Facet-Dependent Reactivity of Fe2O3/CeO2 Nanocomposites: Effect of Ceria Morphology on CO Oxidation
Abstract
1. Introduction
2. Results and Discussion
2.1. Textural and Structural Characteristics (BET/XRD)
2.2. Morphological Characteristics (TEM, SEM-EDS)
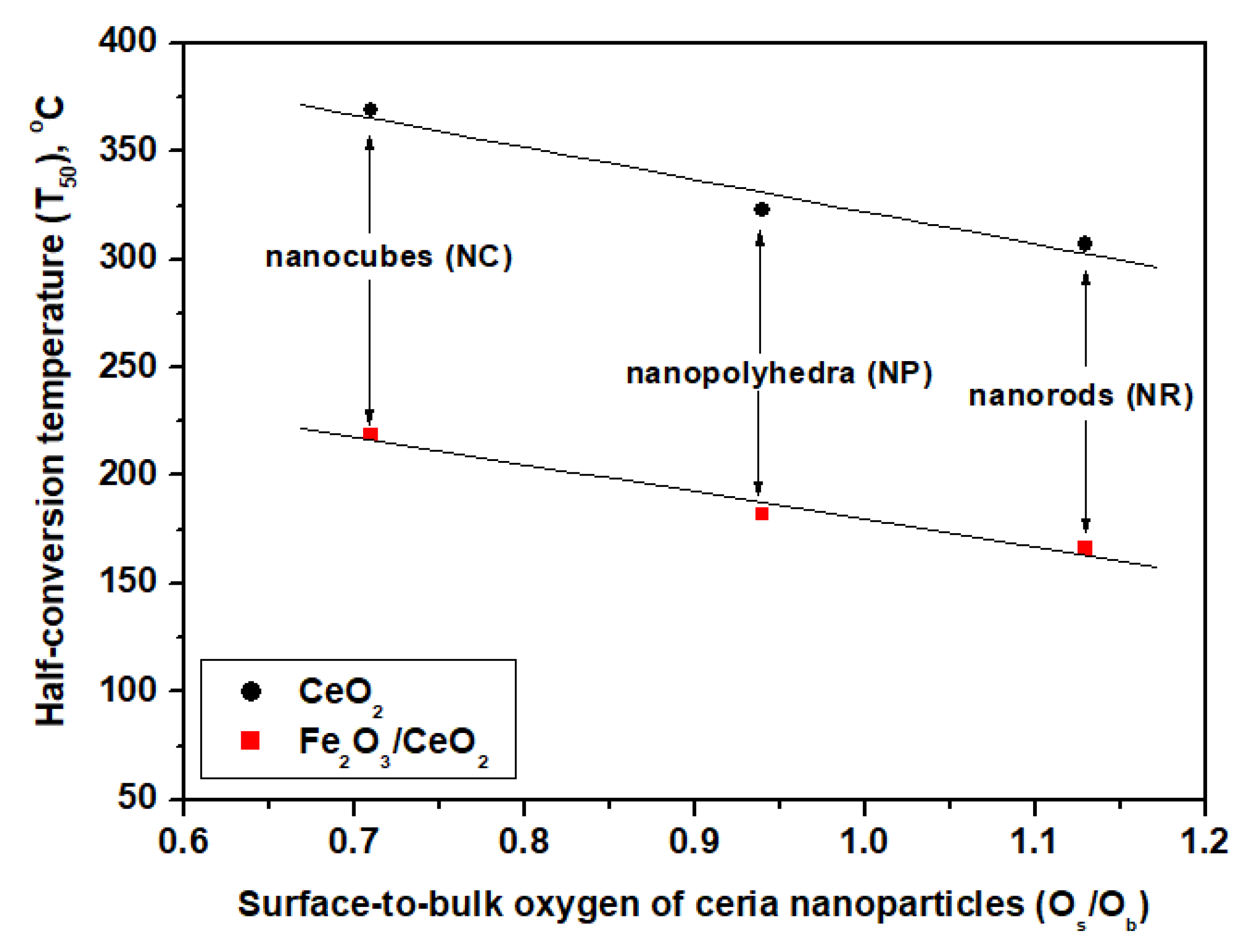
2.3. Redox Properties (H2-TPR)
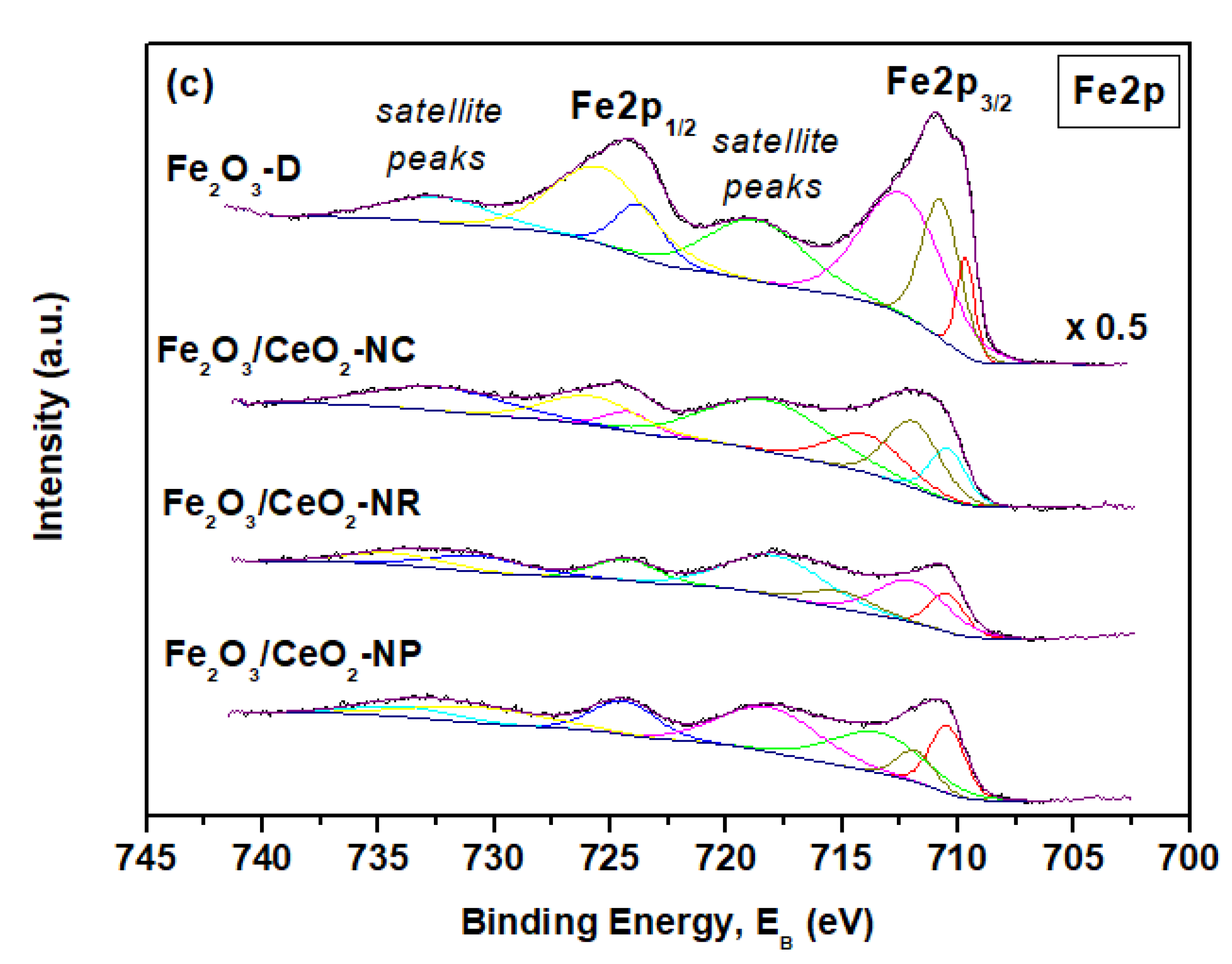
2.4. Surface Properties (XPS)
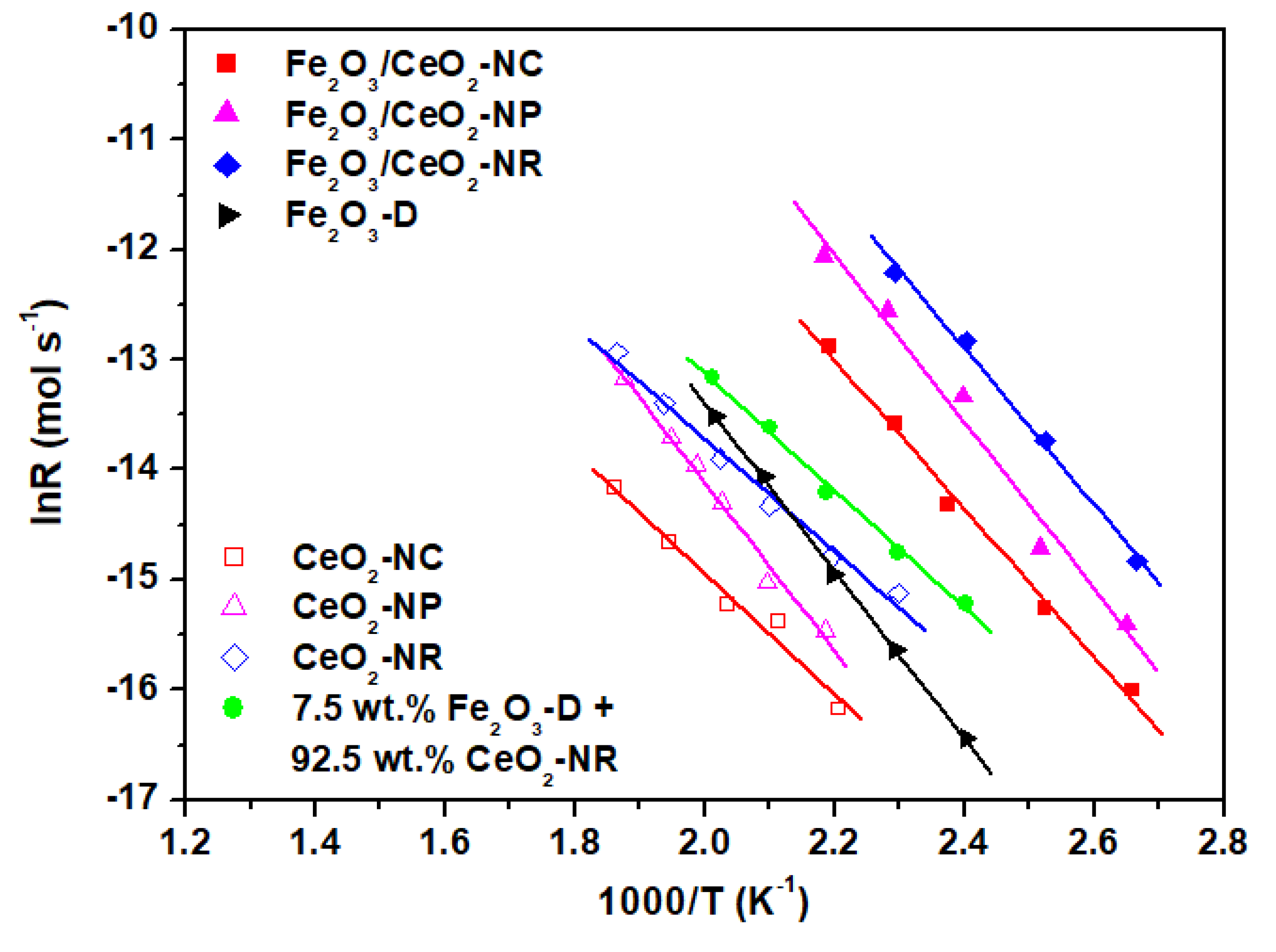
2.5. Catalytic Evaluation Studies
3. Materials and Methods
3.1. Materials Synthesis
3.2. Materials Characterization
3.3. Catalytic Evaluation Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paier, J.; Penschke, C.; Sauer, J. Oxygen defects and surface chemistry of ceria: Quantum chemical studies compared to experiment. Chem. Rev. 2013, 113, 3949–3985. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-X.; Gao, P.-X. Nanostructured cerium oxide: preparation, characterization, and application in energy and environmental catalysis. MRS Commun. 2016, 6, 311–329. [Google Scholar] [CrossRef]
- Sun, C.; Xue, D. Size-dependent oxygen storage ability of nano-sized ceria. Phys. Chem. Chem. Phys. 2013, 15, 14414–14419. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M. Surface Effects in the Reactivity of Ceria: A First Principles Perspective; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780128013403. [Google Scholar]
- Devaiah, D.; Reddy, L.H.; Park, S.E.; Reddy, B.M. Ceria–zirconia mixed oxides: Synthetic methods and applications. Catal. Rev. Sci. Eng. 2018, 60, 177–277. [Google Scholar] [CrossRef]
- Zhang, D.; Du, X.; Shi, L.; Gao, R. Shape-controlled synthesis and catalytic application of ceria nanomaterials. Dalton Trans. 2012, 41, 14455–14475. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. The role of ceria-based nanostructured materials in energy applications. Mater. Today 2014, 17, 349–357. [Google Scholar] [CrossRef]
- Stathopoulos, V.N.; Costa, C.N.; Pomonis, P.J.; Efstathiou, A.M. The CH4/NO/O2 “lean-deNOx” reaction on mesoporous Mn-based mixed oxides. Top. Catal. 2001, 16, 231–235. [Google Scholar] [CrossRef]
- Stathopoulos, V.N.; Petrakis, D.E.; Hudson, M.; Falaras, P.; Neofytides, S.G.; Pomonis, P.J. Novel Mn-based Mesoporous Mixed Oxidic Solids. Stud. Surf. Sci. Catal. 2000, 128, 593–602. [Google Scholar] [CrossRef]
- Stathopoulos, V.N.; Belessi, V.C.; Costa, C.N.; Neophytides, S.; Falaras, P.; Efstathiou, A.M.; Pomonis, P.J. Catalytic activity of high surface area mesoporous Mn-based mixed oxides for the deep oxidation of methane and lean-NOx reduction. Stud. Surf. Sci. Catal. 2000, 130, 1529–1534. [Google Scholar] [CrossRef]
- Stathopoulos, V.N.; Belessi, V.C.; Bakas, T.V.; Neophytides, S.G.; Costa, C.N.; Pomonis, P.J.; Efstathiou, A.M. Comparative study of La–Sr–Fe–O perovskite-type oxides prepared by ceramic and surfactant methods over the CH4 and H2 lean-deNOx. Appl. Catal. B Environ. 2009, 93, 1–11. [Google Scholar] [CrossRef]
- Stathopoulos, V.N.; Belessi, V.C.; Ladavos, A.K. Samarium based high surface area perovskite type oxides SmFe1-XAlXO3 (X = 0.00, 0.50, 0.95). Part II, Catalytic combustion of CH4. React. Kinet. Catal. Lett. 2001, 72, 49–55. [Google Scholar] [CrossRef]
- Corberán, V.C.; Rives, V.; Stathopoulos, V. Chapter 7—Recent Applications of Nanometal Oxide Catalysts in Oxidation Reactions. In Advanced Nanomaterials for Catalysis and Energy; Synthesis, Characterization and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 227–293. ISBN 9780128148075. [Google Scholar]
- Qiao, Z.A.; Wu, Z.; Dai, S. Shape-controlled ceria-based nanostructures for catalysis applications. ChemSusChem 2013, 6, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Matte, L.P.; Kilian, A.S.; Luza, L.; Alves, M.C.M.; Morais, J.; Baptista, D.L.; Dupont, J.; Bernardi, F. Influence of the CeO2 Support on the Reduction Properties of Cu/CeO2 and Ni/CeO2 Nanoparticles. J. Phys. Chem. C 2015, 119, 26459–26470. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Djinović, P.; Tchernychova, E.; Tkachenko, O.P.; Kustov, L.M.; Pintar, A. Nanoshaped CuO/CeO2 Materials: Effect of the Exposed Ceria Surfaces on Catalytic Activity in N2O Decomposition Reaction. ACS Catal. 2015, 5, 5357–5365. [Google Scholar] [CrossRef]
- Lykaki, M.; Pachatouridou, E.; Carabineiro, S.A.C.; Iliopoulou, E.; Andriopoulou, C.; Kallithrakas-Kontos, N.; Boghosian, S.; Konsolakis, M. Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl. Catal. B Environ. 2018, 230, 18–28. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Overbury, S.H. On the structure dependence of CO oxidation over CeO2 nanocrystals with well-defined surface planes. J. Catal. 2012, 285, 61–73. [Google Scholar] [CrossRef]
- Vilé, G.; Colussi, S.; Krumeich, F.; Trovarelli, A.; Pérez-Ramírez, J. Opposite face sensitivity of CeO2 in hydrogenation and oxidation catalysis. Angew. Chem. Int. Ed. 2014, 53, 12069–12072. [Google Scholar] [CrossRef] [PubMed]
- Aneggi, E.; Wiater, D.; De Leitenburg, C.; Llorca, J.; Trovarelli, A. Shape-dependent activity of ceria in soot combustion. ACS Catal. 2014, 4, 172–181. [Google Scholar] [CrossRef]
- Cargnello, M.; Doan-Nguyen, V.V.T.; Gordon, T.R.; Diaz, R.E.; Stach, E.A.; Gorte, R.J.; Fornasiero, P.; Murray, C.B. Control of Metal Nanocrystal Size Reveals Metal-Support Interface Role for Ceria Catalysts. Science 2013, 341, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Mistry, H.; Roldan Cuenya, B. Tailoring the Catalytic Properties of Metal Nanoparticles via Support Interactions. J. Phys. Chem. Lett. 2016, 7, 3519–3533. [Google Scholar] [CrossRef] [PubMed]
- Qiu, N.; Zhang, J.; Wu, Z. Peculiar surface–interface properties of nanocrystalline ceria–cobalt oxides with enhanced oxygen storage capacity. Phys. Chem. Chem. Phys. 2014, 16, 22659–22664. [Google Scholar] [CrossRef] [PubMed]
- Konsolakis, M.; Carabineiro, S.A.C.; Papista, E.; Marnellos, G.E.; Tavares, P.B.; Agostinho Moreira, J.; Romaguera-Barcelay, Y.; Figueiredo, J.L. Effect of preparation method on the solid state properties and the deN2O performance of CuO-CeO2 oxides. Catal. Sci. Technol. 2015, 5, 3714–3727. [Google Scholar] [CrossRef]
- Konsolakis, M. The role of Copper–Ceria interactions in catalysis science: Recent theoretical and experimental advances. Appl. Catal. B Environ. 2016, 198, 49–66. [Google Scholar] [CrossRef]
- Wu, K.; Sun, L.D.; Yan, C.H. Recent Progress in Well-Controlled Synthesis of Ceria-Based Nanocatalysts towards Enhanced Catalytic Performance. Adv. Energy Mater. 2016, 6, 1600501. [Google Scholar] [CrossRef]
- Devaiah, D.; Thrimurthulu, G.; Smirniotis, P.G.; Reddy, B.M. Nanocrystalline alumina-supported ceria-praseodymia solid solutions: Structural characteristics and catalytic CO oxidation. RSC Adv. 2016, 6, 44826–44837. [Google Scholar] [CrossRef]
- Devaiah, D.; Tsuzuki, T.; Thirupathi, B.; Smirniotis, P.G.; Reddy, B.M. Ce0.80M0.12Sn0.08O2–δ (M = Hf, Zr, Pr, and La) ternary oxide solid solutions with superior properties for CO oxidation. RSC Adv. 2015, 5, 30275–30285. [Google Scholar] [CrossRef]
- Zhu, X.; Du, Y.; Wang, H.; Wei, Y.; Li, K.; Sun, L. Chemical interaction of Ce-Fe mixed oxides for methane selective oxidation. J. Rare Earths 2014, 32, 824–830. [Google Scholar] [CrossRef]
- Bao, H.; Chen, X.; Fang, J.; Jiang, Z.; Huang, W. Structure-activity Relation of Fe2O3–CeO2 Composite Catalysts in CO Oxidation. Catal. Lett. 2008, 125, 160–167. [Google Scholar] [CrossRef]
- Wang, W.; Li, W.; Guo, R.; Chen, Q.; Wang, Q.; Pan, W.; Hu, G. A CeFeOx catalyst for catalytic oxidation of NO to NO2. J. Rare Earths 2016, 34, 876–881. [Google Scholar] [CrossRef]
- Tan, L.; Tao, Q.; Gao, H.; Li, J.; Jia, D.; Yang, M. Preparation and catalytic performance of mesoporous ceria-base composites CuO/CeO2, Fe2O3/CeO2 and La2O3/CeO2. J. Porous Mater. 2017, 24, 795–803. [Google Scholar] [CrossRef]
- Massa, P.; Dafinov, A.; Cabello, F.M.; Fenoglio, R. Catalytic wet peroxide oxidation of phenolic solutions over Fe2O3/CeO2 and WO3/CeO2 catalyst systems. Catal. Commun. 2008, 9, 1533–1538. [Google Scholar] [CrossRef]
- Han, J.; Meeprasert, J.; Maitarad, P.; Nammuangruk, S.; Shi, L.; Zhang, D. Investigation of the Facet-Dependent Catalytic Performance of Fe2O3/CeO2 for the Selective Catalytic Reduction of NO with NH3. J. Phys. Chem. C 2016, 120, 1523–1533. [Google Scholar] [CrossRef]
- Prieto-Centurion, D.; Eaton, T.R.; Roberts, C.A.; Fanson, P.T.; Notestein, J.M. Catalytic reduction of NO with H2 over redox-cycling Fe on CeO2. Appl. Catal. B Environ. 2015, 168–169, 68–76. [Google Scholar] [CrossRef]
- Nadar, A.; Banerjee, A.M.; Pai, M.R.; Pai, R.V.; Meena, S.S.; Tewari, R.; Tripathi, A.K. Catalytic properties of dispersed iron oxides Fe2O3/MO2 (M = Zr, Ce, Ti and Si) for sulfuric acid decomposition reaction: Role of support. Int. J. Hydrogen Energy 2018, 43, 37–52. [Google Scholar] [CrossRef]
- Perez-Alonso, F.J.; Melián-Cabrera, I.; López Granados, M.; Kapteijn, F.; Fierro, J.L.G. Synergy of FexCe1-xO2 mixed oxides for N2O decomposition. J. Catal. 2006, 239, 340–346. [Google Scholar] [CrossRef]
- Shen, Q.; Lu, G.; Du, C.; Guo, Y.; Wang, Y.; Guo, Y.; Gong, X. Role and reduction of NOx in the catalytic combustion of soot over iron—ceria mixed oxide catalyst. Chem. Eng. J. 2013, 218, 164–172. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Hillary, B.; Amin, M.H.; Rockstroh, N.; Bentrup, U.; Brückner, A.; Bhargava, S.K. Heterostructured Copper-Ceria and Iron-Ceria Nanorods: Role of Morphology, Redox, and Acid Properties in Catalytic Diesel Soot Combustion. Langmuir 2018, 34, 2663–2673. [Google Scholar] [CrossRef]
- Lu, F.; Jiang, B.B.; Wang, J.; Huang, Z.; Liao, Z.; Yang, Y. Insights into the improvement effect of Fe doping into the CeO2 catalyst for vapor phase ketonization of carboxylic acids. Mol. Catal. 2018, 444, 22–33. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Wei, Y.; Yan, D. Transformation of methane into synthesis gas using the redox property of Ce-Fe mixed oxides: Effect of calcination temperature. Int. J. Hydrogen Energy 2011, 36, 3471–3482. [Google Scholar] [CrossRef]
- Bao, H.; Qian, K.; Fang, J.; Huang, W. Fe-doped CeO2 solid solutions: Substituting-site doping versus interstitial-site doping, bulk doping versus surface doping. Appl. Surf. Sci. 2017, 414, 131–139. [Google Scholar] [CrossRef]
- Reddy, A.S.; Chen, C.Y.; Chen, C.C.; Chien, S.H.; Lin, C.J.; Lin, K.H.; Chen, C.L.; Chang, S.C. Synthesis and characterization of Fe/CeO2 catalysts: Epoxidation of cyclohexene. J. Mol. Catal. A Chem. 2010, 318, 60–67. [Google Scholar] [CrossRef]
- Torrente-Murciano, L.; Chapman, R.S.L.; Narvaez-Dinamarca, A.; Mattia, D.; Jones, M.D. Effect of nanostructured ceria as support for the iron catalysed hydrogenation of CO2 into hydrocarbons. Phys. Chem. Chem. Phys. 2016, 18, 15496–15500. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, C. Synthesis, characterization and catalytic wet air oxidation property of mesoporous Ce1-xFexO2 mixed oxides. Mater. Chem. Phys. 2015, 155, 223–231. [Google Scholar] [CrossRef]
- Trens, P.; Stathopoulos, V.; Hudson, M.J.; Pomonis, P. Synthesis and characterization of packed mesoporous tungsteno-silicates: application to the catalytic dehydrogenation of 2-propanol. Appl. Catal. A Gen. 2004, 263, 103–108. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Zarinkamar, M. Synthesis of nano-sized ceria (CeO2) particles via a cerium hydroxy carbonate precursor and the effect of reaction temperature on particle morphology. J. Ultrafine Grained Nanostructured Mater. 2015, 48, 5–10. [Google Scholar] [CrossRef]
- Sharma, V.; Eberhardt, K.M.; Sharma, R.; Adams, J.B.; Crozier, P.A. A spray drying system for synthesis of rare-earth doped cerium oxide nanoparticles. Chem. Phys. Lett. 2010, 495, 280–286. [Google Scholar] [CrossRef]
- Sahoo, T.R.; Armandi, M.; Arletti, R.; Piumetti, M.; Bensaid, S.; Manzoli, M.; Panda, S.R.; Bonelli, B. Pure and Fe-doped CeO2 nanoparticles obtained by microwave assisted combustion synthesis: Physico-chemical properties ruling their catalytic activity towards CO oxidation and soot combustion. Appl. Catal. B Environ. 2017, 211, 31–45. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Papista, E.; Marnellos, G.E.; Tavares, P.B.; Maldonado-Hódar, F.J.; Konsolakis, M. Catalytic decomposition of N2O on inorganic oxides: Effect of doping with Au nanoparticles. Mol. Catal. 2017, 436, 78–89. [Google Scholar] [CrossRef]
- Trpkov, D.; Panjan, M.; Kopanja, L.; Tadić, M. Hydrothermal synthesis, morphology, magnetic properties and self-assembly of hierarchical α-Fe2O3 (hematite) mushroom-, cube- and sphere-like superstructures. Appl. Surf. Sci. 2018, 457, 427–438. [Google Scholar] [CrossRef]
- Zhu, X.; Wei, Y.; Wang, H.; Li, K. Ce-Fe oxygen carriers for chemical-looping steam methane reforming. Int. J. Hydrogen Energy 2013, 38, 4492–4501. [Google Scholar] [CrossRef]
- Ma, S.; Chen, S.; Zhu, M.; Zhao, Z.; Hu, J.; Wu, M.; Toan, S.; Xiang, W. Enhanced sintering resistance of Fe2O3/CeO2 oxygen carrier for chemical looping hydrogen generation using core-shell structure. Int. J. Hydrogen Energy 2019, 44, 6491–6504. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Wei, Y.; Yan, D. Syngas production from methane and air via a redox process using Ce-Fe mixed oxides as oxygen carriers. Appl. Catal. B Environ. 2010, 97, 361–372. [Google Scholar] [CrossRef]
- Channei, D.; Inceesungvorn, B.; Wetchakun, N.; Phanichphant, S.; Nakaruk, A.; Koshy, P.; Sorrell, C.C. Photocatalytic activity under visible light of Fe-doped CeO2 nanoparticles synthesized by flame spray pyrolysis. Ceram. Int. 2013, 39, 3129–3134. [Google Scholar] [CrossRef]
- Hosseini, S.Y.; Khosravi-Nikou, M.R.; Shariati, A. Production of hydrogen and syngas using chemical looping technology via cerium-iron mixed oxides. Chem. Eng. Process. Process Intensif. 2019, 139, 23–33. [Google Scholar] [CrossRef]
- Qiao, D.; Lu, G.; Liu, X.; Guo, Y.; Wang, Y.; Guo, Y. Preparation of Ce1-xFexO2 solid solution and its catalytic performance for oxidation of CH4 and CO. J. Mater. Sci. 2011, 46, 3500–3506. [Google Scholar] [CrossRef]
- Li, D.; Li, K.; Xu, R.; Wang, H.; Tian, D.; Wei, Y.; Zhu, X.; Zeng, C.; Zeng, L. Ce1-xFexO2-δ catalysts for catalytic methane combustion: Role of oxygen vacancy and structural dependence. Catal. Today 2018, 318, 73–85. [Google Scholar] [CrossRef]
- Désaunay, T.; Bonura, G.; Chiodo, V.; Freni, S.; Couzinié, J.P.; Bourgon, J.; Ringuedé, A.; Labat, F.; Adamo, C.; Cassir, M. Surface-dependent oxidation of H2 on CeO2 surfaces. J. Catal. 2013, 297, 193–201. [Google Scholar] [CrossRef]
- Alvarez, P.; Araya, P.; Rojas, R.; Guerrero, S.; Aguila, G. Activity of alumina supported Fe catalysts for N2O decomposition: effects of the iron content and thermal treatment. J. Chil. Chem. Soc. 2017, 62, 3752–3759. [Google Scholar] [CrossRef]
- Zieliński, J.; Zglinicka, I.; Znak, L.; Kaszkur, Z. Reduction of Fe2O3 with hydrogen. Appl. Catal. A Gen. 2010, 381, 191–196. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Jia, Q.; Takriff, M.S. Catalytic decomposition of methane over rare earth metal (Ce and La) oxides supported iron catalysts. Appl. Surf. Sci. 2019, 467, 236–248. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, X.; Su, D.; Wang, Z.; Chang, J.; Ma, C. NO reduction by CO over copper catalyst supported on mixed CeO2 and Fe2O3: Catalyst design and activity test. Appl. Catal. B Environ. 2018, 239, 485–501. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Song, Z.; Zhao, H.; Xing, Y.; Zhao, M.; Zhao, J.; Ma, Z.; Zhang, P.; Tsubaki, N. Promotion of surface acidity and surface species of doped Fe and SO42− over CeO2 catalytic for NH3-SCR reaction. Mol. Catal. 2019, 463, 1–7. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, Q.; Wang, X.; Ma, K.; Bai, X.; Tan, S.; Tian, Y.; Ding, T.; Zheng, L.; Zhang, J.; et al. Enhanced catalytic performance for CO preferential oxidation over CuO catalysts supported on highly defective CeO2 nanocrystals. Appl. Surf. Sci. 2017, 422, 932–943. [Google Scholar] [CrossRef]
- Piumetti, M.; Andana, T.; Bensaid, S.; Russo, N.; Fino, D.; Pirone, R. Study on the CO Oxidation over Ceria-Based Nanocatalysts. Nanoscale Res. Lett. 2016, 11, 165. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M.; Overbury, S.H. Probing defect sites on CeO2 nanocrystals with well-defined surface planes by Raman spectroscopy and O2 adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef]
- Hillary, B.; Sudarsanam, P.; Amin, M.H.; Bhargava, S.K. Nanoscale Cobalt-Manganese Oxide Catalyst Supported on Shape-Controlled Cerium Oxide: Effect of Nanointerface Configuration on Structural, Redox, and Catalytic Properties. Langmuir 2017, 33, 1743–1750. [Google Scholar] [CrossRef]
- Andana, T.; Piumetti, M.; Bensaid, S.; Veyre, L.; Thieuleux, C.; Russo, N.; Fino, D.; Quadrelli, E.A.; Pirone, R. CuO nanoparticles supported by ceria for NOx-assisted soot oxidation: insight into catalytic activity and sintering. Appl. Catal. B Environ. 2017, 216, 41–58. [Google Scholar] [CrossRef]
- Konsolakis, M.; Ioakimidis, Z.; Kraia, T.; Marnellos, G.E. Hydrogen Production by Ethanol Steam Reforming (ESR) over CeO2 Supported Transition Metal (Fe, Co, Ni, Cu) Catalysts: Insight into the Structure-Activity Relationship. Catalysts 2016, 6, 39. [Google Scholar] [CrossRef]
- Wang, H.; Jin, B.; Wang, H.; Ma, N.; Liu, W.; Weng, D.; Wu, X.; Liu, S. Study of Ag promoted Fe2O3@CeO2 as superior soot oxidation catalysts: The role of Fe2O3 crystal plane and tandem oxygen delivery. Appl. Catal. B Environ. 2018, 237, 251–262. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Z.; Wang, D.; Chen, D.; Chen, F.; Li, X. Morphology and Crystal-Plane Effects of Fe/W-CeO2 for Selective Catalytic Reduction of NO with NH3. Catalysts 2019, 9, 288. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, R.; Peng, W.; Tang, G.; Gao, X. Inverse CeO2-Fe2O3 catalyst for superior low-temperature CO conversion efficiency. Appl. Surf. Sci. 2017, 416, 911–917. [Google Scholar] [CrossRef]
- Kim, K.; Han, J.W. Mechanistic study for enhanced CO oxidation activity on (Mn,Fe) co-doped CeO2 (111). Catal. Today 2017, 293–294, 82–88. [Google Scholar] [CrossRef]
- Said, A.E.A.A.; Abd El-Wahab, M.M.M.; Goda, M.N. Synthesis and characterization of pure and (Ce, Zr, Ag) doped mesoporous CuO-Fe2O3 as highly efficient and stable nanocatalysts for CO oxidation at low temperature. Appl. Surf. Sci. 2016, 390, 649–665. [Google Scholar] [CrossRef]
- Chen, H.T.; Chang, J.G. Computational investigation of CO adsorption and oxidation on iron-modified cerium oxide. J. Phys. Chem. C 2011, 115, 14745–14753. [Google Scholar] [CrossRef]
- Lykaki, M.; Papista, E.; Kaklidis, N.; Carabineiro, S.A.C.; Konsolakis, M. Ceria Nanoparticles’ Morphological Effects on the N2O Decomposition Performance of Co3O4/CeO2 Mixed Oxides. Catalysts 2019, 9, 233. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Xu, J.; Harmer, J.; Li, G.; Chapman, T.; Collier, P.; Longworth, S.; Tsang, S.C. Size dependent oxygen buffering capacity of ceria nanocrystals. Chem. Commun. 2010, 46, 1887–1889. [Google Scholar] [CrossRef]
- Barthos, R.; Hegyessy, A.; Klébert, S.; Valyon, J. Vanadium dispersion and catalytic activity of Pd/VOx/SBA-15 catalysts in the Wacker oxidation of ethylene. Microporous Mesoporous Mater. 2015, 207, 1–8. [Google Scholar] [CrossRef][Green Version]
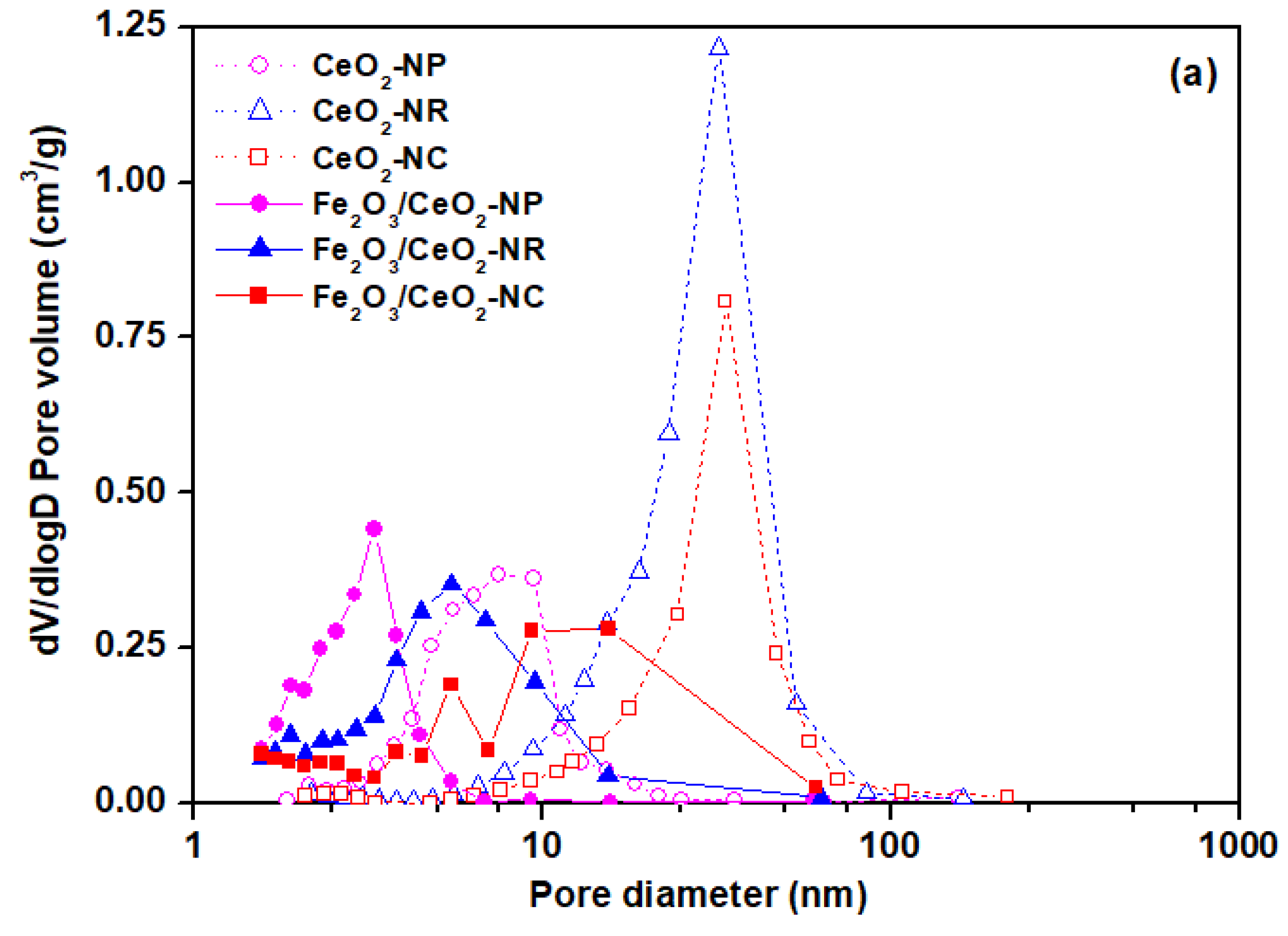

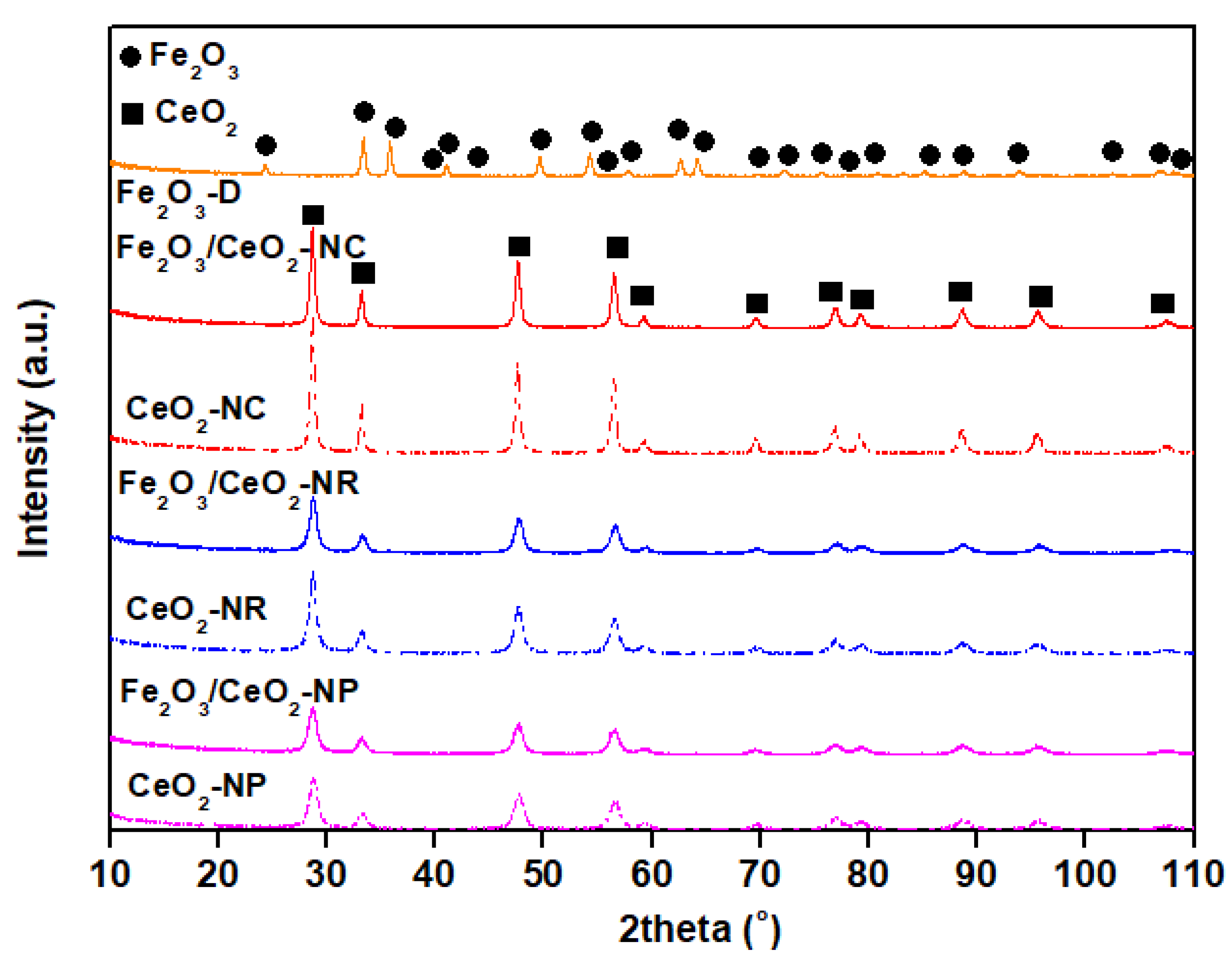
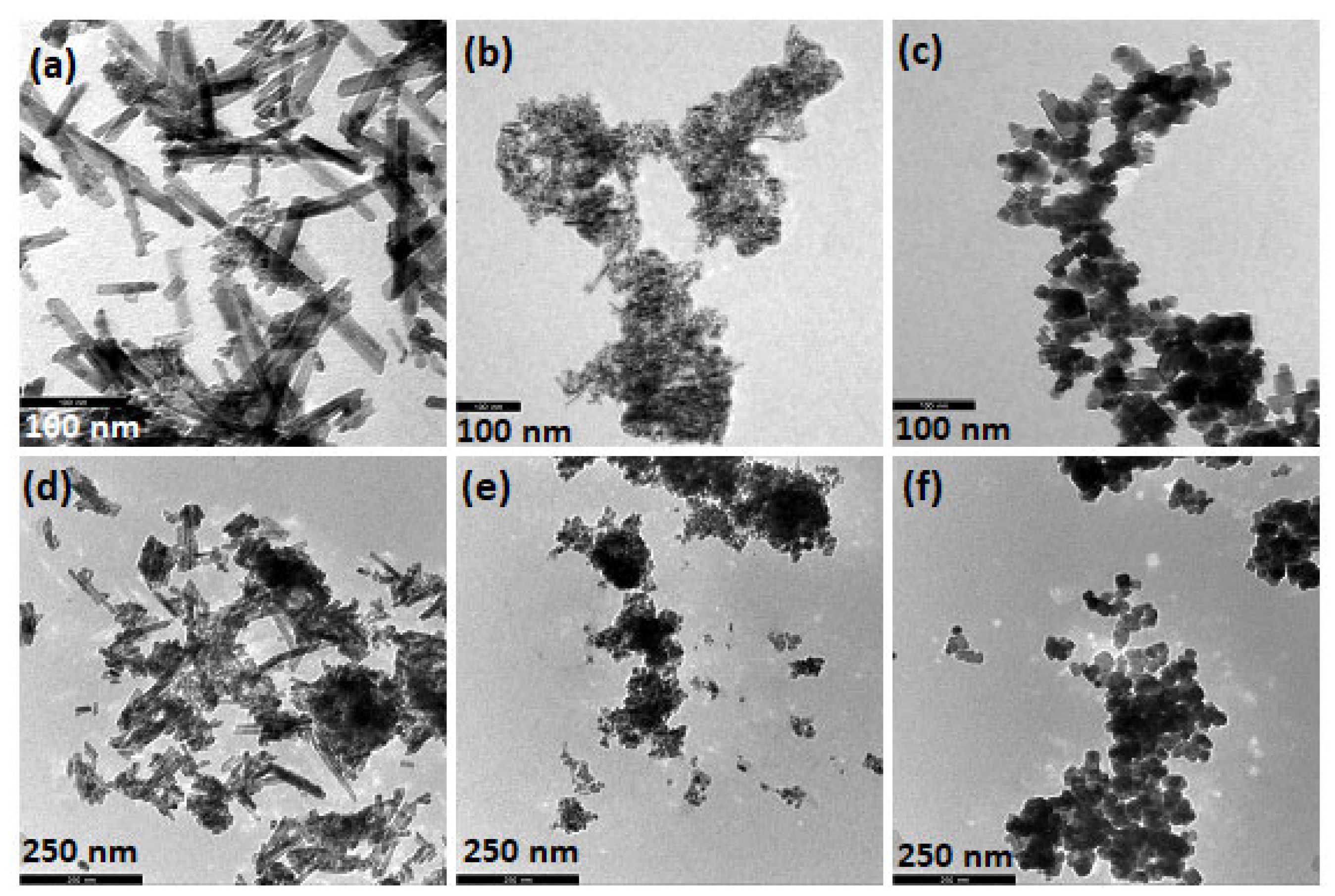

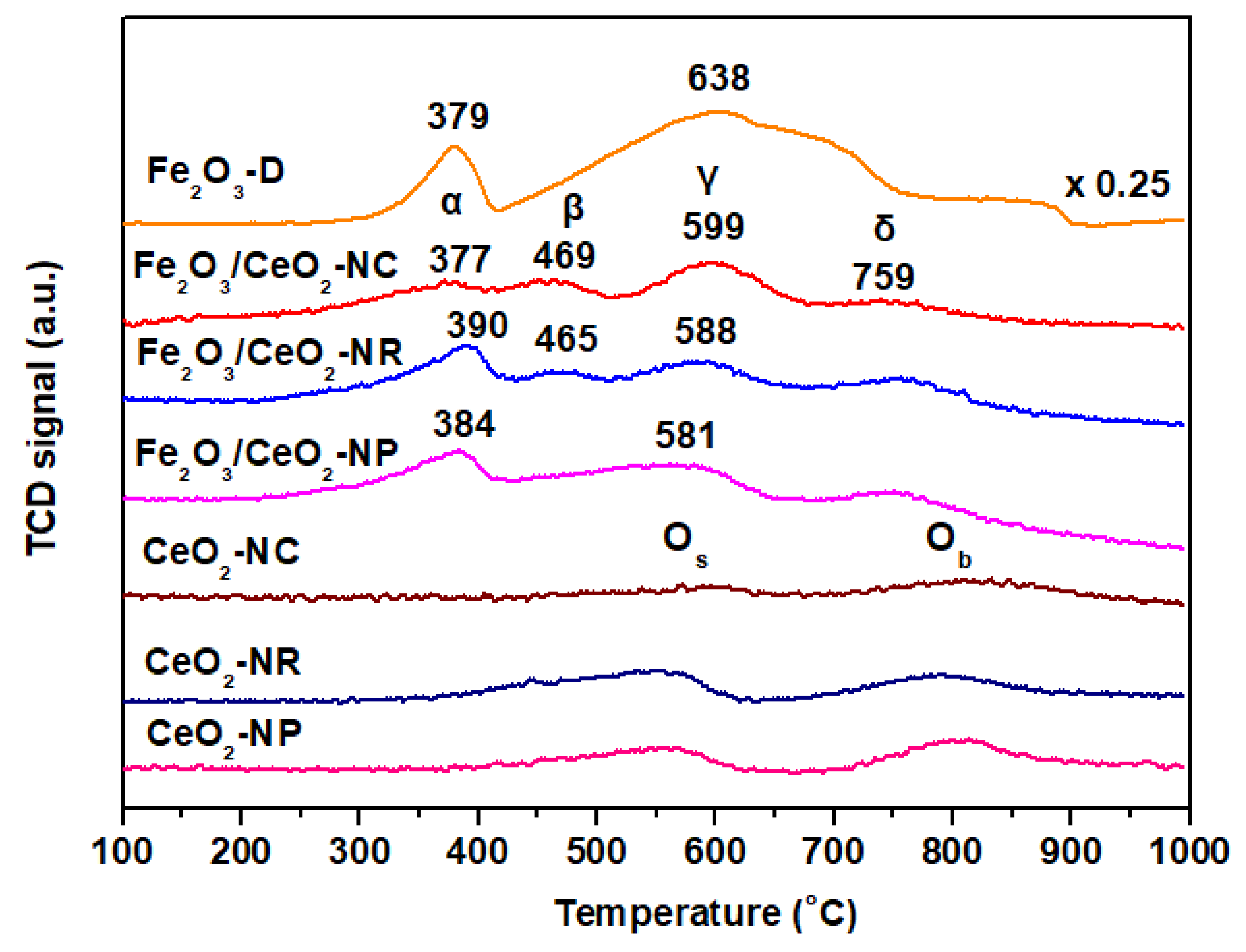


| Sample | BET Analysis | XRD Analysis | |||
|---|---|---|---|---|---|
| BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | Crystallite Size (nm), DXRD 1 | ||
| CeO2 | Fe2O3 | ||||
| CeO2-NR | 79.3 | 0.48 | 24.2 | 15.0 | - |
| CeO2-NP | 87.9 | 0.17 | 7.9 | 11.0 | - |
| CeO2-NC | 37.3 | 0.26 | 27.4 | 27.0 | - |
| Fe2O3/CeO2-NR | 68.6 | 0.19 | 11.3 | 9.7 | 7.2 |
| Fe2O3/CeO2-NP | 64.2 | 0.12 | 7.6 | 8.5 | 16.5 |
| Fe2O3/CeO2-NC | 32.2 | 0.19 | 23.3 | 16.8 | 52.3 |
| Fe2O3-D | 27.0 | 0.15 | 22.3 | - | 23.3 |
| Sample | H2 consumption (mmol H2/g) a | Os/Ob Ratio | Peak Temperature (°C) | |||||
| Os Peak | Ob Peak | Total | Os Peak | Ob Peak | ||||
| CeO2-NR | 0.59 | 0.52 | 1.11 | 1.13 | 545 | 788 | ||
| CeO2-NP | 0.48 | 0.51 | 0.99 | 0.94 | 555 | 804 | ||
| CeO2-NC | 0.41 | 0.58 | 0.99 | 0.71 | 589 | 809 | ||
| H2 Consumption (mmol H2/g) a | H2 Excess (mmol H2/g) b | Peak α | Peak β | Peak γ | Peak δ | |||
| Fe2O3/CeO2-NR | 3.42 | 1.42 | 390 | 465 | 588 | 759 | ||
| Fe2O3/CeO2-NP | 2.93 | 0.93 | 384 | - | 581 | 759 | ||
| Fe2O3/CeO2-NC | 2.47 | 0.47 | 377 | 469 | 599 | 759 | ||
| Fe2O3-D | 17.59 | - | 379 | - | 638 | - | ||
| Sample | Fe2+ (%) | Ce3+ (%) | OI/OII | Fe/(Fe + Ce) |
|---|---|---|---|---|
| CeO2-NR | - | 24.3 | 2.13 | - |
| CeO2-NP | - | 25.3 | 2.04 | - |
| CeO2-NC | - | 23.3 | 1.99 | - |
| Fe2O3/CeO2-NR | 14.4 | 25.3 | 2.52 | 0.28 |
| Fe2O3/CeO2-NP | 13.3 | 26.5 | 2.25 | 0.34 |
| Fe2O3/CeO2-NC | 13.1 | 28.5 | 1.84 | 0.43 |
| Sample | Ea (kJ mol−1) (± 0.1) | R2 (from Fitting Procedure) |
|---|---|---|
| CeO2-NR | 44.2 | 0.9943 |
| 46.7 | 0.9906 | |
| CeO2-NC | 49.8 | 0.9916 |
| Fe2O3/CeO2-NR | 53.5 | 0.9875 |
| Fe2O3/CeO2-NP | 55.7 | 0.9853 |
| Fe2O3/CeO2-NC | 58.6 | 0.9959 |
| Fe2O3-D | 63.3 | 0.9995 |
| Fe2O3-D + CeO2-NR | 48.1 | 0.9983 |
| Sample | CO Conversion (%) | Specific Activity | |
|---|---|---|---|
| r (nmol g−1 s−1) | r (nmol m−2 s−1) | ||
| Fe2O3/CeO2-NC | 2.40 | 26 | 0.80 |
| Fe2O3/CeO2-NP | 4.46 | 48 | 0.75 |
| Fe2O3/CeO2-NR | 11.69 | 126 | 1.84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lykaki, M.; Stefa, S.; Carabineiro, S.A.C.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Facet-Dependent Reactivity of Fe2O3/CeO2 Nanocomposites: Effect of Ceria Morphology on CO Oxidation. Catalysts 2019, 9, 371. https://doi.org/10.3390/catal9040371
Lykaki M, Stefa S, Carabineiro SAC, Pandis PK, Stathopoulos VN, Konsolakis M. Facet-Dependent Reactivity of Fe2O3/CeO2 Nanocomposites: Effect of Ceria Morphology on CO Oxidation. Catalysts. 2019; 9(4):371. https://doi.org/10.3390/catal9040371
Chicago/Turabian StyleLykaki, Maria, Sofia Stefa, Sόnia A. C. Carabineiro, Pavlos K. Pandis, Vassilis N. Stathopoulos, and Michalis Konsolakis. 2019. "Facet-Dependent Reactivity of Fe2O3/CeO2 Nanocomposites: Effect of Ceria Morphology on CO Oxidation" Catalysts 9, no. 4: 371. https://doi.org/10.3390/catal9040371
APA StyleLykaki, M., Stefa, S., Carabineiro, S. A. C., Pandis, P. K., Stathopoulos, V. N., & Konsolakis, M. (2019). Facet-Dependent Reactivity of Fe2O3/CeO2 Nanocomposites: Effect of Ceria Morphology on CO Oxidation. Catalysts, 9(4), 371. https://doi.org/10.3390/catal9040371










