Esterification of Palm Fatty Acid Distillate for Biodiesel Production Catalyzed by Synthesized Kenaf Seed Cake-Based Sulfonated Catalyst
Abstract
1. Introduction
2. Results and Discussion
2.1. Surface Area Enhancements via Initial Chemical Activation
2.2. Catalyst Characterization
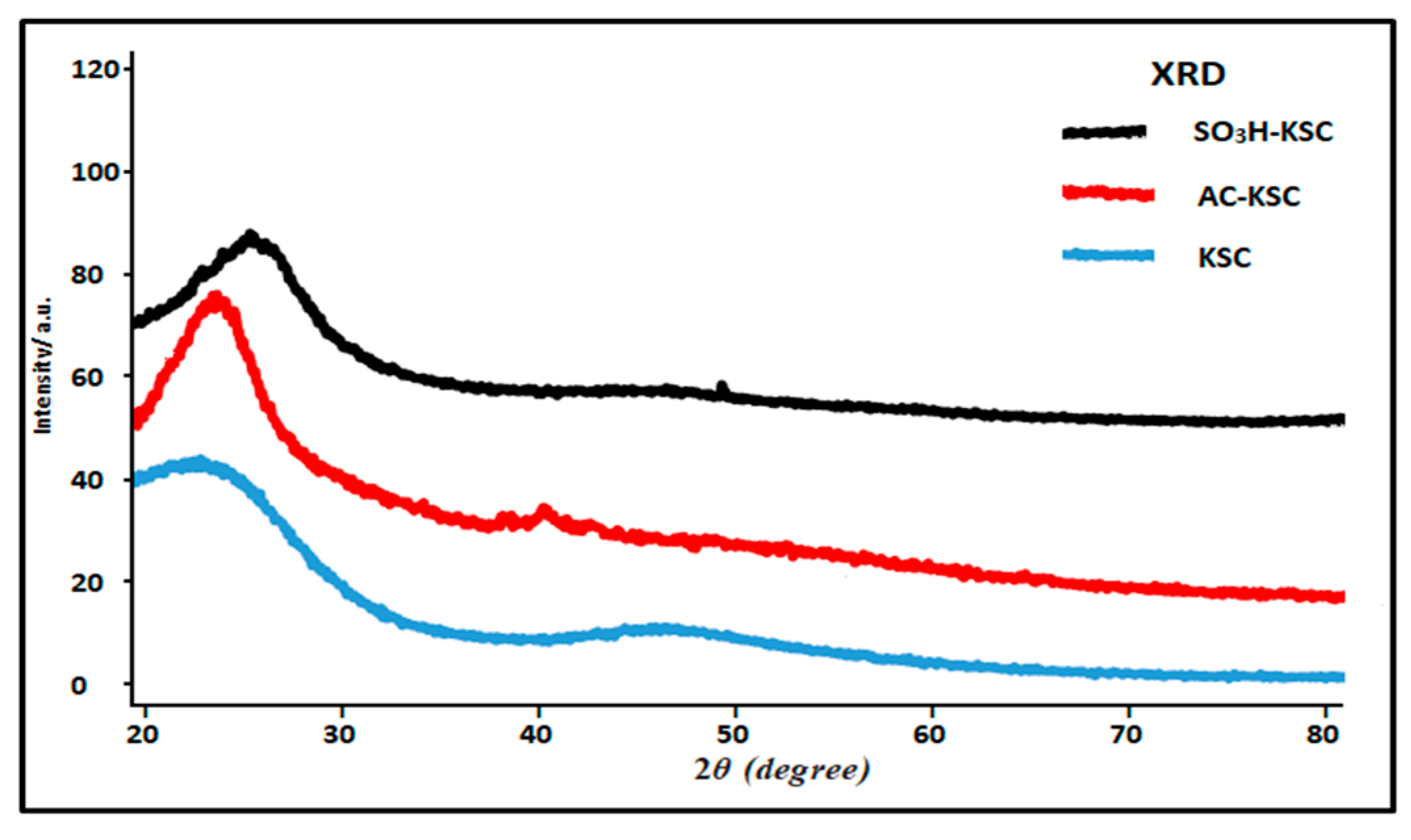
2.2.1. Phase Identification
2.2.2. Morphology
2.2.3. Acid Density
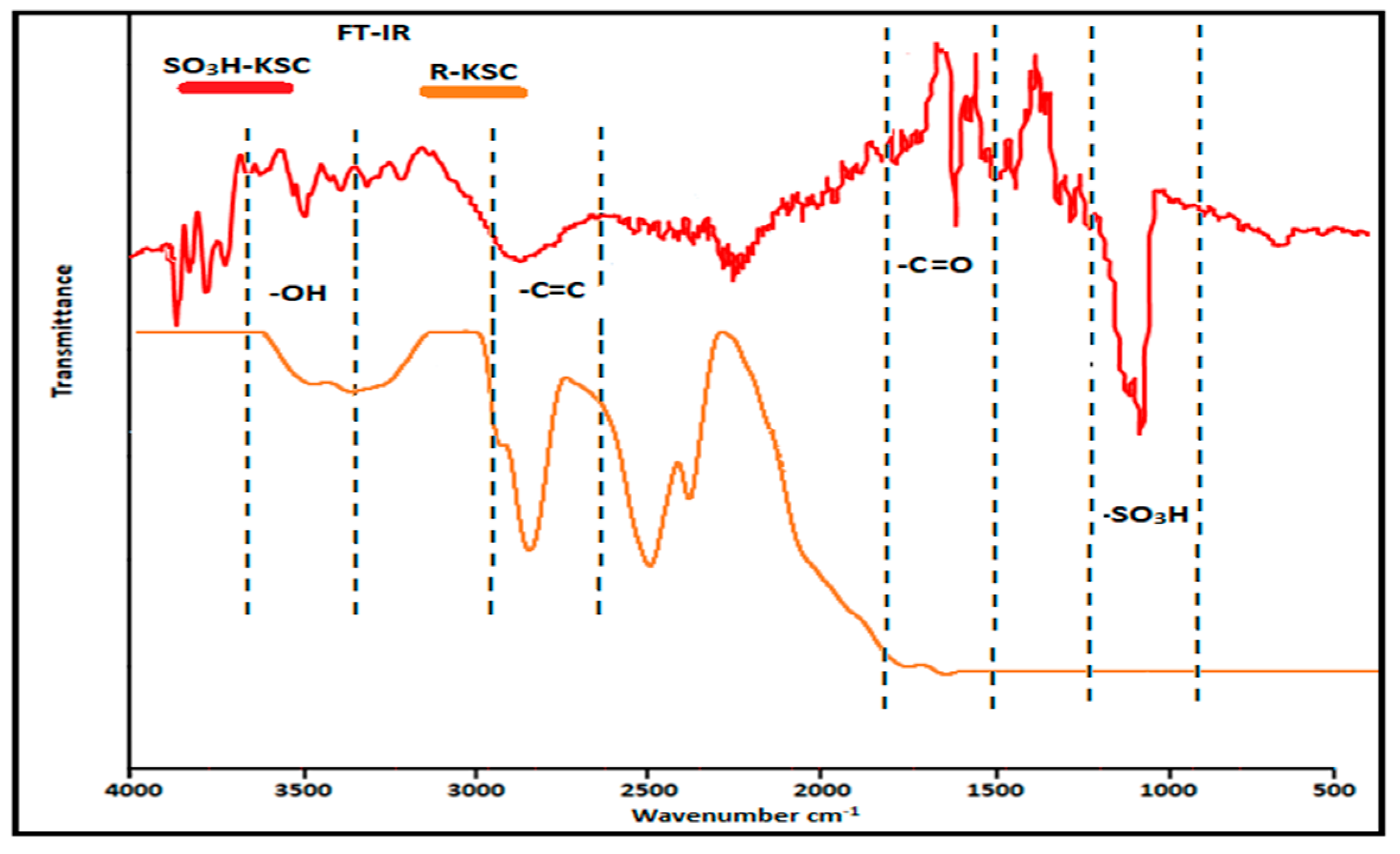
2.2.4. FT-IR Analysis
2.2.5. Thermal Stability
2.2.6. Surface Properties
2.3. Composition of PFAD and FAME
2.4. Esterification Optimization Variables
2.4.1. Reaction Temperature
2.4.2. Reaction Time
2.4.3. Molar Ratio of Methanol: PFAD
2.4.4. Catalyst Concentration
2.5. FAME Yield at Optimum Conditions
2.6. SO3H-KSC Reusability
3. Materials and Methods
3.1. Materials
3.2. Catalyst Synthesis
3.3. Catalyst Characterization
3.4. Experimental Setup for Esterification
3.5. FAME Analysis
3.6. Catalyst Reusability Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Soltani, S.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H. Biodiesel production in the presence of sulfonated mesoporous ZnAl2O4 catalyst via esterification of palm fatty acid distillate (PFAD). Fuel 2016, 178, 253–262. [Google Scholar] [CrossRef]
- Shuit, S.H.; Yee, K.F.; Lee, K.T.; Subhash, B.; Tan, S.H. Evolution towards the utilisation of functionalised carbon nanotubes as a new generation catalyst support in biodiesel production: An overview. RSC Adv. 2013, 3, 9070–9094. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel production via non-catalytic SCF method and biodiesel fuel characteristics. Energy Convers. Manag. 2006, 47, 2271–2282. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887. [Google Scholar] [CrossRef] [PubMed]
- Sunita, G.; Devassy, B.M.; Vinu, A.; Sawant, D.P.; Balasubramanian, V.V.; Halligudi, S.B. Synthesis of biodiesel over zirconia-supported isopoly and heteropoly tungstate catalysts. Catal. Commun. 2008, 9, 696–702. [Google Scholar] [CrossRef]
- Gupta, A.R.; Rathod, V.K. Waste cooking oil and waste chicken eggshells derived solid base catalyst for the biodiesel production: Optimization and kinetics. Waste Manag. 2018, 79, 169–178. [Google Scholar] [CrossRef]
- Peng-Lim, B.; Ganesan, S.; Maniam, G.P.; Khairuddean, M. Sequential conversion of high free fatty acid oils into biodiesel using a new catalyst system. Energy 2012, 46, 132–139. [Google Scholar] [CrossRef]
- Carlucci, C.; Degennaro, L.; Luisi, R. Titanium dioxide as a catalyst in biodiesel production. Catalysts 2019, 9, 75. [Google Scholar] [CrossRef]
- Yang, B.; Leclercq, L.; Clacens, J.M.; Nardello-Rataj, V. Acidic/amphiphilic silica nanoparticles: New eco-friendly pickering interfacial catalysis for biodiesel production. Green Chem. 2017, 19, 4552–4562. [Google Scholar] [CrossRef]
- Thanh, L.T.; Okitsu, K.; Van Boi, L.; Maeda, Y. Catalytic technologies for biodiesel fuel production and utilization of glycerol: A review. Catalysts 2012, 2, 191–222. [Google Scholar] [CrossRef]
- Tahery, Y.; Shukor, N.A.A.; Abdul-Hamid, H. Growth characteristics and biomass production of kenaf. Afr. J. Biotechnol. 2011, 10, 13756–13761. [Google Scholar] [CrossRef][Green Version]
- Khalil, H.A.; Yusra, A.I.; Bhat, A.H.; Jawaid, M. Cell wall ultrastructure, anatomy, lignin distribution, and chemical composition of Malaysian cultivated kenaf fiber. Ind. Crops Prod. 2010, 31, 113–121. [Google Scholar] [CrossRef]
- Akinfalabi, S.I.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H. Synthesis of biodiesel from palm fatty acid distillate using sulfonated palm seed cake catalyst. Renew. Energy 2017, 111, 611–619. [Google Scholar] [CrossRef]
- Chin, L.H.; Abdullah, A.Z.; Hameed, B.H. Sugar cane bagasse as solid catalyst for synthesis of methyl esters from palm fatty acid distillate. Chem. Eng. J. 2012, 183, 104–107. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Chen, Y.; Zhu, X. Biodiesel production from waste cooking oil using a heterogeneous catalyst from pyrolyzed rice husk. Bioresour. Technol. 2014, 154, 345–348. [Google Scholar] [CrossRef]
- Mundi, I. Malaysia Palm Oil Production by Year. 2017. Available online: http://bepi.mpob.gov.my/index.php/en/statistics/production/177-production-2017/792-production-of-crude-oil-palm-2017.html (accessed on 3 March 2019).
- Chongkhong, S.; Tongurai, C.; Chetpattananondh, P.; Bunyakan, C. Biodiesel production by esterification of palm fatty acid distillate. Biomass Bioenergy 2007, 31, 563–568. [Google Scholar] [CrossRef]
- Roschat, W.; Siritanon, T.; Yoosuk, B.; Promarak, V. Rice husk-derived sodium silicate as a highly efficient and low-cost basic heterogeneous catalyst for biodiesel production. Energy Convers. Manag. 2016, 119, 453–462. [Google Scholar] [CrossRef]
- Shehu-Ibrahim, A.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H. Appraisal of sulphonation processes to synthesize palm waste biochar catalysts for the esterification of palm fatty acid distillate. Catalysts 2019, 9, 184. [Google Scholar] [CrossRef]
- Omri, A.; Benzina, M. Characterization of activated carbon prepared from a new raw lignocellulosic material: Ziziphus spina-christi seeds. J. Soc. Chim. Tunis. 2012, 14, 175–183. [Google Scholar]
- Shamsuddin, M.S.; Yusoff, N.R.N.; Sulaiman, M.A. Synthesis and characterization of activated carbon produced from kenaf core fiber using H3PO4 activation. Procedia Chem. 2016, 19, 558–565. [Google Scholar] [CrossRef]
- Al-Jaberi, S.H.H.; Rashid, U.; Fairs, A.-D.; Abdulkareem-Alsultan, G.; Taufiq-Yap, Y.H. Synthesis of MnO-NiO-SO4−2/ZrO2 solid acid catalyst for methyl ester production from palm fatty acid distillate. Energy Convers. Manag. 2017, 139, 166–174. [Google Scholar] [CrossRef]
- Lokman, M.I.; Rashid, U.; Taufiq-Yap, Y.H.; Yunus, R. Methyl ester production from palm fatty acid distillate using sulfonated glucose-derived acid catalyst. Renew. Energy 2015, 81, 347–354. [Google Scholar] [CrossRef]
- Kastner, J.R.; Miller, J.; Geller, D.P.; Locklin, J.; Keith, L.H.; Johnson, T. Catalytic esterification of fatty acids using solid acid catalysts generated from biochar and activated carbon. Catal. Today 2012, 190, 122–132. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Ayodele, O.; Xin, J.; Zhang, S.; Yan, D. Effective conversion of non-edible oil with high free fatty acid into biodiesel by sulphonated carbon catalyst. Appl. Energy 2014, 114, 819–826. [Google Scholar] [CrossRef]
- Okamura, M.; Takagaki, A.; Toda, M.; Kondo, J.N.; Domen, K.; Tatsumi, T.; Hara, M.; Hayashi, S. Acid-catalyzed reactions on flexible polycyclic aromatic carbon in amorphous carbon. Chem. Mater. 2006, 18, 3039–3045. [Google Scholar] [CrossRef]
- Ezebor, F.; Khairuddean, M.; Abdullah, A.Z.; Boey, P.L. Esterification of oily-FFA and transesterification of high FFA waste oils using novel palm trunk and bagasse-derived catalysts. Energy Convers. Manag. 2014, 88, 1143–1150. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Aziz, A.M.; Al-Tamer, M.H. Biodiesel production from Silybum marianum L. seed oil with high FFA content using sulfonated carbon catalyst for esterification and base catalyst for transesterification. Energy Convers. Manag. 2016, 108, 255–265. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Kaiprommarat, S.; Kongparakul, S.; Reubroycharoen, P.; Guan, G.; Nguyen, M.H.; Samart, C. Green biodiesel production from waste cooking oil using an environmentally benign acid catalyst. Waste Manag. 2016, 52, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Z.; Li, W.; Shi, C.; Wang, Y. Preparation and characterization of biomass carbon-based solid acid catalyst for the esterification of oleic acid with methanol. Bioresour. Technol. 2013, 133, 618–621. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Hayyan, A.; Alam, M.Z.; Mirghani, M.E.; Kabbashi, N.A.; Hakimi, N.I.N.M.; Siran, Y.M.; Tahiruddin, S. Reduction of high content of free fatty acid in sludge palm oil via acid catalyst for biodiesel production. Fuel Process. Technol. 2011, 92, 920–924. [Google Scholar] [CrossRef]
- Guldhe, A.; Moura, C.V.; Singh, P.; Rawat, I.; Moura, E.M.; Sharma, Y.; Bux, F. Conversion of microalgal lipids to biodiesel using chromium-aluminum mixed oxide as a heterogeneous solid acid catalyst. Renew. Energy 2017, 105, 175–182. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Vernersson, T.; Bonelli, P.R.; Cerrella, E.G.; Cukierman, A.L. Arundo donax cane as a precursor for activated carbons preparation by phosphoric acid activation. Bioresour. Technol. 2002, 83, 95–104. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; De Rossi, S.; Faticanti, M.; Porta, P. Methane combustion and CO oxidation on LaAl1−xMnxO3 perovskite-type oxide solid solutions. Appl. Catal. B Environ. 2003, 43, 397–406. [Google Scholar] [CrossRef]
- Konwar, L.J.; Das, R.; Thakur, A.J.; Salminen, E.; Mäki-Arvela, P.; Kumar, N.; Deka, D. Biodiesel production from acid oils using sulfonated carbon catalyst derived from oil-cake waste. J. Mol. Catal. A Chem. 2014, 388, 167–176. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, L. Acid-catalyzed esterification of Zanthoxylum bungeanum seed oil with high free fatty acids for biodiesel production. Bioresour. Technol. 2008, 99, 8995–8998. [Google Scholar] [CrossRef] [PubMed]
- Yujaroen, D.; Goto, M.; Sasaki, M.; Shotipruk, A. Esterification of palm fatty acid distillate (PFAD) in supercritical methanol: Effect of hydrolysis on reaction reactivity. Fuel 2009, 88, 2011–2016. [Google Scholar] [CrossRef]
- Guo, F.; Xiu, Z.L.; Liang, Z.X. Synthesis of biodiesel from acidified soybean soapstock using a lignin-derived carbonaceous catalyst. Appl. Energy 2012, 98, 47–52. [Google Scholar] [CrossRef]
- Alhassan, F.H.; Yunus, R.; Rashid, U.; Sirat, K.; Islam, A.; Lee, H.V.; Taufiq-Yap, Y.H. Production of biodiesel from mixed waste vegetable oils using Ferric hydrogen sulphate as an effective reusable heterogeneous solid acid catalyst. Appl. Catal. A Gen. 2013, 456, 182–187. [Google Scholar] [CrossRef]
- Nahil, M.A.; Williams, P.T. Pore characteristics of activated carbons from the phosphoric acid chemical activation of cotton stalks. Biomass Bioenergy 2012, 37, 142–149. [Google Scholar] [CrossRef]
- Malaysian Palm Oil Board (MPOB). 2016. Available online: www.mpob.gov.my (accessed on 3 March 2019).
- Duvekot, C. Determination of Total FAME and Linoleic Acid Methyl Ester in Biodiesel According to EN-14103; Agilent Technologies: Santa Clara, CA, USA, 2011. [Google Scholar]







| Sample | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| KSC | 23.01 | 0.02 | 0.93 |
| AC-KSC | 375.18 | 0.39 | 3.07 |
| SO3H-KSC | 365.63 | 0.31 | 2.89 |
| Sample | C | O | P | S | Total Acidity (mmol g−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| a Weight (%) | b Atomicity | a Weight (%) | b Atomicity | a Weight (%) | b Atomicity | a Weight (%) | b Atomicity | ||
| KSC | 67.51 | 71.17 | 32.29 | 28.63 | 0.1 | 0.1 | 0.08 | 0.1 | 0.13 |
| AC-KSC | 84.91 | 87.14 | 10.51 | 10.66 | 4.37 | 2.10 | 0.21 | 0.1 | 8.37 |
| SO3H-KSC | 60.65 | 78.53 | 29.17 | 16.14 | 4.84 | 1.42 | 5.34 | 3.91 | 14.32 |
| Properties | PFAD | Methods |
|---|---|---|
| Saponification value | 210.15 | AOCS Cd-3-25 |
| Iodine value | 51.07 | AOCS Cd 1-25 |
| Free Fatty Acid content (%) | 90 | AOCS Ca 5a-40 |
| Acid Value (mg KOH) (g sample)−1 | 180 | |
| Moisture content (%) | 0.11 | AOCS Aa 3-38 |
| Fatty acid composition (wt.%) | PFAD | FAME |
| Palmitic acid, C16:0 | 49.23 | 54.02 |
| Oleic acid, C18:1 | 37.91 | 33.43 |
| Linoleic acid, C18:2 | 7.87 | 8.12 |
| Stearic acid, C18:0 | 3.75 | 2.84 |
| Myristic acid, C14:0 | 1.04 | 1.44 |
| Triglycerides | 6.00 | - |
| Diglycerides | 3.00 | - |
| Monoglycerides | 0.50 | - |
| Others | 0.2 | 0.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinfalabi, S.-I.; Rashid, U.; Shean, T.Y.C.; Nehdi, I.A.; Sbihi, H.M.; Gewik, M.M. Esterification of Palm Fatty Acid Distillate for Biodiesel Production Catalyzed by Synthesized Kenaf Seed Cake-Based Sulfonated Catalyst. Catalysts 2019, 9, 482. https://doi.org/10.3390/catal9050482
Akinfalabi S-I, Rashid U, Shean TYC, Nehdi IA, Sbihi HM, Gewik MM. Esterification of Palm Fatty Acid Distillate for Biodiesel Production Catalyzed by Synthesized Kenaf Seed Cake-Based Sulfonated Catalyst. Catalysts. 2019; 9(5):482. https://doi.org/10.3390/catal9050482
Chicago/Turabian StyleAkinfalabi, Shehu-Ibrahim, Umer Rashid, Thomas Yaw Choong Shean, Imededdine Arbi Nehdi, Hassen Mohamed Sbihi, and Mohamed Mossad Gewik. 2019. "Esterification of Palm Fatty Acid Distillate for Biodiesel Production Catalyzed by Synthesized Kenaf Seed Cake-Based Sulfonated Catalyst" Catalysts 9, no. 5: 482. https://doi.org/10.3390/catal9050482
APA StyleAkinfalabi, S.-I., Rashid, U., Shean, T. Y. C., Nehdi, I. A., Sbihi, H. M., & Gewik, M. M. (2019). Esterification of Palm Fatty Acid Distillate for Biodiesel Production Catalyzed by Synthesized Kenaf Seed Cake-Based Sulfonated Catalyst. Catalysts, 9(5), 482. https://doi.org/10.3390/catal9050482







