Effect of Organic Assistant on the Performance of Ceria-Based Catalysts for the Selective Catalytic Reduction of NO with Ammonia
Abstract
:1. Introduction
2. Results and Discussion
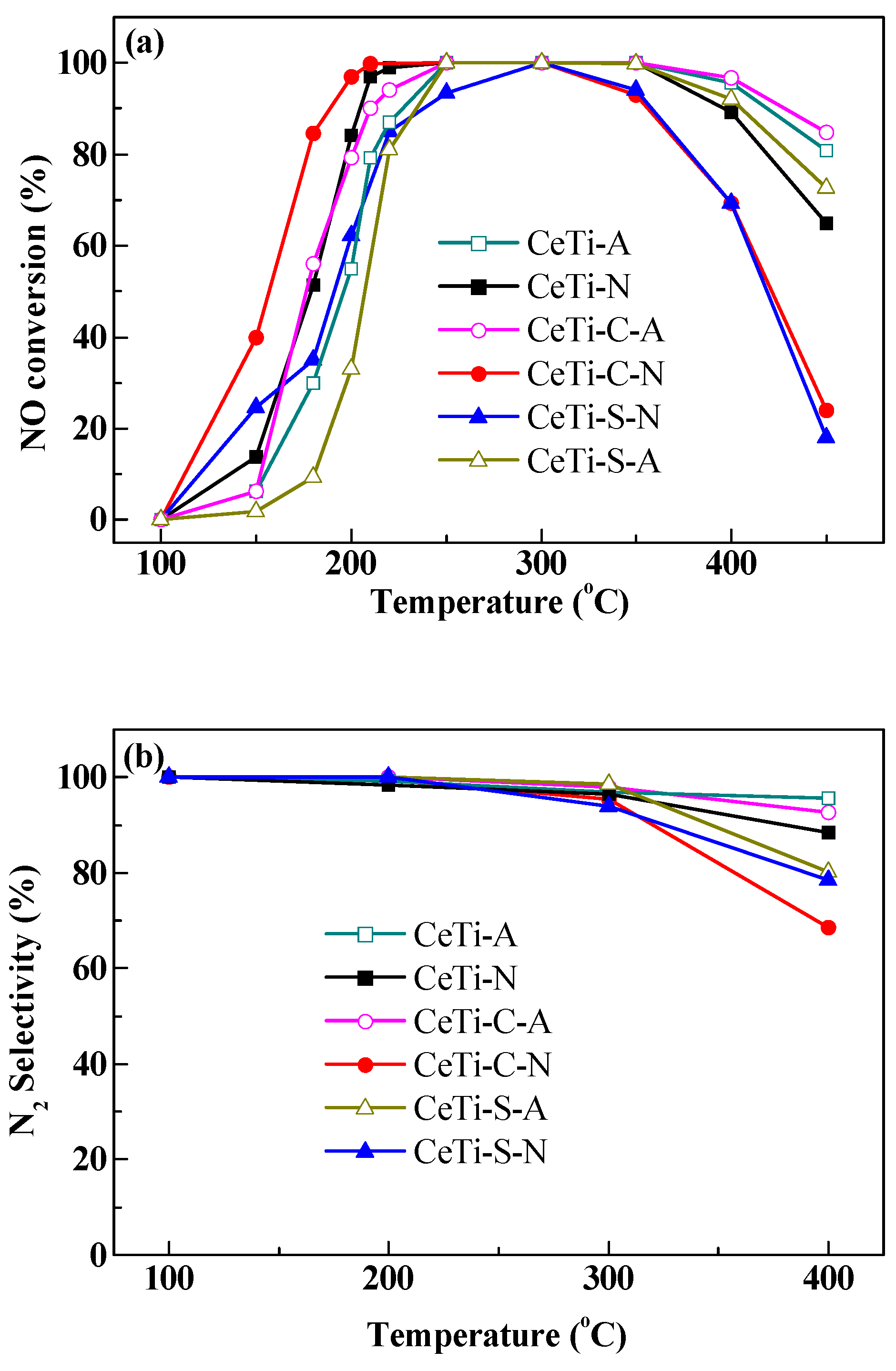
2.1. Catalytic Activity
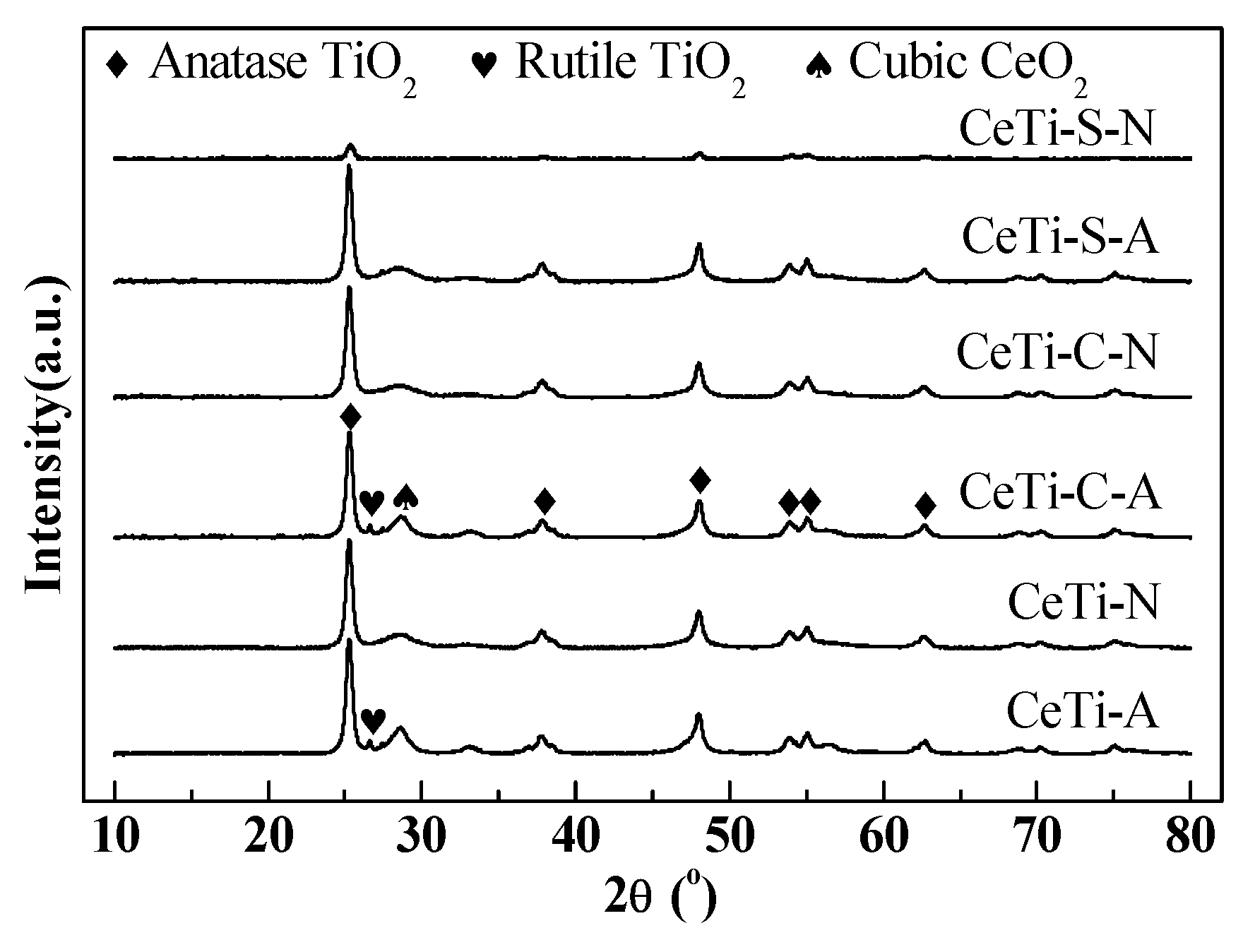
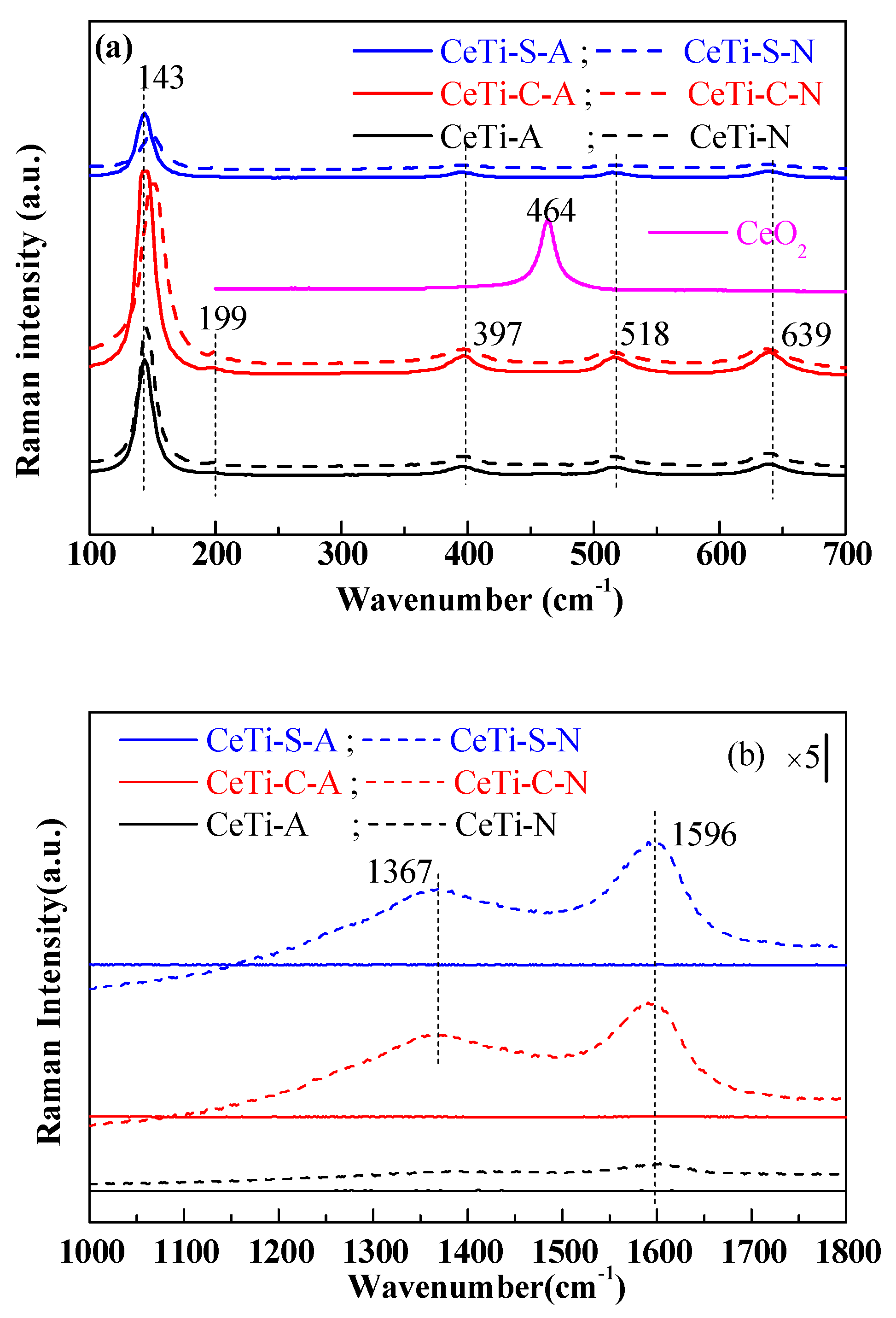
2.2. Structural and Textural Characterization of Various Catalysts
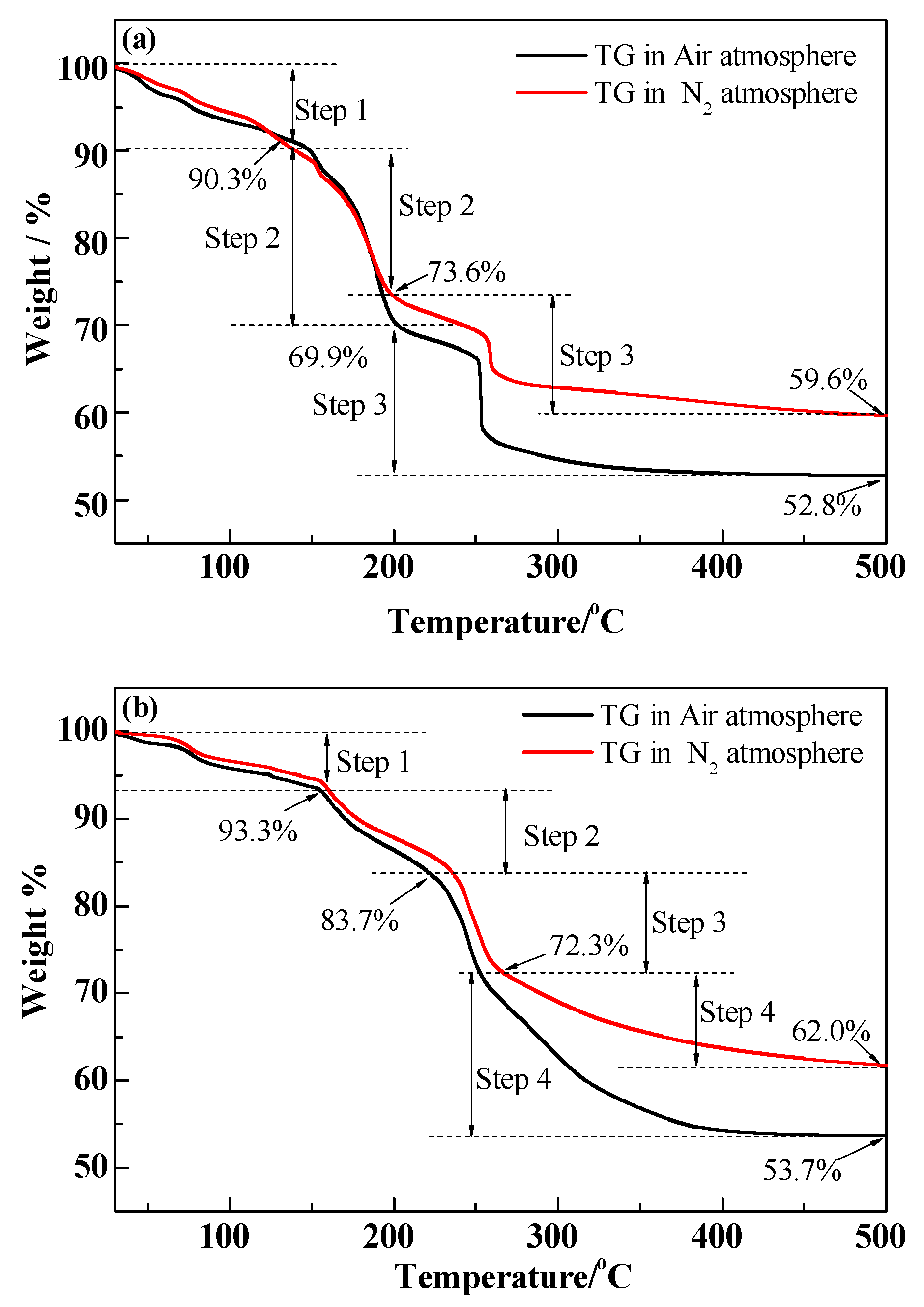
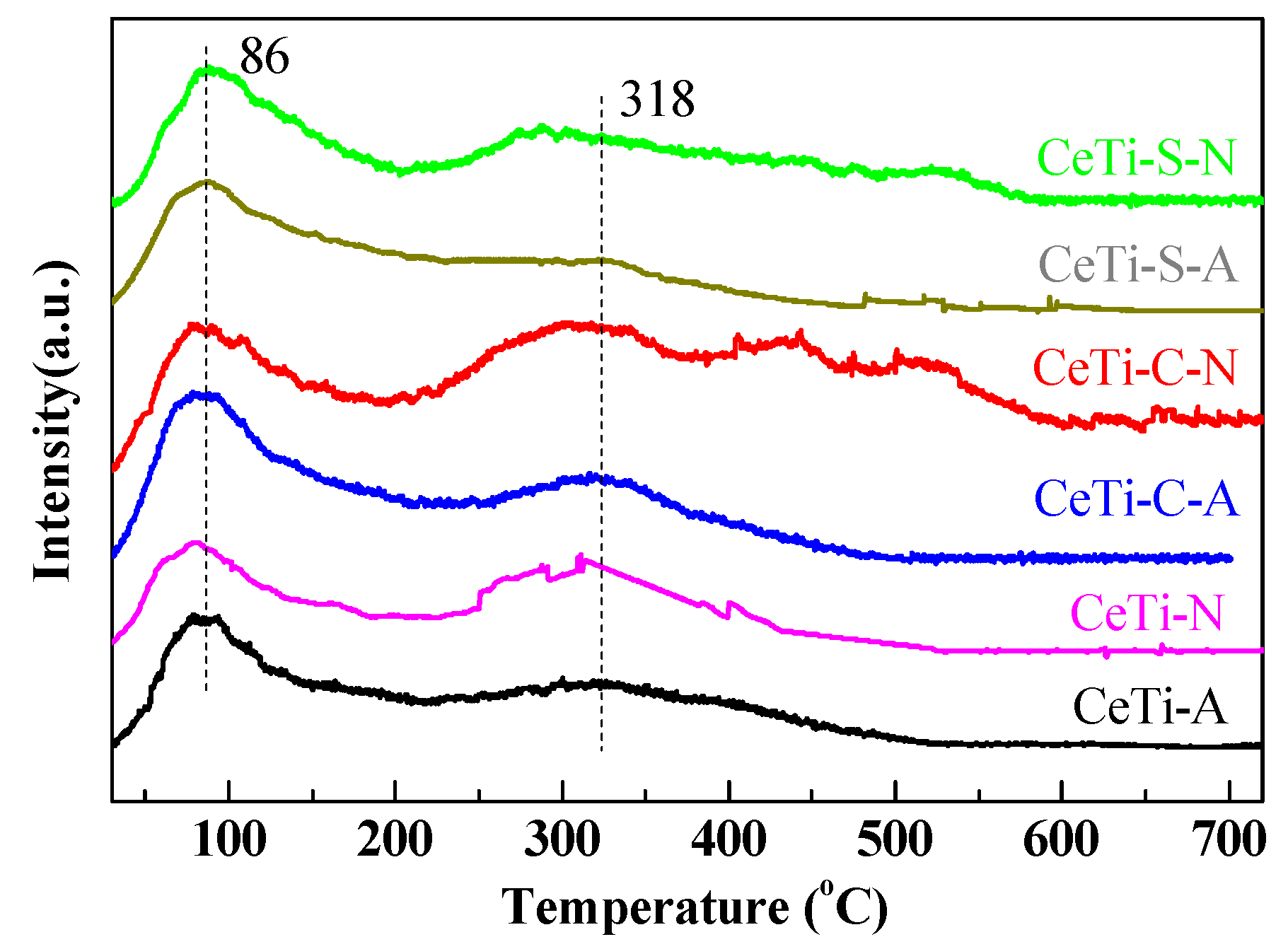
2.3. Measurements of the Decomposition Process of Organic Assistants (TG and FT-IR)
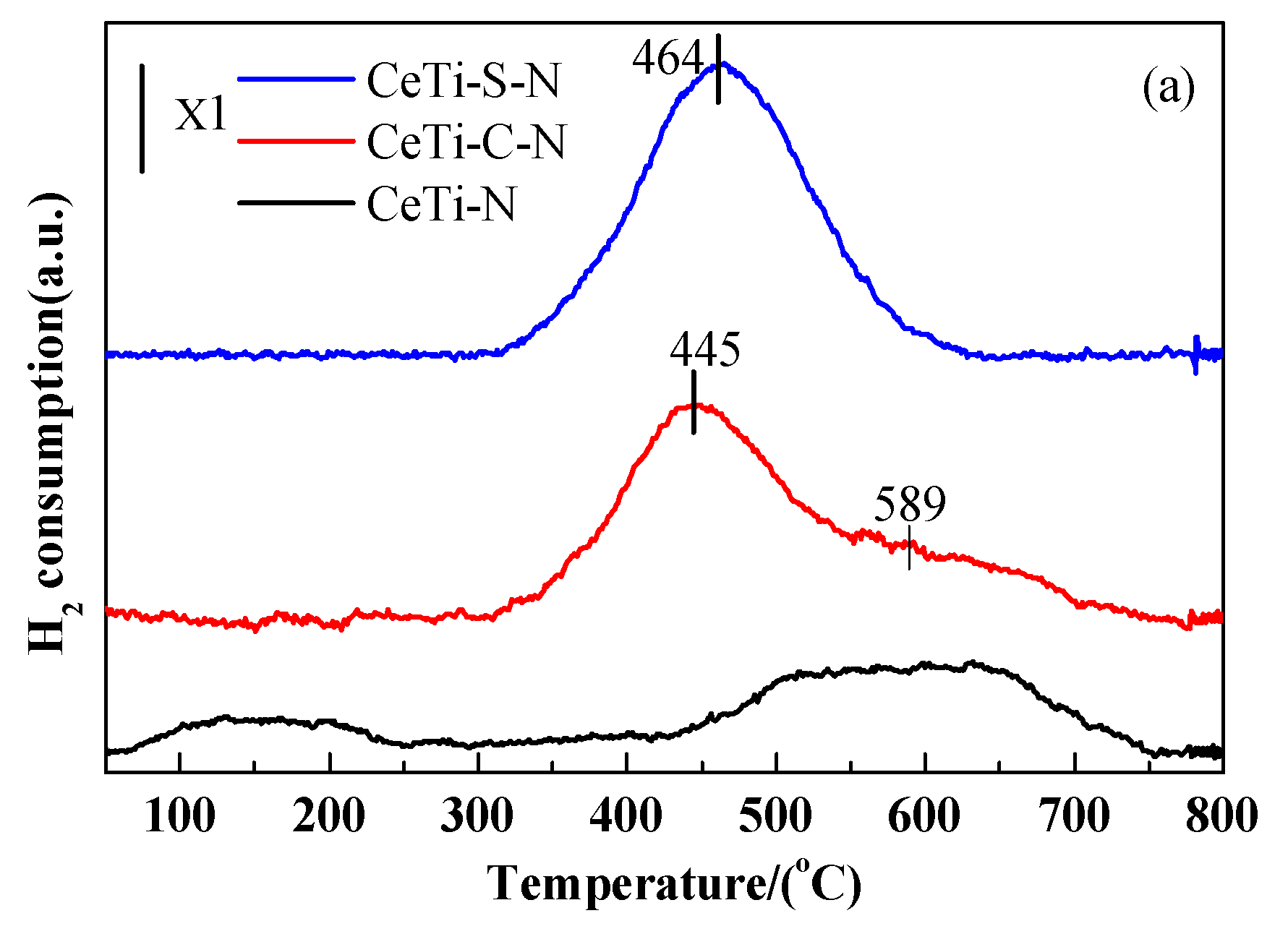
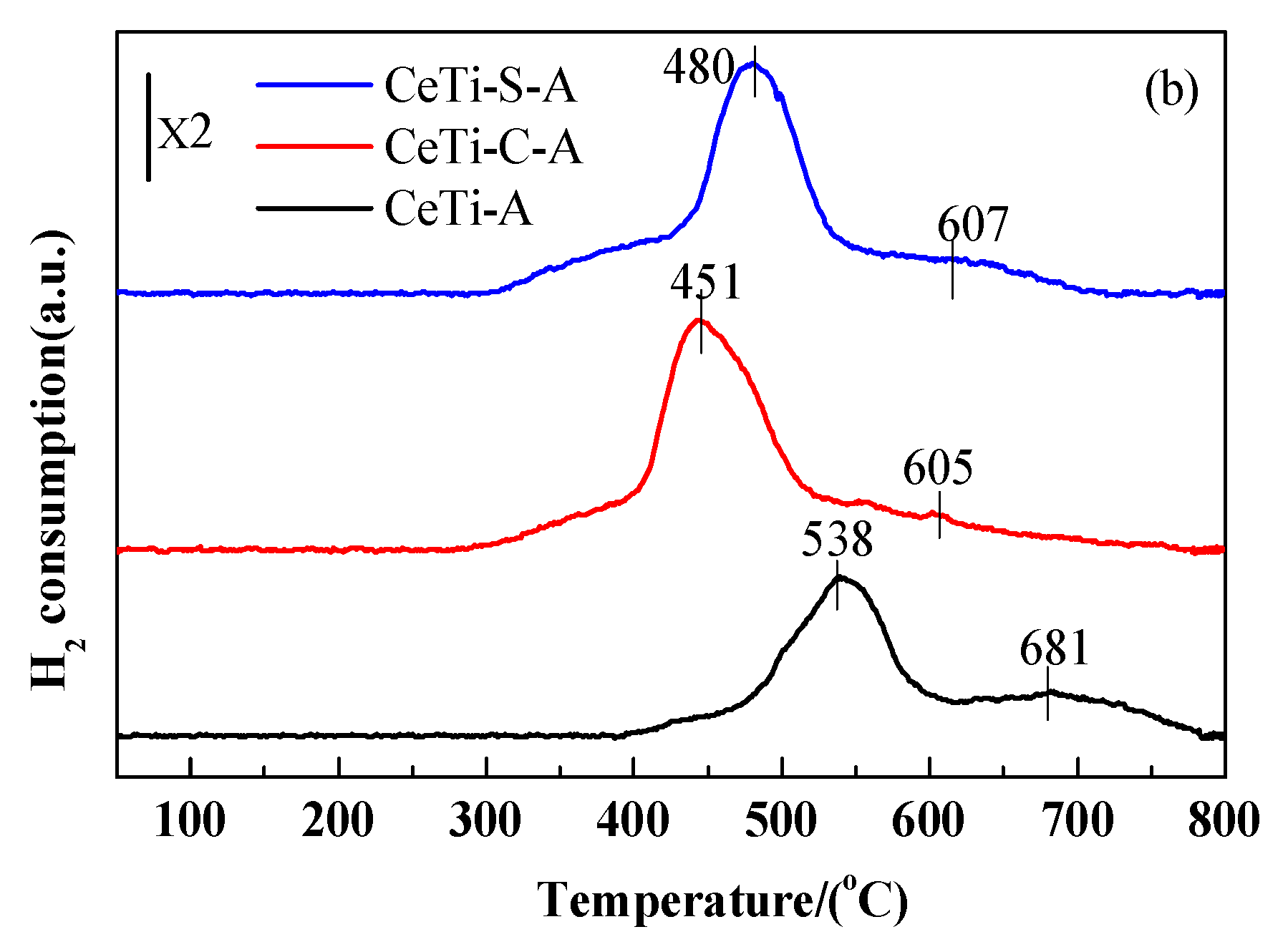
2.4. Redox Properties, Surface Acidity and SO2 Molecular Adsorption on Surface (H2-TPR,NH3-TPD and SO2-TPD)
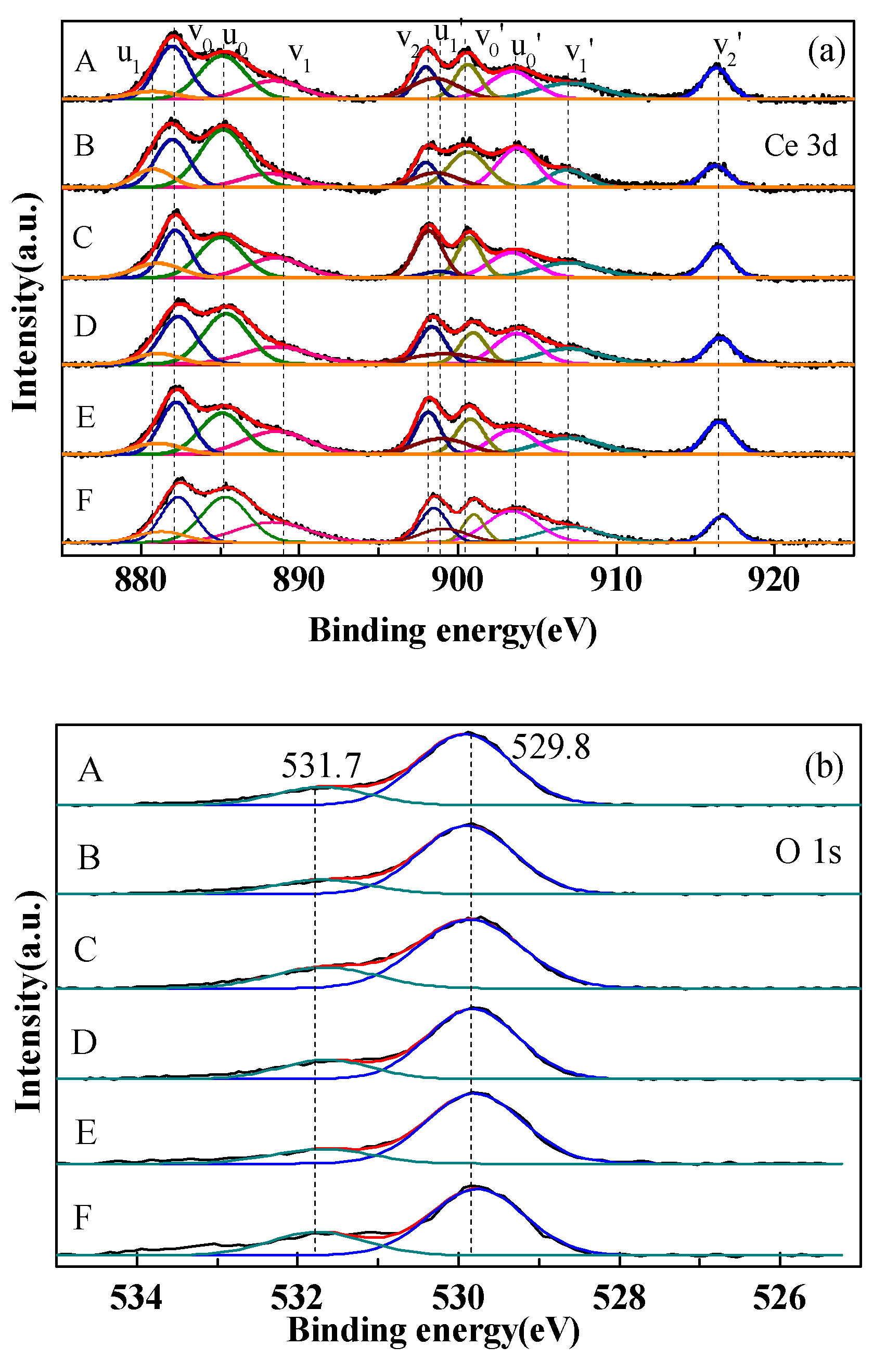
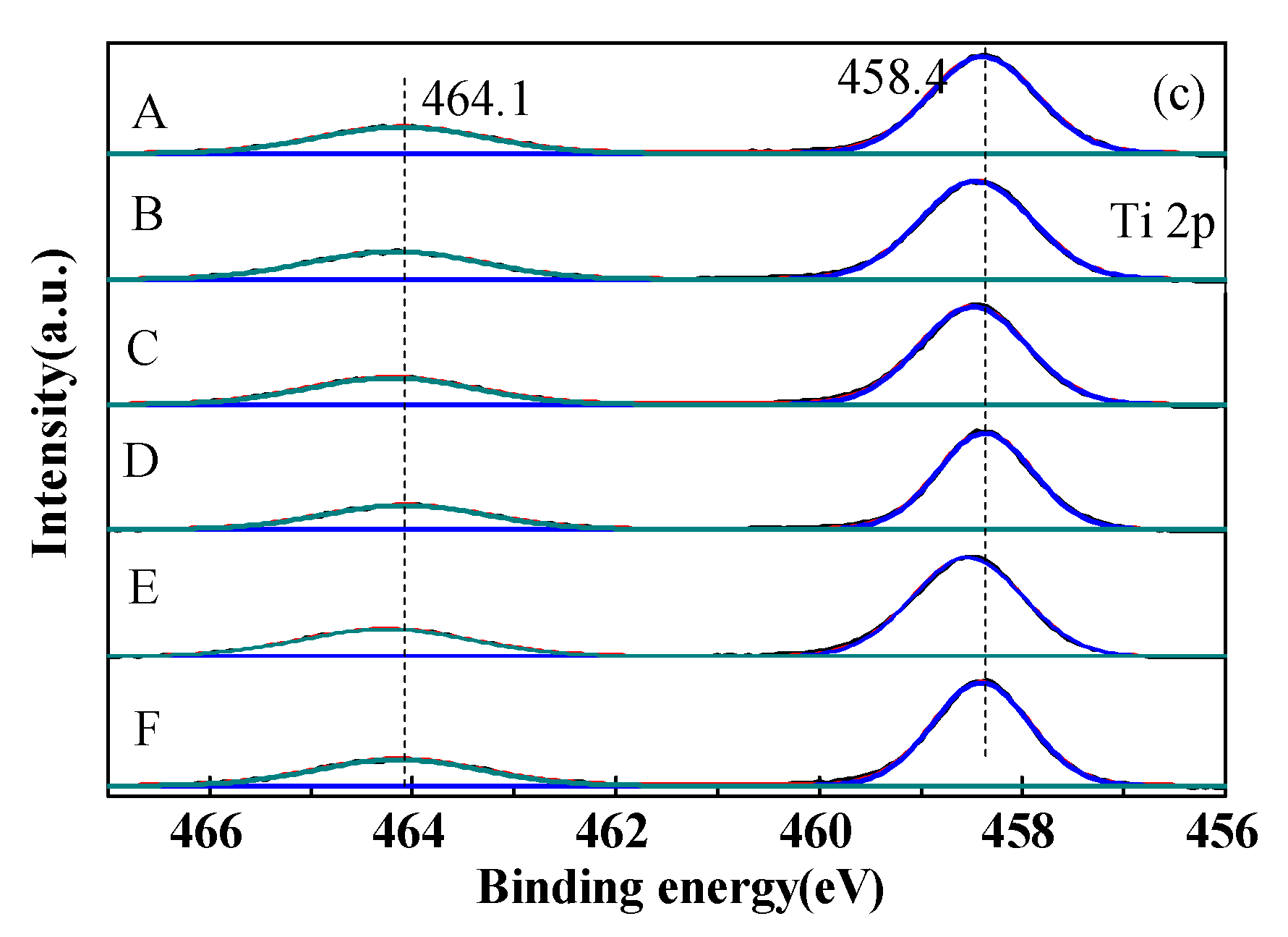
2.5. Surface Chemical State Analysis (XPS)
3. Experimental
3.1. Preparation of CeO2/TiO2 Catalysts
3.2. NH3-SCR Catalytic Activity Test
3.3. Characterization of the Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef] [PubMed]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Devadas, M.; Kröcher, O.; Elsener, M.; Wokaun, A.; Söger, N.; Pfeifer, M.; Demel, Y.; Mussmann, L. Influence of NO2 on the selective catalytic reduction of NO with ammonia over Fe-ZSM5. Appl. Catal. B 2006, 67, 187–196. [Google Scholar] [CrossRef]
- Roy, S.; Hegde, M.S.; Madras, G. Catalysis for NOx abatement. Appl. Energy 2009, 86, 2283–2297. [Google Scholar] [CrossRef]
- Granger, P.; Parvulescu, V.I. Catalytic NOx abatement systems for mobile sources: From three-way to lean burn after-treatment technologies. Chem. Rev. 2011, 111, 3155–3207. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yu, Y.; He, H. Environmentally-benign catalysts for the selective catalytic reduction of NOx from diesel engines: Structure–activity relationship and reaction mechanism aspects. Chem. Commun. 2014, 50, 8445–8463. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yan, N.; Qu, Z.; Liu, W.; Mei, J.; Huang, W.; Zhao, S. Gaseous heterogeneous catalytic reactions over Mn-based oxides for environmental applications—A critical review. Environ. Sci. Technol. 2017, 51, 8879–8892. [Google Scholar] [CrossRef]
- France, L.J.; Yang, Q.; Li, W.; Chen, Z.; Guang, J.; Guo, D.; Wang, L.; Li, X. Ceria modified FeMnOx —Enhanced performance and sulphur resistance for low-temperature SCR of NOx. Appl. Catal. B 2017, 206, 203–215. [Google Scholar] [CrossRef]
- Xu, W.; Yu, Y.; Zhang, C.; He, H. Selective catalytic reduction of NO by NH3 over a Ce/TiO2 catalyst. Catal. Commun. 2008, 9, 1453–1457. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Cao, Y.; Yao, X.; Ge, C.; Gao, F.; Deng, Y.; Tang, C.; Dong, L. Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B 2015, 165, 589–598. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. A superior catalyst for low-temperature NO reduction with NH3. Chem. Commun. 2003, 34, 848–849. [Google Scholar] [CrossRef]
- Shan, W.; Liu, F.; He, H.; Shi, X.; Zhang, C. Novel cerium-tungsten mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Chem. Commun. 2011, 47, 8046–8056. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, H.; Lin, D. Ceria-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2016, 6, 1248–1264. [Google Scholar] [CrossRef]
- Song, L.; Chao, J.; Fang, Y.; Hong, H.; Jian, L.; Qiu, W.; Zhang, G. Promotion of ceria for decomposition of ammonia bisulfate over V2O5-MoO3/TiO2 catalyst for selective catalytic reduction. Chem. Eng. J. 2016, 303, 275–281. [Google Scholar] [CrossRef]
- Zhang, L.; Zou, W.; Ma, K.; Cao, Y.; Xiong, Y.; Wu, S.; Tang, C.; Gao, F.; Dong, L. Sulfated temperature effects on the catalytic activity of CeO2 in NH3-selective catalytic reduction conditions. J. Phys. Chem. C 2015, 119, 1155–1163. [Google Scholar] [CrossRef]
- Shan, W.; Liu, F.; He, H.; Shi, X.; Zhang, C. Cheminform abstract: The remarkable improvement of a CeTi based catalyst for NOx abatement, prepared by a homogeneous precipitation method. ChemCatChem 2011, 3, 1286–1289. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, Y.; Fu, Y.; Zhong, Y.; Luo, Z.; Cen, K. Preparation and characterization of CeO2/TiO2 catalysts for selective catalytic reduction of NO with NH3. Catal. Commun. 2010, 11, 465–469. [Google Scholar] [CrossRef]
- Li, P.; Xin, Y.; Li, Q.; Wang, Z.; Zhang, Z.; Zheng, L. Ce–Ti amorphous oxides for selective catalytic reduction of NO with NH3: Confirmation of Ce–O–Ti active sites. Environ. Sci. Technol. 2012, 46, 9600–9605. [Google Scholar] [CrossRef]
- Michalowmauke, K.A.; Lu, Y.; Kowalski, K.; Graule, T.; Nachtegaal, M.; Kröcher, O.; Ferri, D. Flame-made WO3/CeOx-TiO2 catalysts for selective catalytic reduction of NOx by NH3. ACS Catal. 2015, 5, 5657–5672. [Google Scholar] [CrossRef]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef] [PubMed]
- Ralphs, K.; Hardacre, C.; James, S.L. Application of heterogeneous catalysts prepared by mechanochemical synthesis. Chem. Soc. Rev. 2013, 42, 7701–7718. [Google Scholar] [CrossRef]
- Yan, J.; Qiu, W.; Song, L.; Chen, Y.; Su, Y.; Bai, G.; Zhang, G.; He, H. Ligand-assisted mechanochemical synthesis of ceria-based catalysts for the selective catalytic reduction of NO by NH3. Chem. Commun. 2017, 53, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Qiu, W.; Song, L.; Zhu, H.; Chen, Y.; Li, S.; Sun, Y.; Bai, G.; Zhang, G.; He, H. Promoting effect of organic ligand on the performance of ceria for the selective catalytic reduction of NO by NH3. ChemistrySelect 2018, 3, 2683–2691. [Google Scholar] [CrossRef]
- Ma, L.; Seo, C.; Nahata, M.; Chen, X.; Li, J.; Schwank, J. Shape dependence and sulfate promotion of CeO2 for selective catalytic reduction of NOx with NH3. Appl. Catal. B 2018, 232, 246–259. [Google Scholar] [CrossRef]
- Weerd, C.D.; Gomez, L.; Zhang, H.; Buma, W.J.; Nedelcu, G.; Kovalenko, M.V.; Gregorkiewicz, T. Energy transfer between inorganic perovskite nanocrystals. J. Phys. Chem. C 2016, 120, 1066–1076. [Google Scholar] [CrossRef]
- Kaneko, K. Determination of pore size and pore size distribution: 1. adsorbents and catalysts. J. Membr. Sci. 1994, 96, 59–89. [Google Scholar] [CrossRef]
- Weber, W.H.; Hass, K.C.; Mcbride, J.R. Raman study of CeO2: Second-order scattering, lattice dynamics, and particle-size effects. Phys. Rev. B 1993, 48, 178–185. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, S.; Wang, Y.; Liu, G.; Zhong, Q. The effects of calcination atmosphere on the catalytic performance of Ce-doped TiO2 catalysts for selective catalytic reduction of NO with NH3. RSC Adv. 2017, 7, 23348–23354. [Google Scholar] [CrossRef]
- Zhang, W.; He, Y.; Zhang, M.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Sivadas, D.L.; Vijayan, S.; Rajeev, R.; Ninan, K.N.; Prabhakaran, K. Nitrogen-enriched microporous carbon derived from sucrose and urea with superior CO2 capture performance. Carbon 2016, 109, 7–18. [Google Scholar] [CrossRef]
- Zhang, J.; Ohara, S.; Umetsu, M.; Naka, T.; Hatakeyama, Y.; Adschiri, T. Colloidal ceria nanocrystals: A tailor-made crystal morphology in supercritical water. Adv. Mater. 2007, 19, 203–206. [Google Scholar] [CrossRef]
- Arakawa, K.; Matsuda, S.; Kinoshita, H. SOX poisoning mechanism of NOX selective reduction catalysts. Appl. Surf. Sci. 1997, 121–122, 382–386. [Google Scholar] [CrossRef]
- Boaro, M.; Vicario, M.; Leitenburg, C.; Dolcetti, G.; Trovarelli, A. The use of temperature-programmed and dynamic/transient methods in catalysis: Characterization of ceria-based, model three-way catalysts. Catal. Today 2003, 77, 407–417. [Google Scholar] [CrossRef]
- Zhan, S.; Shi, Q.; Zhang, Y.; Li, Y.; Tian, Y. Preparation of novel CeMo(x) hollow microspheres for low-temperature SCR removal of NOx with NH3. RSC Adv. 2016, 6, 59185–59194. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Li, C. Adsorption and activation of NH3 during selective catalytic reduction of NO by NH3. Chin. J. Catal. 2006, 27, 636–646. [Google Scholar] [CrossRef]
- Hair, M.; Hertl, W. Acidity of surface hydroxyl groups. J. Phys. Chem. C 1970, 74, 91–94. [Google Scholar] [CrossRef]
- Ballinger, T.; Yates, J. IR spectroscopic detection of Lewis acid sites on alumina using adsorbed carbon monoxide. Correlation with aluminum-fydroxyl group removal. Langmuir 1991, 7, 3041–3045. [Google Scholar] [CrossRef]
- Zhu, M.; Lai, J.; Tumuluri, U.; Wu, Z.; Wachs, I.E. Nature of active sites and surface intermediates during SCR of NO with NH3 by supported V2O5–WO3/TiO2 catalysts. J. Am. Chem. Soc. 2017, 139, 15624–15627. [Google Scholar] [CrossRef]
- Ramis, G.; Li, Y.; Busca, G.; Turco, M.; Kotur, E.; Willey, R. Adsorption activiation, and oxidation of ammonia over SCR catalysts. J. Catal. 1995, 157, 523–535. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Ke, R.; Hao, J. Improvement of catalytic activity and sulfur-resistance of Ag/TiO2-Al2O3 for NO reduction with propene under lean burn conditions. Appl. Catal. B 2008, 80, 202–213. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Y.; Li, W.; Jin, R.; Tang, S.; Hu, W. Adsorption and interaction of H2S/SO2 on TiO2. Catal. Today 1999, 50, 39–47. [Google Scholar]
- Zhang, R.; Zhong, Q.; Zhao, W.; Yu, L.; Qu, H. Promotional effect of fluorine on the selective catalytic reduction of NO with NH3 over CeO2-TiO2 catalyst at low temperature. Appl. Surf. Sci. 2014, 289, 237–244. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Li, J.; Ma, L.; Arandiyan, H.; Du, Y.; Xu, J.; Hao, J. Enhancement of activity and sulfur resistance of CeO2 supported on TiO2-SiO2 for the selective catalytic reduction of NO by NH3. Environ. Sci. Technol. 2012, 46, 6182–6189. [Google Scholar] [CrossRef] [PubMed]
- Sellick, D.R.; Aranda, A.; Garcia, T.; Lopez, J.M.; Solsona, B.; Mastral, A.M.; Morgan, D.J.; Carley, A.F.; Taylor, S.H. Influence of the preparation method on the activity of ceria zirconia mixed oxides for naphthalene total oxidation. Appl. Catal. B 2013, 132–133, 98–106. [Google Scholar] [CrossRef]
- Xiong, Y.; Tang, C.; Yao, X.; Zhang, L.; Li, L.; Wang, X.; Deng, Y.; Gao, F.; Dong, L. Effect of metal ions doping (M = Ti4+,Sn4+) on the catalytic performance of MnOx/CeO2 catalyst for low temperature selective catalytic reduction of NO with NH3. Appl. Catal. A 2015, 495, 206–216. [Google Scholar] [CrossRef]

| Samples | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| CeTi-A | 50 | 0.11 | 13.0 |
| CeTi-N | 44 | 0.11 | 12.9 |
| CeTi-C-A | 77 | 0.19 | 10.6 |
| CeTi-C-N | 77 | 0.17 | 9.1 |
| CeTi-S-A | 74 | 0.2 | 12.8 |
| CeTi-S-N | 108 | 0.13 | 5.7 |
| TiO2 | 77 | 0.43 | 16.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Li, S.; Qiu, W.; Chen, Y.; Cheng, J.; Sun, Y.; Bai, G.; Song, L.; Zhang, G.; He, H. Effect of Organic Assistant on the Performance of Ceria-Based Catalysts for the Selective Catalytic Reduction of NO with Ammonia. Catalysts 2019, 9, 357. https://doi.org/10.3390/catal9040357
Huang X, Li S, Qiu W, Chen Y, Cheng J, Sun Y, Bai G, Song L, Zhang G, He H. Effect of Organic Assistant on the Performance of Ceria-Based Catalysts for the Selective Catalytic Reduction of NO with Ammonia. Catalysts. 2019; 9(4):357. https://doi.org/10.3390/catal9040357
Chicago/Turabian StyleHuang, Xing, Shining Li, Wenge Qiu, Yun Chen, Jie Cheng, Yanming Sun, Guangmei Bai, Liyun Song, Guizhen Zhang, and Hong He. 2019. "Effect of Organic Assistant on the Performance of Ceria-Based Catalysts for the Selective Catalytic Reduction of NO with Ammonia" Catalysts 9, no. 4: 357. https://doi.org/10.3390/catal9040357
APA StyleHuang, X., Li, S., Qiu, W., Chen, Y., Cheng, J., Sun, Y., Bai, G., Song, L., Zhang, G., & He, H. (2019). Effect of Organic Assistant on the Performance of Ceria-Based Catalysts for the Selective Catalytic Reduction of NO with Ammonia. Catalysts, 9(4), 357. https://doi.org/10.3390/catal9040357






