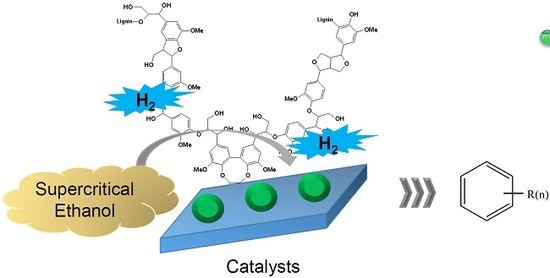
Catalytic Cleavage of Ether Bond in a Lignin Model Compound over Carbon-Supported Noble Metal Catalysts in Supercritical Ethanol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
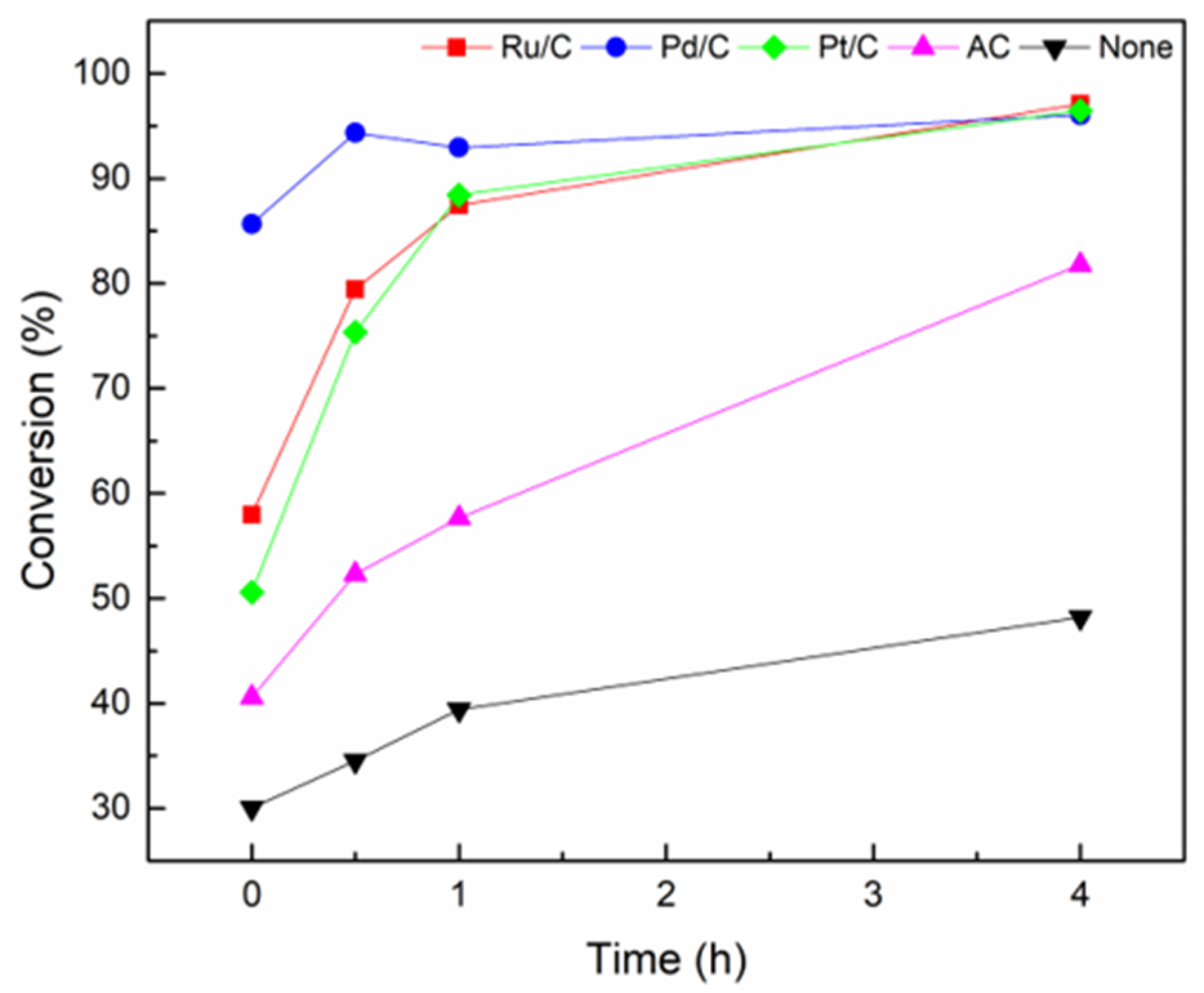
2.2. Conversion Rates and Reactivity over Noble Metal-Based Catalysts in Supercritical Ethanol
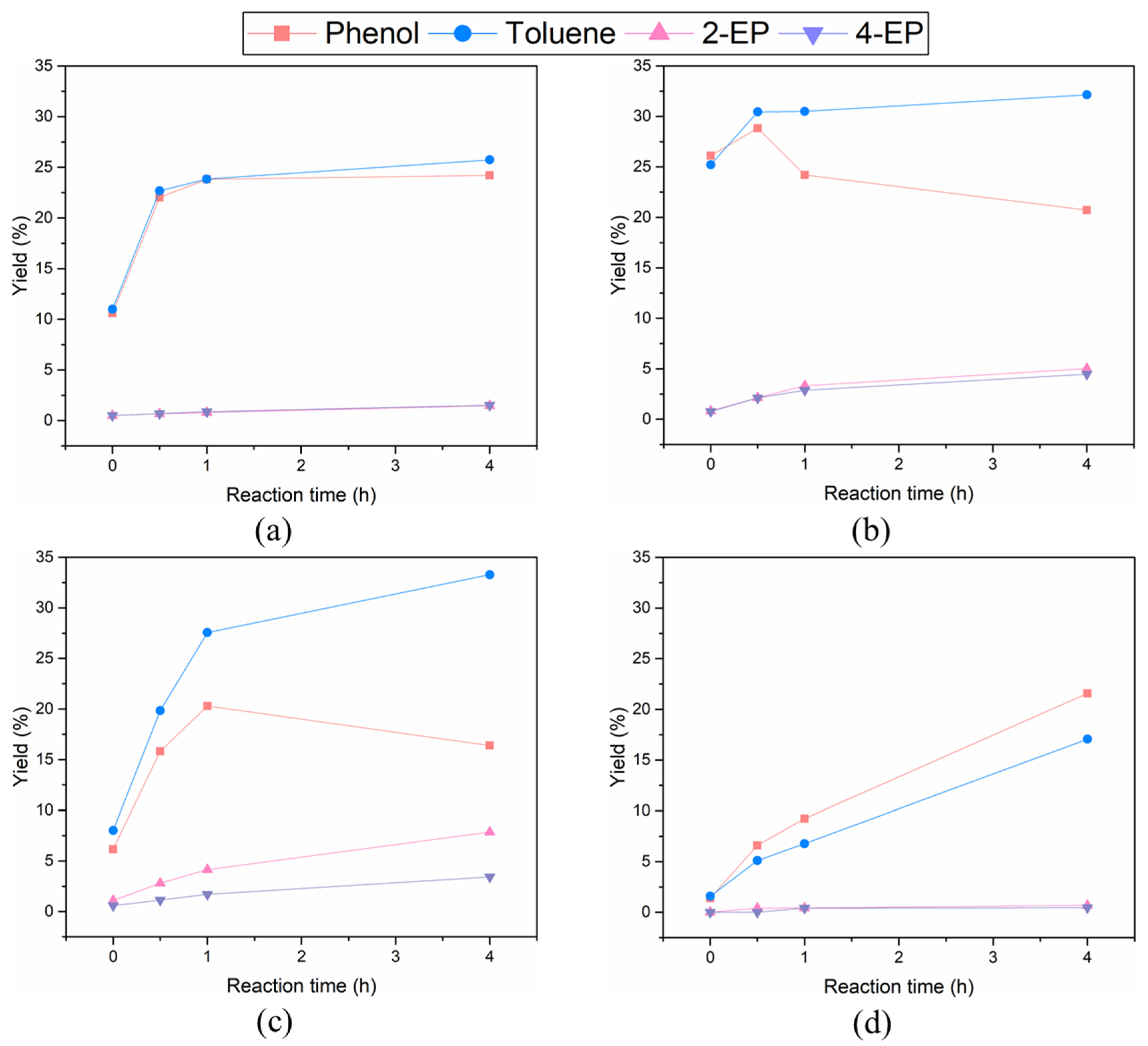
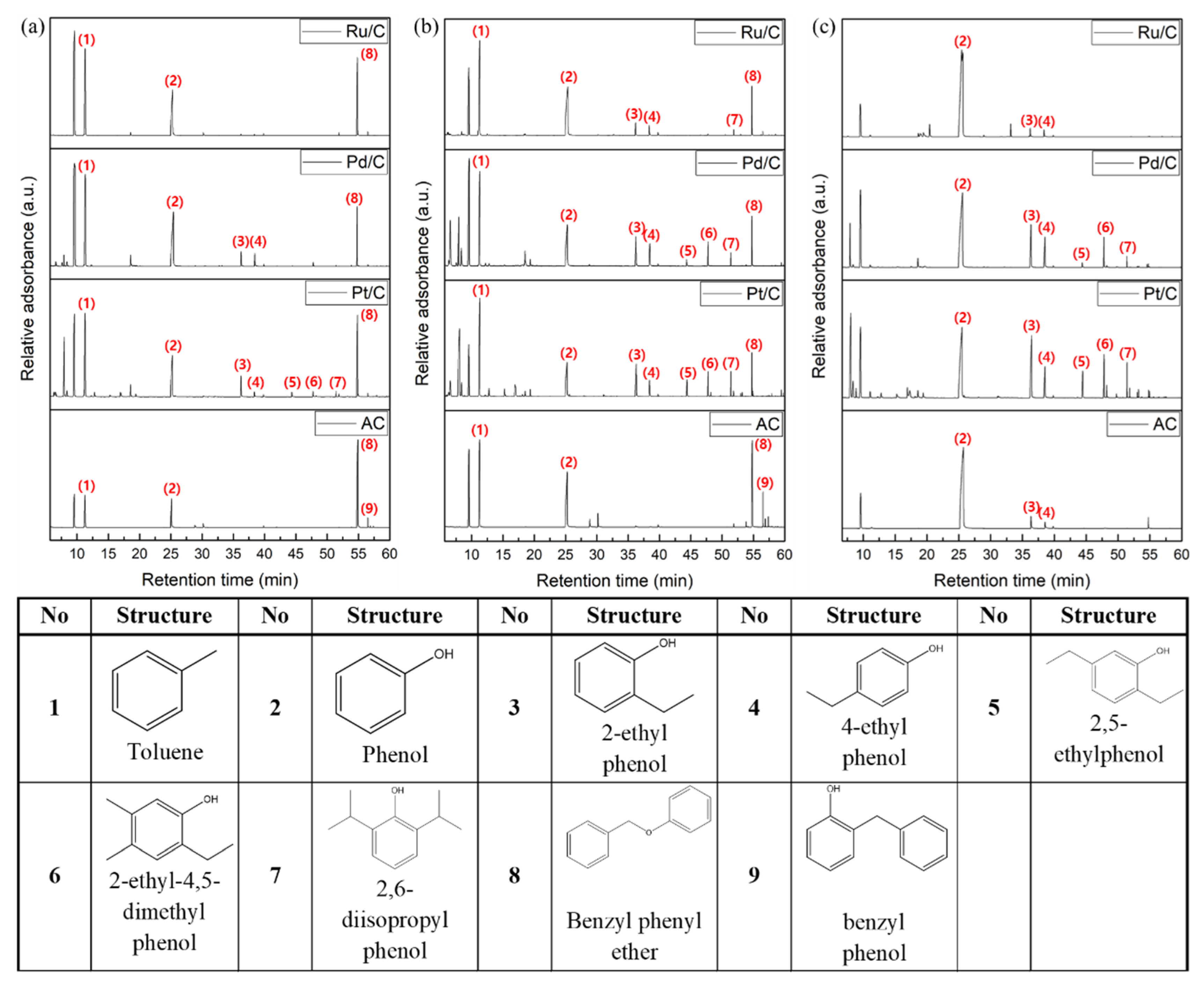
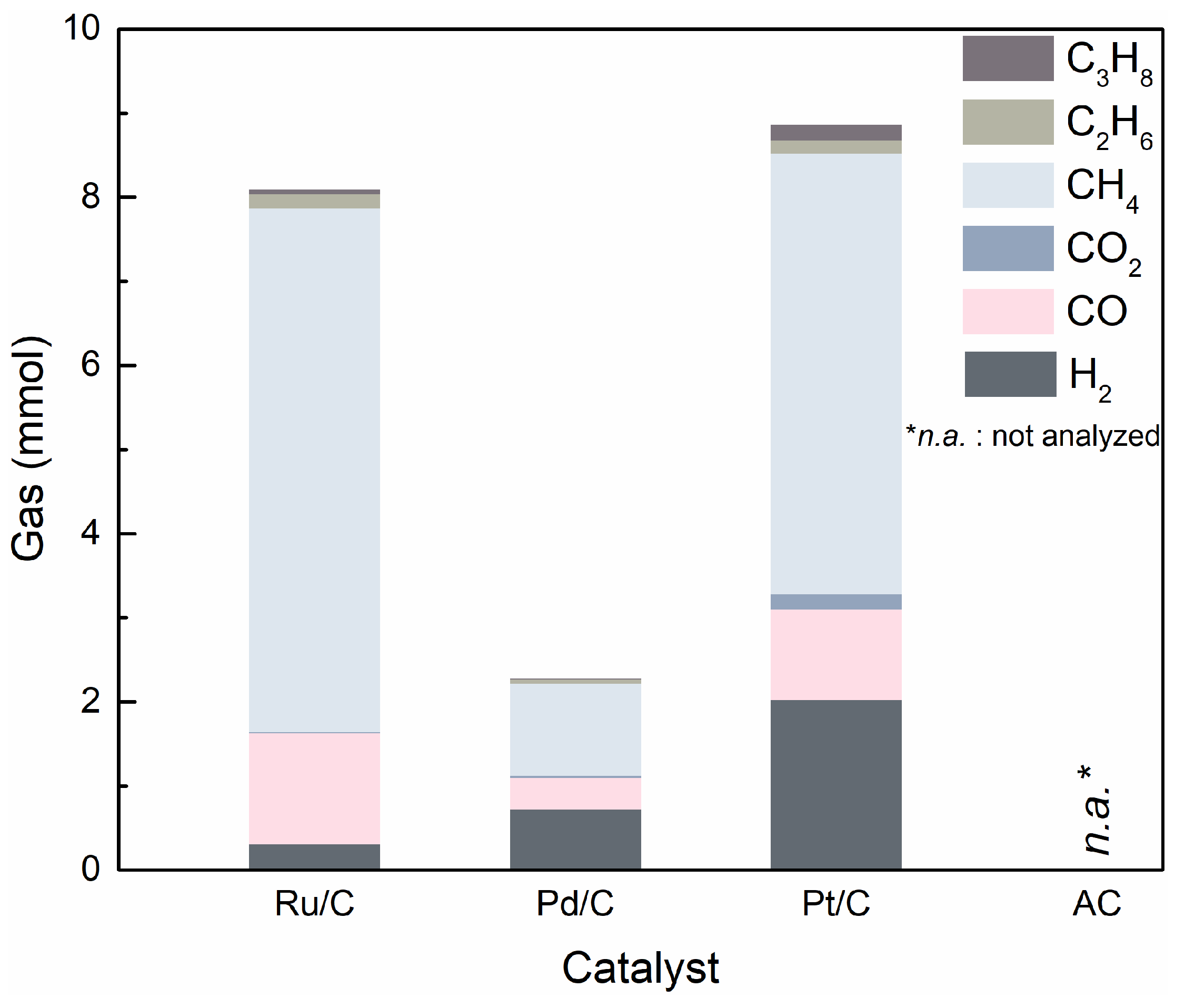
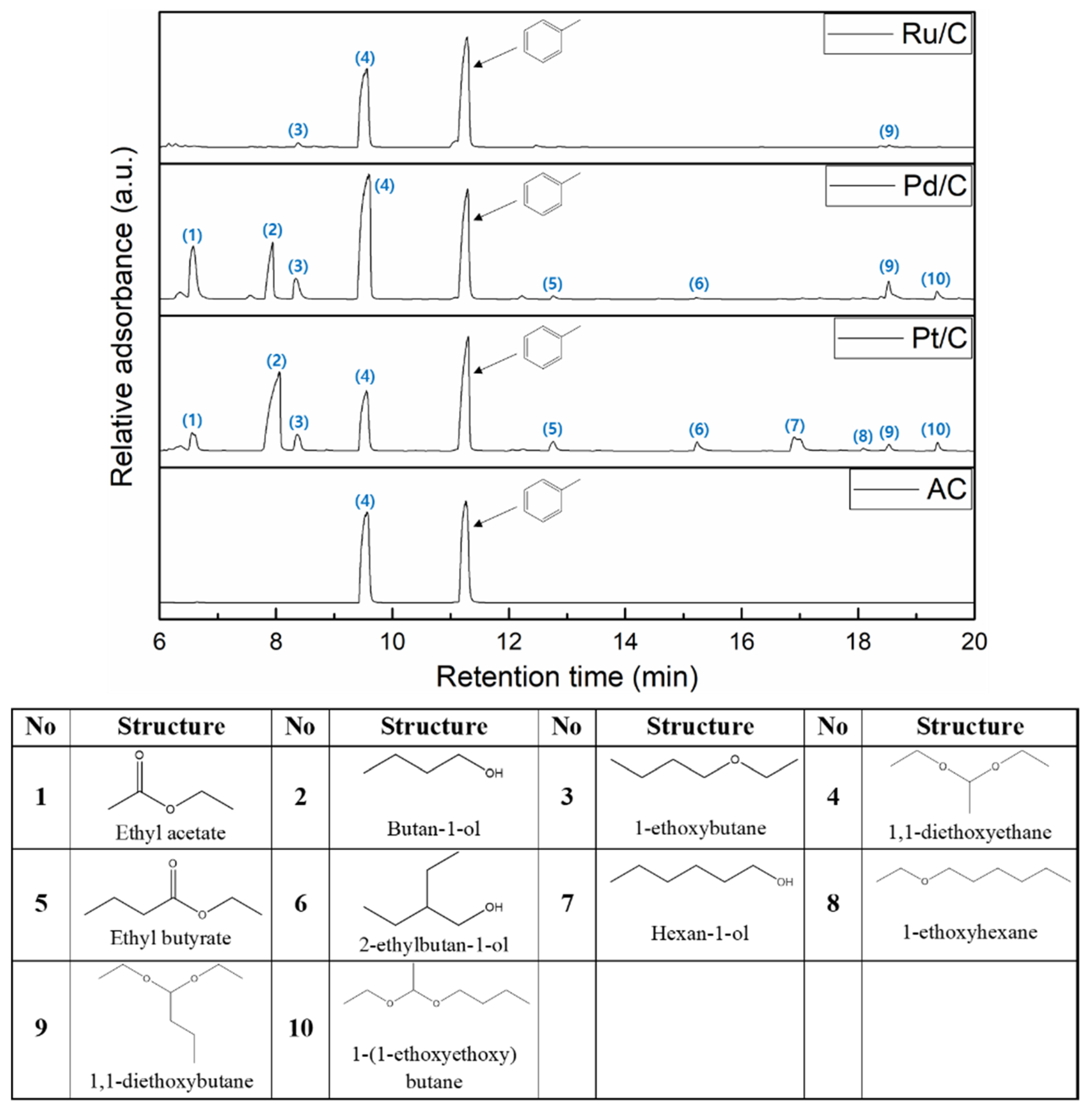
2.3. The Effect of Noble Metal over Activated Carbon Support on Product Distribution
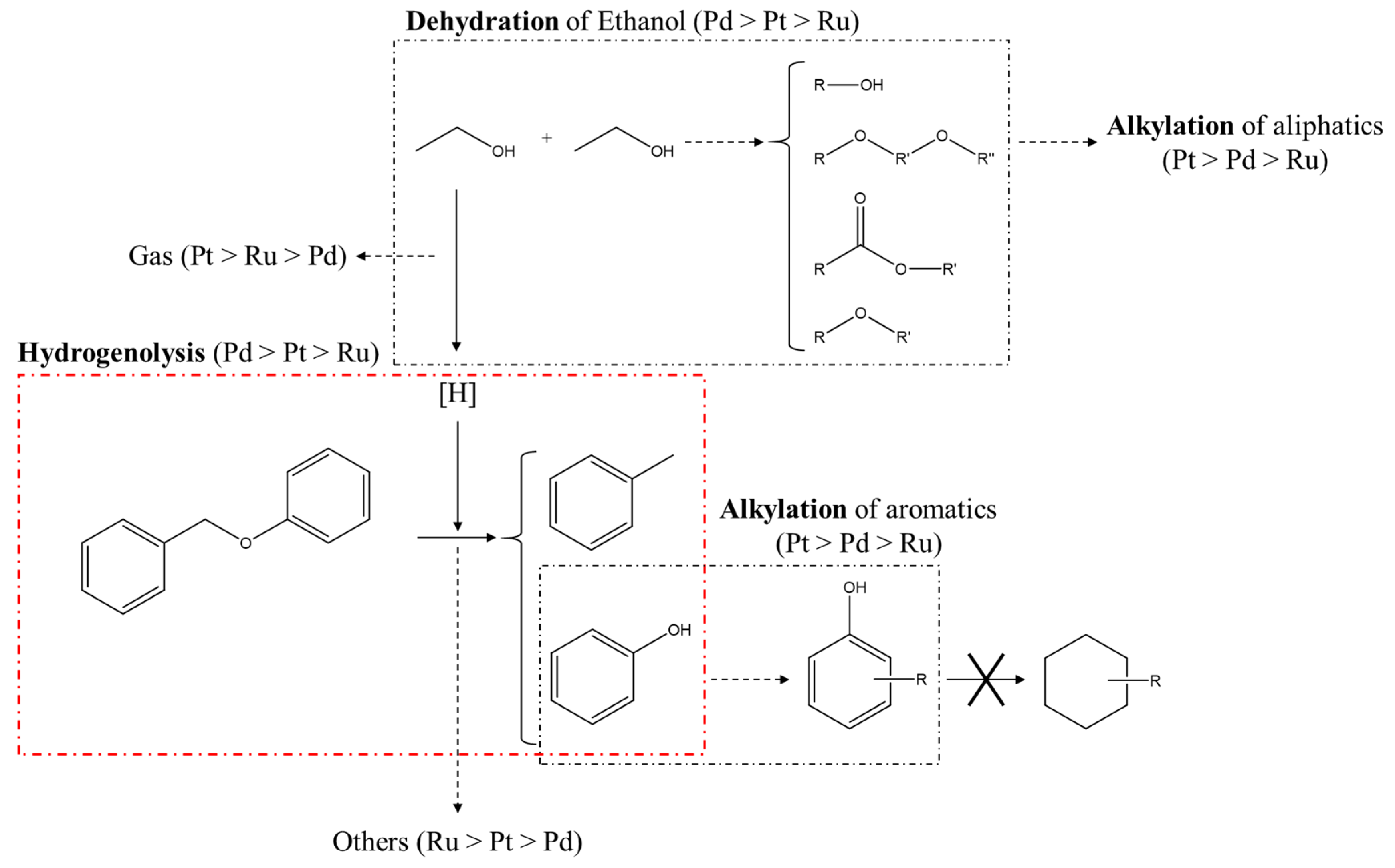
2.4. The Plausible Pathways Of BPE Decomposition
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Characterization
3.4. Experimental Setup and Procedure
3.5. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zakzeski, J.; Jongerius, A.L.; Bruijnincx, P.C.; Weckhuysen, B.M. Catalytic lignin valorization process for the production of aromatic chemicals and hydrogen. ChemSusChem 2012, 5, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, R.; Monteil-Rivera, F.; Lavoie, J.M. Conversion of lignin to aromatic-based chemicals (l-chems) and biofuels (l-fuels). Bioresour. Technol. 2012, 121, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant. Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Deuss, P.J.; Barta, K.; de Vries, J.G. Homogeneous catalysis for the conversion of biomass and biomass-derived platform chemicals. Catal. Sci. Technol. 2014, 4, 1174–1196. [Google Scholar] [CrossRef]
- Zhou, S.; Pecha, B.; van Kuppevelt, M.; McDonald, A.G.; Garcia-Perez, M. Slow and fast pyrolysis of douglas-fir lignin: Importance of liquid-intermediate formation on the distribution of products. Biomass Bioenergy 2014, 66, 398–409. [Google Scholar] [CrossRef]
- Yan, F.; Ma, R.; Ma, X.; Cui, K.; Wu, K.; Chen, M.; Li, Y. Ethanolysis of kraft lignin to platform chemicals on a MoC 1-x /Cu-MgAlO z catalyst. Appl. Catal. B Environ. 2017, 202, 305–313. [Google Scholar] [CrossRef]
- Ma, X.; Ma, R.; Hao, W.; Chen, M.; Yan, F.; Cui, K.; Tian, Y.; Li, Y. Common pathways in ethanolysis of kraft lignin to platform chemicals over molybdenum-based catalysts. ACS Catal. 2015, 5, 4803–4813. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, Y. Effects of aromatic cycle oils on performance of residue hydrotreating. Korean J. Chem. Eng. 2013, 30, 1985–1989. [Google Scholar] [CrossRef]
- Collias, D.I.; Harris, A.M.; Nagpal, V.; Cottrell, I.W.; Schultheis, M.W. Biobased terephthalic acid technologies: A literature review. Ind. Biotechnol. 2014, 10, 91–105. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin depolymerization and conversion: A review of thermochemical methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Boonyasuwat, S.; Omotoso, T.; Resasco, D.E.; Crossley, S.P. Conversion of guaiacol over supported Ru catalysts. Catal. Lett. 2013, 143, 783–791. [Google Scholar] [CrossRef]
- Nimmanwudipong, T.; Aydin, C.; Lu, J.; Runnebaum, R.C.; Brodwater, K.C.; Browning, N.D.; Block, D.E.; Gates, B.C. Selective hydrodeoxygenation of guaiacol catalyzed by platinum supported on magnesium oxide. Catal. Lett. 2012, 142, 1190–1196. [Google Scholar] [CrossRef]
- Nimmanwudipong, T.; Runnebaum, R.C.; Block, D.E.; Gates, B.C. Catalytic reactions of guaiacol: Reaction network and evidence of oxygen removal in reactions with hydrogen. Catal. Lett. 2011, 141, 779–783. [Google Scholar] [CrossRef]
- Schlosberg, R.H.; Davis, W.H.; Ashe, T.R. Pyrolysis studies of organic oxygenates. 2. Benzyl phenyl ether pyrolysis under batch autoclave conditions. Fuel 1981, 60, 201–204. [Google Scholar] [CrossRef]
- He, J.; Zhao, C.; Lercher, J.A. Ni-catalyzed cleavage of aryl ethers in the aqueous phase. J. Am. Chem. Soc. 2012, 134, 20768–20775. [Google Scholar] [CrossRef]
- Wu, B.C.; Klein, M.T.; Sandler, S.I. Influence of supercritical fluid solvent density on benzyl phenyl ether pyrolysis: Indications of diffusional limitations. Energy Fuels 1991, 5, 453–458. [Google Scholar] [CrossRef]
- Wu, B.C.; Klein, M.T.; Sandler, S.I. The benzylphenylether thermolysis mechanism: Insights from phase behavior. AIChE J. 1990, 36, 1129–1136. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, J.K.; Kang, K.H.; Song, J.C.; Song, I.K. Selective cleavage of CO bond in benzyl phenyl ether to aromatics over Pd–Fe bimetallic catalyst supported on ordered mesoporous carbon. Appl. Catal. A Gen. 2015, 498, 142–149. [Google Scholar] [CrossRef]
- Løhre, C.; Barth, T.; Kleinert, M. The effect of solvent and input material pretreatment on product yield and composition of bio-oils from lignin solvolysis. J. Anal. Appl. Pyrol. 2016, 119, 208–216. [Google Scholar] [CrossRef]
- Gosselink, R.J.; Teunissen, W.; van Dam, J.E.; de Jong, E.; Gellerstedt, G.; Scott, E.L.; Sanders, J.P. Lignin depolymerisation in supercritical carbon dioxide/acetone/water fluid for the production of aromatic chemicals. Bioresour. Technol. 2012, 106, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Jae, J.; Myung, S.; Sung, B.H.; Dong, J.I.; Park, Y.K. Investigation into the lignin decomposition mechanism by analysis of the pyrolysis product of pinus radiata. Bioresour. Technol. 2016, 219, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.-X.; Zong, Z.-M.; Yan, H.-L.; Li, Z.-K.; Wei, X.-Y. Improvement of organonitrogen compounds in methanol-soluble portion from supercritical methanolysis of pretreated rice straw with trichoderma sp. Ah. Fuel 2017, 205, 100–108. [Google Scholar] [CrossRef]
- Barta, K.; Warner, G.R.; Beach, E.S.; Anastas, P.T. Depolymerization of organosolv lignin to aromatic compounds over Cu-doped porous metal oxides. Green Chem. 2014, 16, 191–196. [Google Scholar] [CrossRef]
- Warner, G.; Hansen, T.S.; Riisager, A.; Beach, E.S.; Barta, K.; Anastas, P.T. Depolymerization of organosolv lignin using doped porous metal oxides in supercritical methanol. Bioresour. Technol. 2014, 161, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Kim, C.S.; Kim, Y.; Kim, J. High-yield and high-calorific bio-oil production from concentrated sulfuric acid hydrolysis lignin in supercritical ethanol. Fuel 2016, 172, 238–247. [Google Scholar] [CrossRef]
- Güvenatam, B.; Heeres, E.H.J.; Pidko, E.A.; Hensen, E.J.M. Lewis-acid catalyzed depolymerization of protobind lignin in supercritical water and ethanol. Catal. Today 2016, 259, 460–466. [Google Scholar] [CrossRef]
- Zabeti, M.; Wan Daud, W.M.A.; Aroua, M.K. Activity of solid catalysts for biodiesel production: A review. Fuel Process. Technol. 2009, 90, 770–777. [Google Scholar] [CrossRef]
- Jackson, M.A.; Compton, D.L.; Boateng, A.A. Screening heterogeneous catalysts for the pyrolysis of lignin. J. Anal. Appl. Pyrol. 2009, 85, 226–230. [Google Scholar] [CrossRef]
- De, S.; Balu, A.M.; van der Waal, J.C.; Luque, R. Biomass-derived porous carbon materials: Synthesis and catalytic applications. ChemCatChem 2015, 7, 1608–1629. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Kusunoki, Y.; Miyazawa, T.; Kunimori, K.; Tomishige, K. Highly active metal-acid bifunctional catalyst system for hydrogenolysis of glycerol under mild reaction conditions. Catal. Commun. 2005, 6, 645–649. [Google Scholar] [CrossRef]
- Lee, H.S.; Jae, J.; Ha, J.M.; Suh, D.J. Hydro- and solvothermolysis of kraft lignin for maximizing production of monomeric aromatic chemicals. Bioresour. Technol. 2016, 203, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Guo, J.; Wang, X.; Wang, J.; Fan, W. Alcoholysis: A promising technology for conversion of lignocellulose and platform chemicals. ChemSusChem 2017, 10, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Konnerth, H.; Zhang, J.; Ma, D.; Prechtl, M.H.G.; Yan, N. Base promoted hydrogenolysis of lignin model compounds and organosolv lignin over metal catalysts in water. Chem. Eng. Sci. 2015, 123, 155–163. [Google Scholar] [CrossRef]
- Resende, F.L.; Savage, P.E. Effect of metals on supercritical water gasification of cellulose and lignin. Ind. Eng. Chem. Res. 2010, 49, 2694–2700. [Google Scholar] [CrossRef]
- Yan, N.; Zhao, C.; Dyson, P.J.; Wang, C.; Liu, L.T.; Kou, Y. Selective degradation of wood lignin over noble-metal catalysts in a two-step process. ChemSusChem 2008, 1, 626–629. [Google Scholar]
- Wildschut, J.; Mahfud, F.H.; Venderbosch, R.H.; Heeres, H.J. Hydrotreatment of fast pyrolysis oil using heterogeneous noble-metal catalysts. Ind. Eng. Chem. Res. 2009, 48, 10324–10334. [Google Scholar] [CrossRef]
- Erdőhelyi, A.; Raskó, J.; Kecskés, T.; Tóth, M.; Dömök, M.; Baán, K. Hydrogen formation in ethanol reforming on supported noble metal catalysts. Catal. Today 2006, 116, 367–376. [Google Scholar] [CrossRef]
- Horikawa, Y.; Uchino, Y.; Sako, T. Alkylation and acetal formation using supercritical alcohol without catalyst. Chem. Lett. 2003, 32, 232–233. [Google Scholar] [CrossRef]
- Huang, X.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Ethanol as capping agent and formaldehyde scavenger for efficient depolymerization of lignin to aromatics. Green Chem. 2015, 17, 4941–4950. [Google Scholar] [CrossRef]
- Huang, X.; Koranyi, T.I.; Boot, M.D.; Hensen, E.J. Catalytic depolymerization of lignin in supercritical ethanol. ChemSusChem 2014, 7, 2276–2288. [Google Scholar] [CrossRef] [PubMed]
- Koda, K.; Matsu-ura, T.; Obora, Y.; Ishii, Y. Guerbet reaction of ethanol ton-butanol catalyzed by iridium complexes. Chem. Lett. 2009, 38, 838–839. [Google Scholar] [CrossRef]
- Scalbert, J.; Thibault-Starzyk, F.; Jacquot, R.; Morvan, D.; Meunier, F. Ethanol condensation to butanol at high temperatures over a basic heterogeneous catalyst: How relevant is acetaldehyde self-aldolization? J. Catal. 2014, 311, 28–32. [Google Scholar] [CrossRef]
- Ndou, A. Dimerisation of ethanol to butanol over solid-base catalysts. Appl. Catal. A Gen. 2003, 251, 337–345. [Google Scholar] [CrossRef]
- Riittonen, T.; Toukoniitty, E.; Madnani, D.K.; Leino, A.-R.; Kordas, K.; Szabo, M.; Sapi, A.; Arve, K.; Wärnå, J.; Mikkola, J.-P. One-pot liquid-phase catalytic conversion of ethanol to 1-butanol over aluminium oxide—The effect of the active metal on the selectivity. Catalysts 2012, 2, 68–84. [Google Scholar] [CrossRef]
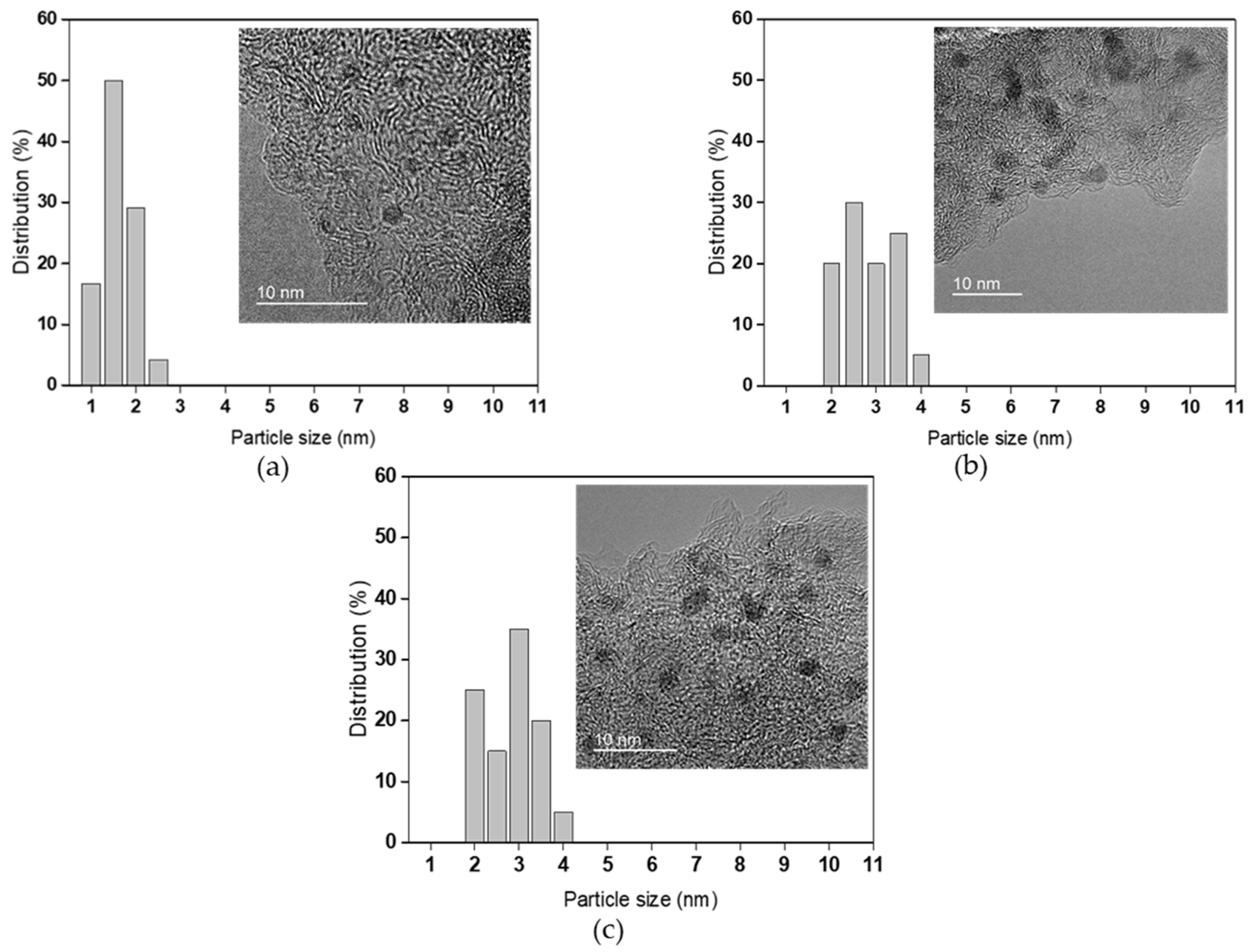
| Catalyst | N2 Physisorption | ||||
|---|---|---|---|---|---|
| Surface Area (m2/g) 1 | Micropore Area (m2/g) 2 | External Surface Area (m2/g) 2 | Pore Volume (cm3/g) 3 | Pore Diameter (nm) 3 | |
| Ru/C | 789.51 | 463.30 | 326.21 | 0.61 | 3.09 |
| Pd/C | 953.70 | 524.26 | 429.44 | 0.68 | 2.84 |
| Pt/C | 834.12 | 449.17 | 384.95 | 0.61 | 2.94 |
| AC | 1032.47 | 596.05 | 436.42 | 0.72 | 2.81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Jeong, S.; Ban, C.; Kim, H.; Kim, D.H. Catalytic Cleavage of Ether Bond in a Lignin Model Compound over Carbon-Supported Noble Metal Catalysts in Supercritical Ethanol. Catalysts 2019, 9, 158. https://doi.org/10.3390/catal9020158
Yang S, Jeong S, Ban C, Kim H, Kim DH. Catalytic Cleavage of Ether Bond in a Lignin Model Compound over Carbon-Supported Noble Metal Catalysts in Supercritical Ethanol. Catalysts. 2019; 9(2):158. https://doi.org/10.3390/catal9020158
Chicago/Turabian StyleYang, Seungdo, Soyeon Jeong, Chunghyeon Ban, Hyungjoo Kim, and Do Heui Kim. 2019. "Catalytic Cleavage of Ether Bond in a Lignin Model Compound over Carbon-Supported Noble Metal Catalysts in Supercritical Ethanol" Catalysts 9, no. 2: 158. https://doi.org/10.3390/catal9020158
APA StyleYang, S., Jeong, S., Ban, C., Kim, H., & Kim, D. H. (2019). Catalytic Cleavage of Ether Bond in a Lignin Model Compound over Carbon-Supported Noble Metal Catalysts in Supercritical Ethanol. Catalysts, 9(2), 158. https://doi.org/10.3390/catal9020158