Synergistic Effect of Photocatalytic Degradation of Hexabromocyclododecane in Water by UV/TiO2/persulfate
Abstract
1. Introduction
2. Results and Discussions
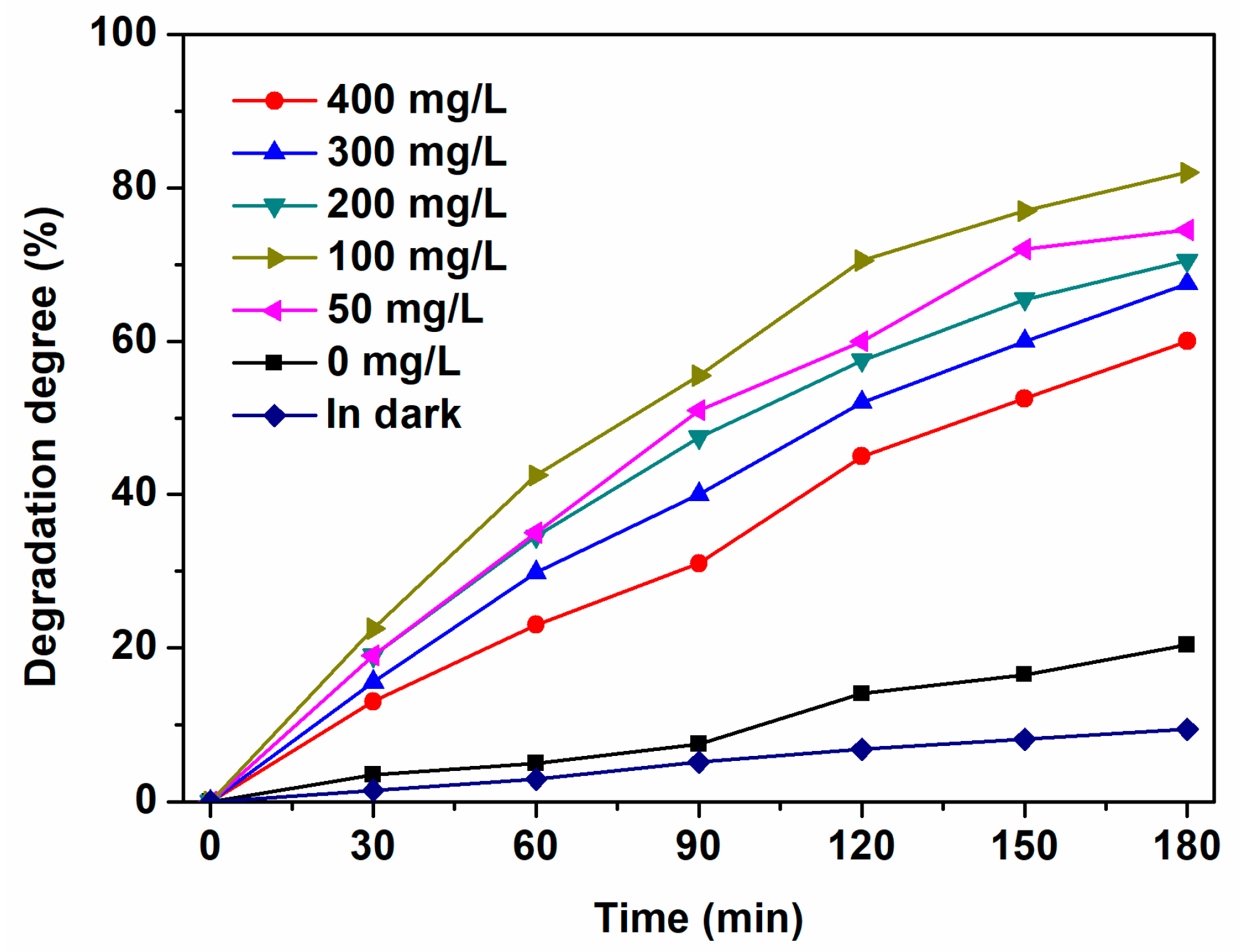
2.1. Determination of TiO2 Dosages
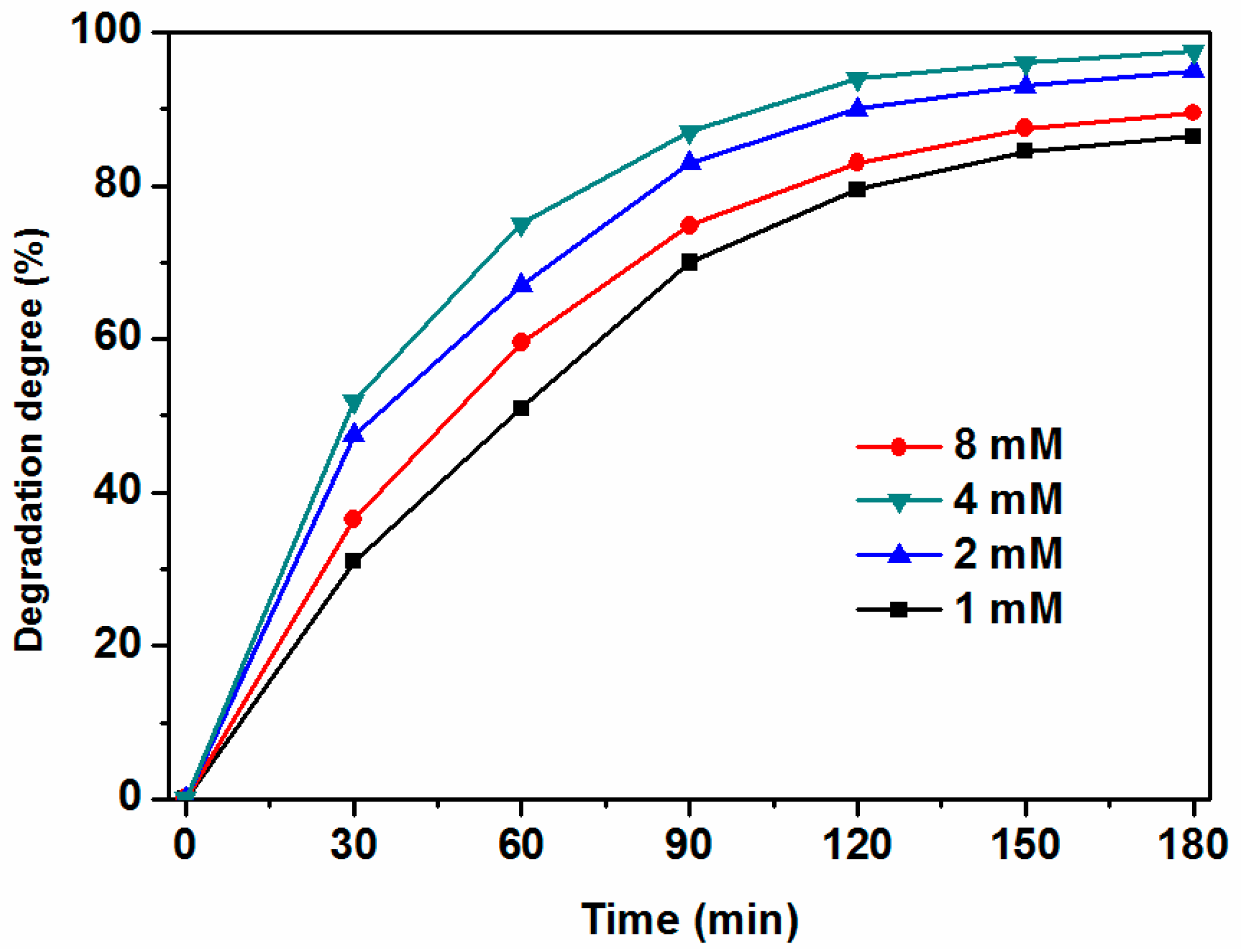
2.2. Effect of KPS Dosage
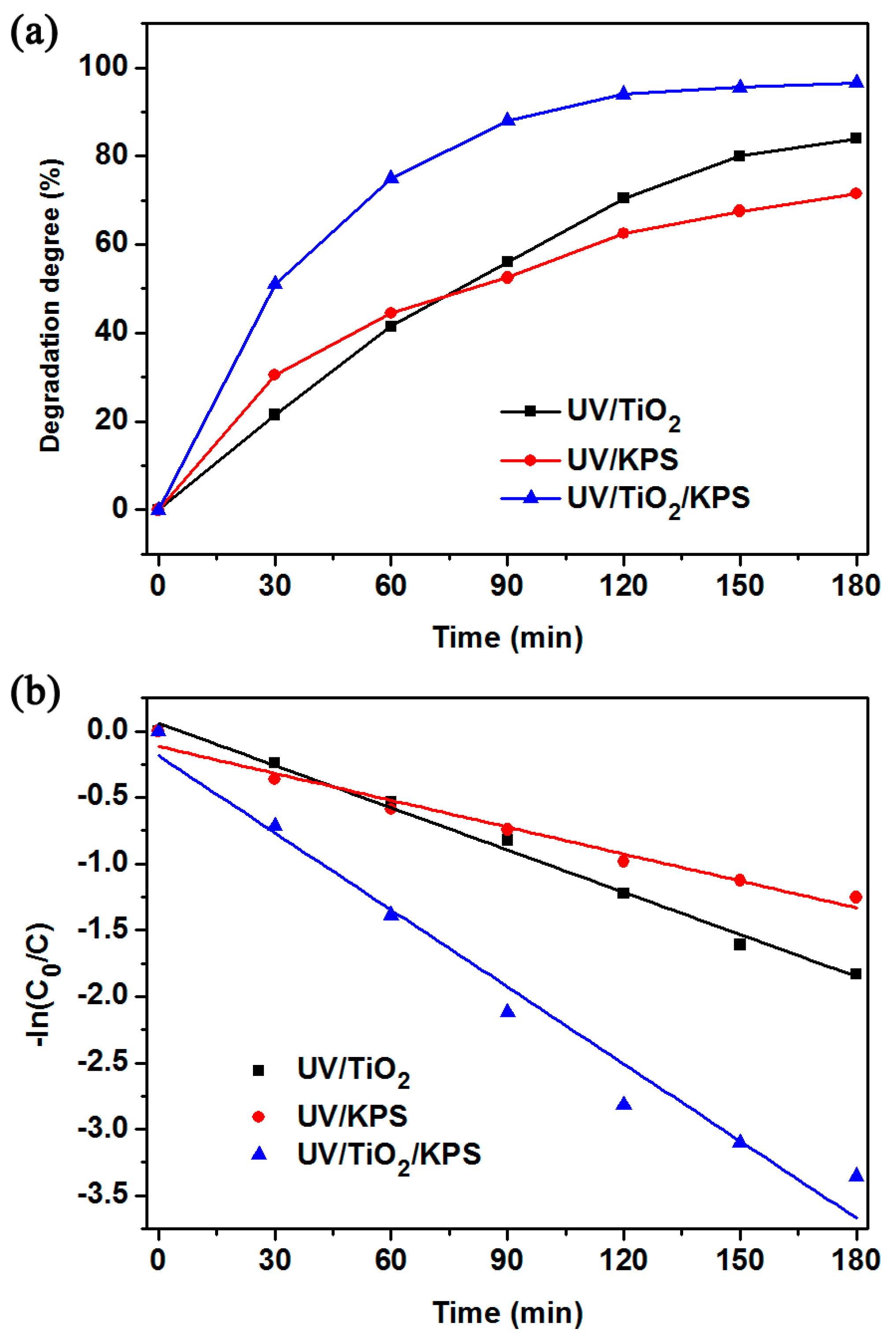
2.3. Kinetic Analysis of Different Reaction Systems
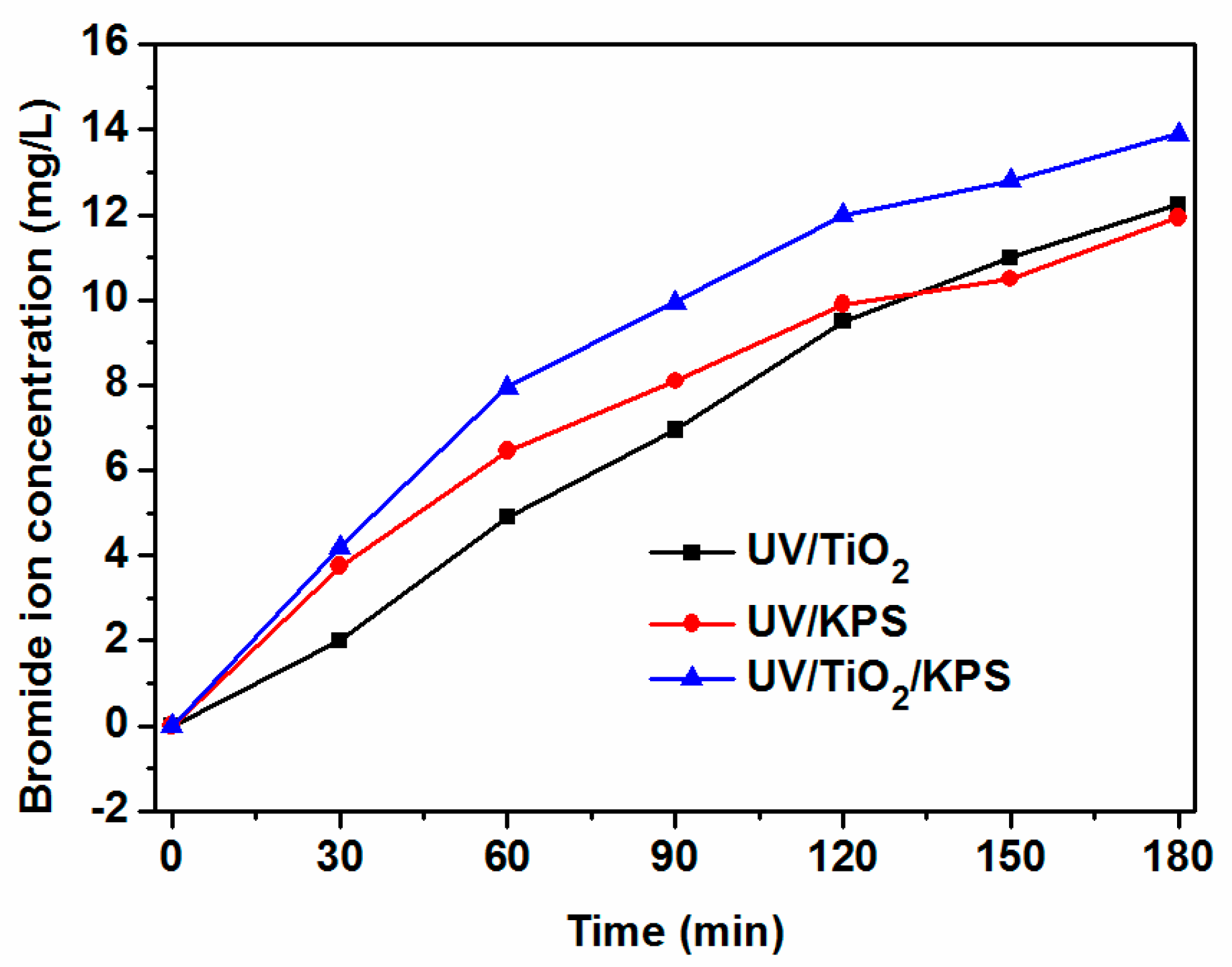
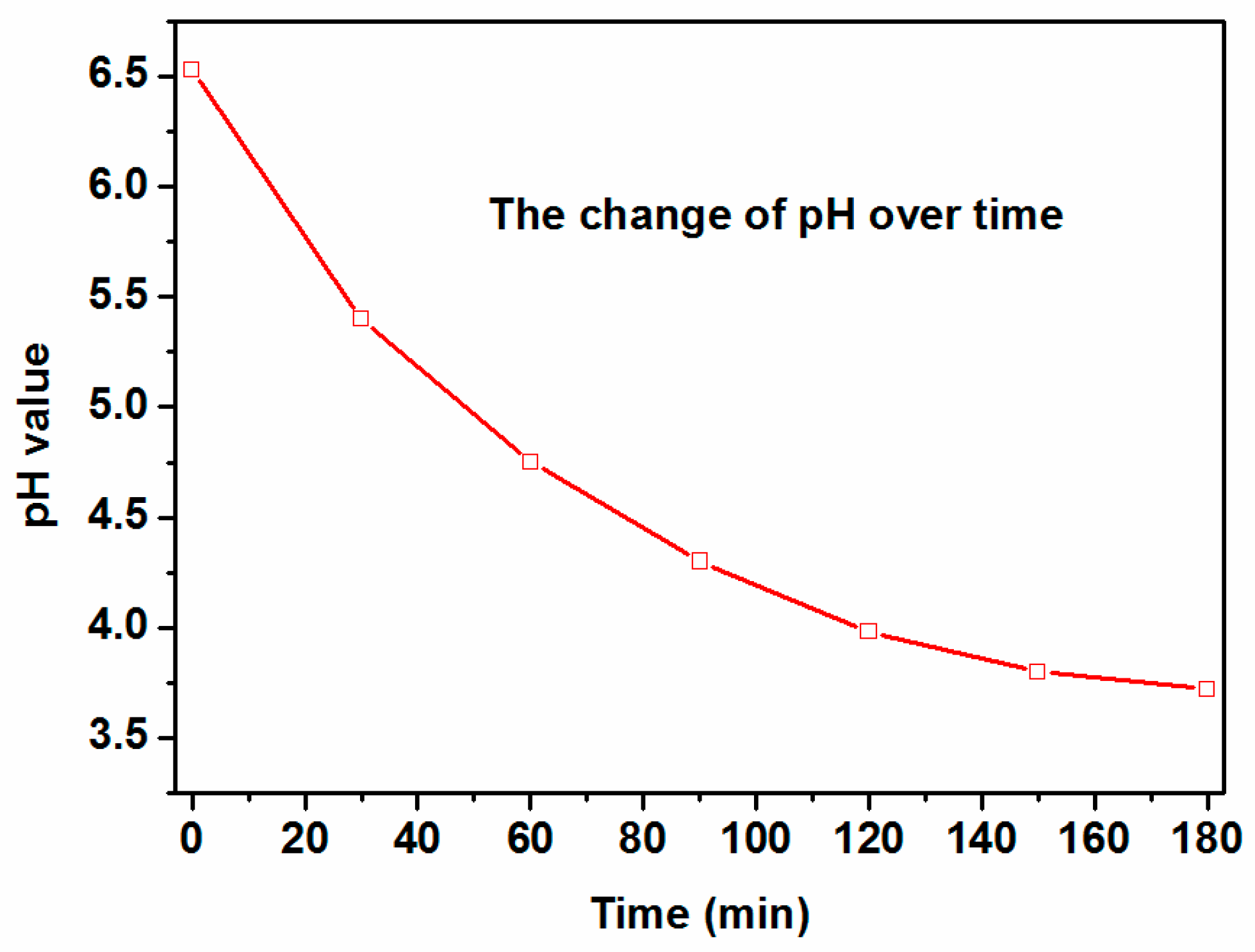
2.4. The Mineralization Degree of HBCD
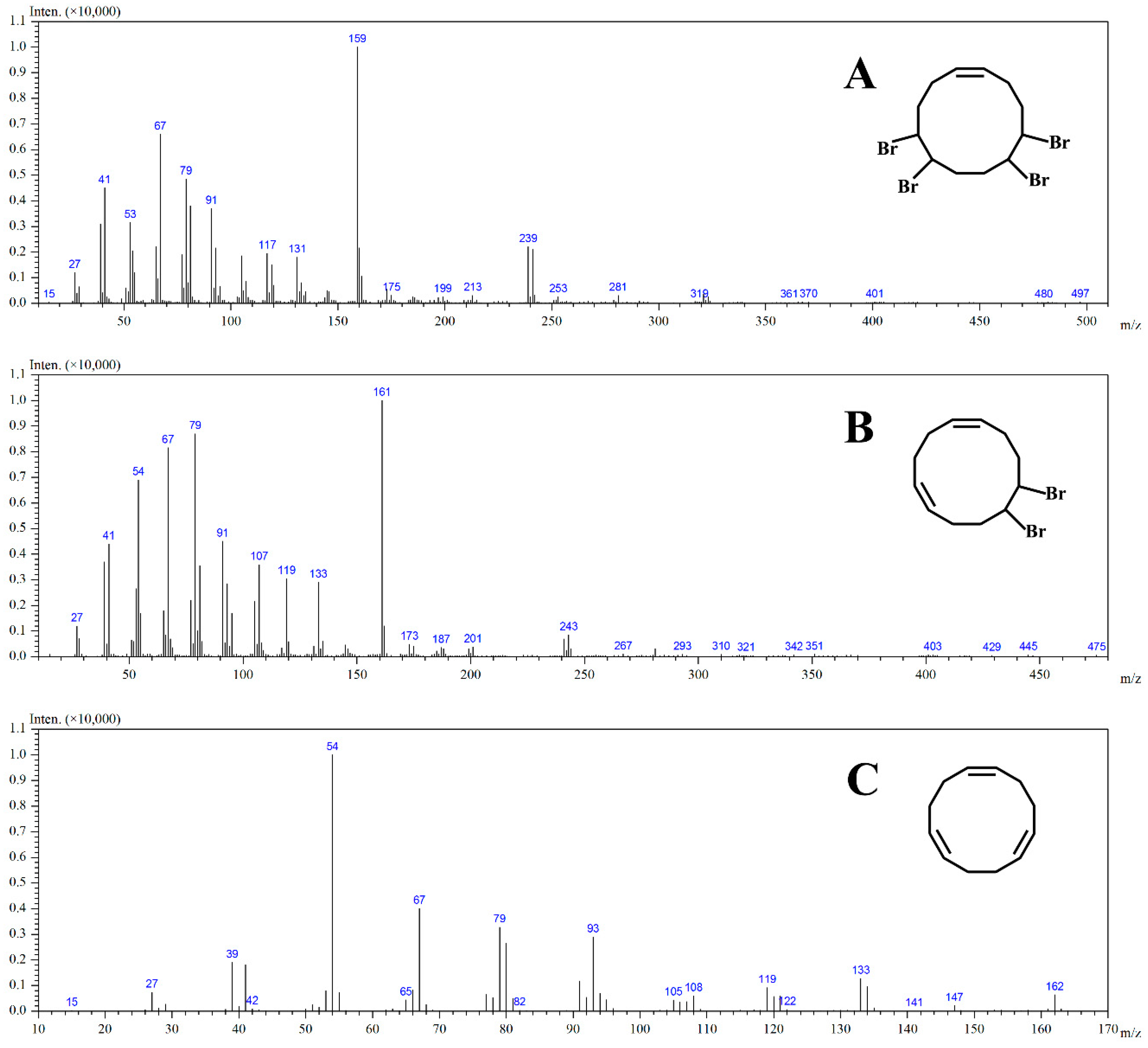
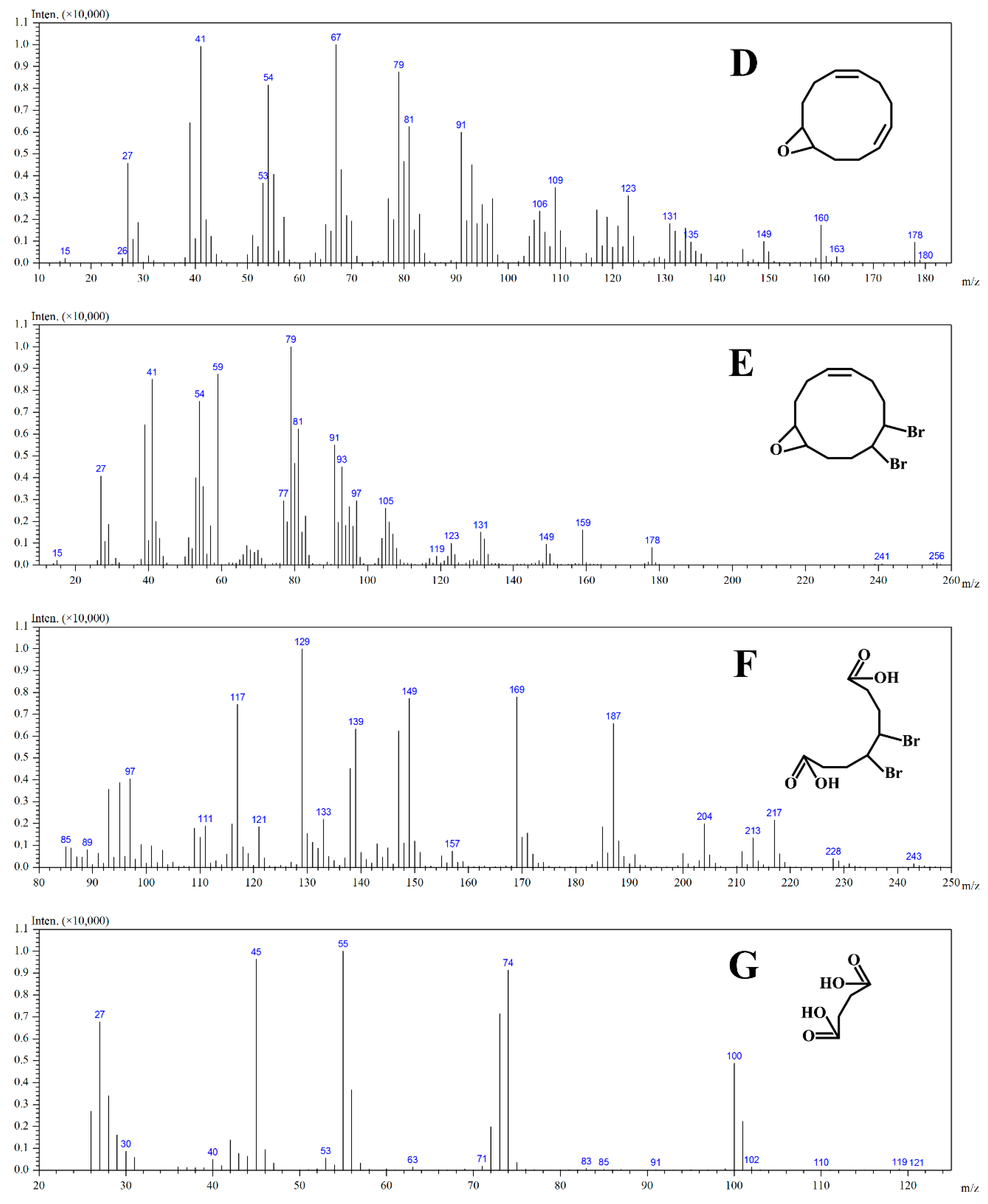
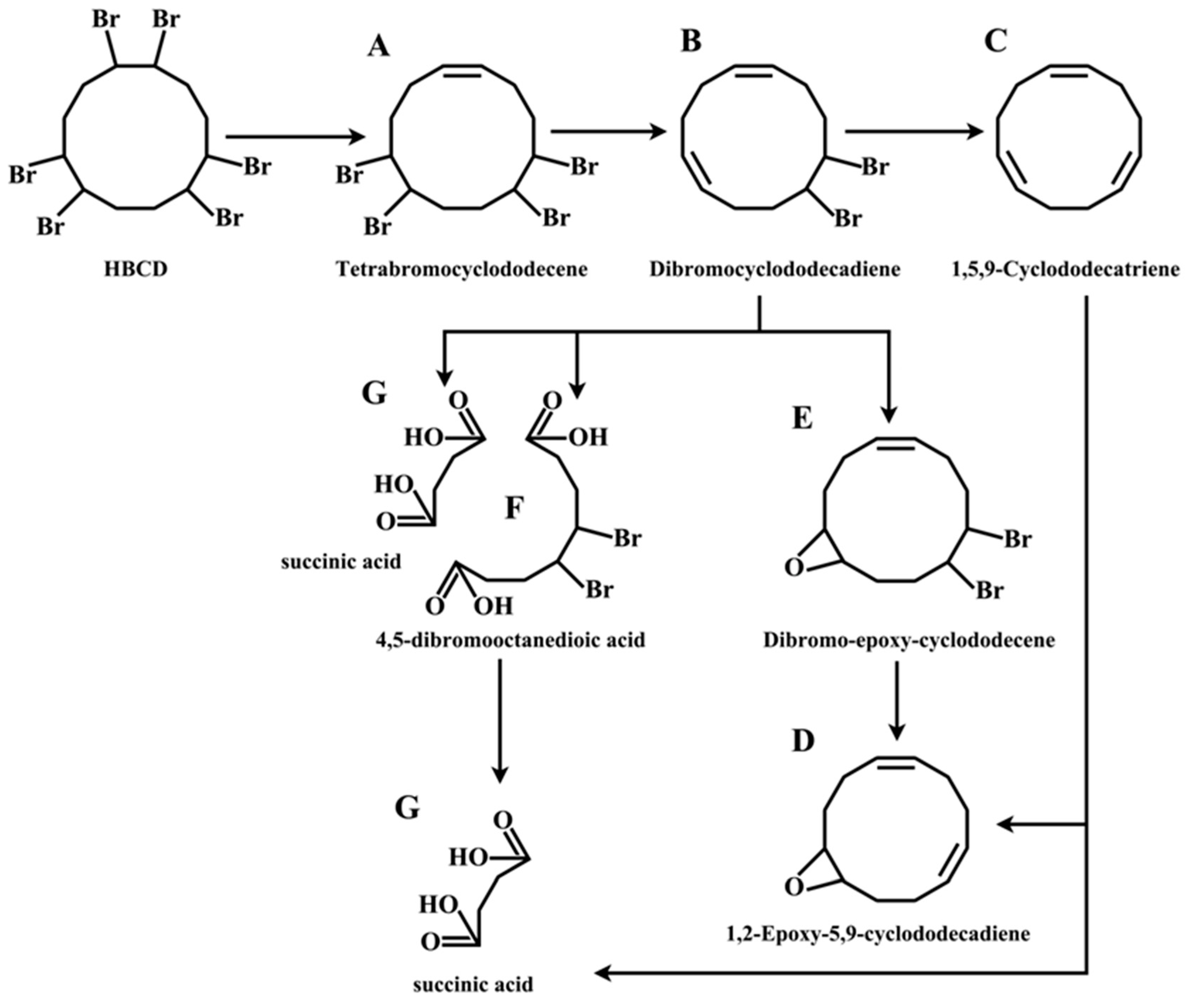
2.5. The Mechanism of Photodegradation of HBCD
3. Materials and Methods
3.1. Reagents
3.2. Photodegradation of HBCD
3.3. Analysis Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Almughamsi, H.; Whalen, M.M. Hexabromocyclododecane and tetrabromobisphenol A alter secretion of interferon gamma (IFN-gamma) from human immune cells. Arch. Toxicol. 2016, 90, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Alaee, M.; Arias, P.; Sjodin, A.; Bergman, A. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef]
- Jeannerat, D.; Pupier, M.; Schweizer, S.; Mitrev, Y.N.; Favreau, P.; Kohler, M. Discrimination of hexabromocyclododecane from new polymeric brominated flame retardant in polystyrene foam by nuclear magnetic resonance. Chemosphere 2016, 144, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Weber, R.; Liu, J.G.; Hu, J.X. Long-term emissions of hexabromocyclododecane as a chemical of concern in products in China. Environ. Int. 2016, 91, 291–300. [Google Scholar] [CrossRef]
- Hunziker, R.W.; Gonsior, S.; Macgregor, J.A.; Desjardins, D. Fate and effect of hexabromocyclododecane in the environment. Organohalogen Compd. 2004, 66, 2300–2305. [Google Scholar]
- Stiborova, H.; Vrkoslavova, J.; Pulkrabova, J.; Poustka, J.; Hajslova, J.; Demnerova, K. Dynamics of brominated flame retardants removal in contaminated wastewater sewage sludge under anaerobic conditions. Sci. Total Environ. 2015, 533, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, E.R.; Baumberger, C.P.; Peverly, A.A.; Peters, D.G. Electrochemical reduction of 1, 2, 5, 6, 9, 10-hexabromocyclododecane at carbon and silver cathodes in dimethylformamide. J. Electroanal. Chem. 2014, 713, 136–142. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, J.; Wang, H.; Liu, K.; Yu, G.; Deng, S.B.; Wang, B. Mechanochemical degradation of hexabromocyclododecane and approaches for the remediation of its contaminated soil. Chemosphere 2014, 116, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Takigami, H.; Watanabe, M.; Kajiwara, N. Destruction behavior of hexabromocyclododecanes during incineration of solid waste containing expanded and extruded polystyrene insulation foams. Chemosphere 2014, 116, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Jondreville, C.; Cariou, R.; Meda, B.; Dominguez-Romero, E.; Omer, E.; Dervilly-Pinel, G.; Le Bizec, B.; Travel, A.; Baeza, E. Accumulation of a-hexabromocyclododecane (alpha-HBCDD) in tissues of fast- and slow-growing broilers (Gallus domesticus). Chemosphere 2017, 178, 424–431. [Google Scholar] [CrossRef][Green Version]
- Guo, Y.G.; Lou, X.Y.; Xiao, D.X.; Xu, L.; Wang, Z.H.; Liu, J.S. Sequential reduction-oxidation for photocatalytic degradation of tetrabromobisphenol A: Kinetics and intermediates. J. Hazard. Mater. 2014, 241–242, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Saien, J.; Ojaghloo, Z.; Soleymani, A.R.; Rasoulifard, M.H. Homogeneous and heterogeneous AOPs for rapid degradation of Triton X-100 in aqueous media via UV light, nano titania hydrogen peroxide and potassium persulfate. Chem. Eng. J. 2011, 167, 172–182. [Google Scholar] [CrossRef]
- Salari, D.; Niaei, A.; Aber, S.; Rasoulifard, M.H. The photooxidative destruction of CI basic yellow 2 using UV/S2O82− process in a rectangular continuous photoreactor. J. Hazard. Mater. 2009, 166, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Q.H.; Xie, H.Y.; Guo, J.; Lyu, H.L.; Li, Y.G.; Sun, Z.G.; Wang, H.Z.; Guo, Z.H. Electrospun titania nanofibers segregated by graphene oxide for improved visible light photocatalysis. Appl. Catal. B Environ. 2017, 201, 470–478. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.G.; Xie, H.Y.; Wang, H.Z.; Zhang, Q.H. Efficient mineralization of toluene by W-doped TiO2 nanofibers under visible light irradiation. J. Nanosci. Nanotechnol. 2015, 15, 2944–2951. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.G.; Zhang, Q.H.; Shi, G.Y.; Wang, H.Z. Fast synthesis of highly dispersed anatase TiO2 nanocrystals in a microfluidic reactor. Chem. Lett. 2011, 40, 1371–1373. [Google Scholar] [CrossRef]
- Aronne, A.; Fantauzzi, M.; Imparato, C.; Atzei, D.; De Stefano, L.; D′Errico, G.; Sannino, F.; Rea, I.; Pirozzi, D.; Elsener, B.; et al. Electronic properties of TiO2-based materials characterized by high Ti3+ self-doping and low recombination rate of electron–hole pairs. RSC Adv. 2017, 7, 2373–2381. [Google Scholar] [CrossRef]
- Sannino, F.; Pernice, P.; Imparato, C.; Aronne, A.; D′Errico, G.; Minieri, L.; Perfetti, M.; Pirozzi, D. Hybrid TiO2–acetylacetonate amorphous gel-derived material with stably adsorbed superoxide radical active in oxidative degradation of organic pollutants. RSC Adv. 2015, 5, 93831–93839. [Google Scholar] [CrossRef]
- Sannino, F.; Pernice, P.; Minieri, L.; Gamandona, G.A.; Aronne, A.; Pirozzi, D. Oxidative Degradation of Different Chlorinated Phenoxyalkanoic Acid Herbicides by a Hybrid ZrO2 Gel-Derived Catalyst without Light Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 256–263. [Google Scholar] [CrossRef]
- Guo, Y.G.; Zhou, J.; Lou, X.Y.; Liu, R.L.; Xiao, D.X.; Fang, C.L.; Wang, Z.H.; Liu, J.S. Enhanced degradation of Tetrabromobisphenol A in water by a UV/base/persulfate system: Kinetics and intermediates. Chem. Eng. J. 2014, 254, 538–544. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants of inorganic radicals in aqueous-solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Xu, J.; Meng, W.; Zhang, Y.; Lei, L.; Guo, C.S. Photocatalytic degradation of tetrabromobisphenol A by mesoporous BiOBr: Efficacy, products and pathway. Appl. Catal. B Environ. 2011, 107, 355–362. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Zhang, Y.B.; Quan, X.; Chen, S. Enhanced oxidation of 4-chlorophenol using sulfate radicals generated from zero-valent iron and peroxydisulfate at ambient temperature. Sep. Purif. Technol. 2010, 71, 302–307. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.F.; Zhang, L.; Xie, H.Y. Rapid degradation of tetrabromobisphenol A under the UV/TiO2/KPS systems in alkaline aqueous solutions. Res. Chem. Intermed. 2018. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ghanbari, F.; Moradi, M. Photocatalysis assisted by peroxymonosulfate and persulfate for benzotriazole degradation: Effect of pH on sulfate and hydroxyl radicals. Water Sci. Technol. 2015, 72, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.Y.; Zhu, L.P.; Wang, L.L.; Chen, S.W.; Yang, D.D.; Yang, L.J.; Gao, G.L.; Yuan, H. Photodegradation of benzene by TiO2 nanoparticles prepared by flame CVD process. Particuology 2011, 9, 75–79. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, S.L.; Yin, M.L.; Pi, L.L.; Zeng, J.; Yi, B. Parameters effect on photocatalytic kinetics of carbofuran in TiO2 aqueous solution. China Environ. Sci. 2013, 33, 82–87. [Google Scholar]
- Varanasi, L.; Coscarelli, E.; Khaksari, M.; Mazzoleni, L.R.; Minakata, D. Transformations of dissolved organic matter induced by UV photolysis, hydroxyl radicals, chlorine radicals, and sulfate radicals in aqueous-phase UV-Based advanced oxidation processes. Water Res. 2018, 135, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jain, T.; Ishida, K.; Liu, H.Z. A mechanistic understanding of the degradation of trace organic contaminants by UV/hydrogen peroxide, UV/persulfate and UV/free chlorine for water reuse. Environ. Sci. Water Res. Technol. 2017, 3, 128–138. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J. Hazard. Mater. 2010, 173, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Barontini, F.; Cozzani, V.; Cuzzola, A.; Petarca, L. Investigation of hexabromocyclododecane thermal degradation pathways by gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.N.; Chen, L.; Wu, F.; Wang, J.; Yang, F. Debromination of hexabromocyclododecane in aqueous solutions by UV-C irradiation. Fresenius Environ. Bull. 2012, 21, 107–111. [Google Scholar]
- Yu, Y.; Zhou, D.; Wu, F. Mechanism and products of the photolysis of hexabromocyclododecane in acetonitrile–water solutions under a UV-C lamp. Chem. Eng. J. 2015, 281, 892–899. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Zhang, X.H.; Sojinu, O.S. Thermodynamics and photochemical properties of alpha, beta, and gamma-hexabromocyclododecanes: A theoretical study. Chemosphere 2010, 80, 150–156. [Google Scholar]
- Tso, C.P.; Shih, Y.H. The transformation of hexabromocyclododecane using zerovalent iron nanoparticle aggregates. J. Hazard. Mater. 2014, 277, 76–83. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wang, L.; Fang, X.; Zhang, L.; Li, J.; Xie, H. Synergistic Effect of Photocatalytic Degradation of Hexabromocyclododecane in Water by UV/TiO2/persulfate. Catalysts 2019, 9, 189. https://doi.org/10.3390/catal9020189
Li Q, Wang L, Fang X, Zhang L, Li J, Xie H. Synergistic Effect of Photocatalytic Degradation of Hexabromocyclododecane in Water by UV/TiO2/persulfate. Catalysts. 2019; 9(2):189. https://doi.org/10.3390/catal9020189
Chicago/Turabian StyleLi, Qiang, Lifang Wang, Xuhui Fang, Li Zhang, Jingjiu Li, and Hongyong Xie. 2019. "Synergistic Effect of Photocatalytic Degradation of Hexabromocyclododecane in Water by UV/TiO2/persulfate" Catalysts 9, no. 2: 189. https://doi.org/10.3390/catal9020189
APA StyleLi, Q., Wang, L., Fang, X., Zhang, L., Li, J., & Xie, H. (2019). Synergistic Effect of Photocatalytic Degradation of Hexabromocyclododecane in Water by UV/TiO2/persulfate. Catalysts, 9(2), 189. https://doi.org/10.3390/catal9020189



