L.; Tronconi, E.; Forzatti, P.; Ghiotti, G.; Psaro, R.; Recchia, S. NOx Adsorption Study over Pt–Ba/Alumina Catalysts: FT-IR and Reactivity Study. Top. Catal. 2004, 30, 181–186. [Google Scholar] [CrossRef]
- Scotti, A.; Nova, I.; Tronconi, E.; Castoldi, L.; Lietti, L.; Forzatti, P. Kinetic Study of Lean NOx Storage over the Pt−Ba/Al2O3 System. Ind. Eng. Chem. Res. 2004, 43, 4522–4534. [Google Scholar] [CrossRef]
- Forzatti, P.; Castoldi, L.; Lietti, L.; Nova, I.; Tronconi, E. Chapter 6 Identification of the reaction networks of the NOx storage/reduction in lean NOx trap systems. In Studies in Surface Science and Catalysis; Granger, P., Pârvulescu, V.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 171, pp. 175–208. [Google Scholar]
- Lietti, L.; Daturi, M.; Blasin-Aubé, V.; Ghiotti, G.; Prinetto, F.; Forzatti, P. Relevance of the Nitrite Route in the NOx Adsorption Mechanism over Pt–Ba/Al2O3 NOx Storage Reduction Catalysts Investigated by using Operando FTIR Spectroscopy. ChemCatChem 2012, 4, 55–58. [Google Scholar] [CrossRef]
- Prinetto, F.; Ghiotti, G.; Nova, I.; Lietti, L.; Tronconi, E.; Forzatti, P. FT-IR and TPD Investigation of the NOx Storage Properties of BaO/Al2O3 and Pt−BaO/Al2O3 Catalysts. J. Phys. Chem. B 2001, 105, 12732–12745. [Google Scholar] [CrossRef]
- Lindholm, A.; Currier, N.W.; Dawody, J.; Hidayat, A.; Li, J.; Yezerets, A.; Olsson, L. The influence of the preparation procedure on the storage and regeneration behavior of Pt and Ba based NOx storage and reduction catalysts. Appl. Catal. B Environ. 2009, 88, 240–248. [Google Scholar] [CrossRef]
- Svedberg, P.; Jobson, E.; Erkfeldt, S.; Andersson, B.; Larsson, M.; Skoglundh, M. Influence of the storage material on the storage of NO x at low temperatures. Top. Catal. 2004, 30, 199–206. [Google Scholar] [CrossRef]
- De Abreu Goes, J.; Kristoffersson, A.; Wentworth, T.; Olsson, L. Detailed Characterization Studies of Vehicle and Rapid Aged Commercial Lean NOx Trap Catalysts. Ind. Eng. Chem. Res. 2018. [Google Scholar] [CrossRef]
- Uy, D.; Wiegand, K.A.; O’Neill, A.E.; Dearth, M.A.; Weber, W.H. In Situ UV Raman Study of the NOx Trapping and Sulfur Poisoning Behavior of Pt/Ba/γ-Al2O3 Catalysts. J. Phys. Chem. B 2002, 106, 387–394. [Google Scholar] [CrossRef]
- Abdulhamid, H.; Fridell, E.; Dawody, J.; Skoglundh, M. In situ FTIR study of SO2 interaction with Pt/BaCO3/Al2O3 NOx storage catalysts under lean and rich conditions. J. Catal. 2006, 241, 200–210. [Google Scholar] [CrossRef]
- Waqif, M.; Saur, O.; Lavalley, J.C.; Perathoner, S.; Centi, G. Nature and mechanism of formation of sulfate species on copper/alumina sorbent-catalysts for sulfur dioxide removal. J. Phys. Chem. 1991, 95, 4051–4058. [Google Scholar] [CrossRef]
- Xie, S.X.; Yu, Y.B.; Wang, J.; He, H. Effect Of SO2 on the performance of Ag-Pd/Al2O3 for the selective catalytic reduction of NOx with C2H5OH. J. Environ. Sci. 2006, 18, 973–978. [Google Scholar] [CrossRef]
- Bounechada, D.; Fouladvand, S.; Kylhammar, L.; Pingel, T.; Olsson, E.; Skoglundh, M.; Gustafson, J.; Di Michiel, M.; Newton, M.A.; Carlsson, P.-A. Mechanisms behind sulfur promoted oxidation of methane. Phys. Chem. Chem. Phys. 2013, 15, 8648–8661. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.B.; Sheinker, V.N.; White, M.G. Adsorption and Reaction of Sulfur Dioxide on Alumina and Sodium-Impregnated Alumina. J. Phys. Chem. 1996, 100, 7550–7557. [Google Scholar] [CrossRef]
- Wilburn, M.S.; Epling, W.S. SO2 adsorption and desorption characteristics of bimetallic Pd-Pt catalysts: Pd:Pt ratio dependency. Catal. Today 2017. [Google Scholar] [CrossRef]
- Li, Y.; Shen, M.; Wang, J.; Wan, T.; Wang, J. Influence of sulfation and regeneration on Pt/Al2O3 for NO oxidation. Catal. Sci. Technol. 2015, 5, 1731–1740. [Google Scholar] [CrossRef]
- Mowery, D.L.; McCormick, R.L. Deactivation of alumina supported and unsupported PdO methane oxidation catalyst: the effect of water on sulfate poisoning. Appl. Catal. B Environ. 2001, 34, 287–297. [Google Scholar] [CrossRef]
- Yu, T.-C.; Shaw, H. The effect of sulfur poisoning on methane oxidation over palladium supported on γ-alumina catalysts. Appl. Catal. B Environ. 1998, 18, 105–114. [Google Scholar] [CrossRef]
- Colussi, S.; Arosio, F.; Montanari, T.; Busca, G.; Groppi, G.; Trovarelli, A. Study of sulfur poisoning on Pd/Al2O3 and Pd/CeO2/Al2O3 methane combustion catalysts. Catal. Today 2010, 155, 59–65. [Google Scholar] [CrossRef]
- Wilburn, M.S.; Epling, W.S. Sulfur deactivation and regeneration of mono- and bimetallic Pd-Pt methane oxidation catalysts. Appl. Catal. B Environ. 2017, 206, 589–598. [Google Scholar] [CrossRef]
- Lampert, J.K.; Kazi, M.S.; Farrauto, R.J. Palladium catalyst performance for methane emissions abatement from lean burn natural gas vehicles. Appl. Catal. B Environ. 1997, 14, 211–223. [Google Scholar] [CrossRef]
- Ohtsuka, H. The selective catalytic reduction of nitrogen oxides by methane on noble metal-loaded sulfated zirconia. Appl. Catal. B Environ. 2001, 33, 325–333. [Google Scholar] [CrossRef]
- Gélin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B Environ. 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Lapisardi, G.; Gélin, P.; Kaddouri, A.; Garbowski, E.; Da Costa, S. Pt–Pd bimetallic catalysts for methane emissions abatement. Top. Catal. 2007, 42, 461–464. [Google Scholar] [CrossRef]
- Sadokhina, N.; Smedler, G.; Nylén, U.; Olofsson, M.; Olsson, L. Deceleration of SO2 poisoning on PtPd/Al2O3 catalyst during complete methane oxidation. Appl. Catal. B Environ. 2018, 236, 384–395. [Google Scholar] [CrossRef]
- Knözinger, H.; Ratnasamy, P. Catalytic Aluminas: Surface Models and Characterization of Surface Sites. Catal. Rev. 1978, 17, 31–70. [Google Scholar] [CrossRef]
- Hamzehlouyan, T.; Sampara, C.S.; Li, J.; Kumar, A.; Epling, W.S. Kinetic study of adsorption and desorption of SO2 over γ-Al2O3 and Pt/γ-Al2O3. Appl. Catal. B Environ. 2016, 181, 587–598. [Google Scholar] [CrossRef]
- Wang, J.-A.; Li, C.-L. SO2 adsorption and thermal stability and reducibility of sulfates formed on the magnesium–aluminate spinel sulfur-transfer catalyst. Appl. Surf. Sci. 2000, 161, 406–416. [Google Scholar] [CrossRef]
- Schneider, W.F.; Li, J.; Hass, K.C. Combined Computational and Experimental Investigation of SOx Adsorption on MgO. J. Phys. Chem. B 2001, 105, 6972–6979. [Google Scholar] [CrossRef]
- Kylhammar, L.; Carlsson, P.-A.; Skoglundh, M. Sulfur promoted low-temperature oxidation of methane over ceria supported platinum catalysts. J. Catal. 2011, 284, 50–59. [Google Scholar] [CrossRef]
- Kylhammar, L.; Carlsson, P.-A.; Ingelsten, H.H.; Grönbeck, H.; Skoglundh, M. Regenerable ceria-based SOx traps for sulfur removal in lean exhausts. Appl. Catal. B Environ. 2008, 84, 268–276. [Google Scholar] [CrossRef]
- Li, J.; Theis, J.; Chun, W.; Goralski, C.; Kudla, R.; Ura, J.; Watkins, W.; Chattha, M.; Hurley, R. Sulfur Poisoning and Desulfation of the Lean NOx Trap. SAE Tech. Pap. 2001. [Google Scholar] [CrossRef]
- Boaro, M.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A.; Graziani, M. Oxygen Storage Behavior of Ceria–Zirconia-Based Catalysts in the Presence of SO2. Top. Catal. 2001, 16, 299–306. [Google Scholar] [CrossRef]
- Hamzehlouyan, T.; Sampara, C.; Li, J.; Kumar, A.; Epling, W. Sulfur Poisoning of a Pt/Al2O3 Oxidation Catalyst: Understanding of SO2, SO3 and H2SO4 Impacts. Top. Catal. 2016, 59, 1028–1032. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, D.H. Sulfation and Desulfation Behavior of Pt-BaO/MgO-Al2O3 NOx Storage Reduction Catalyst. J. Nanosci. Nanotechnol. 2016, 16, 4411–4416. [Google Scholar] [CrossRef] [PubMed]
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks, J.E. Overview of the Fundamental Reactions and Degradation Mechanisms of NOx Storage/Reduction Catalysts. Catal. Rev. 2004, 46, 163–245. [Google Scholar] [CrossRef]
- Navarro, R.M.; Pawelec, B.; Trejo, J.M.; Mariscal, R.; Fierro, J.L.G. Hydrogenation of Aromatics on Sulfur-Resistant PtPd Bimetallic Catalysts. J. Catal. 2000, 189, 184–194. [Google Scholar] [CrossRef]
- Rades, T.; Pak, C.; Polisset-Thfoin, M.; Ryoo, R.; Fraissard, J. Characterization of bimetallic NaY-supported Pt-Pd catalyst by EXAFS, TEM and TPR. Catal. Lett. 1994, 29, 91–103. [Google Scholar] [CrossRef]



| Sample | Total NOx Ads (μmol) | NO Des. (μmol) | NO2 Des. (μmol) | T20 NOx (°C) | T90 NOx (°C) |
|---|---|---|---|---|---|
| Pt/Al2O3 | 37.3 | 13.8 | 18.2 | 141 | 417 |
| Pd/Al2O3 | 38.9 | 15.6 | 18.1 | 146 | 417 |
| Pt/Pd/Al2O3 | 37.2 | 11.9 | 20.8 | 132 | 406 |
| Pt/Mg/Al2O3 | 35.3 | 15.4 | 11.5 | 142 | 438 |
| Pd/Mg/Al2O3 | 34.5 | 15 | 11.5 | 142 | 435 |
| Pt/Pd/Mg/Al2O3 | 37.1 | 17.3 | 10 | 160 | 442 |
| Pt/Mg/CeO2/Al2O3 | 35.5 | 16.6 | 9.4 | 165 | 424 |
| Pd/Mg/CeO2/Al2O3 | 35.8 | 16.8 | 8.7 | 191 | 435 |
| Pt/Pd/Mg/CeO2/Al2O3 | 36 | 17 | 7.5 | 184 | 428 |
| Sample | H2 Consumption (μmol) | T20 SO2 (°C) | T90 SO2 (°C) |
|---|---|---|---|
| Pt/Al2O3 | 52 | 401 | 652 |
| Pd/Al2O3 | 38.3 | 386.9 | 661.9 |
| Pt/Pd/Al2O3 | 46.2 | 409.4 | 653.1 |
| Pt/Mg/Al2O3 | 48.8 | 487.8 | 689.7 |
| Pd/Mg/Al2O3 | 24 | 351.2 | 694.7 |
| Pt/Pd/Mg/Al2O3 | 50.7 | 508.3 | 713.8 |
| Pt/Mg/CeO2/Al2O3 | 62.8 | 482.5 | 714.6 |
| Pd/Mg/CeO2/Al2O3 | 57.7 | 475.3 | 674.3 |
| Pt/Pd/Mg/CeO2/Al2O3 | 61.3 | 506 | 701.7 |
| Sample | TPD1 | TPD2 | ||||
|---|---|---|---|---|---|---|
| NOx Ads (μmol) | NO Des. (μmol) | NO2 Des. (μmol) | NOx Ads (μmol) | NO Des. (μmol) | NO2 Des. (μmol) | |
| Pt/Al2O3 | 37.3 | 13.8 | 18.2 | 35.7 | 17.4 | 11.3 |
| Pd/Al2O3 | 38.9 | 15.6 | 18.1 | 37.4 | 17.5 | 13.1 |
| Pt/Pd/Al2O3 | 37.2 | 11.9 | 20.8 | 36 | 16.1 | 13.9 |
| Pt/Mg/Al2O3 | 35.3 | 15.4 | 11.5 | 34.4 | 16.9 | 7.7 |
| Pd/Mg/Al2O3 | 34.5 | 15 | 11.5 | 34.1 | 13 | 11.1 |
| Pt/Pd/Mg/Al2O3 | 37.1 | 17.3 | 10 | 36.8 | 18.8 | 6.9 |
| Pt/Mg/CeO2/Al2O3 | 35.5 | 16.6 | 9.4 | 36.7 | 17.9 | 4.2 |
| Pd/Mg/CeO2/Al2O3 | 35.8 | 16.8 | 8.7 | 37.2 | 17.5 | 4.1 |
| Pt/Pd/Mg/CeO2/Al2O3 | 36 | 17 | 7.5 | 37 | 17.6 | 3 |
| Group | Sample | Pt | Pd | Al2O3 | Mg | CeO2 |
|---|---|---|---|---|---|---|
| PGM-Al2O3 | Pt/Al2O3 | 2 | - | 98 | - | - |
| Pd/Al2O3 | - | 1.09 | 98.9 | - | - | |
| Pt/Pd/Al2O3 | 1.6 | 0.2 | 98.2 | - | - | |
| Mg-Al2O3 | Pt/Mg/Al2O3 | 2 | - | 88 | 10 | - |
| Pd/Mg/Al2O3 | - | 1.09 | 88.9 | 10 | - | |
| Pt/Pd/Mg/Al2O3 | 1.6 | 0.2 | 88.2 | 10 | - | |
| Mg-Ce-Al2O3 | Pt/Mg/CeO2/Al2O3 | 2 | - | 68 | 10 | 20 |
| Pd/Mg/CeO2/Al2O3 | - | 1.09 | 68.9 | 10 | 20 | |
| Pt/Pd/Mg/CeO2/Al2O3 | 1.6 | 0.2 | 68.2 | 10 | 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Abreu Goes, J.E.; Kristoffersson, A.; Olsson, L. Sulfur Poisoning Effects on Modern Lean NOx Trap Catalysts Components. Catalysts 2019, 9, 492. https://doi.org/10.3390/catal9060492
De Abreu Goes JE, Kristoffersson A, Olsson L. Sulfur Poisoning Effects on Modern Lean NOx Trap Catalysts Components. Catalysts. 2019; 9(6):492. https://doi.org/10.3390/catal9060492
Chicago/Turabian StyleDe Abreu Goes, Jesus Emmanuel, Annika Kristoffersson, and Louise Olsson. 2019. "Sulfur Poisoning Effects on Modern Lean NOx Trap Catalysts Components" Catalysts 9, no. 6: 492. https://doi.org/10.3390/catal9060492
APA StyleDe Abreu Goes, J. E., Kristoffersson, A., & Olsson, L. (2019). Sulfur Poisoning Effects on Modern Lean NOx Trap Catalysts Components. Catalysts, 9(6), 492. https://doi.org/10.3390/catal9060492