Hydroconversion of Aromatic Hydrocarbons over Bimetallic Catalysts
Abstract
:1. Introduction
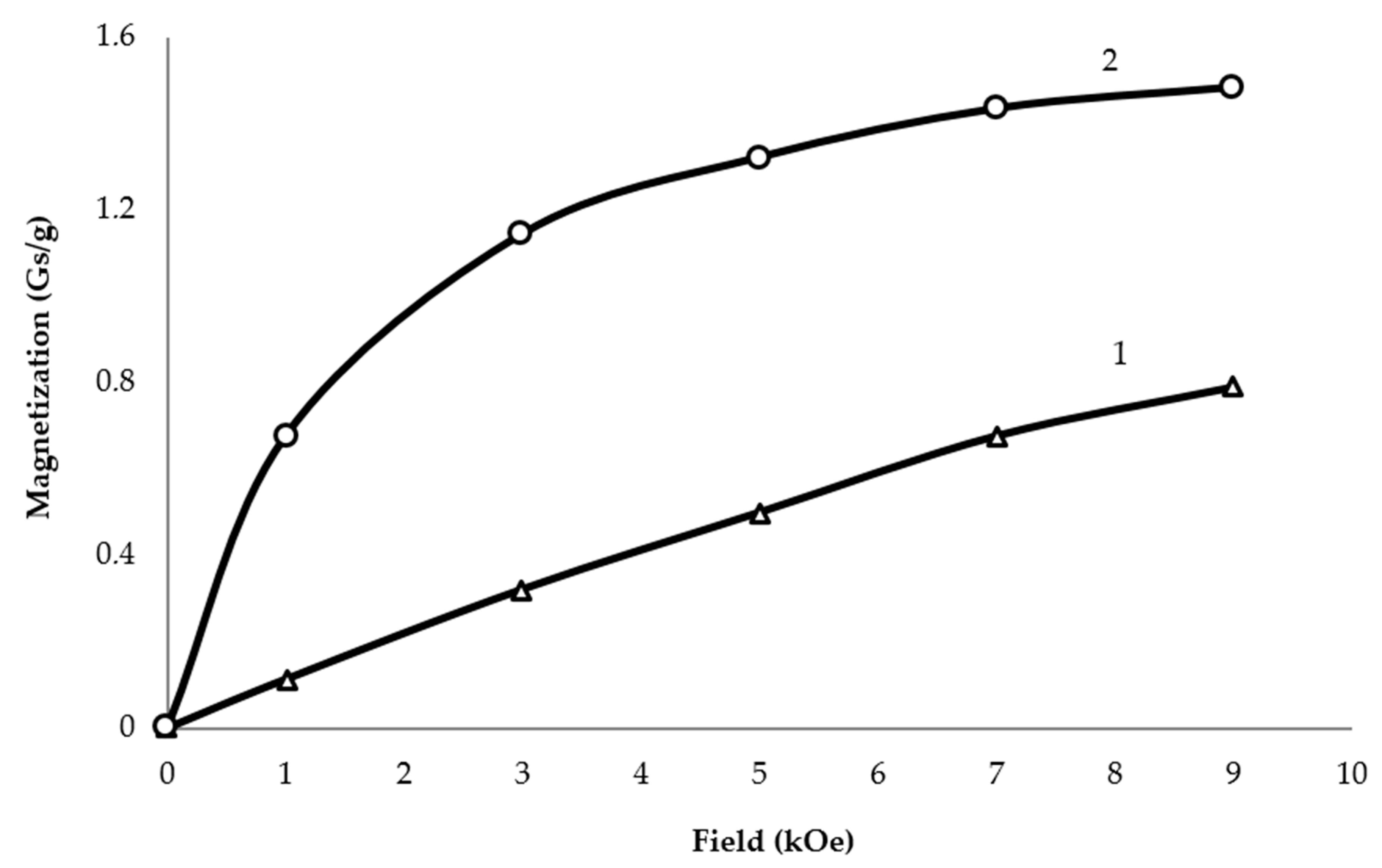
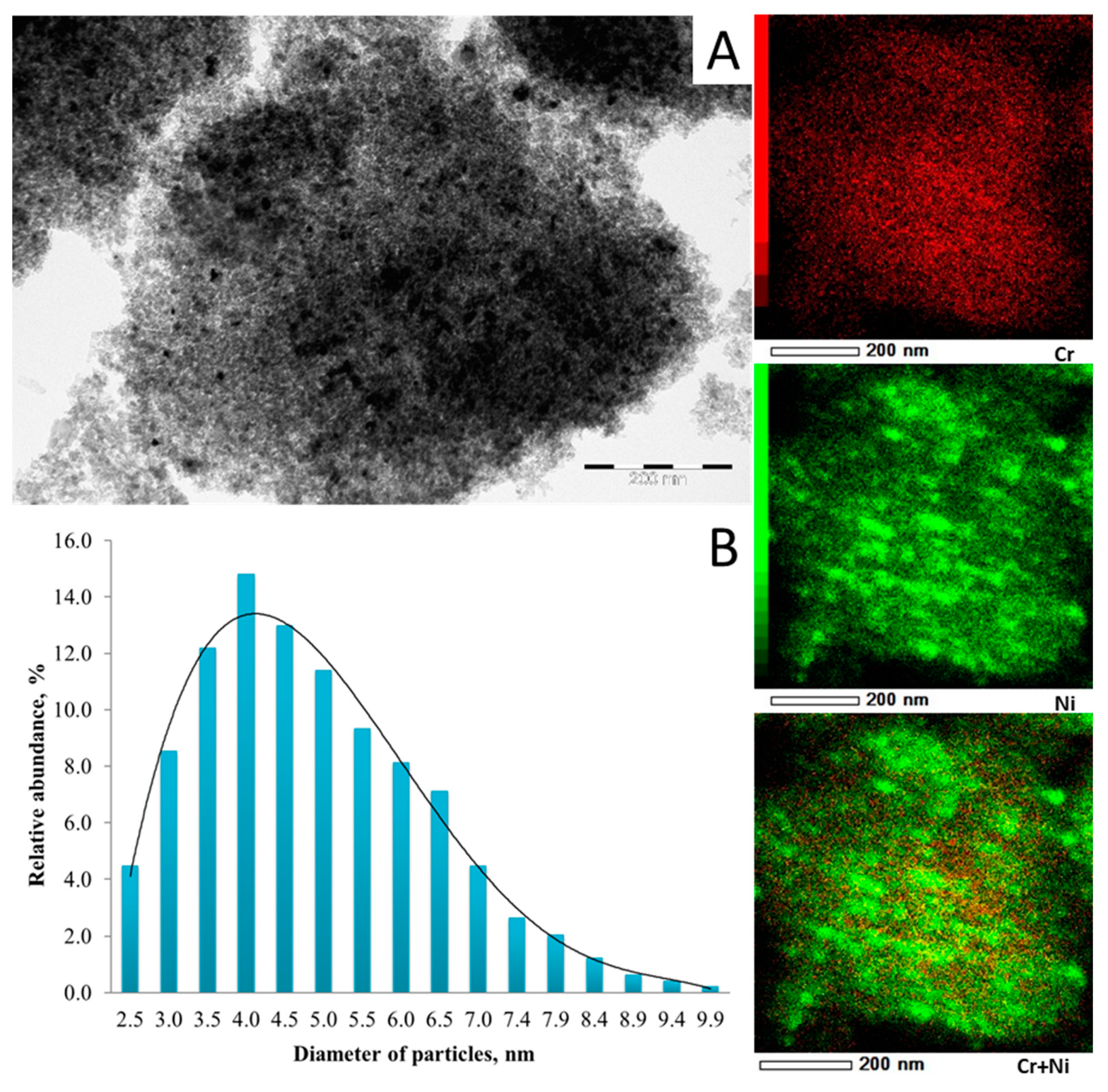
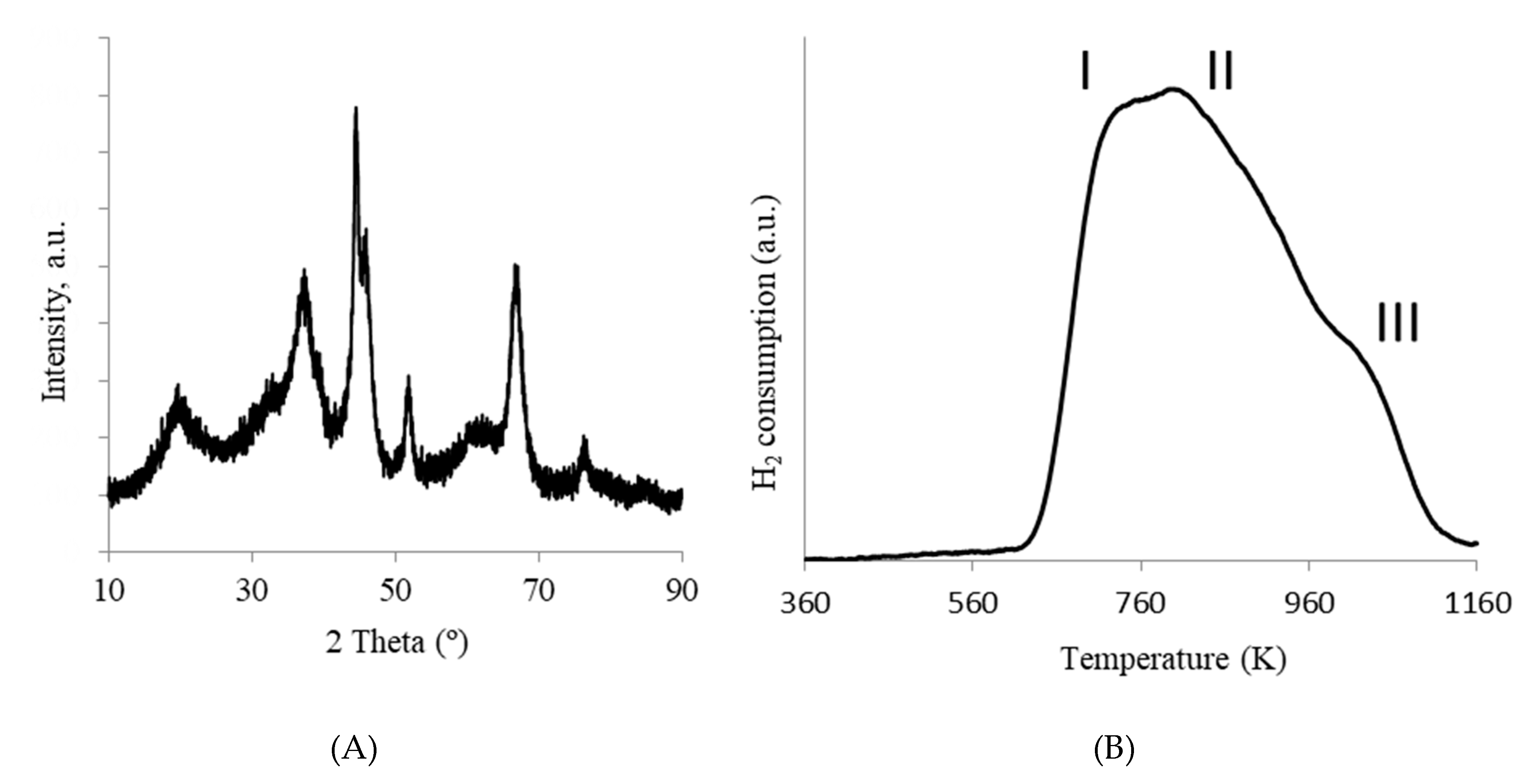
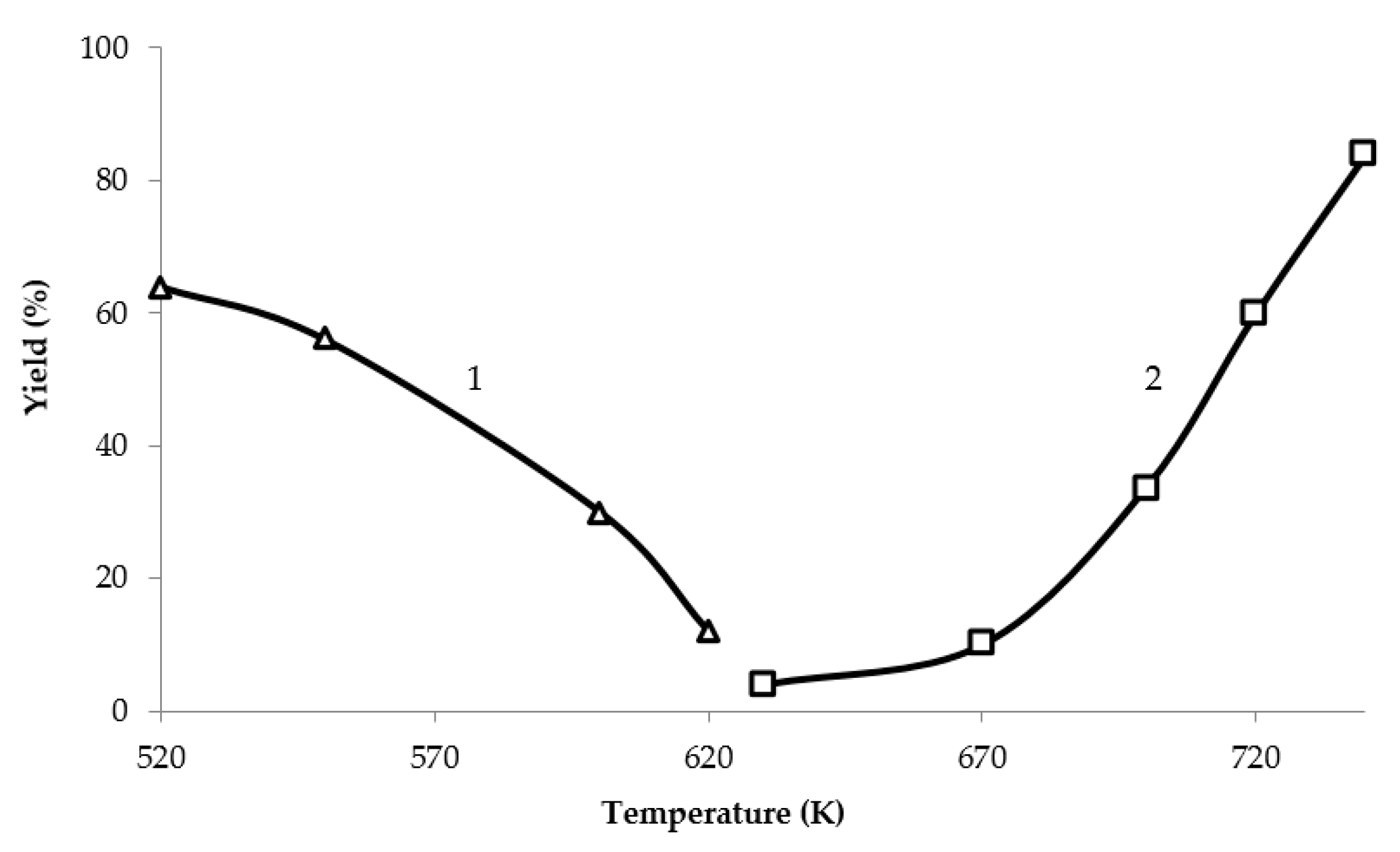
2. Results and Discussion
3. Materials and Methods
3.1. Chemical Reagents
- Microspherical γ-Al2O3 (SKTB grade) grain size 0.5–0.8 mm, S = 210 m2/g
- Hexacarbonyl chromium and bis-arene chromium chemically pure (Reachim)
- LiNO3, Ni(NO3)2·6H2O, H4PMo11VO40—each chemically pure (Aldrich)
- Benzene, toluene—each chemically pure (Reachim)
- Ar, H2, He, N2—spec. pure from gas cylinders being purified in cold traps filled with CaA zeolite.
3.2. Catalyst Preparation
3.3. Analyses and Instrumentations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Franck, H.; Stadelhofer, J. Industrial Aromatic Chemistry: Raw Materials. Processes. Products; Springer: Berlin, Germany, 1988. [Google Scholar]
- Stanislaus, A.; Cooper, B. Aromatic Hydrogenation Catalysis: A Review. Cat. Rev. Sci. Eng. 1994, 36, 75–123. [Google Scholar] [CrossRef]
- Kiperman, S.; Cerveny, L. Catalytic hydrogenation. Stud. Surf. Sci. Catal. 1986, 27, 18. [Google Scholar]
- Schoenmaker-Stolk, M.; Verwijs, J.; Don, J.; Scholten, J. The catalytic hydrogenation of benzene over supported metal catalysts: II. Gas-phase hydrogenation of benzene over copper-on-silica. Appl. Catal. 1987, 29, 91–105. [Google Scholar] [CrossRef]
- Gongbing, Z.; Yanlu, D.; Jiang, L.; He, D.; Yanga, Y.; Zhoua, X. Effect of support composition on the structural and catalytic properties of Ru/AlOOH–SiO2 catalysts for benzene selective hydrogenation. Catal. Sci. Technol. 2018, 8, 1435–1446. [Google Scholar]
- Goundani, K.; Papadopoulou, C.; Kordulis, C. Benzene elimination from reformate gasoline by high pressure hydrogenation in a fixed-bed reactor. React. Kinet. Catal. Lett. 2004, 82, 149. [Google Scholar] [CrossRef]
- Almering, M.; Rock, K.; Judzis, A. Reducing benzene in gasoline. Cost-Effective Solutions for the Reduction of Benzene in Gasoline, Particularly with Regard to MSAT II Requirement. Digitalrefining 2008, 7. [Google Scholar]
- Sassykova, L. Development of Catalysts for the Hydrogenation of the Aromatic Ring in Gasolines. Chem. Biochem. Eng. Q. 2017, 31, 447–453. [Google Scholar] [CrossRef]
- Abu Bakar, N.H.H.; Bettahar, M.M.; Abu Bakar, M.; Monteverdia, S.; Ismail, J. Low temperature activation of Pt/Ni supported MCM-41 catalysts for hydrogenation of benzene. J. Mol. Catal. A Chem. 2010, 333, 11–19. [Google Scholar] [CrossRef]
- Stanley, J.N.G.; Worthington, K.; Heinroth, F.; Masters, A.F.; Maschmeyer, T. Designing nanoscopic, fluxional bimetallic Pt–Ru alloy hydrogenation catalysts for improved sulfur tolerance. Catal. Today 2011, 178, 164–171. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, C. Enhancing Sulfur Tolerance of Pd Catalysts by Hydrogen Spillover with Two Different Zeolite Supports for Low-Temperature Hydrogenation of Aromatics. Energy Fuels 2014, 28, 6788–6792. [Google Scholar] [CrossRef]
- Tang, T.; Yin, C.; Wang, L.; Ji, Y.; Xiao, F.-S. Good sulfur tolerance of a mesoporous Beta zeolite-supported palladium catalyst in the deep hydrogenation of aromatics. J. Catal. 2008, 257, 125–133. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Boronoev, M.; Kulikov, L.; Terenina, M. Mesoporous organo-inorganic hybrid materials as hydrogenation catalysts. Pure Appl. Chem. 2017, 89, 1157–1166. [Google Scholar] [CrossRef]
- Kniazeva, M.; Maximov, A. Effect of Additives on the Activity of Nickel–Tungsten Sulfide Hydroconversion Catalysts Prepared in Situ from Oil-Soluble Precursors. Catalysts 2018, 8, 644. [Google Scholar] [CrossRef]
- Coumans, A.E.; Poduval, D.G.; van Veen, J.A.; Hensen, E.J.M. The nature of the sulfur tolerance of amorphous silica-alumina supported NiMo(W) sulfide and Pt hydrogenation catalysts. Appl. Catal. A 2012, 411, 51–59. [Google Scholar] [CrossRef]
- Comyns, A. Encyclopedic Dictionary of Named Processes in Chemical Technology, 4th ed.; CRC Press: Boca Raton, FL, USA, 21 February 2014. [Google Scholar]
- Franck, H.-G.; Stadelhofer, J.W. Industrial Aromatic Chemistry: Raw Materials Processes Products; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Assafi, M.; Duprez, D. Steam-dealkylation and hydrodealkylation of meta-cresol on potassium-promoted rhodium and nickel catalysts. Appl. Catal. A 1992, 80, 23. [Google Scholar] [CrossRef]
- Vinokurov, V.; Glotov, A.; Chudakov, Y.; Stavitskaya, A.; Ivanov, E.; Gushchin, P.; Zolotukhina, A.; Maximov, A.; Karakhanov, E.; Lvov, Y. Core Shell Ruthenium-Halloysite Nanocatalysts for Hydrogenation of Phenol. Ind. Eng. Chem. Res. 2017, 56, 14043–14052. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Zolotukhina, A.; Mamadli, A.; Vutolkina, A.; Ivanov, A. Dendrimer-stabilized Ru nanoparticles immobilized in organo-silica materials for hydrogenation of phenols. Catalysts 2017, 7, 86. [Google Scholar] [CrossRef]
- Song, Y.; Seo, G.; Ihm, S. Hydrodealkylation reaction of ethylbenzene over a supported Ni-W catalyst. Appl. Catal. A 1992, 83, 75. [Google Scholar] [CrossRef]
- Verma, R.K. Aromatics Upgrading Technologies, PEP Report 25. Available online: https://cdn.ihs.com/www/pdf/RP25E-toc.pdf (accessed on 22 December 2018).
- Brunelle, J.-P. Hydrodealkylation of Alkylaromatic Hydrocarbons. U.S. Patent 4138443, 6 February 1979. [Google Scholar]
- Courty, P.; Page, J.; Sugier, A. Process for Steam Dealkylation of Aromatic Hydrocarbons. U.S. Patent 4199437, 22 April 1980. [Google Scholar]
- Lim, D.; Jang, J.; Kim, T.; Shim, S.; Baeck, S.-H. Selective hydrodealkylation of C9+ aromatics to benzene, toluene, and xylenes (BTX) over a Pt/H-ZSM-5 catalyst. J. Mol. Catal. A Chem. 2015, 407, 147. [Google Scholar] [CrossRef]
- Stytsenko, V. Surface modified bimetallic catalysts: Preparation, characterization, and applications. Appl. Catal. A. 1995, 126, 1–26. [Google Scholar] [CrossRef]
- Gale, W.F.; Totemeier, T.C. Smithells Metals Reference Book; Butterworths: London, UK, 2003. [Google Scholar]
- Bozort, R. Ferromagnetism; Wiley-IEEE Press: New York/Piscataway, NJ, USA, 27 August 1993. [Google Scholar]
- Victora, R.H.; Falicov, L.M. Calculated electronic structure of chromium surfaces and chromium monolayers on iron. Phys. Rev. B. 1985, 31, 7335–7343. [Google Scholar] [CrossRef]
- Bohra, M.; Grammatikopoulos, P.; Diaz, R.E.; Singh, V.; Zhao, J.; Bobo, J.-F.; Kuronen, A.; Djurabekova, F.; Nordlund, K.; Sowwanas, M. Surface Segregation in Chromium-Doped NiCr Alloy Nanoparticles and Its Effect on Their Magnetic Behavior. Chem. Mater. 2015, 27, 3216–3225. [Google Scholar] [CrossRef]
- Fidalgo, B.; Arenillas, A.; Menéndez, J.Á. Synergetic effect of a mixture of activated carbon + Ni/Al2O3 used as catalysts for the CO2 reforming of CH4. Appl. Catal. A 2010, 390, 78–83. [Google Scholar] [CrossRef]
- Bates, M.K.; Jia, Q.; Ramaswamy, N.; Allen, R.J.; Mukerjee, S. Composite Ni/NiO-Cr2O3 Catalyst for Alkaline Hydrogen Evolution Reaction. J. Phys. Chem. C 2015, 119, 5467–5477. [Google Scholar] [CrossRef]
- Wen, G.; Xu, Y.; Xu, Z.; Tian, Z. Characterization and Catalytic Properties of the Ni/Al2O3 Catalysts for Aqueous-phase Reforming of Glucose. Catal. Lett. 2009, 129, 250–257. [Google Scholar] [CrossRef]
- Hossein, M.; Shiraz, A.; Rezaei, M.; Meshkani, F. Preparation of nanocrystalline Ni/Al2O3 catalysts with the microemulsion method for dry reforming of methane. Can. J. Chem. Eng. 2016, 94, 1177–1183. [Google Scholar]
- Kanervo, J.; Harlin, M.E.; Krause, A.; Banares, M. Characterisation of alumina-supported vanadium oxide catalysts by kinetic analysis of H2-TPR data. Catal. Today 2003, 78, 171–180. [Google Scholar] [CrossRef]
- Venezia, A.; Parola, V.; Pawelec, B.; Fierrob, J. Hydrogenation of aromatics over Au-Pd/SiO2-Al2O3 catalysts; support acidity effect. Appl. Catal. A Gen. 2004, 264, 43–51. [Google Scholar] [CrossRef]
- Zlotina, N.E. Cand. Sci. (Chem.) Dissertation. Ph.D. Thesis, Inst. Org. Chem. Acad. Sci. USSR, Moscow, Russia, 1967. [Google Scholar]
- Stytsenko, V. Kinetic Descriptions of Heterogeneous Catalytic Processes Using Adsorption Substitution Reactions. Russ. J. Phys. Chem. 2018, 92, 228–238. [Google Scholar] [CrossRef]
- Westsson, E.; Koper, G.J. How to Determine the Core-Shell Nature in Bimetallic Catalyst Particles? Catalysts 2014, 4, 375–396. [Google Scholar] [CrossRef]
- Richardson, J. Principles of Catalyst Development; Plenum: New York, NY, USA, 1989. [Google Scholar]
- Stytsenko, V.; Lee, W.; Lee, J. Catalyst Design for Methacrolein Oxidation to Methacrylic Acid. Kinet. Catal. 2001, 42, 212–216. [Google Scholar] [CrossRef]
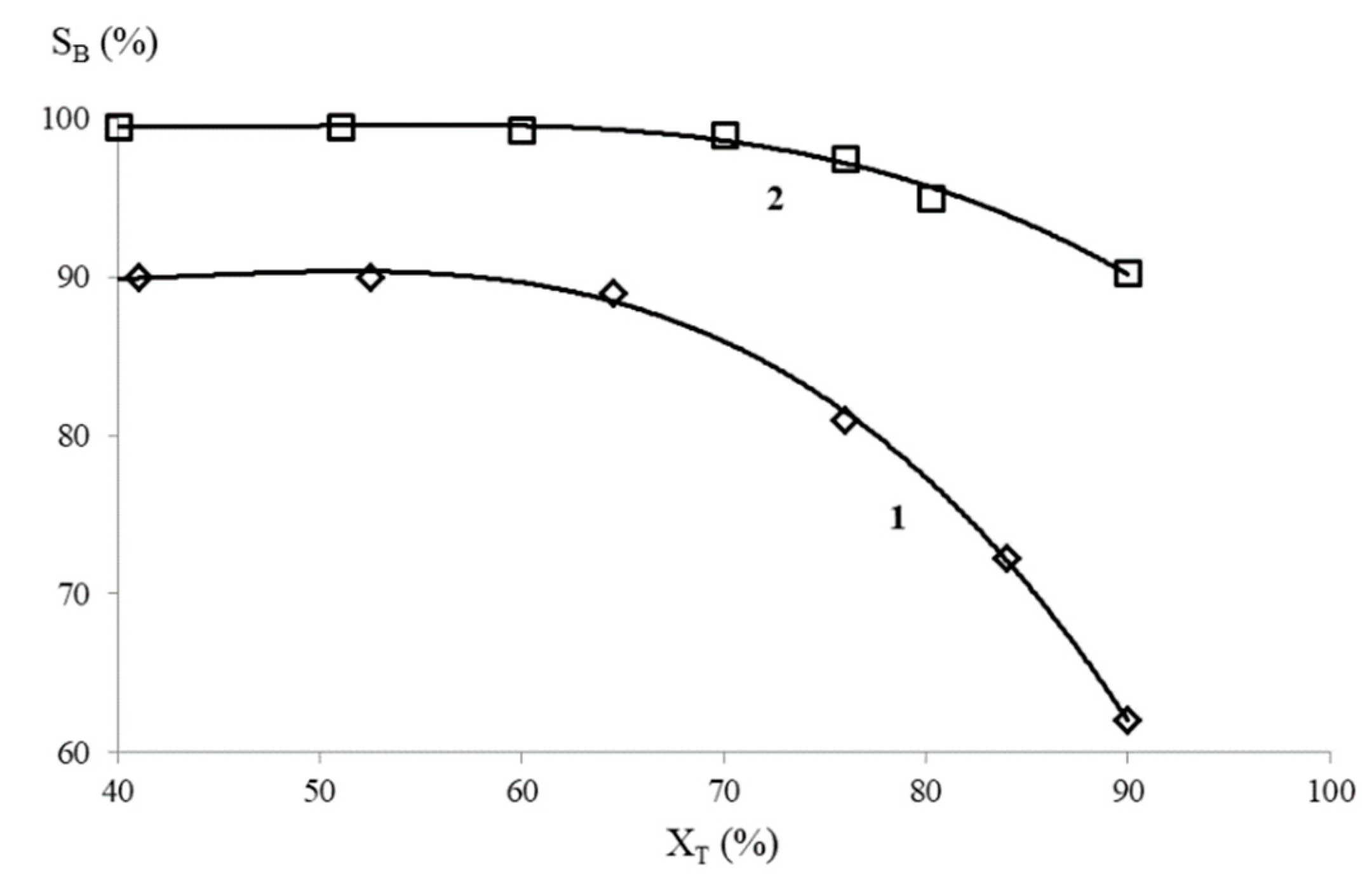
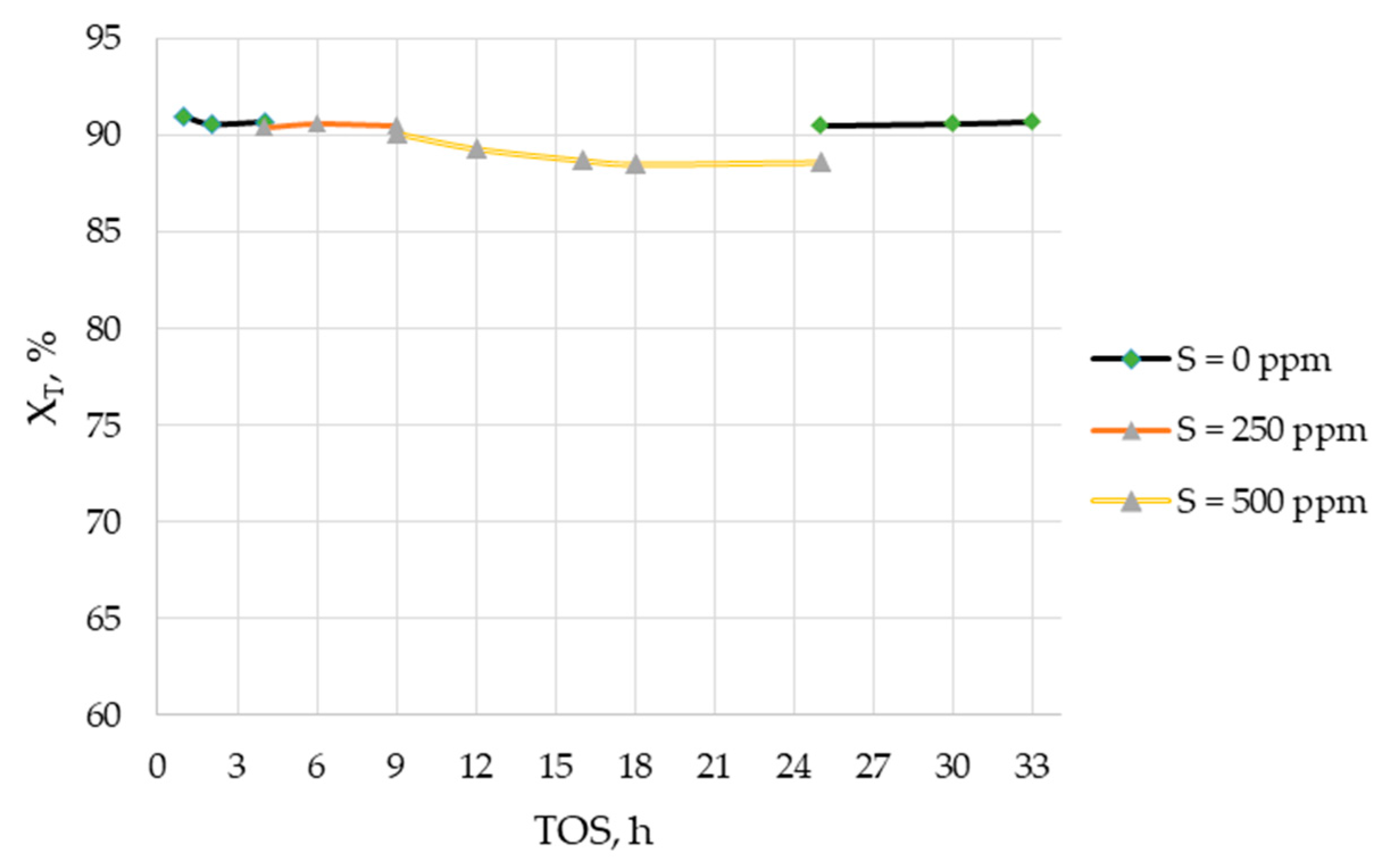
| No. | Catalyst (wt. %) | BET Surface Area, m2/g | O2 Consumption, μmol/g | Ni Specific Surface Area, m2/g |
|---|---|---|---|---|
| 1 | 4 Ni | 130 | 58 | 3.5 |
| 2 | 4 Ni-3 CrA | 131 | 2.5 | 0.15 |
| 3 | 4 Ni-7 CrA | 132 | 2.1 | 0.13 |
| 4 | 4 Ni-3 CrA- 5 HPA * | 135 | 2.7 | 0.16 |
| 5 | 4 Ni-3 CrA-5 LiHPA * | 133 | 2.4 | 0.14 |
| 6 | 10 Ni | 128 | 166 | 9.9 |
| 7 | 10 Ni-1.5 CrCO | 129 | 18.2 | 1.1 |
| 8 | 7 CrA | 132 | 0 | - |
| Sample No. | Catalyst/ArH | T, K | Conversion, % | Activity | |
|---|---|---|---|---|---|
| mol/(kg·h) | 102·s−1 $ | ||||
| 1 | 4 Ni/ Bz | 370 | 4.6 | 5.7 | 2.6 |
| 2 | 4 Ni –3 CrA/ Bz | 370 | 5.6 | 7.0 | 39 |
| 3 | 10 Ni/ Bz | 370 | 6.1 | 7.5 | 1.2 |
| 4 | 10 Ni-1.5CrCO/ Bz | 370 | 12.4 | 15.2 | 22 |
| 5 | 10 Ni-1.5CrCO/ Tol | 370 | 14.0 | 17.1 | 24.8 |
| 6 | 4 Ni–7 CrA/ Bz | 420 | 1.0 | 1.25 | 3.2 |
| 7 | 4 Ni–7 CrA/ Tol | 420 | 1.15 | 1.44 | 3.7 |
| 8 | 4 Ni–3 CrA –5 HPA/ Bz | 370 | 27.1 | 33.7 | 187 |
| 9 | 4 Ni–3 CrA –5 HPA/ Tol | 370 | 30.5 | 38.1 | 211 |
| 10 | CrA/ Bz(Tol) | 370–570 | 0 | 0 | - |
| 11 | HPA/ Bz(Tol) | 370–570 | 0 | 0 | - |
| Metal Loading (%) | Temperature, K | Toluene Conversion, % | Activity (mol/kg·h) | Benzene Selectivity, % |
|---|---|---|---|---|
| 10 Ni-1.5 CrCO | 650 | 99.9 | # | <1 |
| 4 Ni | 680 | 99.9 | # | <1 |
| 4 Ni-3 CrA | 720 | 70.3 | 34.4 | 85.2 |
| 4 Ni-3 CrA-5 | 733 | 77.1 | 37.7 | 80 |
| HPA | 753 | 90.9 | 44.3 | 62# |
| 4 Ni-3 CrA-5 | 733 | 85.3 | 41.6 | 92.3 |
| LiHPA | 753 | 89.8 | 43.8 | 90.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glotov, A.; Stytsenko, V.; Artemova, M.; Kotelev, M.; Ivanov, E.; Gushchin, P.; Vinokurov, V. Hydroconversion of Aromatic Hydrocarbons over Bimetallic Catalysts. Catalysts 2019, 9, 384. https://doi.org/10.3390/catal9040384
Glotov A, Stytsenko V, Artemova M, Kotelev M, Ivanov E, Gushchin P, Vinokurov V. Hydroconversion of Aromatic Hydrocarbons over Bimetallic Catalysts. Catalysts. 2019; 9(4):384. https://doi.org/10.3390/catal9040384
Chicago/Turabian StyleGlotov, Aleksandr, Valentine Stytsenko, Maria Artemova, Michail Kotelev, Evgenii Ivanov, Pavel Gushchin, and Vladimir Vinokurov. 2019. "Hydroconversion of Aromatic Hydrocarbons over Bimetallic Catalysts" Catalysts 9, no. 4: 384. https://doi.org/10.3390/catal9040384
APA StyleGlotov, A., Stytsenko, V., Artemova, M., Kotelev, M., Ivanov, E., Gushchin, P., & Vinokurov, V. (2019). Hydroconversion of Aromatic Hydrocarbons over Bimetallic Catalysts. Catalysts, 9(4), 384. https://doi.org/10.3390/catal9040384





