Band Gap Modulation of Tantalum(V) Perovskite Semiconductors by Anion Control
Abstract
:1. Introduction
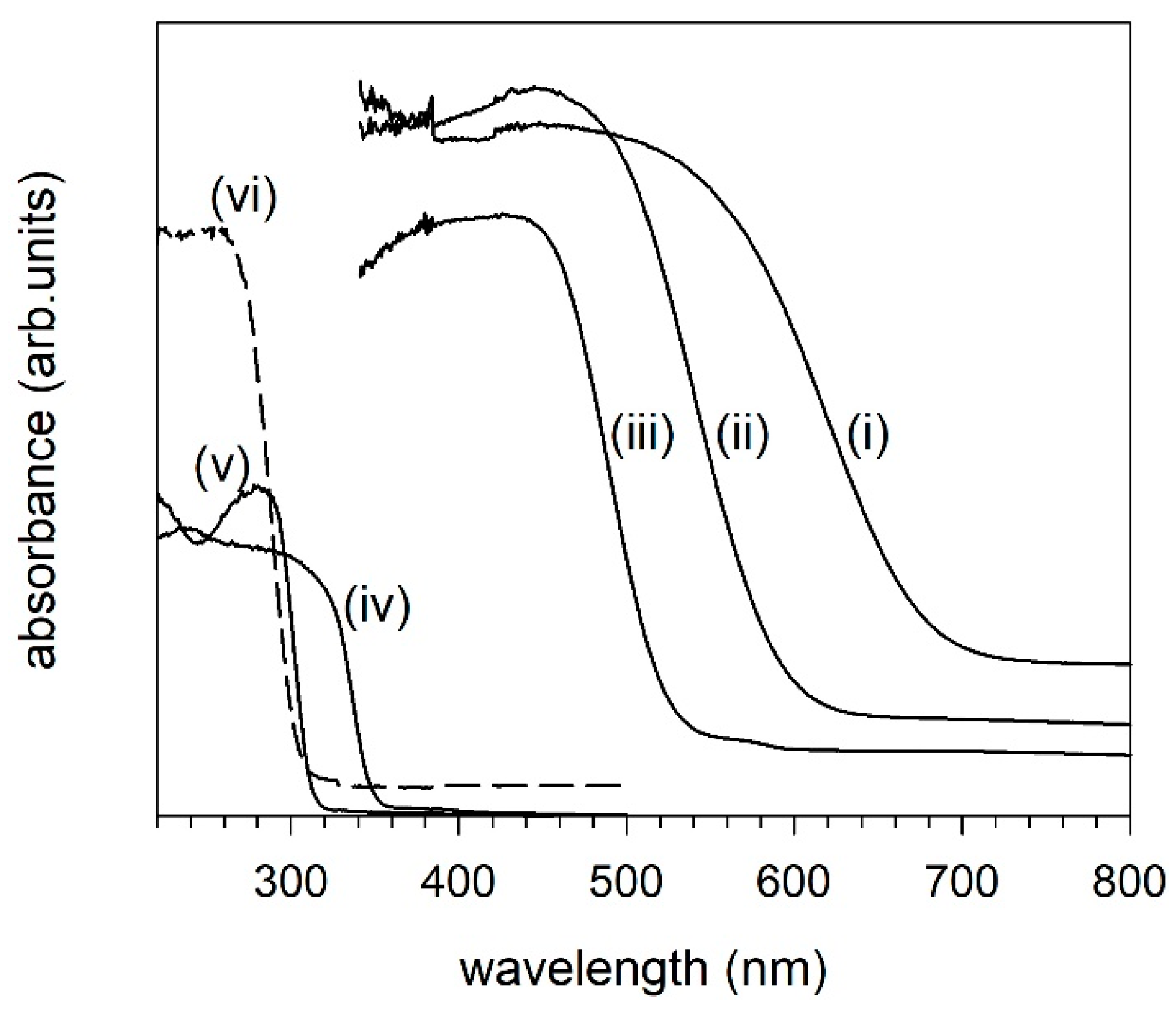
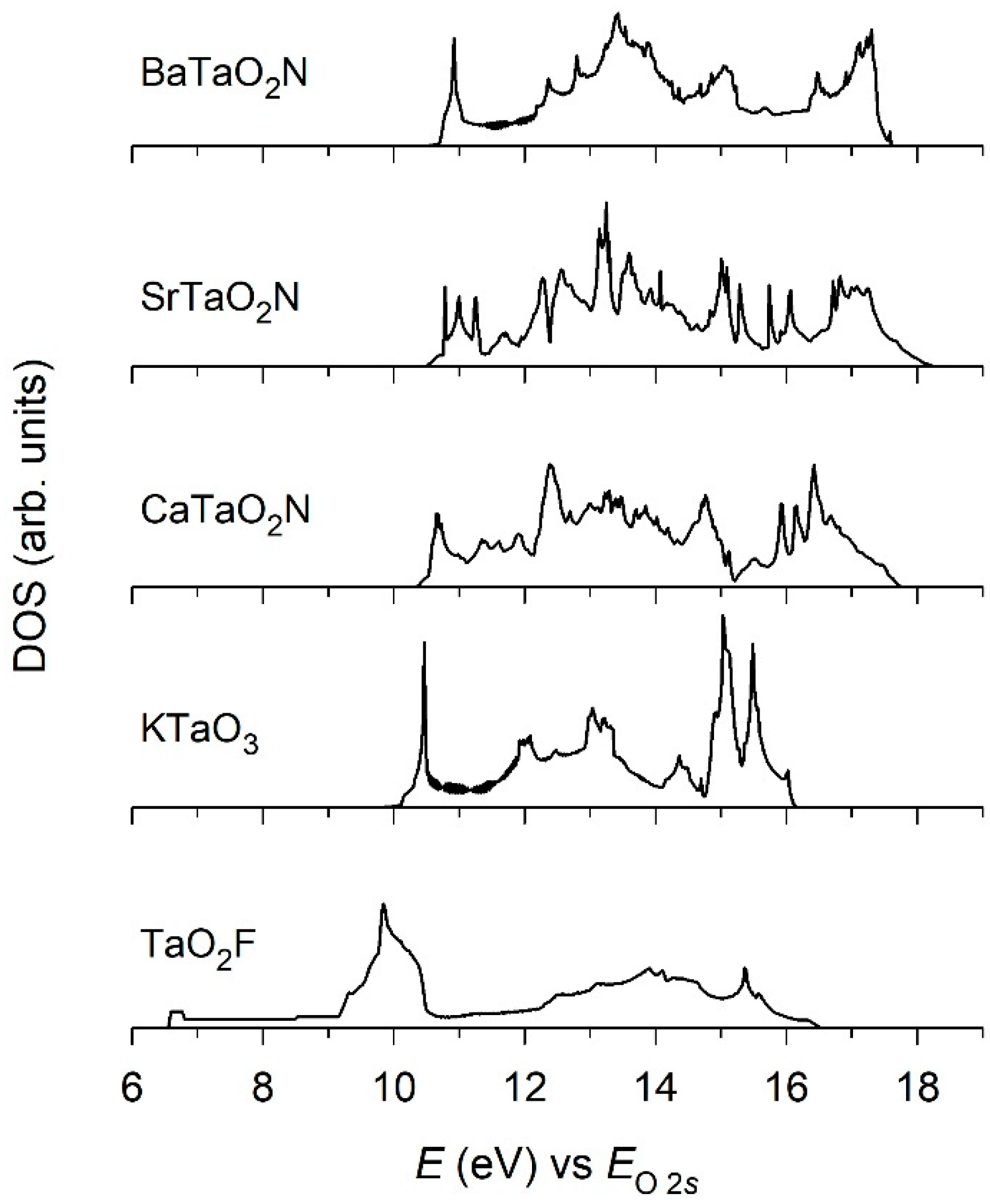
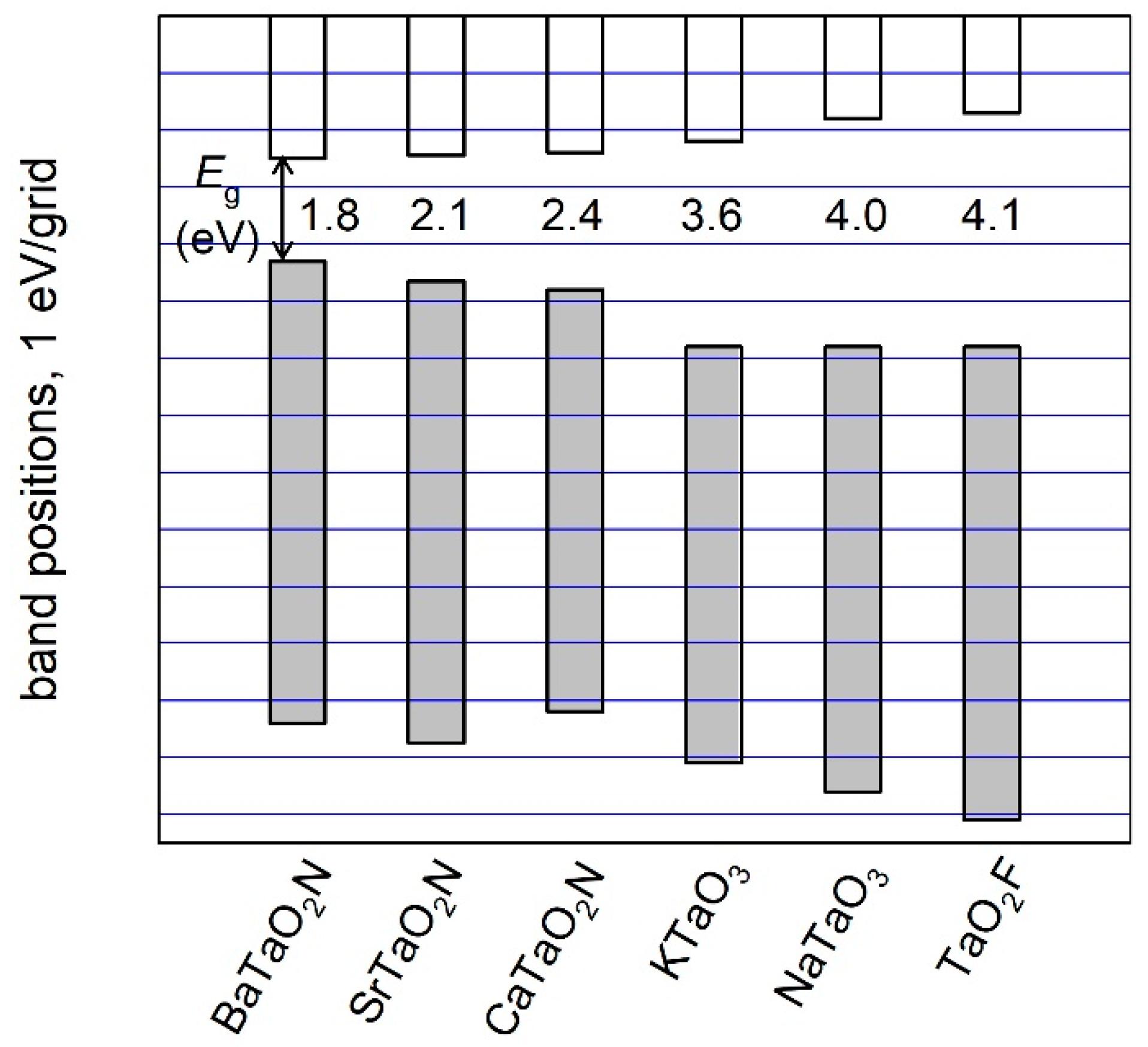
2. Results and Discussion
3. Materials and Methods
3.1. Sample Syntheses and Crystal Structure
3.2. Electronic Structure and Photocatalytic Property
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Higashi, M.; Abe, R.; Takata, T.; Domen, K. Photocatalytic Overall Water Splitting under Visible Light Using ATaO2N (A = Ca, Sr, Ba) and WO3 in a IO3−/I− Shuttle Redox Mediated System. Chem. Mater. 2009, 21, 1543–1549. [Google Scholar] [CrossRef]
- Takata, T.; Pan, C.; Domen, K. Recent progress in oxynitride photocatalysts for visible-light-driven water splitting. Sci. Technol. Adv. Mater. 2015, 16, 033506. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lazic, P.; Hautier, G.; Persson, K.; Ceder, G. First principles high throughput screening of oxynitrides for water-splitting photocatalysts. Energy Environ. Sci. 2013, 6, 157–168. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.; Gong, J. Tantalum-based semiconductors for solar water splitting. Chem. Soc. Rev. 2014, 43, 4395–4422. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Xinxin, G. A review of metal oxynitrides for photocatalysis. Inorg. Chem. Front. 2016, 3, 578–590. [Google Scholar] [CrossRef]
- Jansen, M.; Letschert, H.P. Inorganic yellow-red pigments without toxic metals. Nature 2000, 404, 980–982. [Google Scholar] [CrossRef]
- Tessier, F.; Marchand, R. Ternary and higher order rare-earth nitride materials: Synthesis and characterization of ionic-covalent oxynitride powders. J. Solid State Chem. 2003, 171, 143–151. [Google Scholar] [CrossRef]
- Cabana, J.; Dupre, N.; Gillot, F.; Chadwick, A.V.; Grey, C.P.; Palacin, M.R. Synthesis, Short-Range Structure, and Electrochemical Properties of New Phases in the Li−Mn−N−O System. Inorg. Chem. 2009, 48, 5141–5153. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Woodward, P.M.; Baba-Kishi, K.Z.; Tai, C.W. Characterization of the Structural, Optical, and Dielectric Properties of Oxynitride Perovskites AMO2N (A = Ba, Sr, Ca; M = Ta, Nb). Chem. Mater. 2004, 16, 1267–1276. [Google Scholar] [CrossRef]
- Harrison, W.A. Electronic Structure and the Properties of Solids; W. H. Freeman and Company: San Francisco, CA, USA, 1980; p. 50. [Google Scholar]
- Higashi, M.; Abe, R.; Teramura, K.; Takata, T.; Ohtani, B.; Domen, K. Two step water splitting into H2 and O2 under visible light by ATaO2N (A = Ca, Sr, Ba) and WO3 with IO3−/I− shuttle redox mediator. Chem. Phys. Lett. 2008, 452, 120–123. [Google Scholar] [CrossRef]
- Yashima, M.; Yamada, H.; Maeda, K.; Domen, K. Experimental visualization of covalent bonds and structrural disorder in a gallium zince oxynitride photocatalyst (Ga1−xZnx)(N1−xOx): Origin of visible light absorption. Chem. Commun. 2010, 46, 2379–2382. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, T.; Katayama, C.; Moriya, Y.; Minegishi, T.; Katayama, M.; Nishiyama, H.; Yamada, T.; Domen, K. Photocatalytic oxygen evolution using BaNbO2N modified with cobalt oxide under photoexcitation up to 740 nm. Energy Environ. Sci. 2013, 6, 3595–3599. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, S.; Thaweesak, S.; Luo, B.; Wang, L. Tantalum (Oxy)Nitride: Narrow Bandgap Photocatalysts for Solar Hydrogen generation. Engineering 2017, 3, 365–378. [Google Scholar] [CrossRef]
- Mukherji, A.; Seger, B.; Lu, G.Q.; Wang, L. Nitrogen Doped Sr2Ta2O7 Coupled with Graphene Sheets as Photocatalysts for Increased Photocatalytic Hydrogen Production. ACS Nano 2011, 5, 3483–3492. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, J.; Ding, C.; Li, R.; Jin, S.; Wang, D.; Han, H.; Zhang, F.; Li, C. Nitrogen-doped layered oxide Sr5Ta4O15−xNx for water reduction and oxidation under visible light irradiation. J. Mater. Chem. A 2013, 1, 5651–5659. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Kubota, J.; Domen, K. Photocatalytic Water Splitting Using Oxynitride and Nitride Semiconductor Powders for Production of Solar Hydrogen. Electrochem. Soc. Interface 2013, 22, 57–62. [Google Scholar] [CrossRef]
- Matoba, T.; Maeda, K.; Domen, K. Activation of BaTaO2N photocatalyst for enhanced non-sacrificial hydrogen evolution from water under visible light by forming a solid solution with BaZrO3. Chem. Eur. J. 2011, 17, 14731–14735. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Lu, D.; Domen, K. Direct water splitting into hydrogen and oxygen under visible light by using modified TaON photocatalysts with d0 electronic configuration. Chem. Eur. J. 2013, 19, 4986–4991. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Takata, T.; Nakabayashi, M.; Matsumoto, T.; Shibata, N.; Ikuhara, Y.; Domen, K. A complex perovskite-type oxynitride: The first photocatalyst for water splitting operable at up to 600 nm. Angew. Chem. Int. Ed. 2015, 54, 2955–2959. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Pan, C.; Takata, T.; Domen, K. Photocatalytic overall water splitting on the perovskite-type transition metal oxynitride CaTaO2N under visible light irradiation. Chem. Commun. 2015, 51, 7191–7194. [Google Scholar] [CrossRef]
- Shapiro, I.P. Determination of the forbidden zone width from diffuse reflection spectra. Opt. Specktroskopiya 1958, 4, 256–260. [Google Scholar]
- Cox, P.A. The Electronic Structure and Chemistry of Solids; Oxford University Press: Oxford, UK, 1987; pp. 45–72. [Google Scholar]
- Eng, H.W.; Barnes, P.W.; Auer, B.M.; Woodward, P.M. Investigations of the electronic structure of d0 transition metal oxides belonging to the perovskite family. J. Solid State Chem. 2003, 175, 94–109. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Zhurova, E.A.; Ivanov, Y.; Zavodnik, V.; Tsirelson, V. Electron density and atomic displacements in KTaO3. Acta Cryst. B 2000, 56, 594–600. [Google Scholar] [CrossRef]
- Kennedy, B.J.; Prodjosantoso, A.K.; Howard, C.J. Powder neutron diffraction study of the high temperature phase transitions in NaTaO3. J. Phys. Condens. Matter 1999, 11, 6319–6327. [Google Scholar] [CrossRef]
- Frevel, L.K.; Rinn, H.W. The crystal structure of NbO2F and TaO2F. Acta Cryst. 1956, 9, 626–627. [Google Scholar] [CrossRef]
- Larson, A.C.; von Dreele, R.B. General Structure Analysis System (GSAS)—Report LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 1994.
- Toby, B. EXPGUI, a graphical user interface for GSAS. J. Appl. Cryst. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Anderson, O.K.; Jepsen, O. Explicit, First-Principles Tight-Binding Theory. Phys. Rev. Lett. 1984, 53, 2571–2574. [Google Scholar] [CrossRef]
- Anderson, O.K.; Pawlowska, Z.; Jepsen, O. Illustration of the linear-muffin-tin-orbital tight-binding representation: Compact orbitals and charge density in Si. Phys. Rev. B 1986, 34, 5253–5269. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Yang, J.Y.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-I.; Woodward, P.M. Band Gap Modulation of Tantalum(V) Perovskite Semiconductors by Anion Control. Catalysts 2019, 9, 161. https://doi.org/10.3390/catal9020161
Kim Y-I, Woodward PM. Band Gap Modulation of Tantalum(V) Perovskite Semiconductors by Anion Control. Catalysts. 2019; 9(2):161. https://doi.org/10.3390/catal9020161
Chicago/Turabian StyleKim, Young-Il, and Patrick M. Woodward. 2019. "Band Gap Modulation of Tantalum(V) Perovskite Semiconductors by Anion Control" Catalysts 9, no. 2: 161. https://doi.org/10.3390/catal9020161
APA StyleKim, Y.-I., & Woodward, P. M. (2019). Band Gap Modulation of Tantalum(V) Perovskite Semiconductors by Anion Control. Catalysts, 9(2), 161. https://doi.org/10.3390/catal9020161





