Abstract
Propane dehydrogenation (PDH) is the extensive pathway to produce propylene, which is as a very important chemical building block for the chemical industry. Various catalysts have been developed to increase the propylene yield over recent decades; however, an active site of monometallic Pt nanoparticles prevents them from achieving this, due to the interferences of side-reactions. In this context, we describe the use of promoter-free hierarchical Pt/silicalite-1 nanosheets in the PDH application. The Pt dispersion on weakly acidic supports can be improved due to an increase in the metal-support interaction of ultra-small metal nanoparticles and silanol defect sites of hierarchical structures. This behavior leads to highly selective propylene production, with more than 95% of propylene selectivity, due to the complete suppression of the side catalytic cracking. Moreover, the oligomerization as a side reaction is prevented in the presence of hierarchical structures due to the shortening of the diffusion path length.
1. Introduction
Propylene is one of the most important petrochemical building blocks for the production of a diverse range of products, from solvents to plastics and other valuable intermediates, for example, polypropylene, acrylonitrile, acrolein, and acrylic acid. In the present technology, the majority of propylene production is obtained as a byproduct from steam cracking and fluid catalytic cracking of hydrocarbons [1,2]. Because of an increasing demand of propylene and the depletion of petroleum resources, on-purposed propylene production technologies from alternative substrates, such as methanol-to-olefin (MTO), [3,4] metathesis of ethylene and butylene [5] and propane dehydrogenation (PDH) [6] have been intensively developed. Among these technologies, propane dehydrogenation is the most economical and shortest route for propylene production and is commercially available, developed by several licensing processes including the Catofin process from CB&I Lummus, reference [7] and the Oleflex process from Universal Oil Products (or Honeywell UOP) [8]. Because this reaction is a highly endothermic process and equilibrium-limited reaction, the relatively high temperature (above 550 °C) is usually required for promoting the catalytic activity. Thus, this behavior facilitates several side-reactions, in particular, thermal/catalytic cracking and coke formation, resulting in the low propylene selectivity and the rapid deactivation of catalysts [9]. It is, therefore, a great challenge to develop this process, especially in terms of increasing the efficiency of catalysts exhibiting high selectivity and stability in such severe operating conditions for the industrial production of propylene.
A Cr-based alumina supported catalyst is considered as one of the most important catalysts for this reaction due to their promising catalytic activity [6,8] however, the Cr is rather hazardous. An alternative way is to replace by the Pt-based catalysts [10,11,12] and other non-noble metals, including Cu, Fe, Zn and VOx [13,14,15,16,17,18]. Although the Pt-based catalysts exhibit an excellent activity, they still also suffer from disadvantages, such as a short catalyst lifetime due to the coke formation and the sintering of Pt clusters [19].
Several strategies have been applied to improve the catalytic performance of Pt-based catalysts, including the addition of a second metal (e.g., Sn, Zn, Cu, Ce) as promoters [17,20,21,22,23], the development of the synthesis method to obtain small Pt nanoclusters such as the atomic layer deposition (ALD) technique [24] and the development of metal-support interactions to change the electron density of Pt, which eventually leads to reducing the propylene adsorption, and then suppressing the further catalytic cracking [22,25,26,27,28].
A zeolite is one of the most interesting materials to be widely used as a support owing to its outstanding properties, such as a high surface area and a high thermal/hydrothermal stability [29,30]. However, it often suffers from the metal aggregation on outer surfaces after the thermal treatment, resulting in a very low catalytic selectivity to propylene [31,32]. To prevent the metal aggregation, the introduction of a mesoporous system into the microporous zeolite network can greatly improve the dispersion of metal species due to a large increasing number of defect sites [33]. With the benefits of mesoporous or hierarchical structures [34,35], an improved catalytic efficiency has been observed in some reactions, in particular the processes involving bulky molecules, such as hydroisomerization, aromatization and hydrodesulfurization, as compared to a conventional zeolite [36,37,38,39,40].
Indeed, the acidity of supports also affects the propylene selectivity because of the interferences of the catalytic cracking and alkene oligomerization. Therefore, an alternative way is to apply the concept of using the neutralized surface acidity with basic molecules [41] or the siliceous zeolite with a weak acid. However, the lack of alumina in the zeolite framework results in the weak metal-support interaction, eventually leading to the aggregation of metal species [42,43]. Thus, the development of alternative PDH catalysts, which exhibit the interplay between minimizing the acidity and increasing the surface interaction of support and metal species, is still a great challenge.
In the present study, we report the example of a highly selective propane dehydrogenation over monometallic nanoparticles supported on weakly acidic siliceous MFI-type zeolite having the hierarchical nanosheet structure. This catalyst has been chosen since it not only benefits to the shape-selectivity of MFI structure that is preferable to propylene, but also suppresses the side catalytic cracking on acidic surfaces and prevents the aggregation of metal species owing to the presence of silanol defect sites, resulting in increasing the metal-support interaction. Compared to the conventional Pt supported zeolite, another side reaction such as an oligomerization is also suppressed in the presence of hierarchical structures due to shorten diffusion path lengths. In addition, its catalytic performance will be discussed in terms of the roles of metal-support interaction and acidity. Finally, the degree of propylene selectivity can be easily adjusted by changing the loading content of metal and the weight hourly space velocity (WHSV).
2. Results and Discussion
2.1. Structures and Properties of Catalysts
XRD patterns of Pt supported conventional and hierarchical siliceous zeolites represented the MFI structure including that the impregnation of Pt did not destroy the structural property of zeolites. In addition, there were no detectable PtO2 characteristics in XRD patterns of all supports indicating the well dispersion of Pt or the relatively low amount of Pt loading (Figure 1A). SEM images of hierarchical siliceous MFI or silicalite-1 nanosheets, conventional silicalite-1 and γ-alumina exhibited uniform particles with a narrow size distribution (see Figure 1B,D and Figure S1) of 250.0 ± 20.0 nm, 2.5 ± 0.3 µm, and 25.0 ± 5.0 nm, respectively. The diameters of Pt particles supported on silicalite-1 nanosheets, conventional silicalite-1 and γ-alumina were approximately 2.0 ± 0.5, 20.0 ± 10.0 and 0.6 ± 0.2 nm, respectively (Figure S2) as shown in TEM images (Figure 1E–G and Figure S2A). The Pt content was confirmed after a successful impregnation of Pt on the supports by using XRF analysis as shown in Table 1. The Pt loading content on various supports was in the same range of 1 wt.%.
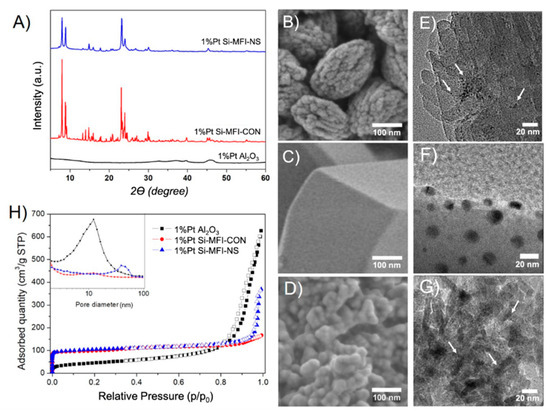
Figure 1.
Characterizations of various Pt supported catalysts: (A) XRD patterns, (B-D) SEM images of Pt supported on hierarchical silicalite-1 nanosheets (1% Pt-Si-MFI-NS), conventional silicalite-1 (1% Pt-Si-MFI-CON) and alumina (1% Pt-Al2O3), respectively, (E–G) TEM images of 1% Pt-Si-MFI-NS, 1% Pt-Si-MFI-CON and 1% Pt-Al2O3, respectively and (H) N2 sorption isotherms and BJH pore size distributions (see inset).

Table 1.
Textural properties of Pt supported catalysts, including hierarchical silicalite-1.
The textural properties of all catalysts were measured using N2 sorption measurements as presented in Figure 1H and Table 1. The isotherm of γ-alumina exhibited an apparent hysteresis loop at P/P0 range of 0.6 to 0.9 corresponding to the type-IV isotherm, suggesting the presence of mesoporous structures [44]. In contrast, the isotherm of the conventional silicalite-1 exhibited only the adsorption at the relatively low pressure (P/P0 < 0.2) due to a microporous structure, corresponding to the type-I isotherm [44]. On the other hand, the silicalite-1 nanosheet exhibited the adsorption at low pressure together with the hysteresis loop at P/P0 in the range of 0.5 to 0.9, indicating the combination of type-I and IV isotherms [44]. The mesopore sizes of γ-alumina and silicalite-1 nanosheets obtained by the BJH model were approximately 10 and 40 nm, respectively, and no mesoporous structure was observed in the case of the conventional silicalite-1. The BET surface area of γ-alumina was relatively low (161 m2·g−1) compared to silicalite-1 (~400 m2·g−1), while the total pore volume was relatively high (up to 0.96 cm3·g−1) due to a high fraction of mesopores (Sext 150 m2·g−1). Considering the siliceous zeolite, the silicalite-1 nanosheets showed a relatively higher total pore volume (0.56 cm3·g−1) than that of the conventional one (0.25 cm3·g−1), while the micropore volume was in the same range (~0.12–0.13 cm3·g−1), indicating the presence of a large amount of meso- and macroporosity. In addition, the silicalite-1 with Pt loading slightly changed the textural properties of support. The surface areas (SBET from 447 to 399 m2·g−1) of Pt supported on the silicalite-1 nanosheets (1% Pt-Si-MFI-NS) and Pt supported on the conventional silicalite-1 (1% Pt-Si-MFI-CON) were decreased by a factor of 11% and 12%, respectively (Table 1).
The alumina support is known to play an important role as metal supported catalysts and provides a significant Pt-support interaction, as compared to a conventional silica support [45]. To verify the reduction behavior of metal-supported catalysts, the influences of different supports on reduced Pt species were characterized by H2-TPR. The Pt supported on γ-alumina exhibited two reduction peaks at a relatively higher temperature (at 385 °C and 460 °C) compared to siliceous zeolites (Figure 2A) and similar to what has been described in previous reports [46,47], indicating the strong interaction between Pt nanoparticles and γ-alumina support. The Pt supported on conventional silicalite-1 exhibited only one peak at 320 °C, suggesting that Pt species on this support were easily reduced to metallic Pt compared γ-alumina support, confirming a weak interaction between a Pt metal and a silica support. Interestingly, in the case of Pt supported on silicalite-1 nanosheets, another reduction peak at higher temperature of 500 °C was observed. It is therefore reasonable to imply that there are some Pt species having a strong interaction with support due to the hierarchical structure, resulting in the higher resistance towards the reduction of Pt species. All H2-TPR results confirmed that the different nature of supports can alter the behaviors of reduced Pt species.
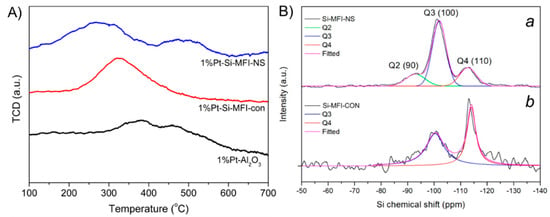
Figure 2.
(A) H2-TPR profiles of Pt supported on different supports, including hierarchical silicalite-1 nanosheets (1% Pt-Si-MFI-NS) (blue), conventional silicalite-1 (1% Pt-Si-MFI-CON) (red) and alumina (1% Pt-Al2O3) (black), and (B) 29Si NMR spectra of (a) hierarchical silicalite-1 nanosheets (Si-MFI-NS) and (b) conventional silicalite-1 (Si-MFI-CON).
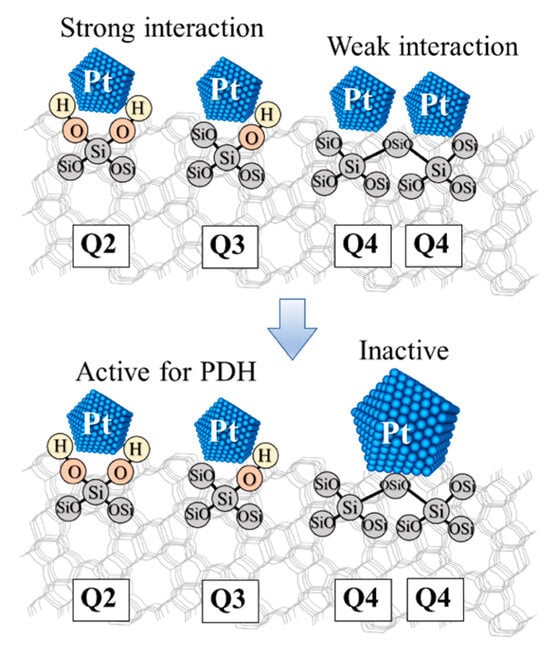
One reason for the strong interaction between Pt and silicalite-1 nanosheets is that the hierarchical siliceous zeolite can provide a large number of defect sites, and therefore improve the interaction with metals. To confirm this hypothesis, the NH3-TPD profiles and 29Si MAS NMR spectra of silicalite-1 nanosheets were also observed, as shown in Figure S3 and Figure 2B. Compared to the conventional silicalite-1, the large number of weak acid sites of Pt supported silicalite-1 nanosheets remarkably increased (1.17 and 0.03 mmol·g−1, for silicalite-1 nanosheets and the conventional silicalite-1, respectively), implying an increase in the surface silanol density due to the hierarchical structures. To further verify the presence of surface silanols of MFI zeolite, the 29Si MAS NMR revealed the environment of Si framework representing as Qn-groups in which n is 1 to 4 according to the number of tetrahedral Si unit as the next-nearest neighbors [48]. Interestingly, the hierarchical silicalite-1 exhibited the higher ratio of Q3 to Q4 (2.90) and the presence of Q2 species, which was the characteristic of silanol surfaces due to a Si atom linked with two O–H groups. In strong contrast to this, the conventional silicalite-1 showed the significantly lower ratio of Q3 to Q4 (1.14) in the absence of Q2 characteristic (see Figure 2B). This relates to the fact that the hierarchical nanosheets can enhance the surface silanol density located on external surfaces.
The electronic properties of Pt species were characterized by XPS analysis. The Pt4f XPS spectra of all catalysts exhibited doublet peaks corresponding to a spin-orbit splitting of Pt4f7/2 and Pt4f5/2 states (Figure S4) [49]. Unfortunately, the binding energy (B.E.) of Pt 4f5/2 was very close to that of Al 2p in case of γ-alumina support [50]. The decomposition of such Pt 4f7/2 and 4f5/2 XPS peaks were used to analyze the predominant species in catalysts (Figure S4 and Table S1). Accordingly, the B.E.s at 71.1–74.4, 72.8–76.1 and 74.3–77.6 eV were assigned to metallic Pt (Pt0), PtIIO and PtIVO2, respectively. The Pt 4f7/2 B.E.s of the Pt supported on hierarchical silicalite-1 nanosheets obviously shifted to a lower binding energy compared with the conventional silicalite-1, indicating that defect sites in hierarchical structures can change the electron density of Pt to be an electron richer due to the strong interaction of metal and support, and thus, this material exhibited the improved metal-support interaction.
To clarify these behaviors, the theoretical study by a computational chemistry approach was used to investigate the electronic properties of catalysts. The interaction of Pt cluster supported on different silicon species (Q4, Q3, and Q2) was investigated as shown in Figure S5 and Table S3. The binding energy of Pt4 cluster supported on the Q3 species (Pt4/Q3-MFI) was approximately −7.0 kcal·mol−1, whereas the binding energy of Pt4 cluster supported on the Q4 species (Pt4/Q4-MFI) was significantly lower (approximately −4.0 kcal·mol−1). Moreover, the Natural Population Analysis (NPA) charge (Table S4) revealed that the electron transfer from a support to Pt4 cluster in the Pt4/Q3-MFI model was more predominant compared to the Pt4/Q4-MFI model. The partial atomic charges of Pt4 cluster were reduced from 0.000 e (bare Pt4 cluster) to −0.290 e and −0.252 e for Pt4/Q3-MFI and Pt4/Q4-MFI models, respectively. The reason of this higher negatively charge over the Pt4/Q3-MFI compared to the Pt4/Q4-MFI related to the improved charge-transfer from defects or silanol surfaces of zeolites to the Pt4 cluster. These observations confirmed the improved interaction of metal-support in the presence of defects or silanol surfaces of the Pt supported on silicalite-1 nanosheet. In addition, the lower adsorption energy of propylene on the Pt4/Q3-MFI model (−29.5 kcal·mol−1) compared to that of the Pt4/Q4-MFI model (−36.1 kcal·mol−1) indicated to a weaken adsorption strength of propylene from active site. It is therefore reasonable to assume that the propylene product is easily desorbed over defects or silanol surfaces, resulting in the suppression of further side reactions. This eventually leads to an increase in overall propylene selectivity over Pt supported silicalite-1 nanosheets.
2.2. Dehydrogenation of Propane over Pt Nanoparticles Supported on Different Supports
The above-mentioned acidity and metal-support interaction makes it clear that the hierarchical silicalite-1 not only improves the metal-support interaction compared to the conventional silicalite-1, but also reduces the acidity of supports with respect to γ-alumina. Therefore, this material is expected to benefit to the catalytic activity and selectivity of propane dehydrogenation.
To illustrate the benefits of Pt supported hierarchical silicalite-1 nanosheets (Pt/MFI-NS) on the propane dehydrogenation (PDH), it clearly demonstrates that the modified catalyst exhibits a relatively high activity with the Turnover Frequency (or TOF) value of 1112.2 h−1 and an improved catalytic stability even after time on stream (TOS) of 12 h (Figure 3A). Interestingly, the high selectivity of propylene above 95% was observed, indicating that the side reactions were diminished on this catalyst. In strong contrast to this, the conventional silicalite-1 (Pt/Si-MFI-con) showed a much lower activity of the propane conversion with the TOF of 157.6 h−1 with the propylene selectivity of 50%. Although the high activity (TOF = 278.4 h−1) was observed over the fresh alumina-based catalyst (Pt/Al2O3), the deactivation rate was also very fast with the 20-fold increase in the deactivation rate constant compared to those of over the Pt supported hierarchical silicalite-1 nanosheets (Pt/Si-MFI-NS) (Figure S6). As expected, the selectivity of propylene also decreased from 80 to 55% after 12 h of TOS.
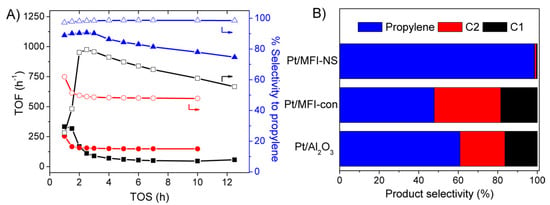
Figure 3.
Catalytic performance of propane dehydrogenation over 1 wt.% of Pt supported on different supports, including hierarchical silicalite-1 nanosheets (Pt/Si-MFI-NS) (blue), conventional silicalite-1 (Pt/Si-MFI-CON) (red), and aluminum oxide (Pt/Al2O3) (black) in terms of (A) TOF of propane conversion (solid symbol) and selectivity to propylene (open symbol) as a function of time-on-stream (TOS) and (B) Product selectivity at 10 h of TOS obtained at weight hourly space velocity (WHSV) of 2.82 h−1.
The distribution of products after 10 h of TOS is shown in Figure 3B. Unfortunately, the Pt/Al2O3 noticeably exhibited a high selectivity to cracked products (methane and ethane) (~40%). The relatively high selectivity of cracked products was also observed over the conventional zeolite (Pt/Si-MFI-CON) (~50%). These results indicated that the nature of supports plays an important role in the selective production of light cracked hydrocarbons. The reason for this increase in cracked product selectivity relates to the fact that the Al2O3 support composes of the high amount of acid sites as shown in NH3-TPD results (see Figure S3), resulting in the promotion of cracking reaction. Compared to the hierarchical silicalite-1 nanosheets, the cracked product selectivity over the conventional silicalite-1 was also remarkably increased. This is most likely due to the fact that the formation of lighter hydrocarbons via C–C bond cleavage is inevitably excluded using Pt-based catalysts [49]. It is well known that the side reactions, such as catalytic cracking of hydrocarbons over Pt-based catalysts are structure-sensitive reactions and they can be accelerated over large Pt clusters [10]. This behavior eventually leads to the formation of a light C–C bond cracking over the conventional silicalite-1, containing large Pt clusters.
2.3. Effects of WHSV and Pt Loading on the Propylene Selectivity
The degree of propylene selectivity can be simply tuned by changing the loading content of metal and the weight hourly space velocity (WHSV). Figure 4A,B demonstrates the conversion, TOF and selectivity of propylene over the hierarchical silicalite-1 nanosheets obtained at different TOS and WHSV. The conversion of propane was in the range of 30–35% and it agreed well with the theoretical equilibrium value at 550 °C is ~37%, [8,15] (Figure 4A). Obviously, at the low WHSV, the selectivity of propylene was decreased to 89% and the selectivity of cracked products was rather high (~11%, Figure 4B). Such an observation suggested that at low WHSV, the side catalytic cracking was preferentially occurred due to a long time of reaction mixtures being in contact with the catalyst. In contrast, it was also found that the highly selective production of propylene (>95%) was observed when the WHSV was greater than 2.8 h−1, indicating that the short contact time suppressed side reactions. In addition, the TOF increased as a function of WHSV; however, at too high WHSV the TOF becomes smaller (Figure 4B).
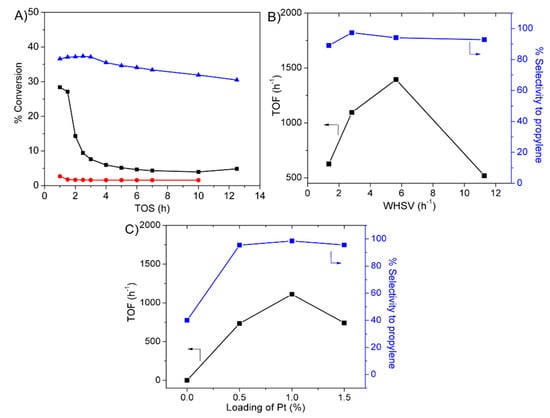
Figure 4.
Catalytic performances of propane dehydrogenation over Pt supported hierarchical silicalite-1 nanosheets with different effects: (A) The conversion of propane as a function of TOS over hierarchical silicalite-1 nanosheets (1% Pt-Si-MFI-NS) (blue), conventional silicalite-1 (1% Pt-Si-MFI-CON) (red) and alumina (1% Pt-Al2O3) (black). (B) TOF as a function of WHSV on 1% Pt-Silicalite-1 nanosheets obtained at TOS of 1 h. (C) Effect of Pt loading on hierarchical Silicalite-1 nanosheets was taken at 3 h of TOS and WHSV of 2.82 h−1.
The conversion of propane and propylene selectivity as a function of Pt loading over the Pt supported hierarchical silicalite-1 nanosheets at WHSV of 2.8 h−1 is presented in Figure 4C. As expected, the silicalite-1 nanosheets without Pt loading, there was no activity and the selectivity of propylene was in a very low level of 45%, demonstrating that the support itself could not serve as the dehydrogenated active site. It was also found that a small amount of methane and ethane derived from catalytic cracking was observed. In strong contrast to this, the Pt supported catalyst exhibited the selectivity of propylene about 95.2%, although the low Pt loading of 0.5 wt.% was used. The different loading of Pt showed a significant influence on the TOF of propane conversion. When the loading of Pt was relatively low (0.5 wt.%), the low TOF (734.3 h−1) of propane conversion was observed. With a further increase in Pt loading to 1 wt.%, the TOF increased and reached the highest value of 1112.2 h−1, while the high propylene selectivity was still observed (95%). At the relatively high Pt loading level of 1.5 wt.%, the TOF becomes smaller (741.2 h−1). This may be explained by the fact that the excessive loading of Pt leads to the aggregation of Pt particles, and consequently the lower catalytic activity. Such an observation suggested that the Pt supported on silicalite-1 nanosheets containing 1 wt.% of Pt exhibited the superior performance in terms of propane activity and propylene selectivity.
In summary, the obtained results indicate that the superior catalytic performance of Pt supported on hierarchical silicalite-1 nanosheets for propane dehydrogenation (PDH) can be achieved to selectively convert propane to propylene without the catalyst deactivation even after the time on stream (TOS) of 12 h. TEM, H2-TPR, 29Si NMR, XPS measurements and DFT calculations confirmed the presence of small active Pt particles supported on silanol or defect surfaces of the hierarchical silicalite-1 nanosheet catalyst. This relates to the fact that the silanol surfaces or defect sites of hierarchical zeolite nanosheets can be greatly enhanced compared to the conventional silicalite-1, eventually resulting in enhancing the interaction of metal and support, thus preventing the aggregation of Pt clusters (Scheme 1).
Scheme 1.
Illustration of the proposed concept of the metal-support interaction effect of Pt supported on silicalite-1 zeolite; the stabilized Pt nanoparticles promoting the selective propane dehydrogenation to propylene.
To obtain the high yield of propylene, the effects of metal loading content and weight hourly space velocity (WHSV) were systematically studied to fine-tune the optimal experimental conditions. It was found that the silicalite-1 nanosheets containing 1 wt.% of Pt with the WHSV of 5.64 h−1 exhibited the highest performance with the TOF of 1394.2 h−1 and propylene selectivity of 96%. In strong contrast to this, the Pt supported conventional silicalite-1 and commercial Pt/Al2O3 catalysts exhibited a very low activity, propylene selectivity and short catalyst lifetime. In particular, they preferentially facilitate the side-reactions, such as catalytic cracking due to the presence of large Pt clusters and high acidity of supports for the Pt supported conventional silicalite-1 and commercial Pt/Al2O3, respectively.
Scheme 1 illustrates the proposed concept of the improved metal-support interaction in Pt supported on hierarchical silicalite-1 to promote the selective propane dehydrogenation to propylene. This is consistent with the view that the modified hierarchical structure enhances the PDH performances by changing the structure of Pt. The high dispersion degree of small Pt particles is responsible for the enhancement of the propane dehydrogenation performances. The smallest size of Pt particles can be obtained in the case of hierarchical silicalite-1 nanosheets due to the high external surface area together with the increasing of silanol or defect surfaces which can enhance the interaction with Pt [51]. In addition, the Pt metal nanoparticles supported on zeolite nanosheets have remained the well-dispersion with the similar size of 2.1 ± 0.7 nm. To confirm this hypothesis, the computational approach was performed to understand the surface interaction and electron transfer in different silanol network systems. It was found that silanol surfaces can greatly enhance the binding energy with Pt clusters, implying that Pt is strongly bound to the hierarchical nanosheet surfaces compared to the conventional one. Therefore, it is reasonable to suppose that the improved interaction of Pt clusters and the silanol groups eventually leads to preventing the aggregation of Pt over hierarchical silicalite-1 nanosheets. In addition, the electronic properties of Pt over different supports were also changed. The negative charge of Pt over the Pt4/Q3-MFI model is relatively larger than that of the Pt4/Q4-MFI model, suggesting that the silanol groups can promote the electron transfer from the supports to Pt cluster. This is consistent with the view that the Q3 species are predominant in the hierarchical silicalite-1 nanosheets, then facilitating the strong interaction between metal and support. The change of electronic properties of Pt on different supports also affects the desorption of propylene as a product. As expected, the silanol surfaces can directly weaken the adsorption of propylene on Pt sites. This leads to an increase in propylene selectivity due to the suppression of further side-reaction. In addition, the hierarchical structure provided a good benefit for promoting the propylene production, resulting in the suppression of the further oligomerization as a side reaction due to the shortening of the diffusion path length of catalysts. These observations may have the benefit of an increase in metal dispersion and it is complementary to the development of the well-dispersed metallic nanoparticles on solid supports in PDH reaction [52].
3. Materials and Methods
3.1. Synthesis of Silicalite-1 Zeolites
Hierarchical silicalite-1 nanosheets (Si-MFI-NS) and conventional silicalite-1 (Si-MFI-CON) were synthesized by using tetra-(n-butyl)phosphonium hydroxide (TBPOH) and tetrapropylammonium hydroxide (TPAOH) as structure directing agents (SDAs), respectively. The hierarchical silicalite-1 nanosheets were crystallized from a gel precursor in the absence of aluminum source by following the modified literature procedures [53]. The molar composition of the precursor was 60SiO2 18TBPOH: 0.75NaOH: 240EtOH: 600H2O. The resultant gel was stirred at room temperature for 12 h and then heated at 130 °C for 2 days. The conventional silicalite-1 was synthesized from a gel precursor with the composition of 10SiO2: 1TPAOH: 1.03NaOH: 240EtOH: 400H2O and crystallized at 180 °C for 3 days. The synthesized products were collected and washed with distilled water until the pH of filtrate less than 9. Finally, the products were calcined in air at 550 °C for 8 h to remove the SDAs.
3.2. Preparation of Pt Supported Silicalite-1 Zeolites
The Pt was loaded on silicalite-1 zeolites by a wet impregnation method. Typically, a 1.2 g of a calcined zeolite catalyst was added to a 10 mL of an aqueous solution of tetraammineplatinum nitrate (Pt (NH3)4(NO3)2) with various Pt loading contents (0.5, 1 and 1.5 wt.%). The slurry was stirred overnight at room temperature. Afterward, the water was carefully removed by the freeze-drying method.
3.3. Characterizations
X-ray diffraction (XRD) patterns of all catalysts were recorded on Bruker (Massachusetts, United States) D8 ADVANCE instrument with CuKα radiation (40 kV, 40 mA). Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images were obtained from JEOL-JSM-7600F (at 2 kV) (Tokyo, Japan) and JEOL-JEM-2100 (at 200 kV) (Tokyo, Japan) microscopes, respectively. The average particle size of Pt and zeolite was estimated from at least 200 particles. N2 sorption techniques were used to examine the textural properties of catalysts. The measurements were carried out on Belmax (MicrotracBEL Corp., Tokyo, Japan) apparatus, and all the prepared samples were degassed at 300 °C for 24 h before the measurement. The specific surface area (SBET) was determined by using the BET theory. The micropore volume (Vmicro), the micropore area (Smicro) and the external surface area (Sext) were calculated from the t-plot method. The total pore volume (Vtotal) was estimated at P/P0 = 0.98. The composition and Pt loading content of prepared catalysts were measured by X-ray fluorescence (XRF) spectrometry performed on a Bruker (Massachusetts, United States) AXS S4 Pioneer instrument. The temperature-programmed reduction of H2 (H2-TPR) and H2 pulse chemisorption were performed on the Belcat II (Tokyo, Japan) chemisorption analyzer equipped with thermal conductivity detectors (TCD). In H2-TPR experiments, the sample was pretreated in Ar (50 mL·min−1) at 300 °C for 1 h and then the temperature was ramped from 50 °C to 700 °C at 5 °C·min−1 under the flow of 2% H2/Ar (50 mL·min−1). For the H2 pulse chemisorption, all samples were heated to 300 °C under the Ar flow (50 mL·min−1). Subsequently, the consecutive pretreatment by O2 and H2 was introduced. Finally, samples were cooled down to 50 °C and the H2 pulse measurement was operated under the flow of 2% (v/v) H2 in Ar with the flow rate of 50 mL·min−1. XPS spectra were obtained using JEOL JPS-9010 (Tokyo, Japan). Non-monochromatic Mg Ka X-rays (1486.6 eV) were used as primary excitation. The C 1s was selected as the reference, which value was accepted equal to 284.7 eV.
3.4. Catalytic Testing
The dehydrogenation of propane to propylene was carried out in a continuous-flow fixed-bed reactor. Prior to the reaction testing, the similar particles size of a catalyst was controlled in the range of 425–850 µm. A 0.2 g of catalyst was placed into the reactor and activated at 550 °C under the flow of H2 (2.4 mL·min−1) for 2 h. Then, the propane in N2 with the molar volume ratio of 1:1 was fed with various flow rates to study the effect of weight hourly space velocity (WHSV). The WHSV was varied from 1.4 to 11.3 h−1 by changing the flow rate of the reactant mixture at constant reactor volume. The turnover frequency (TOF) was calculated according to the following Equation:
where the number of active sites was calculated based on the total number of active metal sites derived from the H2 chemisorption measurement [54]. Dm is the dispersivity of metal. All reactions were carried out at 550 °C, and atmospheric pressure. The analysis of the reaction mixtures was performed by an online gas chromatograph (GC) (Agilent 7820A) equipped with a flame ionization detector (FID) and a GS-GASPRO capillary column (60 m × 0.32 mm) at an interval time of 1 h. To validate the performance of catalysts in the kinetic regime, the experimental criteria due to heat and mass transfer limitation are also discussed as shown in the Supplementary Materials.
4. Conclusions
In conclusion, the results demonstrate the first example, describing the improved metal-support interaction of hierarchical nanosheet structures. A promoter-free Pt on hierarchical silicalite-1 nanosheets exhibited the highly selective production of propylene from propane dehydrogenation above 95% at the propane conversion level of 30%. The observations from TEM, H2-TPR, 29Si NMR, XPS measurements and DFT calculations confirmed that the hierarchical silicalite-1 nanosheet catalyst promoting the presence of small active Pt particles supported on silanol or defect surfaces of the hierarchical silicalite-1 nanosheet catalyst. Therefore, a novel design of supported catalysts with hierarchical structures can promote active species for the highly selective propane dehydrogenation, which has a great potential to be utilized in the important petrochemical catalytic reactions.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4344/9/2/174/s1, Figure S1: Particle size distribution of various supports, Figure S2: (A) TEM images and (B) Particle size distribution of Pt supported on various supports, Figure S3: NH3-TPD profiles of various supports, Figure S4: XPS spectra of Pt supported on different supports, Figure S5: Computational modeling study, Figure S6: Deactivation rate of various catalysts, Table S1: Summary of the contribution of Pt species, Table S2: Summary of metal size and the percentage of metal distribution of Pt supported catalysts obtained by H2 chemisorption technique, Table S3: Selected geometrical parameters of Pt4/Q3-MFI and Pt4/Q4-MFI zeolites and the corresponding adsorption complexes including isolated Pt4, propane and propylene, Table S4: Summarized partial charges determined by the natural atomic orbital (NAO).
Author Contributions
Conceptualization, C.W.; Methodology, W.W., T.Y., P.D., K.R., A.T.; Formal Analysis, T.Y., S.N., T.W.; Investigation, S.P.; Writing—Original Draft Preparation, W.W., T.Y., A.T.; Writing—Review & Editing, W.W., C.W.
Funding
This work was supported in part by grants from Vidyasirimedhi Institute of Science and Technology (VISTEC), Thailand Research Fund (TRF) (MRG6180099), the Office of Higher Education Commission (OHEC), TTSF research project supported by Thailand Toray Science Foundation. In addition, this work has been partially supported by the National Nanotechnology Center (NANOTEC), NSTDA, Ministry of Science and Technology, Thailand, through its program of Research Network NANOTEC (RNN). Furthermore, the authors would like to acknowledge the Frontier Research Center, VISTEC for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Kijima, N.; Hayakawa, T.; Murata, K.; Suzuki, K.; Mizukami, F.; Matano, K.; Konishi, T.; Oikawa, T.; Saito, M.; et al. Catalytic cracking of naphtha to light olefins. Catal. Surv. Jpn. 2001, 4, 157–167. [Google Scholar] [CrossRef]
- Chen, J.Q.; Bozzano, A.; Glover, B.; Fuglerud, T.; Kvisle, S. Recent advancements in ethylene and propylene production using the UOP/hydro MTO process. Catal. Today 2005, 106, 103–107. [Google Scholar] [CrossRef]
- Zhu, X.; Hofmann, J.P.; Mezari, B.; Kosinov, N.; Wu, L.; Qian, Q.; Weckhuysen, B.M.; Asahina, S.; Ruiz-Martínez, J.; Hensen, E.J.M. Trimodal Porous Hierarchical SSZ-13 zeolite with improved catalytic performance in the methanol-to-olefins reaction. ACS Catal. 2016, 6, 2163–2177. [Google Scholar] [CrossRef]
- Mol, J.C. Industrial applications of olefin metathesis. J. Mol. Catal. A 2004, 213, 39–45. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Schoonheydt, R.A. Alkane dehydrogenation over supported chromium oxide catalysts. Catal. Today 1999, 51, 223–232. [Google Scholar] [CrossRef]
- Weiss, A.H. The manufacture of propylene, in refining petroleum for chemicals. Adv. Chemi. 1970, 153–178. [Google Scholar]
- Bhasin, M.M.; McCain, J.H.; Vora, B.V.; Imai, T.; Pujadó, P.R. Dehydrogenation and oxydehydrogenation of paraffins to olefins. Appl. Catal. A 2001, 221, 397–419. [Google Scholar] [CrossRef]
- Gascón, J.; Téllez, C.; Herguido, J.; Menéndez, M. Propane dehydrogenation over a Cr2O3/Al2O3 catalyst: Transient kinetic modeling of propene and coke formation. Appl. Catal. A 2003, 248, 105–116. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Marcinkowski, M.D.; Darby, M.T.; Liu, J.; Wimble, J.M.; Lucci, F.R.; Lee, S.; Michaelides, A.; Flytzani-Stephanopoulos, M.; Stamatakis, M.; Sykes, E.C.H. Pt/Cu single-atom alloys as coke-resistant catalysts for efficient C–H activation. Nat. Chem. 2018, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Han, P.; Li, B.; Zhao, Z. Tunable catalytic performance of single Pt atom on doped graphene in direct dehydrogenation of propane by rational doping: A density functional theory study. J. Phys. Chem. C 2018, 122, 1570–1576. [Google Scholar] [CrossRef]
- Tan, S.; Hu, B.; Kim, W.G.; Pang, S.H.; Moore, J.S.; Liu, Y.; Dixit, R.S.; Pendergast, J.G.; Sholl, D.S.; Nair, S.; Jones, C.W. Propane dehydrogenation over alumina-supported iron/phosphorus catalysts: Structural evolution of iron species leading to high activity and propylene selectivity. ACS Catal. 2016, 6, 5673–5683. [Google Scholar] [CrossRef]
- Liu, G.; Zeng, L.; Zhao, Z.J.; Tian, H.; Wu, T.; Gong, J. Platinum-modified ZnO/Al2O3 for propane dehydrogenation: Minimized platinum usage and improved catalytic stability. ACS Catal. 2016, 6, 2158–2162. [Google Scholar] [CrossRef]
- Schweitzer, N.M.; Hu, B.; Das, U.; Kim, H.; Greeley, J.; Curtiss, L.A.; Stair, P.C.; Miller, J.T.; Hock, A.S. Propylene hydrogenation and propane dehydrogenation by a single-site Zn2+ on silica catalyst. ACS Catal. 2014, 4, 1091–1098. [Google Scholar] [CrossRef]
- Schäferhans, J.; Gómez-Quero, S.; Andreeva, D.V.; Rothenberg, G. Novel and effective copper–aluminum propane dehydrogenation catalysts. Chem. Eur. J. 2011, 17, 12254–12256. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, S.; Jiang, F.; Wang, T.; Ma, X.; Gong, J. Propane dehydrogenation over Pt-Cu bimetallic catalysts: The nature of coke deposition and the role of copper. Nanoscale 2014, 6, 10000–10008. [Google Scholar] [CrossRef]
- Hu, P.; Lang, W.Z.; Yan, X.; Chu, L.F.; Guo, Y.J. Influence of gelation and calcination temperature on the structure-performance of porous VOX-SiO2 solids in non-oxidative propane dehydrogenation. J. Catal. 2018, 358, 108–117. [Google Scholar] [CrossRef]
- Iglesias-Juez, A.; Beale, A.M.; Maaijen, K.; Weng, T.C.; Glatzel, P.; Weckhuysen, B.M. A combined in situ time-resolved UV–Vis, Raman and high-energy resolution X-ray absorption spectroscopy study on the deactivation behavior of Pt and PtSn propane dehydrogenation catalysts under industrial reaction conditions. J. Catal. 2010, 276, 268–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Huang, L.; Xue, M.; Zhang, S. Sn-modified ZSM-5 as support for platinum catalyst in propane dehydrogenation. Ind. Eng. Chem. Res. 2011, 50, 7896–7902. [Google Scholar] [CrossRef]
- Cheung, T.K.; Lange, F.C.; Gates, B.C. Propane conversion catalyzed by sulfated zirconia, iron- and manganese-promoted sulfated zirconia, and USY zeolite. J. Catal. 1996, 159, 99–106. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, B.; Hua, W.; Yue, Y.; Gao, Z. Support effect in dehydrogenation of propane in the presence of CO2 over supported gallium oxide catalysts. J. Catal. 2006, 239, 470–477. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Qiu, A.; Wang, Y.; Xu, Y.; Wu, P. Propane dehydrogenation on PtSn/ZSM-5 catalyst: Effect of tin as a promoter. Catal. Commun. 2006, 7, 860–866. [Google Scholar] [CrossRef]
- Gould, T.D.; Lubers, A.M.; Corpuz, A.R.; Weimer, A.W.; Falconer, J.L.; Medlin, J.W. Controlling nanoscale properties of supported platinum catalysts through atomic layer deposition. ACS Catal. 2015, 5, 1344–1352. [Google Scholar] [CrossRef]
- Santhosh, K.M.; Holmen, A.; Chen, D. The influence of pore geometry of Pt containing ZSM-5, Beta and SBA-15 catalysts on dehydrogenation of propane. Micropor. Mesopor. Mater. 2009, 126, 152–158. [Google Scholar] [CrossRef]
- Jiang, F.; Zeng, L.; Li, S.; Liu, G.; Wang, S.; Gong, J. Propane dehydrogenation over Pt/TiO2–Al2O3 Catalysts. ACS Catal. 2015, 5, 438–447. [Google Scholar] [CrossRef]
- Bednarova, L.; Lyman, C.E.; Rytter, E.; Holmen, A. Effect of support on the size and composition of highly dispersed Pt–Sn particles. J. Catal. 2002, 211, 335–346. [Google Scholar] [CrossRef]
- Deng, L.; Miura, H.; Shishido, T.; Hosokawa, S.; Teramura, K.; Tanaka, T. Strong metal-support interaction between Pt and SiO2 following high-temperature reduction: A catalytic interface for propane dehydrogenation. Chem. Comm. 2017, 53, 6937–6940. [Google Scholar]
- Pérez-Ramírez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef]
- Chen, L.H.; Li, X.Y.; Rooke, J.C.; Zhang, Y.H.; Yang, X.Y.; Tang, Y.; Xiao, F.S.; Su, B.L. Hierarchically structured zeolites: Synthesis, mass transport properties and applications. J. Mater. Chem. 2012, 22, 17381–17403. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Yang, K.; Li, Y.; Wang, Y.; Xu, Y.; Wu, P. Effect of hydrothermal treatment on catalytic properties of PtSnNa/ZSM-5 catalyst for propane dehydrogenation. Micropor. Mesopor. Mater. 2006, 96, 245–254. [Google Scholar] [CrossRef]
- Marafi, M.; Furimsky, E. Hydroprocessing Catalysts Containing noble metals: Deactivation, regeneration, metals reclamation, and environment and safety energy. Fuels 2017, 31, 5711–5750. [Google Scholar] [CrossRef]
- Christensen, C.H.; Schmidt, I.; Carlsson, A.; Johannsen, K.; Herbst, K. Crystals in crystals nanocrystals within mesoporous zeolite single crystals. J. Am. Chem. Soc. 2005, 127, 8098–8102. [Google Scholar] [CrossRef]
- Zhu, X.; Goesten, M.G.; Koekkoek, A.J.J.; Mezari, B.; Kosinov, N.; Filonenko, G.; Friedrich, H.; Rohling, R.; Szyja, B.M.; Gascon, J.; Kapteijn, F.; Hensen, E.J.M. Establishing hierarchy: The chain of events leading to the formation of silicalite-1 nanosheets. Chem. Sci. 2016, 7, 6506–6513. [Google Scholar] [CrossRef] [PubMed]
- Chaikittisilp, W.; Suzuki, Y.; Mukti, R.R.; Suzuki, T.; Sugita, K.; Itabashi, K.; Shimojima, A.; Okubo, T. Formation of hierarchically organized zeolites by sequential intergrowth. Angew. Chem. 2013, 125, 3439–3443. [Google Scholar] [CrossRef]
- Lee, K.; Choi, M. Hierarchically micro-/mesoporous Pt/KL for alkane aromatization: Synergistic combination of high catalytic activity and suppressed hydrogenolysis. J. Catal. 2016, 340, 66–75. [Google Scholar] [CrossRef]
- Sun, Y.; Prins, R. Hydrodesulfurization of 4,6-Dimethyldibenzothiophene over Noble Metals Supported on Mesoporous Zeolites. Angew. Chem. Int. Ed. 2008, 47, 8478–8481. [Google Scholar] [CrossRef]
- Chao, P.H.; Tsai, S.-T.; Chang, S.-L.; Wang, I.; Tsai, T.C. Hexane isomerization over hierarchical Pt/MFI zeolite. Top. Catal. 2010, 53, 231–237. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.; Seo, Y.; Kim, J.C.; Ryoo, R. n-Heptane hydroisomerization over Pt/MFI zeolite nanosheets: Effects of zeolite crystal thickness and platinum location. J. Catal. 2013, 301, 187–197. [Google Scholar] [CrossRef]
- Fernandez, C.; Stan, I.; Gilson, J.P.; Thomas, K.; Vicente, A.; Bonilla, A.; Pérez-Ramírez, J. Hierarchical ZSM-5 zeolites in shape-selective xylene isomerization: Role of mesoporosity and acid site speciation. Chem. Eur. J. 2010, 16, 6224–6233. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Liu, H.; Wang, Y.; Xu, Y.; Wu, P. Effect of La addition on catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation. Appl. Catal. A 2007, 333, 202–210. [Google Scholar] [CrossRef]
- Gates, B.C. Supported metal clusters: Synthesis, structure, and catalysis. Chem. Rev. 1995, 95, 511–522. [Google Scholar] [CrossRef]
- Kulkarni, A.; Lobo-Lapidus, R.J.; Gates, B.C. Metal clusters on supports: Synthesis, structure, reactivity, and catalytic properties. Chem. Comm. 2010, 46, 5997–6015. [Google Scholar] [PubMed]
- Storck, S.; Bretinger, H.; Maier, W.F. Characterization of micro- and mesoporous solids by physisorption methods and pore-size analysis. Appl. Catal. A 1998, 174, 137–146. [Google Scholar] [CrossRef]
- Jacobs, G.; Ji, Y.; Davis, B.H.; Cronauer, D.; Kropf, A.J.; Marshall, C.L. Fischer–Tropsch synthesis: Temperature programmed EXAFS/XANES investigation of the influence of support type, cobalt loading, and noble metal promoter addition to the reduction behavior of cobalt oxide particles. Appl. Catal. A 2017, 333, 177–191. [Google Scholar] [CrossRef]
- Do, P.T.M.; Foster, A.J.; Chen, J.; Lobo, R.F. Bimetallic effects in the hydrodeoxygenation of meta-cresol on γ-Al2O3 supported Pt–Ni and Pt–Co catalysts. Green Chem. 2012, 14, 1388–1397. [Google Scholar] [CrossRef]
- Jeon, S.; Roh, H.-S.; Moon, D.J.; Bae, J.W. Aqueous phase reforming and hydrodeoxygenation of ethylene glycol on Pt/SiO2–Al2O3: Effects of surface acidity on product distribution. RSC Adv. 2016, 6, 68433–68444. [Google Scholar] [CrossRef]
- Maciel, G.E.; Sindorf, D.W. Silicon-29NMR study of the surface of silica gel by cross polarization and magic-angle spinning. J. Am. Chem. Soc. 1980, 102, 7606–7607. [Google Scholar] [CrossRef]
- Vu, B.K.; Song, M.B.; Ahn, I.Y.; Suh, Y.-W.; Suh, D.J.; Kim, W.-I.; Koh, H.-L.; Choi, Y.G.; Shin, E.W. Pt–Sn alloy phases and coke mobility over Pt–Sn/Al2O3 and Pt–Sn/ZnAl2O4 catalysts for propane dehydrogenation. Appl. Catal. A 2011, 400, 25–33. [Google Scholar] [CrossRef]
- Silva, L.P.C.; Terra, L.E.; Coutinho, A.C.S.L.S.; Passos, F.B. Sour water–gas shift reaction over Pt/CeZrO2 catalysts. J. Catal. 2016, 341, 1–12. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, T.; Xu, X.; Xiao, P.; Li, J. Pt nanoparticles supported on SBA-15: Synthesis, characterization and applications in heterogeneous catalysis. Appl. Catal. B 2013, 130, 197–217. [Google Scholar] [CrossRef]
- Akporiaye, D.; Jensen, S.F.; Olsbye, U.; Rohr, F.; Rytter, E.; Rønnekleiv, M.; Spjelkavik, A.I. A novel, Highly efficient catalyst for propane dehydrogenation. Ind. Eng. Chem. Res. 2001, 40, 4741–4748. [Google Scholar] [CrossRef]
- Wannapakdee, W.; Wattanakit, C.; Paluka, V.; Yutthalekha, T.; Limtrakul, J. One-pot synthesis of novel hierarchical bifunctional Ga/HZSM-5 nanosheets for propane aromatization. RSC Adv. 2016, 6, 2875–2881. [Google Scholar] [CrossRef]
- Chang, J.B.; Liu, C.H.; Liu, J.; Zhou, Y.Y.; Gao, X.; Wang, S.D. Green-chemistry Compatible Approach to TiO2-supported PdAu Bimetallic Nanoparticles for Solvent-free 1-Phenylethanol Oxidation under Mild Conditions. Nano-Micro Lett. 2015, 7, 307–315. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).