Effect of Mg/Al2O3 and Calcination Temperature on the Catalytic Decomposition of HFC-134a
Abstract
1. Introduction
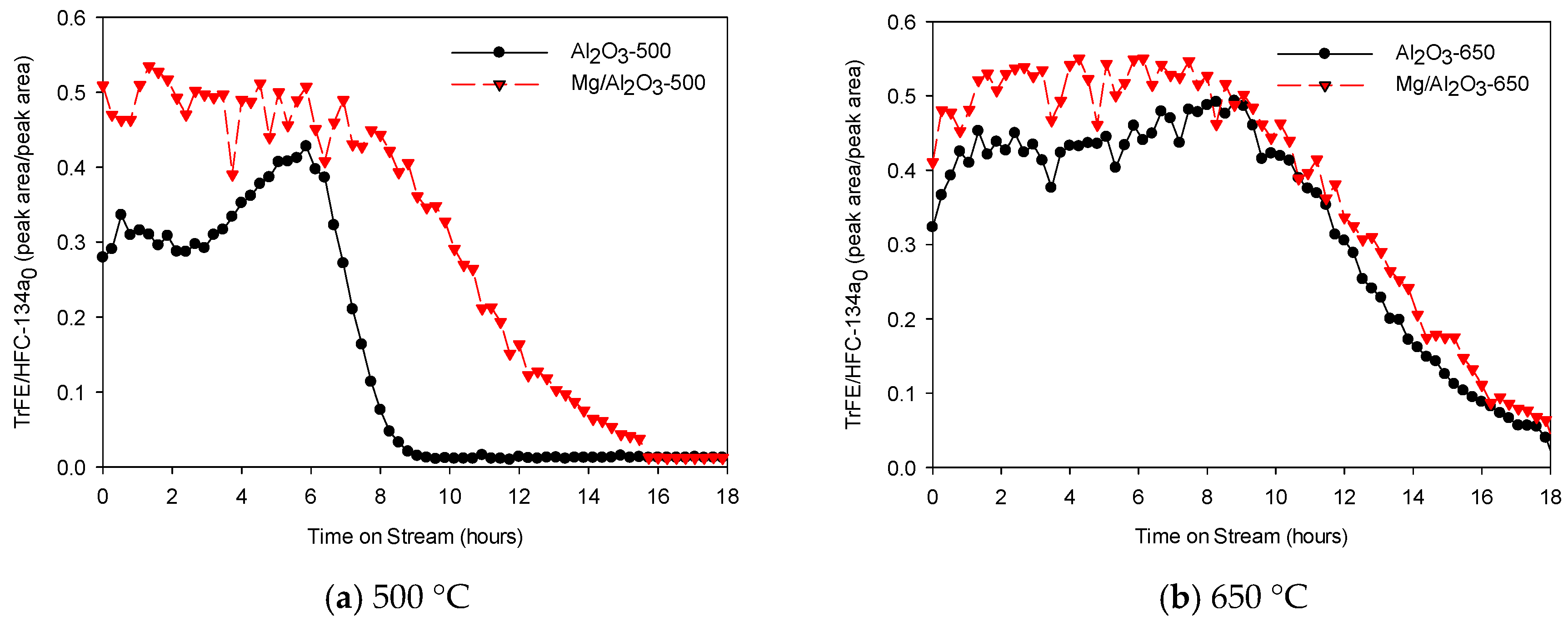
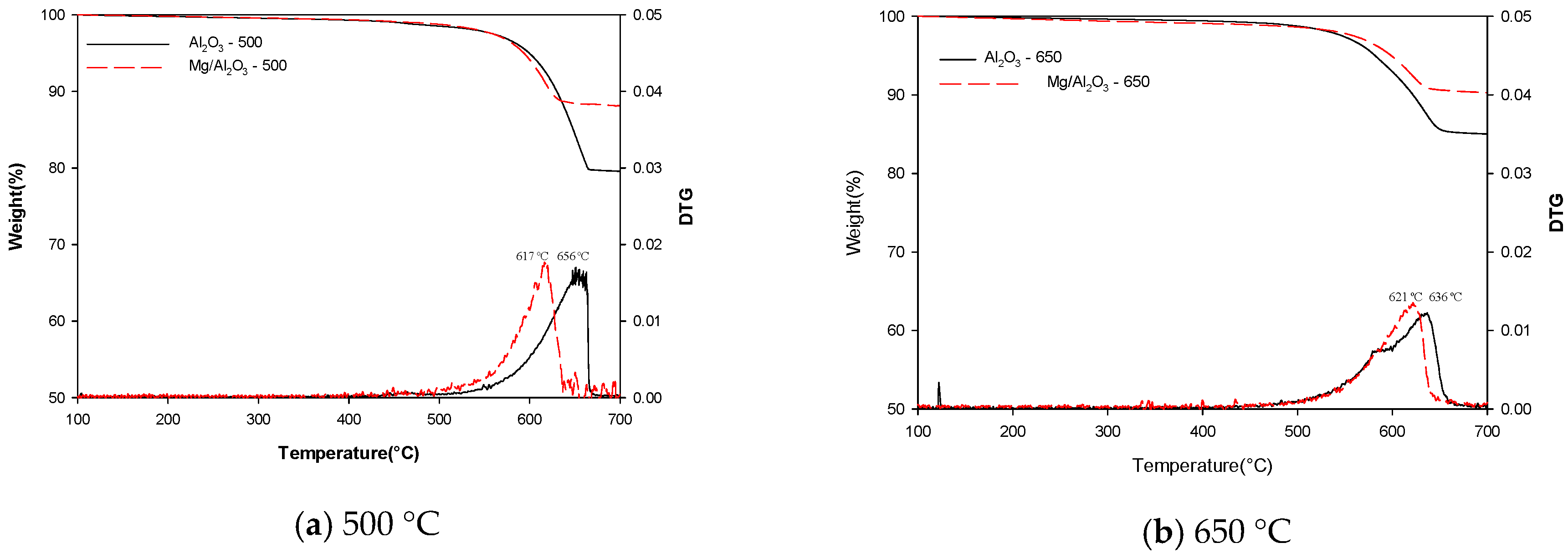
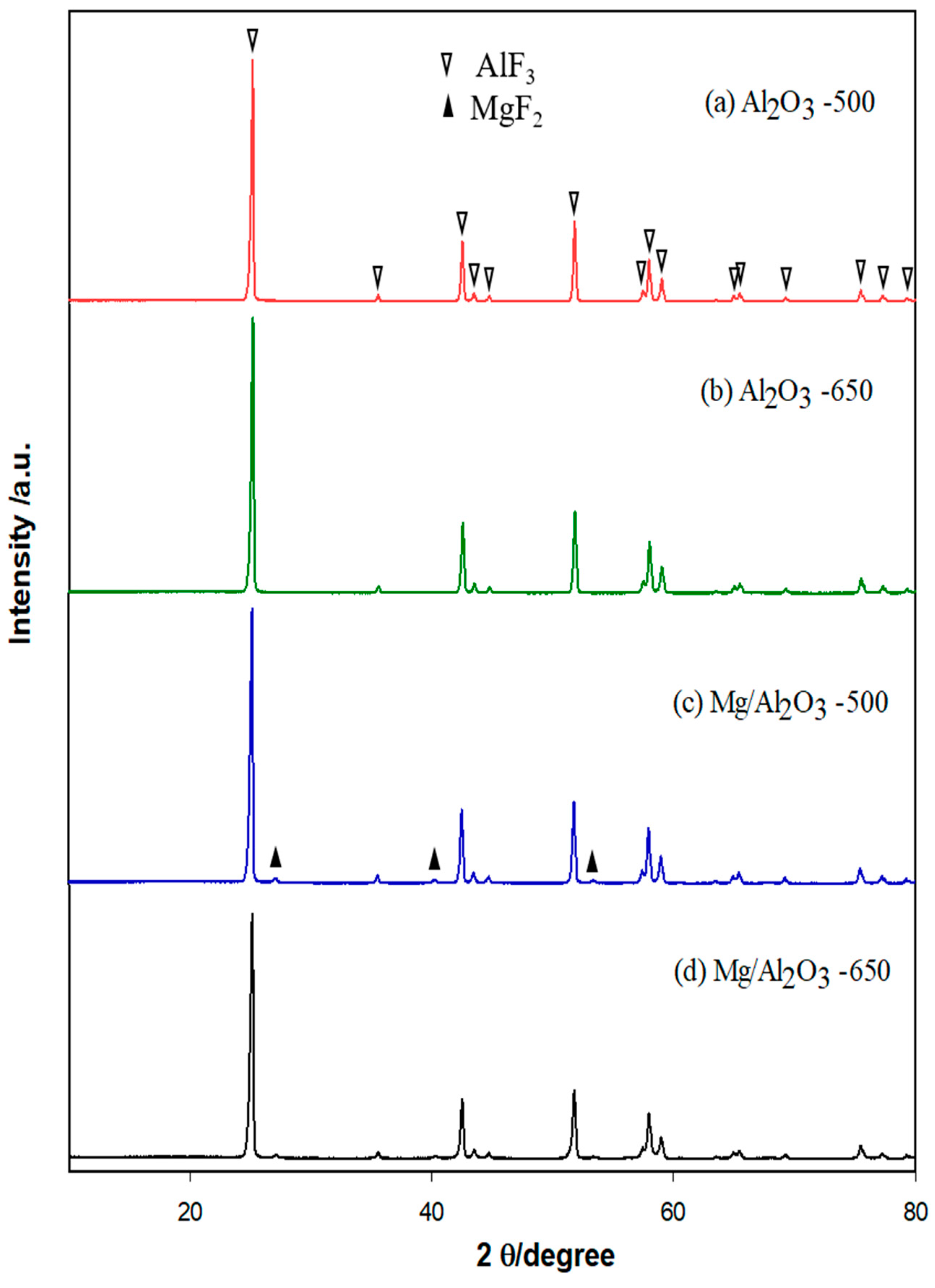
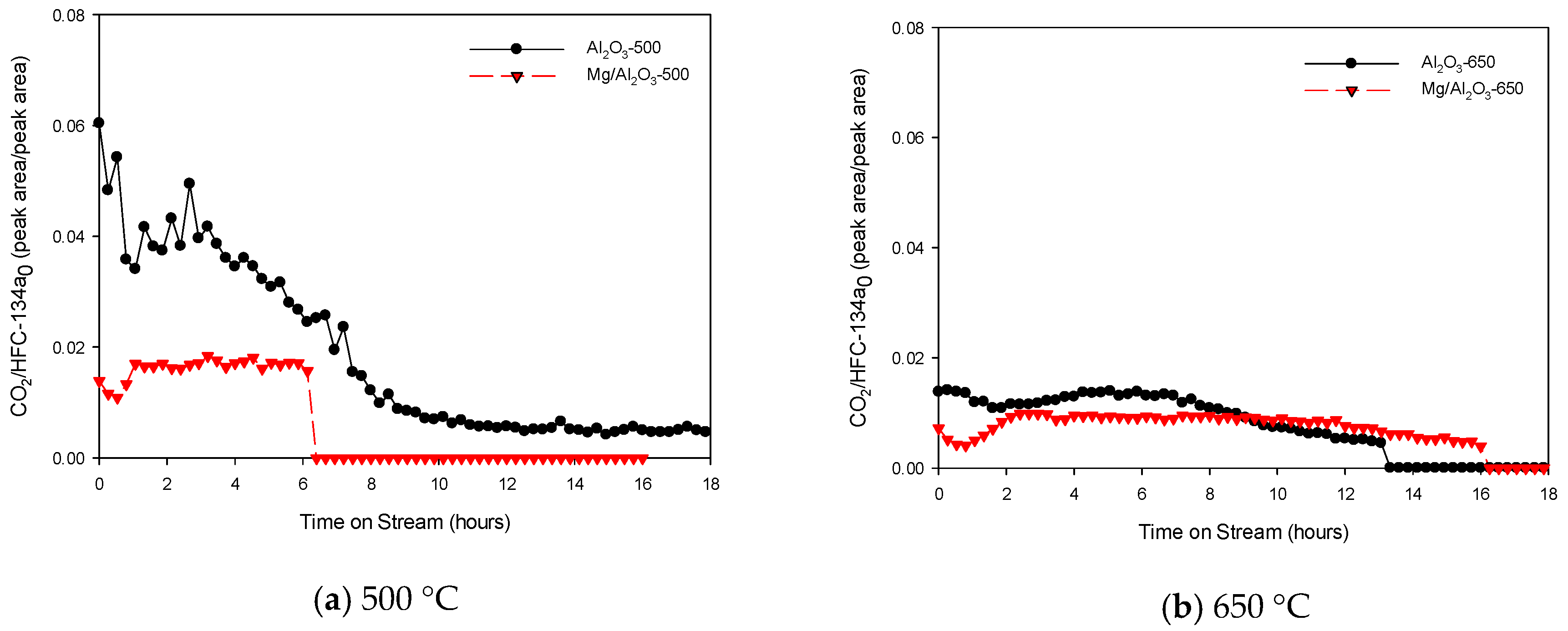
2. Results
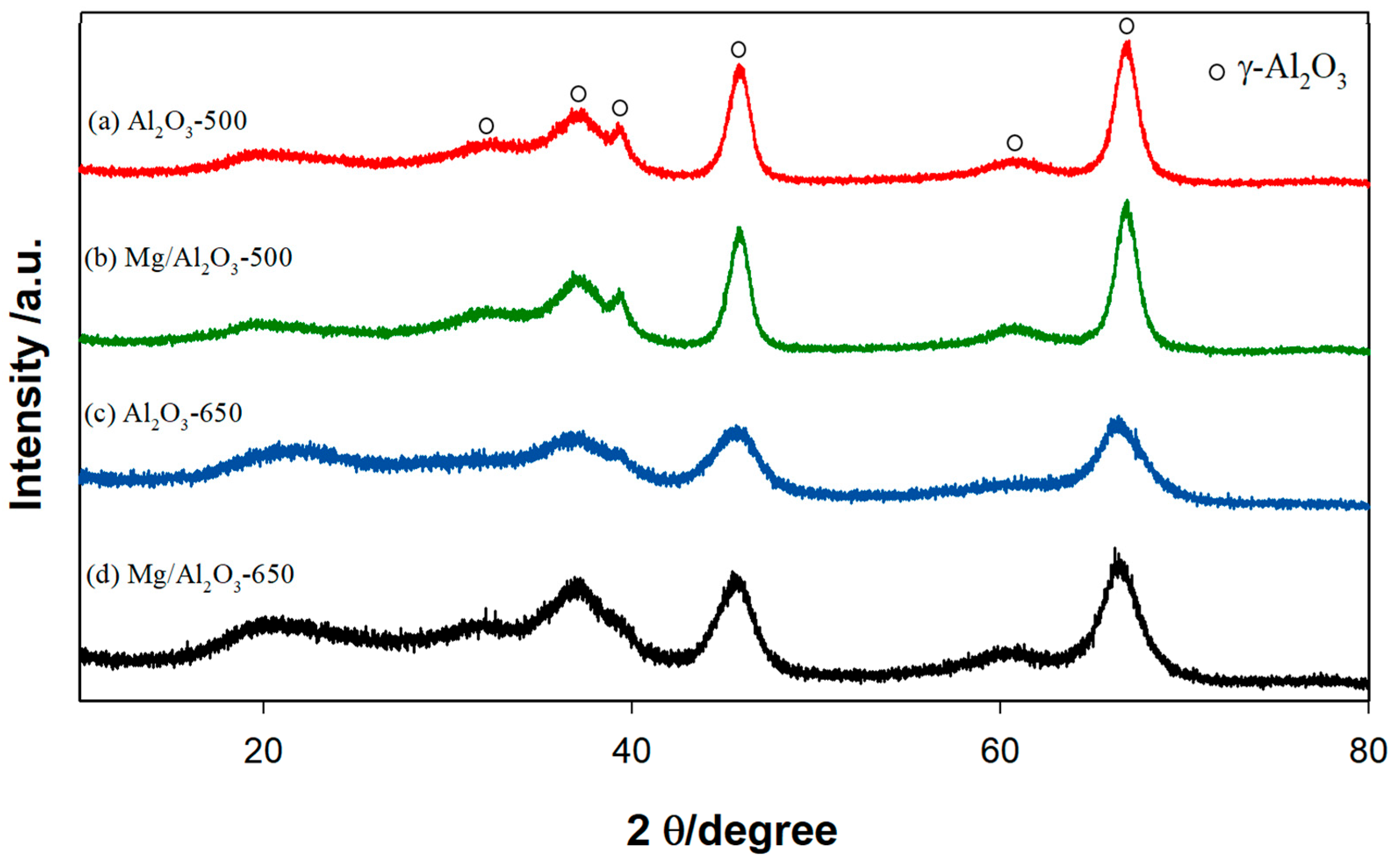
Physicochemical Properties of Catalysts
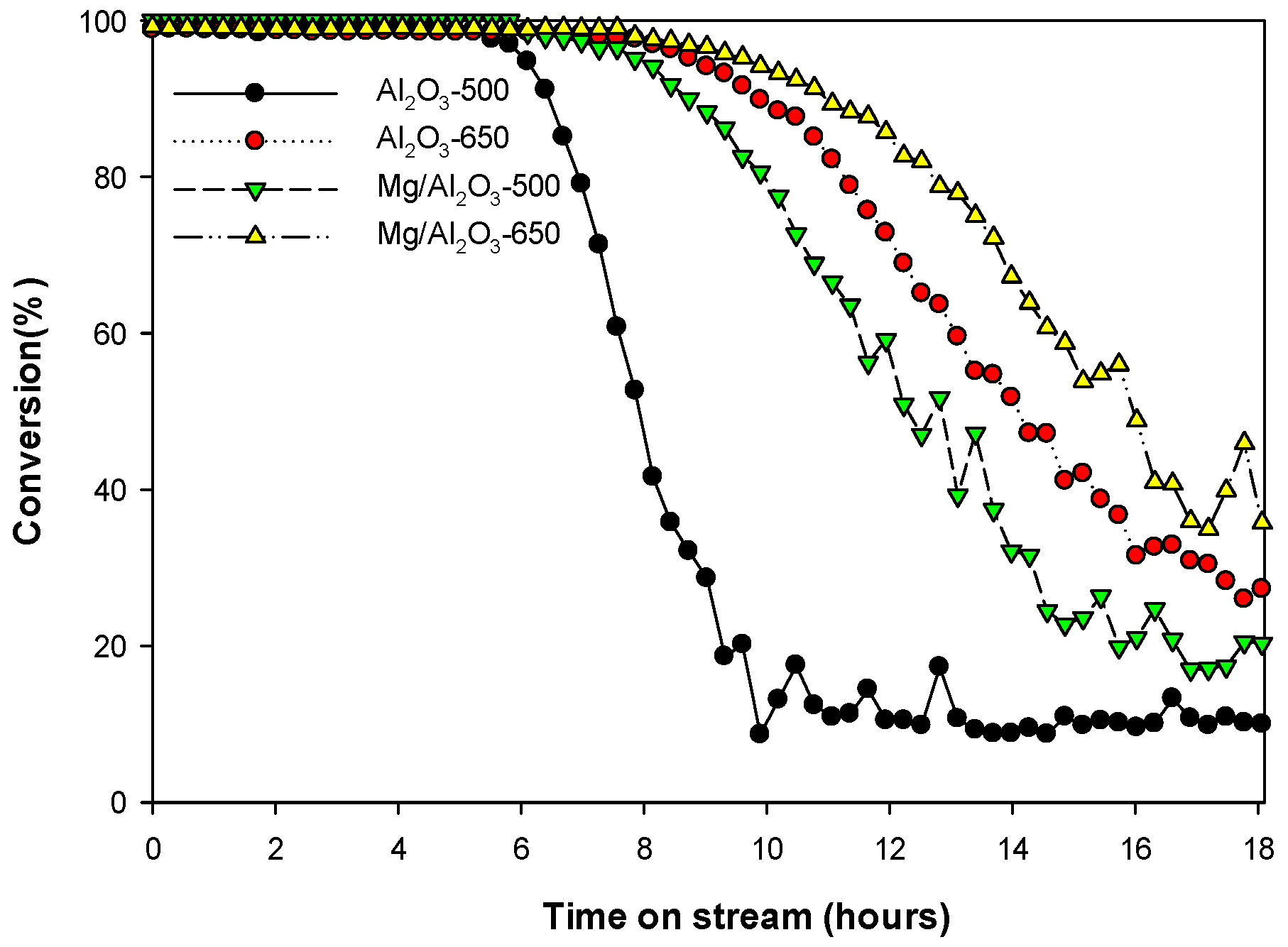
3. Discussion
Catalytic Decomposition of HFC-134a

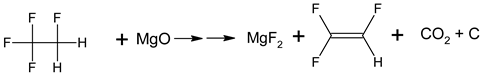
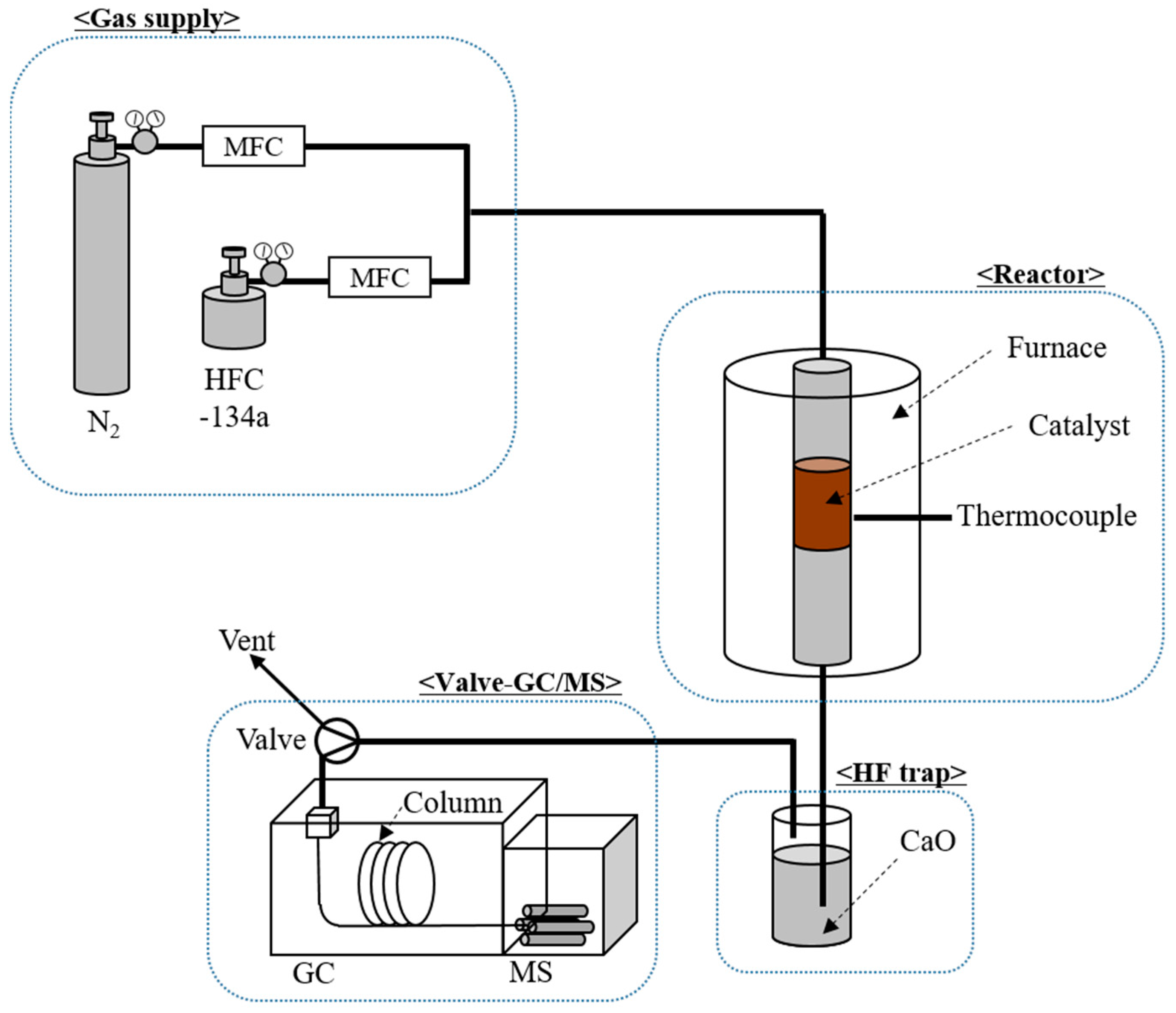
4. Materials and Methods
4.1. HFC-134a and Catalysts
4.2. HFC-134a Decomposition Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- GISTEMP Team. GISS Surface Temperature Analysis (GISTEMP), NASA Goddard Institute for Space Studies. Available online: https://data.giss.nasa.gov/gistemp/ (accessed on 2 November 2018).
- Hansen, J.; Ruedy, R.; Sato, M.; Lo, K. Global surface temperature change. Rev. Geophys. 2010, 48, 1–29. [Google Scholar] [CrossRef]
- Srinivasan, J. Climate Change Greenhouse gases and Aerosols. Resonance 2008, 13, 1146–1155. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L. (Eds.) IPCC Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007; p. 996. [Google Scholar]
- Richter, R.D.; Ming, T.; Davies, P.; Liu, W.; Caillol, S. Removal of non-CO2 greenhouse gases by large-scale atmospheric solar photocatalysis. Prog. Energy Combust. Sci. 2017, 60, 68–96. [Google Scholar] [CrossRef]
- Montreal Protocol- Achievements to Date and Challenges Ahead. Available online: http://ozone.unep.org/en/focus/montreal-protocol-achievements-date-and-challenges-ahead (accessed on 2 November 2018).
- Franklin, J. The atmospheric degradation and impact of 1,1,1,2-tetrafluoroethane (Hydrofluorocarbon 134a). Chemisphere 1993, 27, 1565–1601. [Google Scholar] [CrossRef]
- UNFCCC 1998. The Kyoto Protocol to the United Nations Framework Convention on Climate Change. Available online: https://unfccc.int/resource/docs/convkp/kpeng.pdf (accessed on 2 November 2018).
- Kigali Amendment to the Montreal Protocol. Available online: https://eia-international.org/wp-content/uploads/EIA-Kigali-Ammendment-to-the-Montreal-Protocol-FINAL.pdf (accessed on 2 November 2018).
- Shiojiri, K.; Yanagisawa, Y.; Yamasaki, A.; Kiyono, F. Separation of F-gases (HFC-134a and SF6) from gaseous mixtures with nitrogen by surface diffusion through a porous Vycor glass membrane. J. Membr. Sci. 2006, 282, 442–449. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Mie, T.; Han, J.; He, X.; Qin, L. Investigation of HFC-13a Decomposition by combustion and its kinetic characteristics in a laboratory scale reactor. Environ. Prot. Eng. 2015, 41, 43–150. [Google Scholar]
- Ohm, T.I.; Chae, J.S.; Moon, S.H. Numerical and Experimental Study on the Destruction of Waste Refrigerant (HFCs) in Incinerator. J. Korea Soc. Waste Manag. 2016, 33, 454–460. [Google Scholar] [CrossRef]
- Watanabe, T.; Tsuru, T. Water plasma generation under atmospheric pressure for HFC destruction. Thin Solid Films 2008, 516, 4391–4396. [Google Scholar] [CrossRef]
- Iizuka, A.; Ishizaki, H.; Mizukoshi, A.; Noguchi, M.; Yamasaki, A.; Yanagisawa, Y. Simultaneous Decomposition and Fixation of F-Gases Using Waste Concrete. Ind. Eng. Chem. Res. 2011, 50, 11808–11814. [Google Scholar] [CrossRef]
- Jia, W.; Liu, M.; Lang, X.; Hu, C.; Li, J.; Zhu, Z. Catalytic dehydrofluorination of 1,1,1,2-tetrafluoroethane to synthesize trifluoroethylene over a modified NiO/Al2O3 catalyst. Catal. Sci. Technol. 2015, 5, 3103–3107. [Google Scholar] [CrossRef]
- Takita, Y.; Tanabe, T.; Ito, M.; Ogura, M.; Muraya, T.; Yasuda, S.; Nishiguchi, H.; Ishihara, T. Decomposition of CH2FCF3 (134a) over Metal Phosphate Catalysts. Ind. Eng. Chem. Res. 2002, 41, 2585–2590. [Google Scholar] [CrossRef]
- Han, T.U.; Yoo, B.S.; Kim, Y.M.; Hwang, B.A.; Sudibya, G.L.; Park, Y.K.; Kim, S. Catalytic conversion of 1,1,1,2-tetrafluoroethane (HFC-134a). Korean J. Chem. Eng. 2018, 35, 1611–1619. [Google Scholar] [CrossRef]
- Han, W.; Chen, Y.; Jin, B.; Liu, H.; Yu, H. Catalytic hydrolysis of trifluoroethane over alumina. Greenh. Gases 2014, 4, 121–130. [Google Scholar] [CrossRef]
- Song, J.Y.; Chung, S.H.; Kim, M.S.; Seo, M.G.; Lee, Y.H.; Lee, K.Y.; Kim, J.S. The catalytic decomposition of CF4 over Ce/Al2O3 modified by cerium sulphate precursor. J. Mol. Catal. 2013, 370, 50–55. [Google Scholar] [CrossRef]
- Li, G.L.; Nashiguchi, H.; Ishihara, T.; Moro-oka, Y.; Takita, Y. Catalytic dehydrofluorination of CF3CH3(HFC143a) into CF2CH2(HFC1132a). Appl. Catal. 1998, 16, 309–317. [Google Scholar] [CrossRef]
- Iung, E.; Jeon, S.; Kim, C.U.; Jeong, S.Y.; Park, Y.K.; Jeon, J.K. Hydro-upgrding of n-octadecane over Pt-Mg/HY catalysts. Catal. Today 2016, 265, 124–130. [Google Scholar]
- Jeon, S.H.; You, Y.; Jin, H.; Kim, C.U.; Park, Y.K.; Lee, C.H.; Jeon, J.K. Hydroupgrading of bio-oil over PtMg/KIT-6 catalysts. J. Nanosci. Nanotechnol. 2019, 19, 1126–1129. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Z.; Xu, C.; Duan, A.; Zhang, P. Effects of Calcination Temperature on the Acidity and Catalytic Performances of HZSM-5 Zeolite Catalysts for the Catalytic Cracking of n-Butane. J. Nat. Gas Chem. 2005, 14, 213–220. [Google Scholar]
- Tribalis, A.; Panagiotou, G.D.; Bourikas, K.; Sygellou, L.; Kennou, S.; Ladas, S.; Lycourghiotis, A.; Kordulis, C. Ni Catalysts supported on Modified Alumina for Diesel Steam Reforming. Catalysts 2016, 6, 11. [Google Scholar] [CrossRef]
- Novakovic, T.B.; Rozic, L.S.; Petrovic, S.P.; Vukovic, Z.M.; Mitric, M.N. Study of the effect of Mg(II) addition and annealing conditions on the structure of mesoporous aluminum oxide using Plackett-Burman design. J. Serb. Chem. Soc. 2015, 80, 1–14. [Google Scholar] [CrossRef]
- Nakrela, A.; Benramdane, N.; Bouzidi, A.; Kebbab, Z.; Medles, M.; Mathieu, C. Site location of Al-dopant in ZnO lattice by exploiting the structural and optical characterization of ZnO:Al thin films. Results Phys. 2016, 6, 133–138. [Google Scholar] [CrossRef]
- Wagih, A. Effect of Mg addition on mechanical and thermoelectrical properties of Al-Al2O3 nanocomposite. Trans. Nonferrous Met. Soc. China 2016, 26, 2810–2817. [Google Scholar] [CrossRef]
- Souza, J.J.N.; Meireles, B.R.L.A.; Cordeiro, A.M.T.M.; Santos, I.M.G.; Maria, A.S. Iron-doped Alfa-Alumina applied in the degradation of phenol solutions. Rev. Virtual Quim. 2017, 9, 2539–2550. [Google Scholar] [CrossRef]
- Matori, K.A.; Wah, L.C.; Hashim, M.; Ismail, I.; Mohd Zaid, M.H. Phase transformations of α-Alumina made from waste Aluminium via a precipitation technique. Int. J. Mol. Sci. 2012, 13, 16812–16821. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Wang, Y. NF3 decomposition over Al2O3 reagents without water. J. Nat. Gas Chem. 2011, 20, 418–422. [Google Scholar] [CrossRef]
- Gandhi, M.S.; Mok, Y.S. Effect of packing materials on the decomposition of tetrafluoroethane in a packed bed dielectric barrier discharge plasma reactor. Int. J. Environ. Sci. Technol. 2015, 12, 499–506. [Google Scholar] [CrossRef]
- Jia, W.; Wu, Q.; Lang, X.; Hu, C.; Zhao, G.; Li, J.; Zhu, Z. Influence of Lewis Acidity on Catalytic activity of the porous Alumina for dehydrofluorination of 1,1,1,2-tetrafluoroethane to Trifluoroethylene. Catal. Lett. 2015, 145, 654–661. [Google Scholar] [CrossRef]
- Meng, B.C.; Sun, Z.Y.; Ma, J.P.; Cao, G.P.; Yuan, W.K. Selective Liquid-phase Hydrodechlorination of Chlorotrifluoroethylene over Palladium-Supported Catalysts: Activity and Deactivation. Catal. Lett. 2010, 138, 68–75. [Google Scholar] [CrossRef]
- Ohnishi, R.; Wang, W.L.; Ichikawa, M. Selective hydrodechlorination of CFC-113 on Bi- and Tl-modified palladium catalysts. Appl. Catal. A-Gen. 1994, 113, 29–41. [Google Scholar] [CrossRef]
- Scott, S.P.; Sweetman, M.; Thomson, J.; Fitzegerald, A.G.; Sturrocky, E.J. Catalytic Hydrogenolysis of 1,1,2-Trichlorotrifluoroethane on γ-Al2O3-Supported Palladium/Zinc Oxide Catalyst. J. Catal. 1997, 168, 501–510. [Google Scholar] [CrossRef]
- Mori, T.; Yasuoka, T.; Morikawa, Y. Hydrodechlorination of 1,1,2-trichloro-1,2,2-trifluoroethane (CFC-113) over supported ruthenium and other noble metal catalysts. Catal. Today 2004, 88, 111–120. [Google Scholar] [CrossRef]
- Niwa, M.; Katada, N.; Sawa, M.; Murakami, Y. Temperature-Programmed Desorption of Ammonia with Readsorption based on the derived theoretical equation. J. Phys. Chem. 1995, 99, 8812–8816. [Google Scholar] [CrossRef]
- Speakman, S.A. Introduction to X-Ray Powder Diffraction Data Analysis; Technical Report; Center for Materials Science and Engineering at MIT: Cambridge, MA, USA, 2009; pp. 19–20. [Google Scholar]
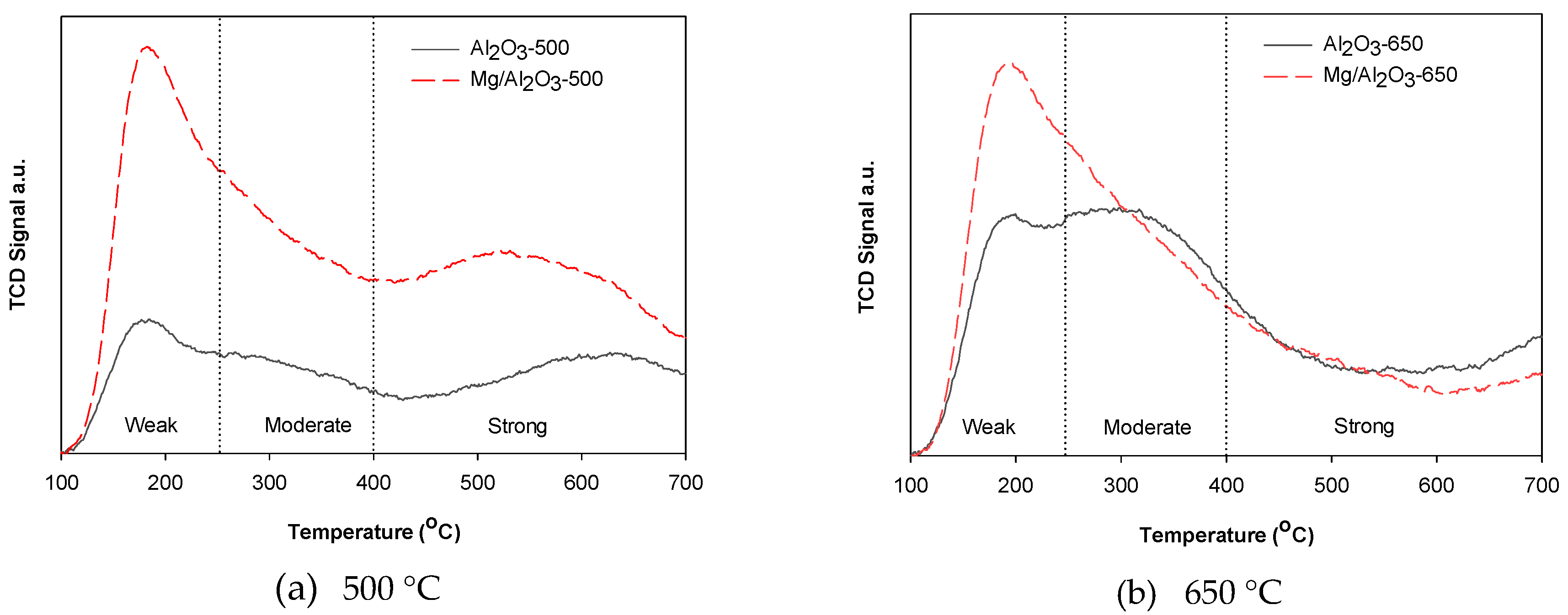
| Catalyst | Weak Acid Amount | Moderate Acid Amount | Strong Acid Amount | Total Acid Amount | Weak Acid Amount/Strong Acid Amount |
|---|---|---|---|---|---|
| Al2O3-500 | 0.26 | 0.25 | 0.47 | 0.98 | 0.55 |
| Mg/Al2O3-500 | 0.77 | 0.66 | 1.06 | 2.49 | 0.73 |
| Al2O3-650 | 0.23 | 0.33 | 0.29 | 0.86 | 0.79 |
| Mg/Al2O3-650 | 0.65 | 0.59 | 0.47 | 1.70 | 1.38 |
| GC | MS | ||
|---|---|---|---|
| Inlet | 260 °C, split ratio 50:1 | Ion source | 230 °C |
| Column | GS-GASPRO, 60 m length × 0.32 mm inner diameter | Quadrupole filter | 150 °C |
| Oven | 50 °C → 20 °C/min → 260 °C | Scan range | m/z 17~600 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrew Swamidoss, C.M.; Sheraz, M.; Anus, A.; Jeong, S.; Park, Y.-K.; Kim, Y.-M.; Kim, S. Effect of Mg/Al2O3 and Calcination Temperature on the Catalytic Decomposition of HFC-134a. Catalysts 2019, 9, 270. https://doi.org/10.3390/catal9030270
Andrew Swamidoss CM, Sheraz M, Anus A, Jeong S, Park Y-K, Kim Y-M, Kim S. Effect of Mg/Al2O3 and Calcination Temperature on the Catalytic Decomposition of HFC-134a. Catalysts. 2019; 9(3):270. https://doi.org/10.3390/catal9030270
Chicago/Turabian StyleAndrew Swamidoss, Caroline Mercy, Mahshab Sheraz, Ali Anus, Sangjae Jeong, Young-Kwon Park, Young-Min Kim, and Seungdo Kim. 2019. "Effect of Mg/Al2O3 and Calcination Temperature on the Catalytic Decomposition of HFC-134a" Catalysts 9, no. 3: 270. https://doi.org/10.3390/catal9030270
APA StyleAndrew Swamidoss, C. M., Sheraz, M., Anus, A., Jeong, S., Park, Y.-K., Kim, Y.-M., & Kim, S. (2019). Effect of Mg/Al2O3 and Calcination Temperature on the Catalytic Decomposition of HFC-134a. Catalysts, 9(3), 270. https://doi.org/10.3390/catal9030270








