Efficient Dye-Sensitized Solar Cells Composed of Nanostructural ZnO Doped with Ti
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.2. Fabrication of Dye-Sensitized Solar Cells
2.2.1. Paste Preparation
2.2.2. Films Fabrication
2.2.3. Dye Loading
2.2.4. Device Fabrication
2.3. Characterization of the Materials and Measurement of the Photovoltaic Performance
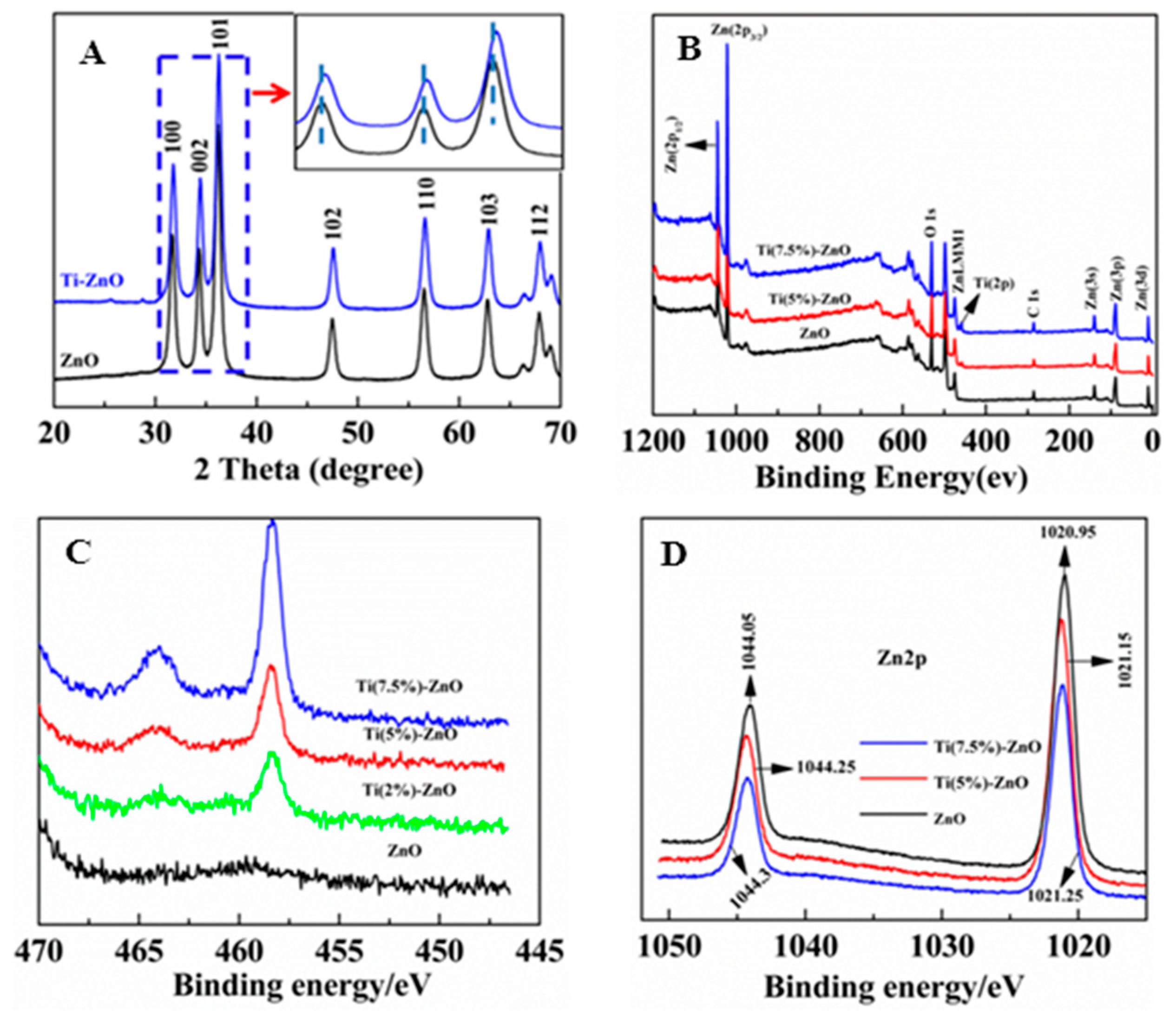
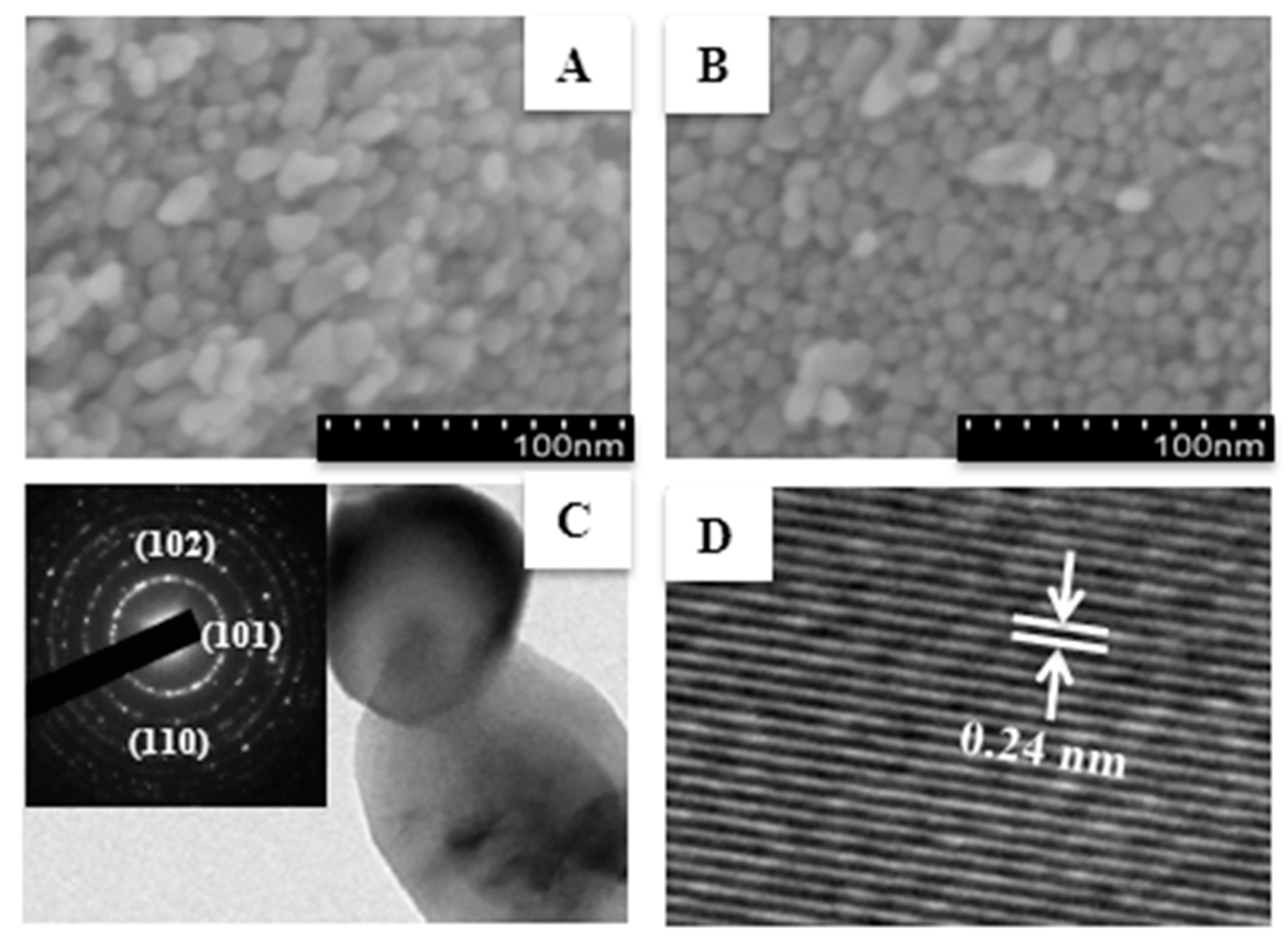
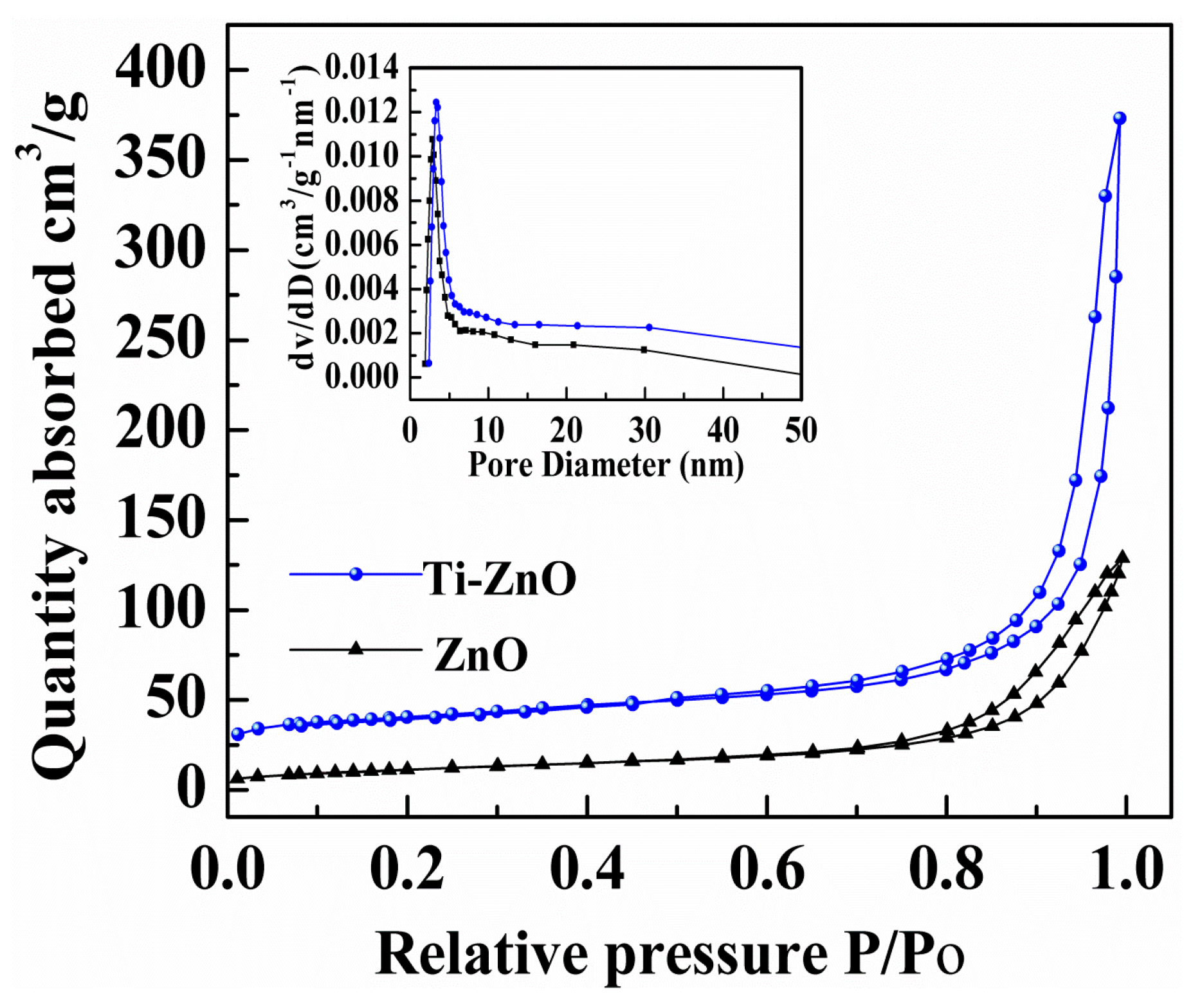
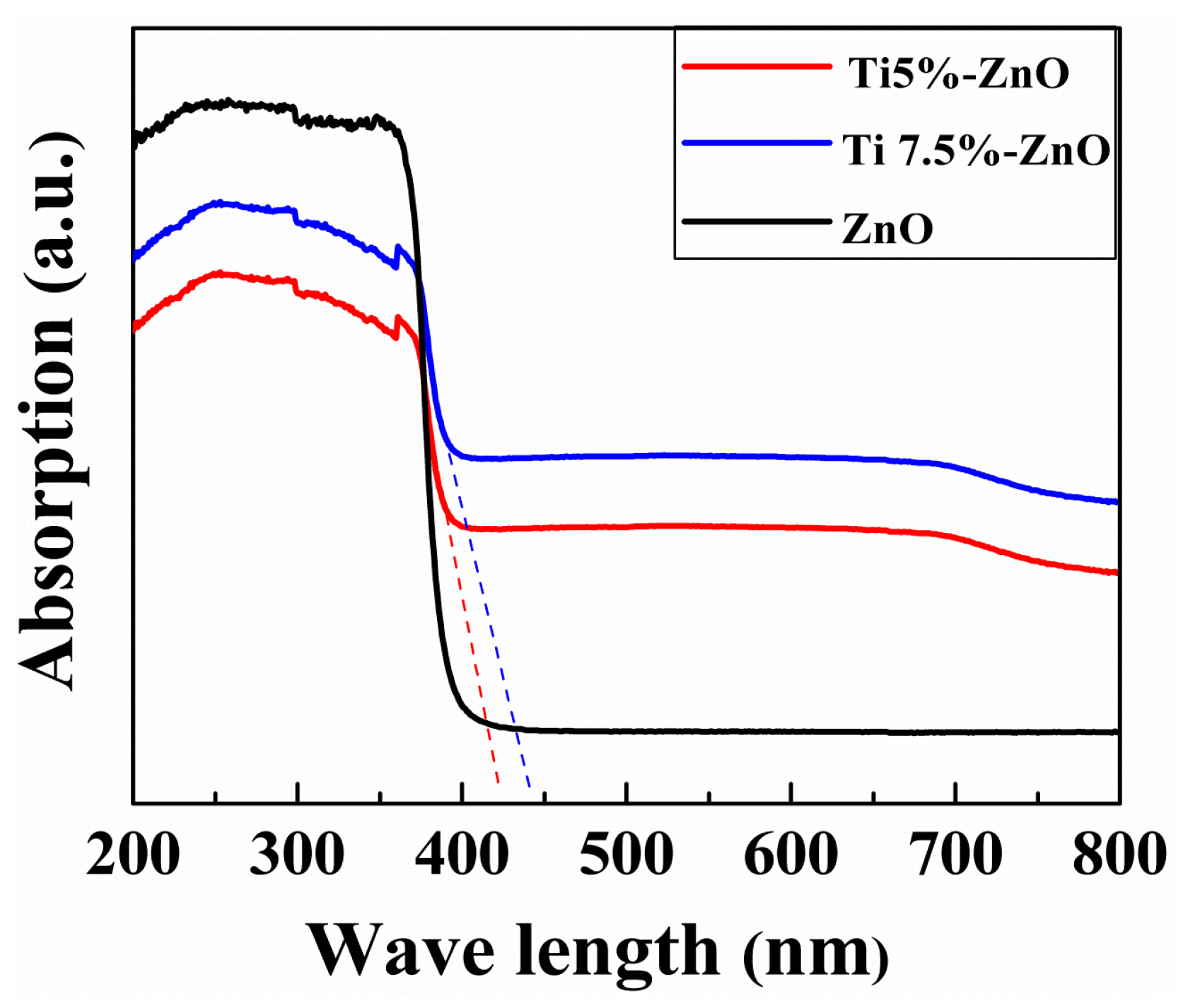
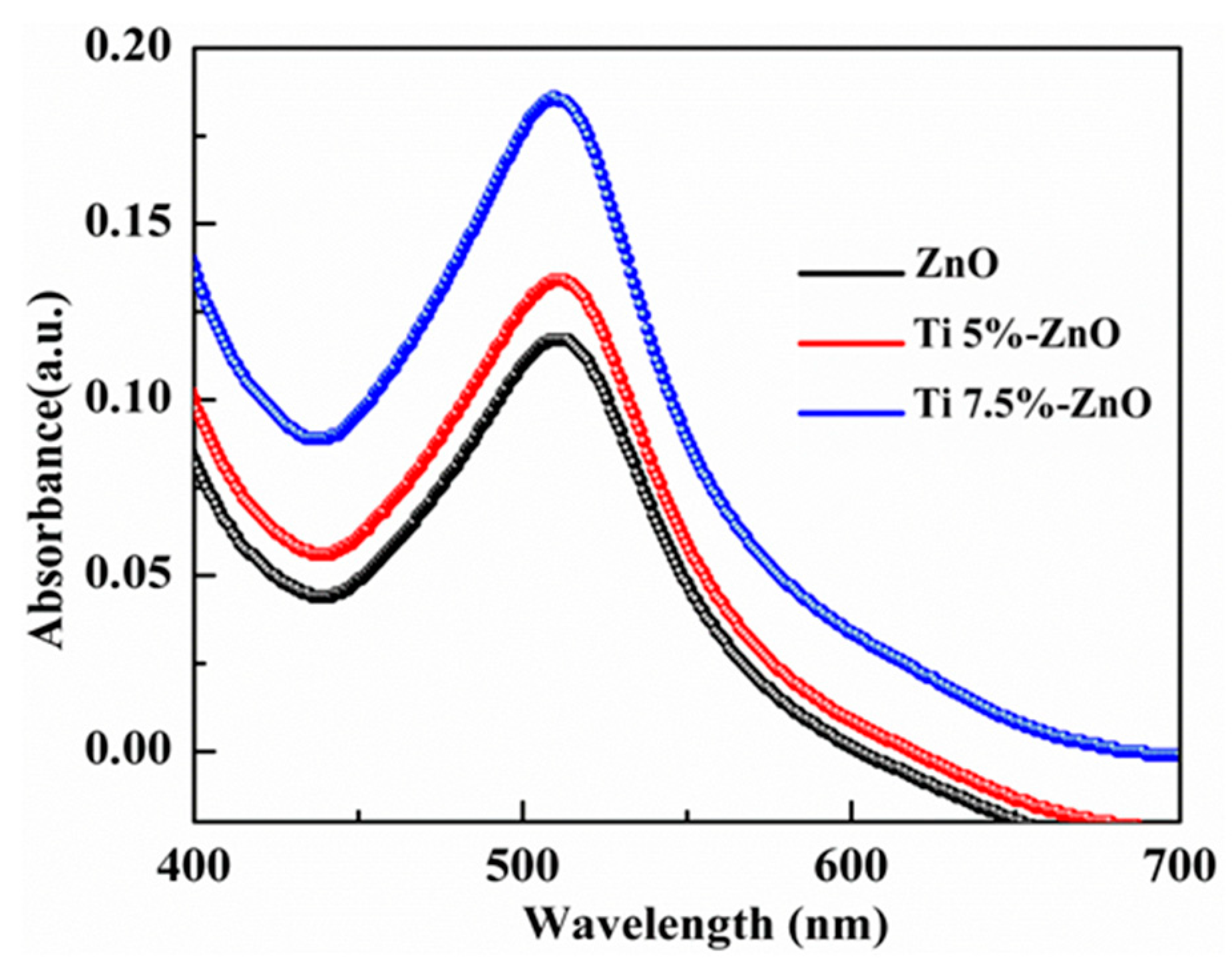
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grätzel, M. Solar Energy Conversion by Dye-Sensitized Photovoltaic Cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- Xiaoguang, M.; Swee Jen, C.; Benhu, F.; Jianyong, O. High-performance dye-sensitized solar cells with gel-coated binder-free carbon nanotube films as counter electrode. Nanotechnology 2010, 21, 395202. [Google Scholar]
- Zhang, W.; Zhu, R.; Ke, L.; Liu, X.; Liu, B.; Ramakrishna, S. Anatase Mesoporous TiO2 Nanofibers with High Surface Area for Solid-State Dye-Sensitized Solar Cells. Small 2010, 6, 2176–2182. [Google Scholar] [CrossRef] [PubMed]
- Poudel, P.; Qiao, Q. One dimensional nanostructure/nanoparticle composites as photoanodes for dye-sensitized solar cells. Nanoscale 2012, 4, 2826–2838. [Google Scholar] [CrossRef]
- Wu, M.; Lin, X.; Wang, T.; Qiu, J.; Ma, T. Low-cost dye-sensitized solar cell based on nine kinds of carbon counter electrodes. Energy Environ. Sci. 2011, 4, 2308–2315. [Google Scholar] [CrossRef]
- Shao, F.; Sun, J.; Gao, L.; Yang, S.; Luo, J. Growth of Various TiO2 Nanostructures for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2011, 115, 1819–1823. [Google Scholar] [CrossRef]
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nat. 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Moser, J.E.; Humphry-Baker, R.; Comte, P.; Aranyos, V.; Hagfeldt, A.; Nazeeruddin, M.K.; Grätzel, M. Stable New Sensitizer with Improved Light Harvesting for Nanocrystalline Dye-Sensitized Solar Cells. Adv. Mater. 2004, 16, 1806–1811. [Google Scholar] [CrossRef]
- Hendry, E.; Koeberg, M.; O’Regan, B.; Bonn, M. Local Field Effects on Electron Transport in Nanostructured TiO2 Revealed by Terahertz Spectroscopy. Nano Lett. 2006, 6, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)–Based Redox Electrolyte Exceed 12 Percent Efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Peng, M.; Cai, X.; Fu, Y.; Yu, X.; Liu, S.; Deng, B.; Hany, K.; Zou, D. Facial synthesis of SnO2 nanoparticle film for efficient fiber-shaped dye-sensitized solar cells. J. Power Sources 2014, 247, 249–255. [Google Scholar] [CrossRef]
- Song, H.; Lee, K.-H.; Jeong, H.; Um, S.H.; Han, G.-S.; Jung, H.S.; Jung, G.Y. A simple self-assembly route to single crystalline SnO2 nanorod growth by oriented attachment for dye sensitized solar cells. Nanoscale 2013, 5, 1188–1194. [Google Scholar] [CrossRef]
- Ramasamy, E.; Lee, J. Ordered Mesoporous SnO2−Based Photoanodes for High-Performance Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 22032–22037. [Google Scholar] [CrossRef]
- Bendall, J.S.; Etgar, L.; Tan, S.C.; Cai, N.; Wang, P.; Zakeeruddin, S.M.; Gratzel, M.; Welland, M.E. An efficient DSSC based on ZnO nanowire photo-anodes and a new D-[small pi]-A organic dye. Energy Environ. Sci. 2011, 4, 2903–2908. [Google Scholar] [CrossRef]
- Xia, J.B.; Li, F.Y.; Yang, S.M.; Huang, C.H. Composite electrode SnO~ 2/TiO~ 2 for dye-sensitized solar cells. Chin. Chem. Lett. 2004, 15, 619–622. [Google Scholar]
- Chen, S.G.; Chappel, S.; Diamant, Y.; Zaban, A. Preparation of Nb2O5 Coated TiO2 Nanoporous Electrodes and Their Application in Dye-Sensitized Solar Cells. Chem. Mater. 2001, 13, 4629–4634. [Google Scholar] [CrossRef]
- Gubbala, S.; Chakrapani, V.; Kumar, V.; Sunkara, M.K. Band-Edge Engineered Hybrid Structures for Dye-Sensitized Solar Cells Based on SnO2 Nanowires. Adv. Funct. Mater. 2008, 18, 2411–2418. [Google Scholar] [CrossRef]
- Prasittichai, C.; Hupp, J.T. Surface modification of SnO2 photoelectrodes in dye-sensitized solar cells: Significant improvements in photovoltage via Al2O3 atomic layer deposition. J. Phys. Chem. Lett. 2010, 1, 1611–1615. [Google Scholar] [CrossRef]
- Qian, J.; Liu, P.; Xiao, Y.; Jiang, Y.; Cao, Y.; Ai, X.; Yang, H. TiO2-coated multilayered SnO2 hollow microspheres for dye-sensitized solar cells. Adv. Mater. 2009, 21, 3663–3667. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef]
- Archana, P.S.; Jose, R.; Vijila, C.; Ramakrishna, S. Improved Electron Diffusion Coefficient in Electrospun TiO2 Nanowires. J. Phys. Chem. C 2009, 113, 21538–21542. [Google Scholar] [CrossRef]
- Hosono, E.; Fujihara, S.; Kimura, T. Synthesis, structure and photoelectrochemical performance of micro/nano-textured ZnO/eosin Y electrodes. Electrochim. Acta 2004, 49, 2287–2293. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Song, L.; Gunawan, P.; Zhong, Z.; She, X.; Su, F. Urchin-like ZnO microspheres synthesized by thermal decomposition of hydrozincite as a copper catalyst promoter for the Rochow reaction. RSC Adv. 2012, 2, 4164–4168. [Google Scholar] [CrossRef]
- Lin, L.; Yang, Y.; Men, L.; Wang, X.; He, D.; Chai, Y.; Zhao, B.; Ghoshroy, S.; Tang, Q. A highly efficient TiO2@ZnO n-p-n heterojunction nanorod photocatalyst. Nanoscale 2013, 5, 588–593. [Google Scholar] [CrossRef]
- Lee, D.; Rho, Y.; Allen, F.I.; Minor, A.M.; Ko, S.H.; Grigoropoulos, C.P. Synthesis of hierarchical TiO2 nanowires with densely-packed and omnidirectional branches. Nanoscale 2013, 5, 11147–11152. [Google Scholar] [CrossRef]
- Herman, I.; Yeo, J.; Hong, S.; Lee, D.; Nam, K.H.; Choi, J.-H.; Hong, W.-H.; Lee, D.; Grigoropoulos, C.P.; Ko, S.H. Hierarchical weeping willow nano-tree growth and effect of branching on dye-sensitized solar cell efficiency. Nanotechnology 2012, 23, 194005. [Google Scholar] [CrossRef]
- Ko, S.H.; Lee, D.; Kang, H.W.; Nam, K.H.; Yeo, J.Y.; Hong, S.J.; Grigoropoulos, C.P.; Sung, H.J. Nanoforest of Hydrothermally Grown Hierarchical ZnO Nanowires for a High Efficiency Dye-Sensitized Solar Cell. Nano Lett. 2011, 11, 666–671. [Google Scholar] [CrossRef]
- Yin, X.; Que, W.; Fei, D.; Xie, H.; He, Z.; Wang, G. Strategies to prepare an efficient photoanode for ZnO nanowires-based CdS–CdSe co-sensitized solar cells. Electrochim. Acta 2013, 89, 561–570. [Google Scholar] [CrossRef]
- Keis, K.; Bauer, C.; Boschloo, G.; Hagfeldt, A.; Westermark, K.; Rensmo, H.; Siegbahn, H. Nanostructured ZnO electrodes for dye-sensitized solar cell applications. J. Photochem. Photobiol. A Chem. 2002, 148, 57–64. [Google Scholar] [CrossRef]
- Han, Z.; Ren, L.; Cui, Z.; Chen, C.; Pan, H.; Chen, J. Ag/ZnO flower heterostructures as a visible-light driven photocatalyst via surface plasmon resonance. Appl. Catal. B: Environ. 2012, 126, 298–305. [Google Scholar] [CrossRef]
- Lee, S.-H.; Han, S.-H.; Jung, H.S.; Shin, H.; Lee, J.; Noh, J.-H.; Lee, S.; Cho, I.-S.; Lee, J.-K.; Kim, J.; et al. Al-Doped ZnO Thin Film: A New Transparent Conducting Layer for ZnO Nanowire-Based Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 7185–7189. [Google Scholar] [CrossRef]
- Kao, C.H.; Wang, J.C.; Lai, C.S.; Huang, C.Y.; Ou, J.C.; Wang, H.Y. Ti-doped Gd2O3 sensing membrane for electrolyte–insulator–semiconductor pH sensor. Thin Solid Films 2012, 520, 3760–3763. [Google Scholar] [CrossRef]
- Wei, M.; Wang, K.; Yanagida, M.; Sugihara, H.; Morris, M.A.; Holmes, J.D.; Zhou, H. Supercritical fluid processing of mesoporous crystalline TiO2 thin films for highly efficient dye-sensitized solar cells. J. Mater. Chem. 2007, 17, 3888–3893. [Google Scholar] [CrossRef]
- Pang, A.; Sun, X.; Ruan, H.; Li, Y.; Dai, S.; Wei, M. Highly efficient dye-sensitized solar cells composed of TiO2@SnO2 core–shell microspheres. Nano Energy 2014, 5, 82–90. [Google Scholar] [CrossRef]
- Rahman, M.u.; Ullah Wazir, H.; Khan, M.; Nosheen, S.; Rahman, S.; Ullah, A. Precursor-Induced Template free hydrothermal synthesis of Faujasite and its Application in Catalytic Pyrolysis. Mater. Res. Express 2017, 4, 055009. [Google Scholar] [CrossRef]
- Xiao, Y.; Han, G.; Wu, J.; Lin, J.-Y. Efficient bifacial perovskite solar cell based on a highly transparent poly(3,4-ethylenedioxythiophene) as the p-type hole-transporting material. J. Power Sources 2016, 306, 171–177. [Google Scholar] [CrossRef]
- Acharya, S.A.; Maheshwari, N.; Tatikondewar, L.; Kshirsagar, A.; Kulkarni, S.K. Ethylenediamine-Mediated Wurtzite Phase Formation in ZnS. Cryst. Growth Des. 2013, 13, 1369–1376. [Google Scholar] [CrossRef]
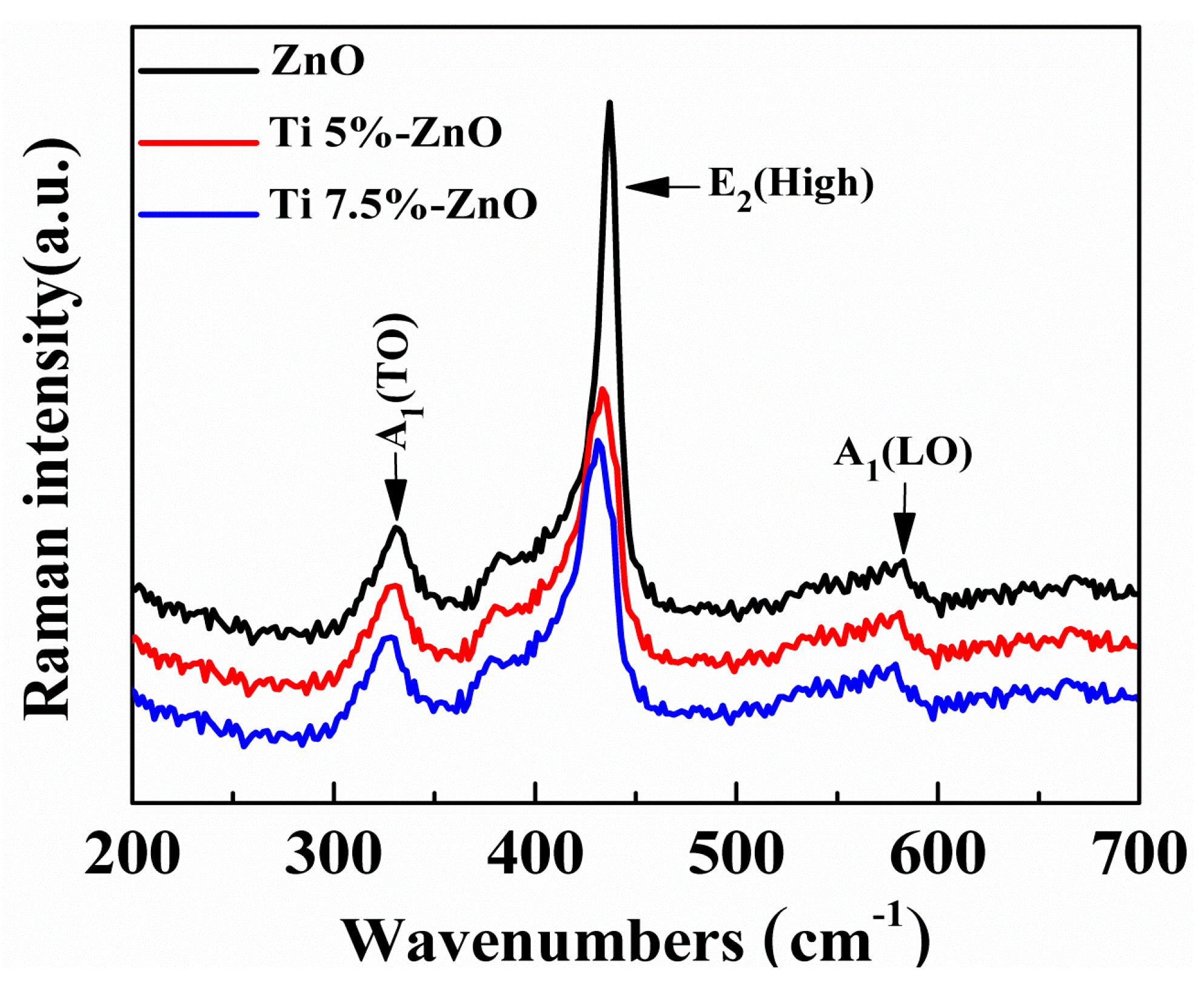
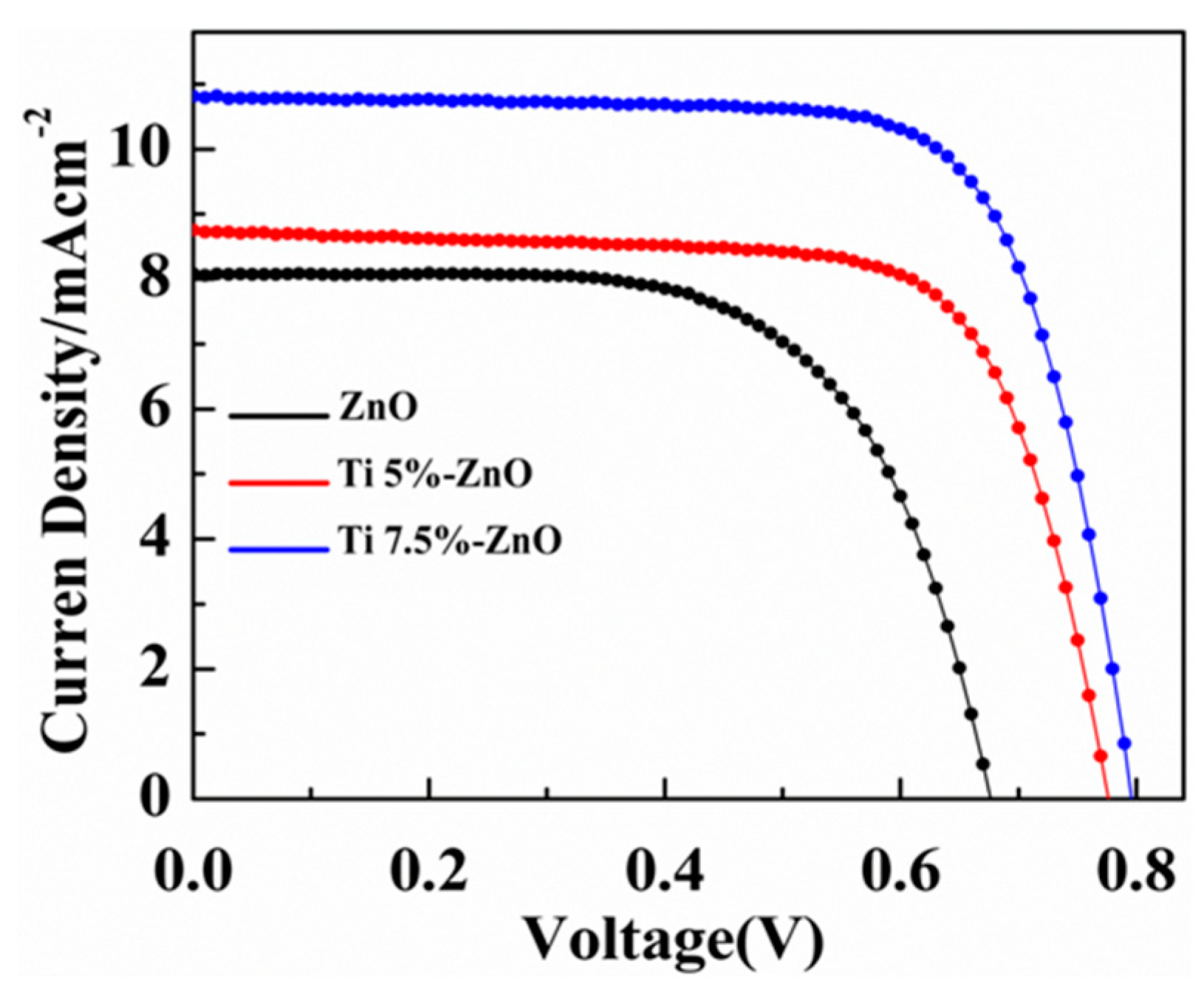
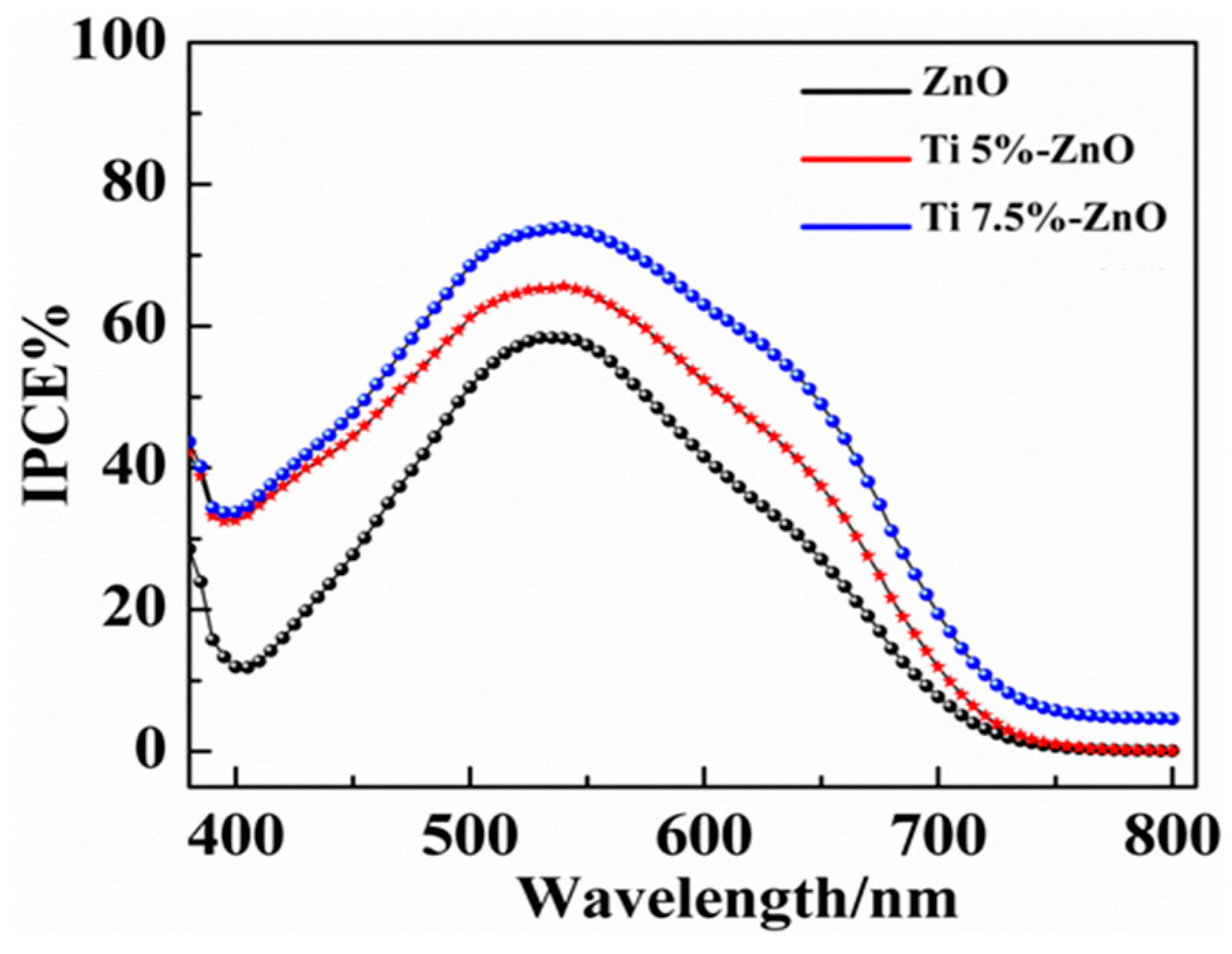
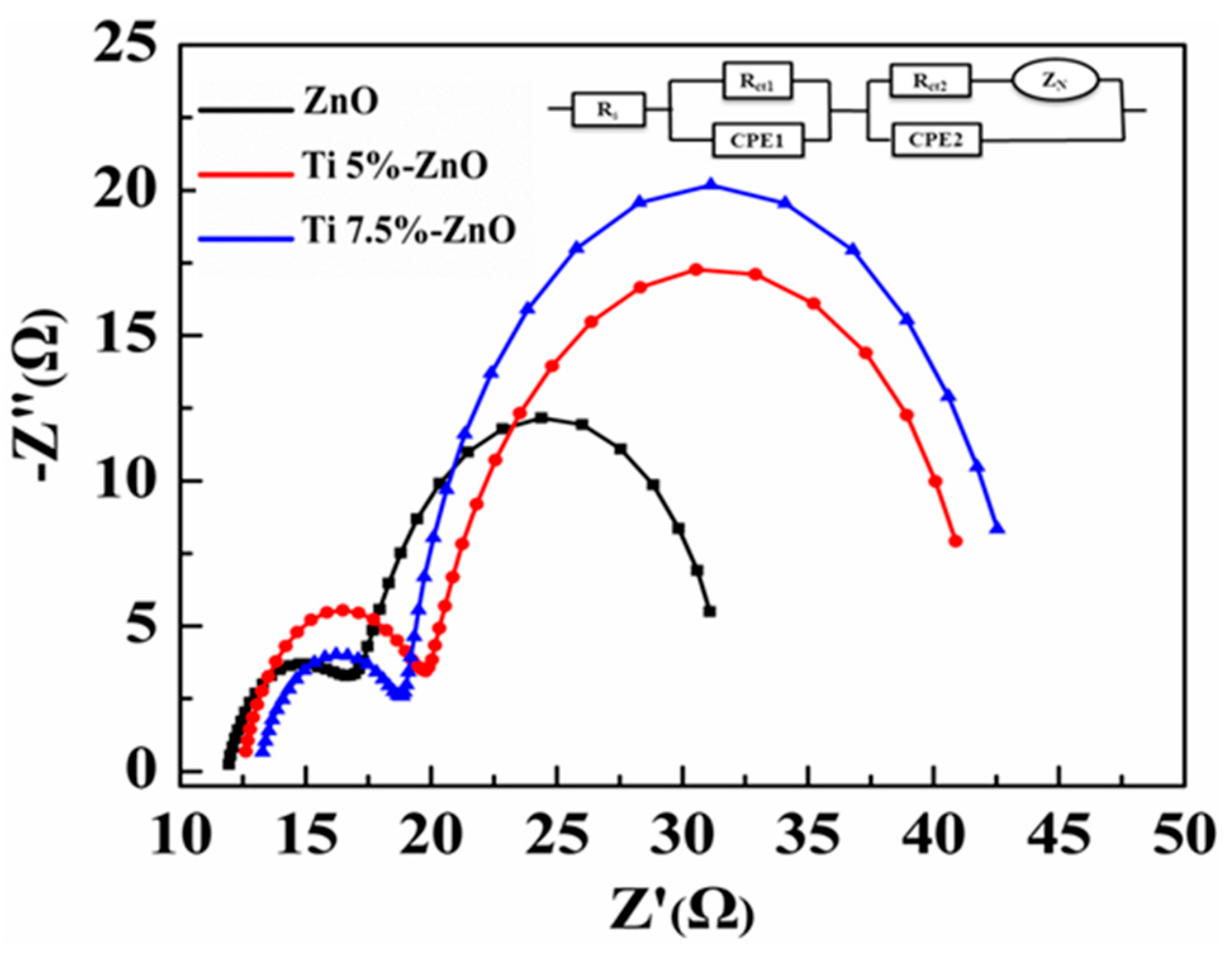
| S/No | Sample Name | Titanium (%) | |
|---|---|---|---|
| Calculated | Observed | ||
| 1 | Ti(1%)-ZnO | 1.0 | 0.96 |
| 2 | Ti(2%)-ZnO | 2.0 | 1.84 |
| 3 | Ti(5%)-ZnO | 5.0 | 4.71 |
| 4 | Ti(7.5%)-ZnO | 7.5 | 7.23 |
| Cell | Photoanode | Thickness (μm) | Jsc (mA cm−2) | Voc (V) | FF | η (%) | Dye Loading (10−7 mol/cm2) |
|---|---|---|---|---|---|---|---|
| 1 | ZnO | 10.9 | 8.01 | 0.68 | 0.64 | 3.82 | 1.10 |
| 2 | Ti(5%)-ZnO | 11.1 | 8.90 | 0.77 | 0.65 | 4.45 | 1.34 |
| 3 | Ti(7.5%)-ZnO | 11.2 | 10.92 | 0.79 | 0.64 | 5.56 | 1.85 |
| Cell | Working Electrode | Rs/Ω | Rct1 | CPE1/F cm−2 | Rct2/Ω | CPE2/F cm−2 | ZN/Ω cm−2 |
|---|---|---|---|---|---|---|---|
| 1 | ZnO | 11.31 | 1.31 | 7.80 × 10−4 | 31.2 | 4.35 × 10−4 | 1.11 × 10−1 |
| 2 | Ti(5%)-ZnO | 12.54 | 3.21 | 5.60 × 10−4 | 43.5 | 2.09 × 10−3 | 2.11 × 10−1 |
| 3 | Ti(7.5%)-ZnO | 13.47 | 3.31 | 3.70 × 10−4 | 46.4 | 5.25 × 10−4 | 2.12 × 10−1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.U.; Wei, M.; Xie, F.; Khan, M. Efficient Dye-Sensitized Solar Cells Composed of Nanostructural ZnO Doped with Ti. Catalysts 2019, 9, 273. https://doi.org/10.3390/catal9030273
Rahman MU, Wei M, Xie F, Khan M. Efficient Dye-Sensitized Solar Cells Composed of Nanostructural ZnO Doped with Ti. Catalysts. 2019; 9(3):273. https://doi.org/10.3390/catal9030273
Chicago/Turabian StyleRahman, Mati Ur, Mingdeng Wei, Fengyan Xie, and Matiullah Khan. 2019. "Efficient Dye-Sensitized Solar Cells Composed of Nanostructural ZnO Doped with Ti" Catalysts 9, no. 3: 273. https://doi.org/10.3390/catal9030273
APA StyleRahman, M. U., Wei, M., Xie, F., & Khan, M. (2019). Efficient Dye-Sensitized Solar Cells Composed of Nanostructural ZnO Doped with Ti. Catalysts, 9(3), 273. https://doi.org/10.3390/catal9030273





