Phase Controlled Synthesis of Pt Doped Co Nanoparticle Composites Using a Metal-Organic Framework for Fischer–Tropsch Catalysis
Abstract
1. Introduction
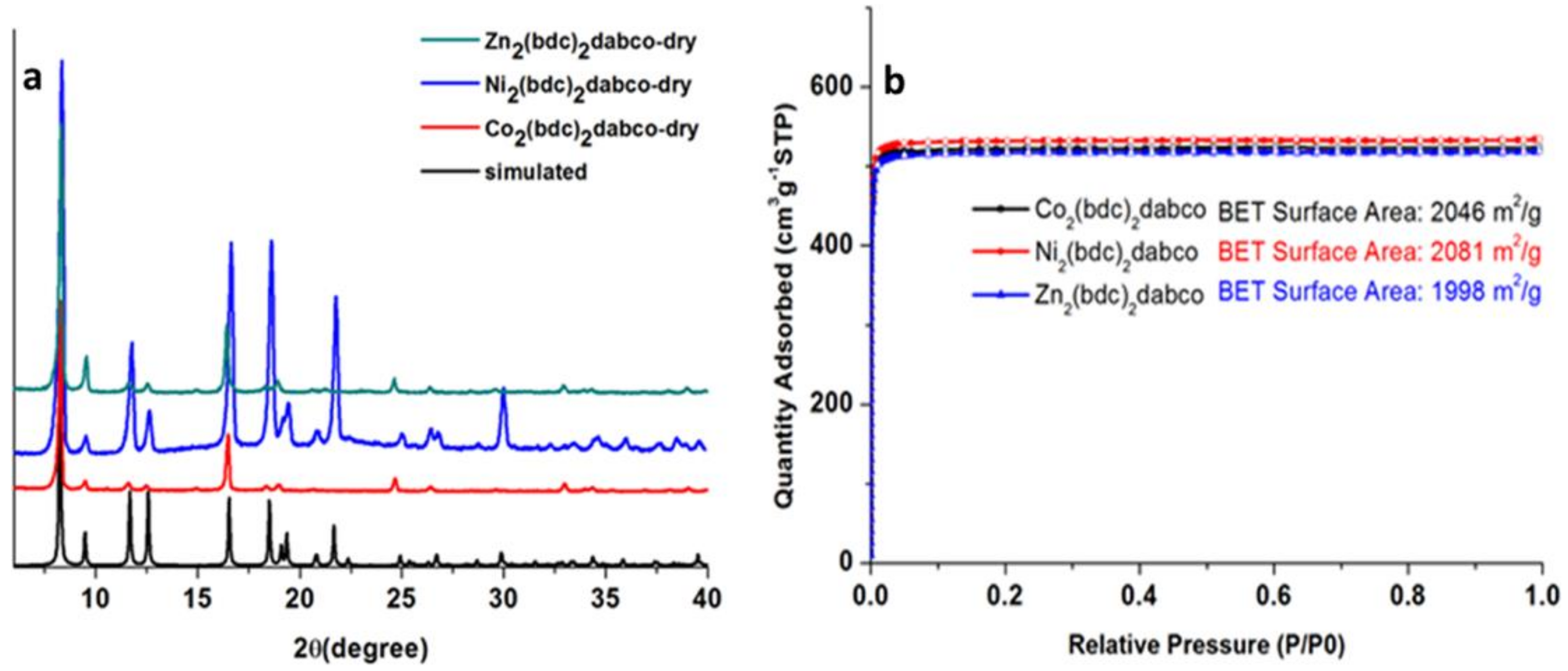
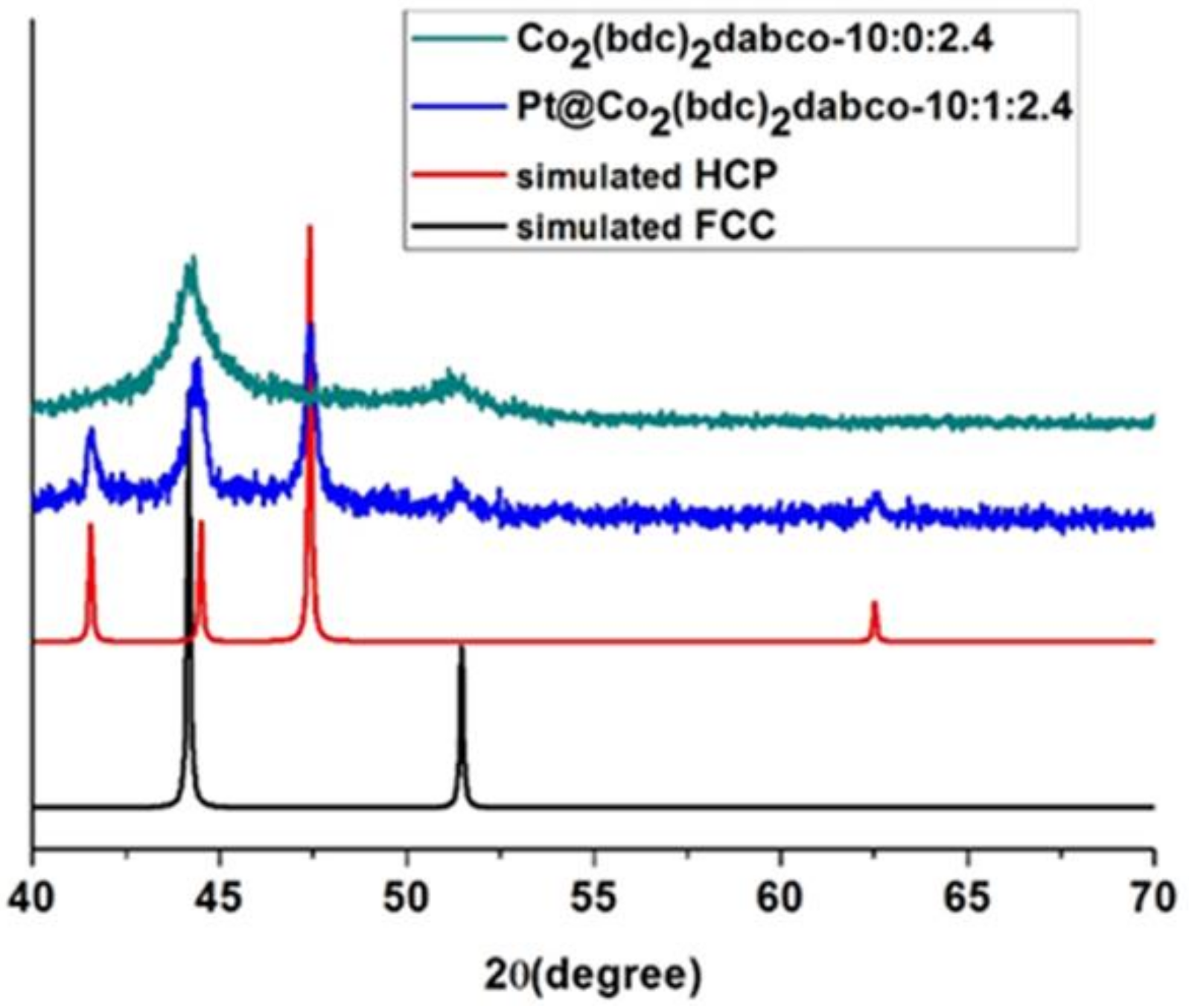
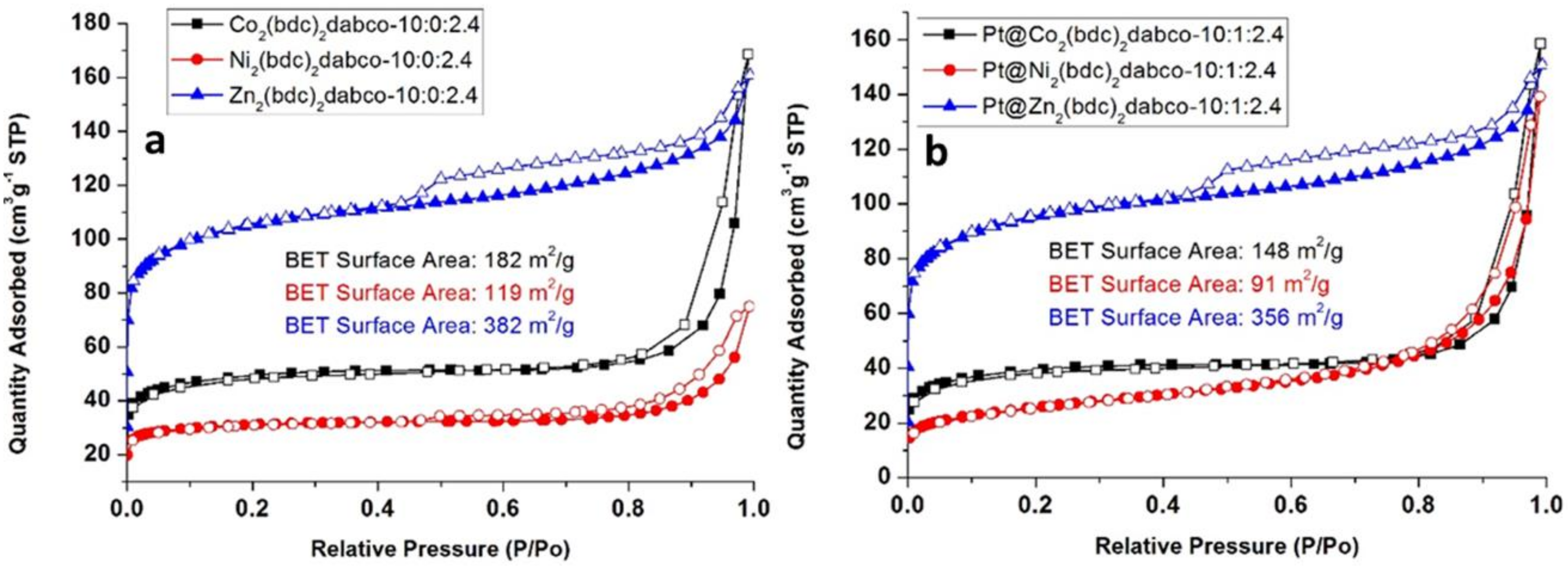
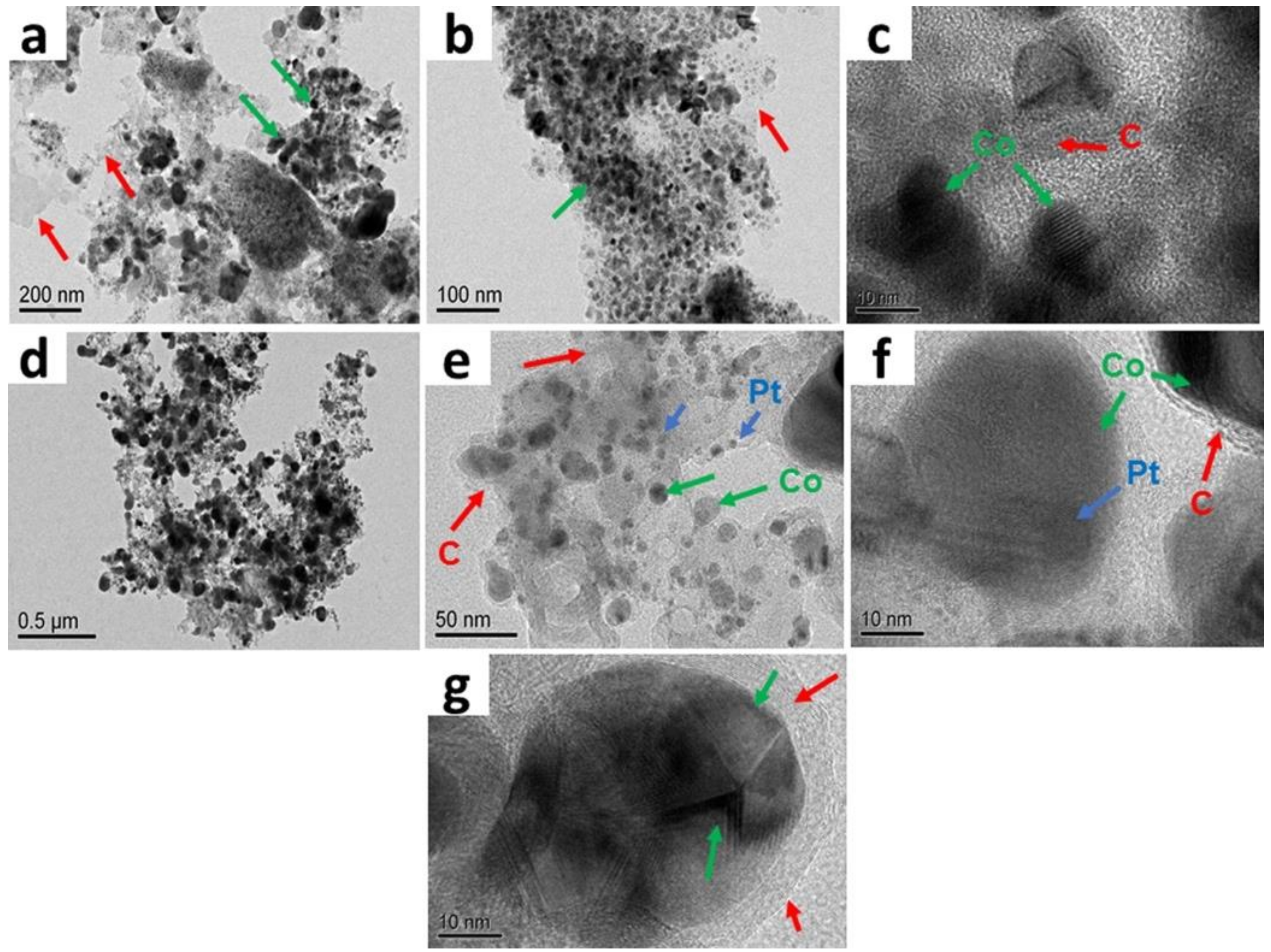
2. Results
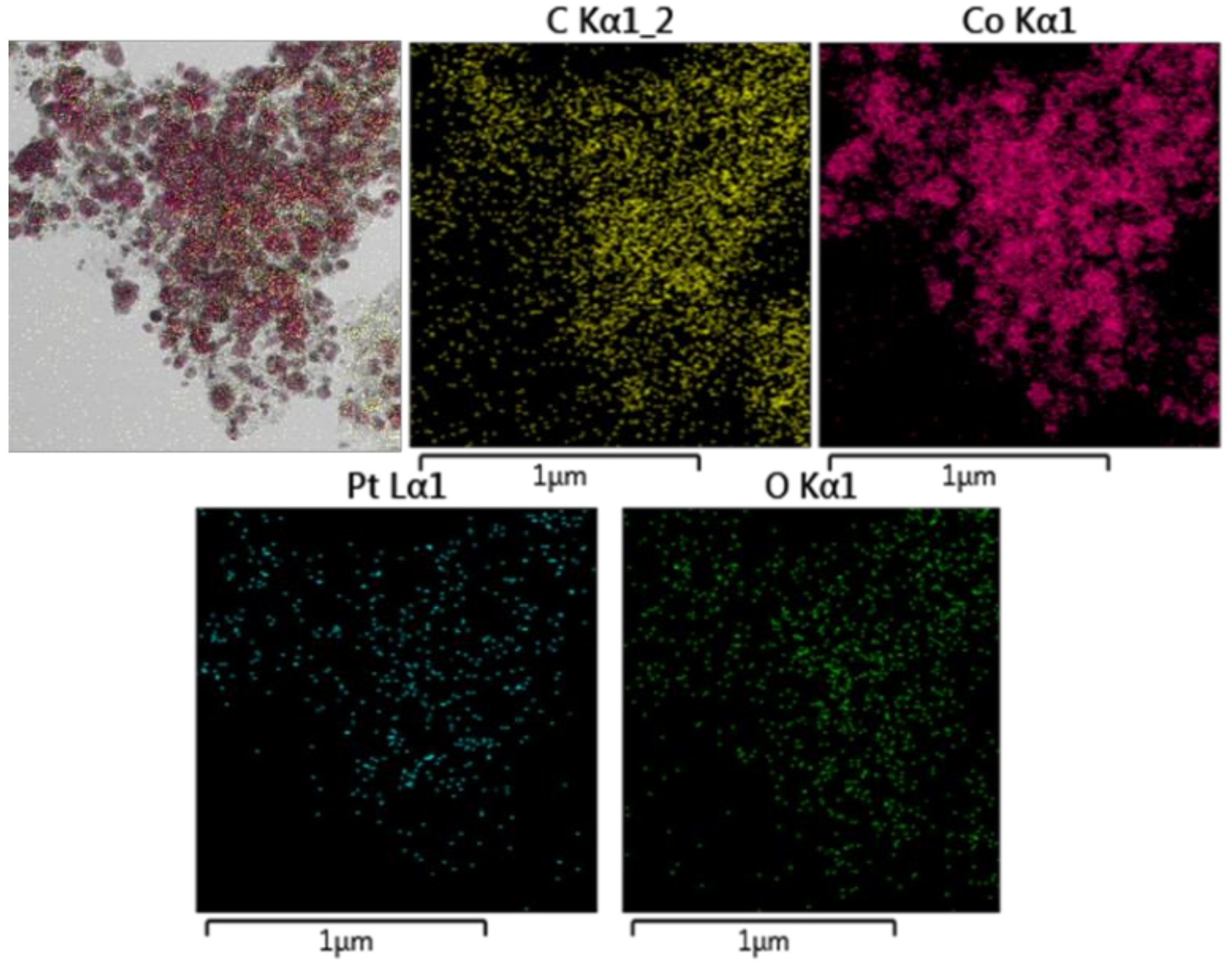
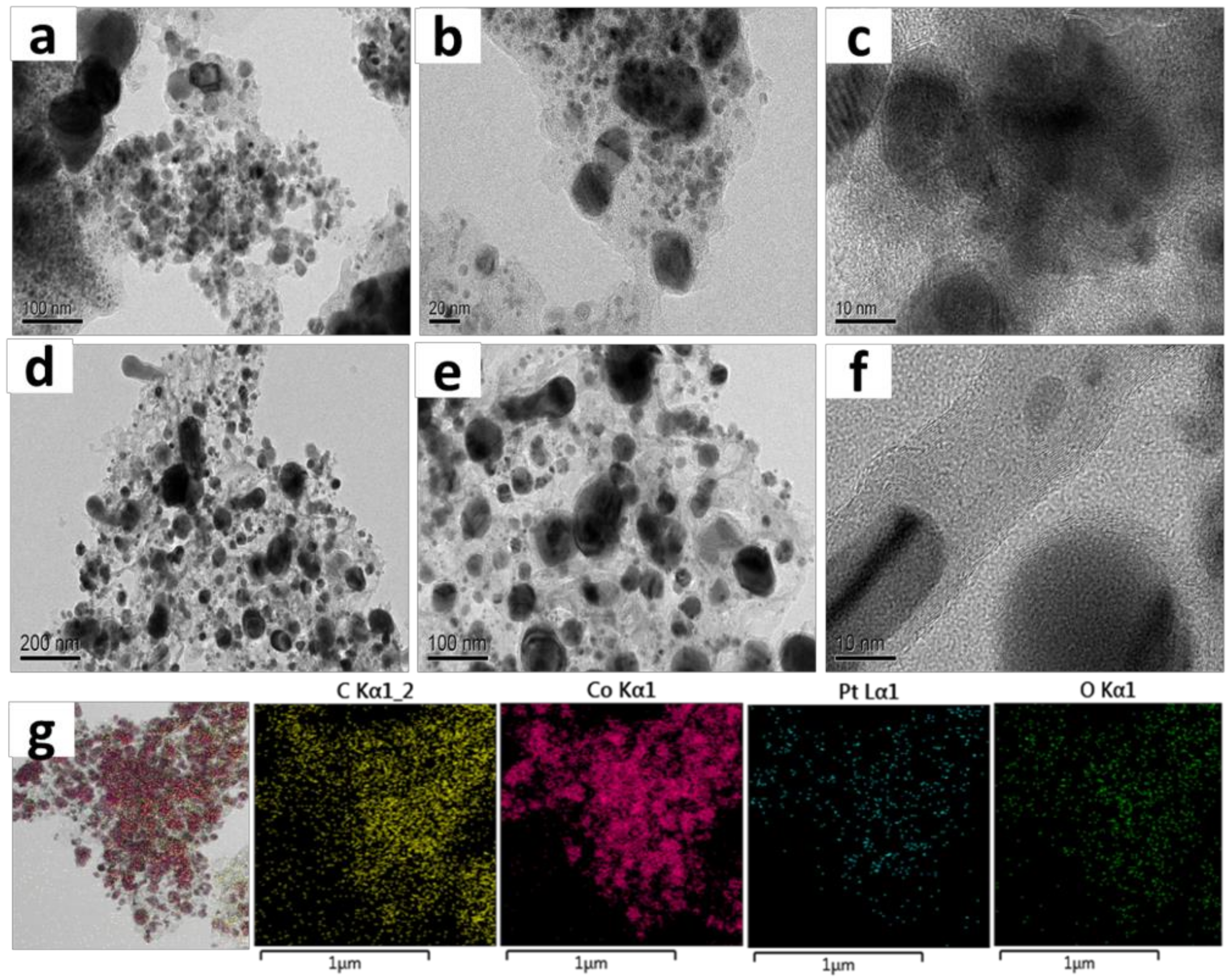
Catalyst Characterization
3. Discussion
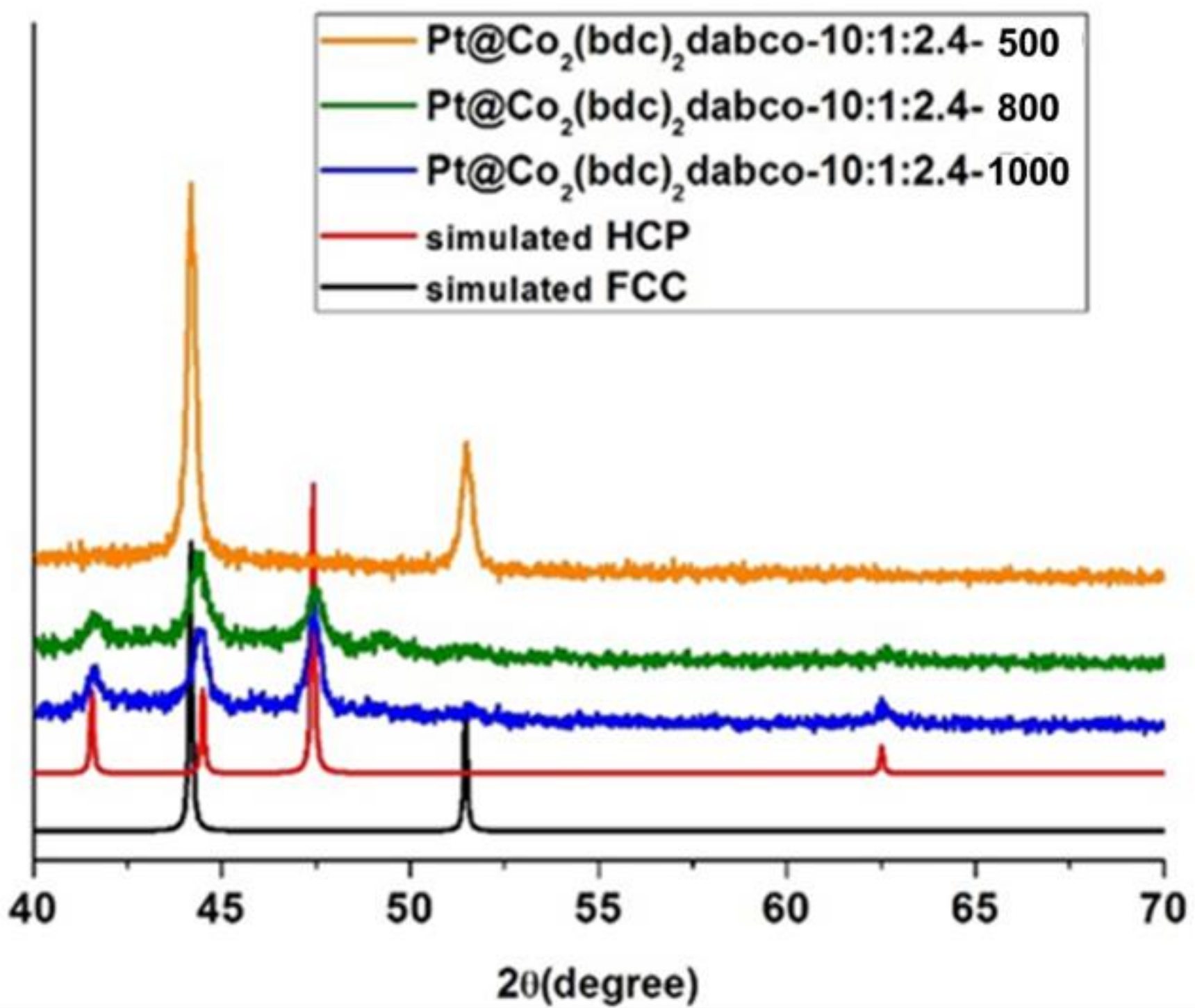
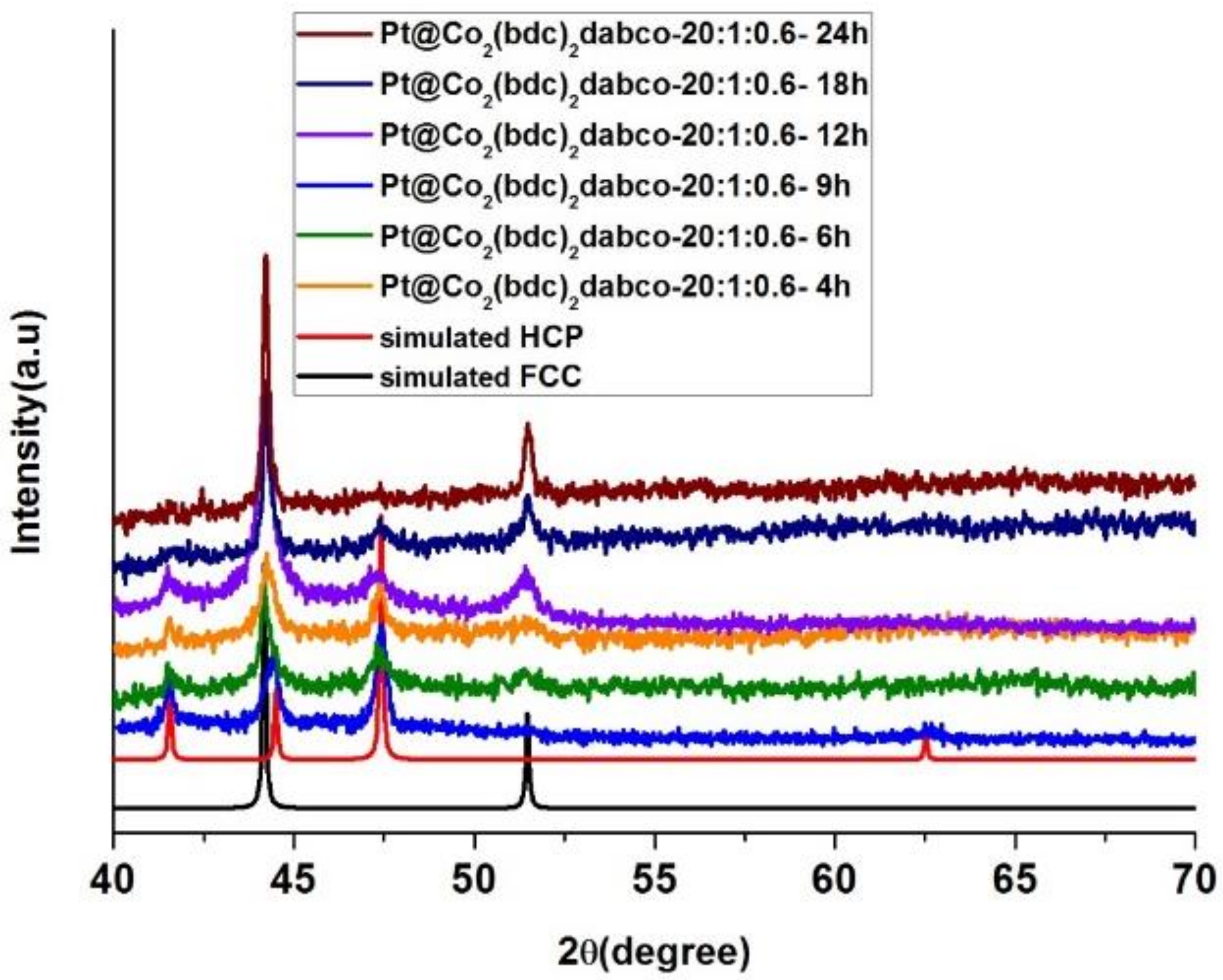
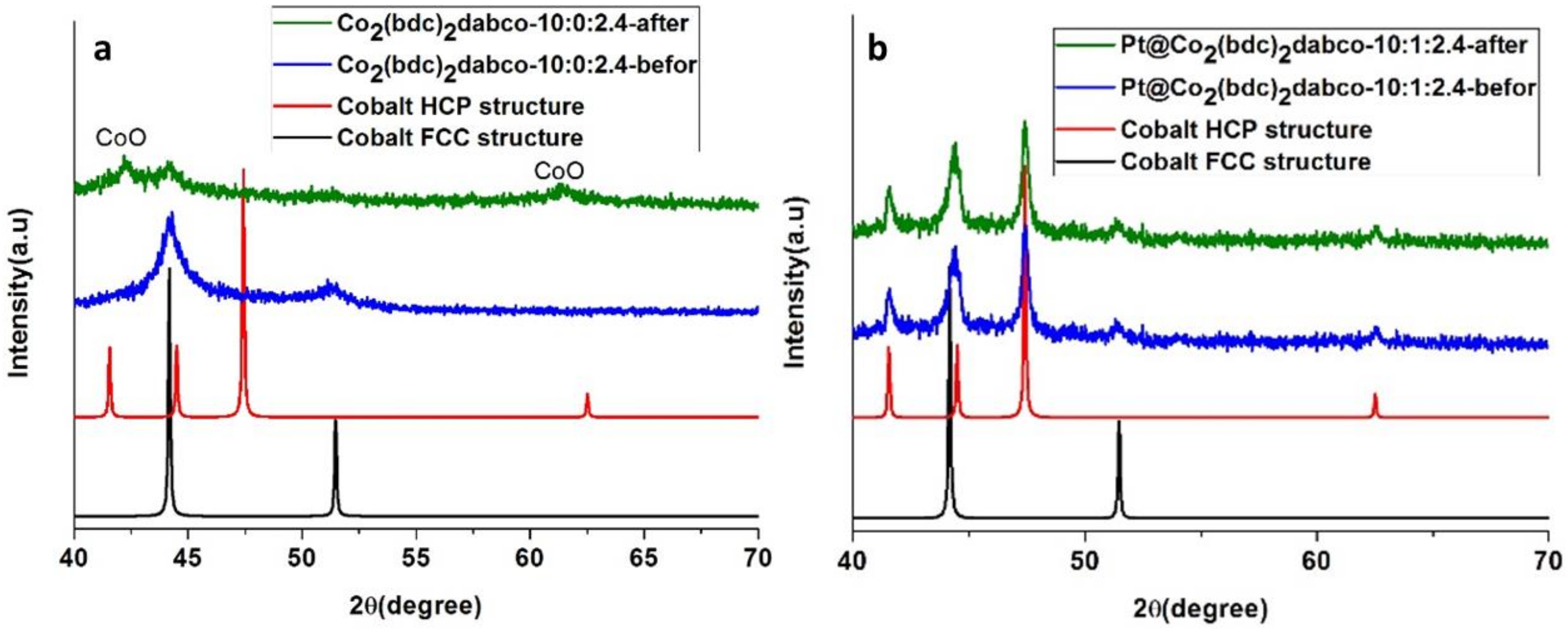
3.1. Cobalt Phase Transition
3.1.1. Effect of NaBH4
3.1.2. Effect of temperature
3.1.3. Effect of Carbonization Time
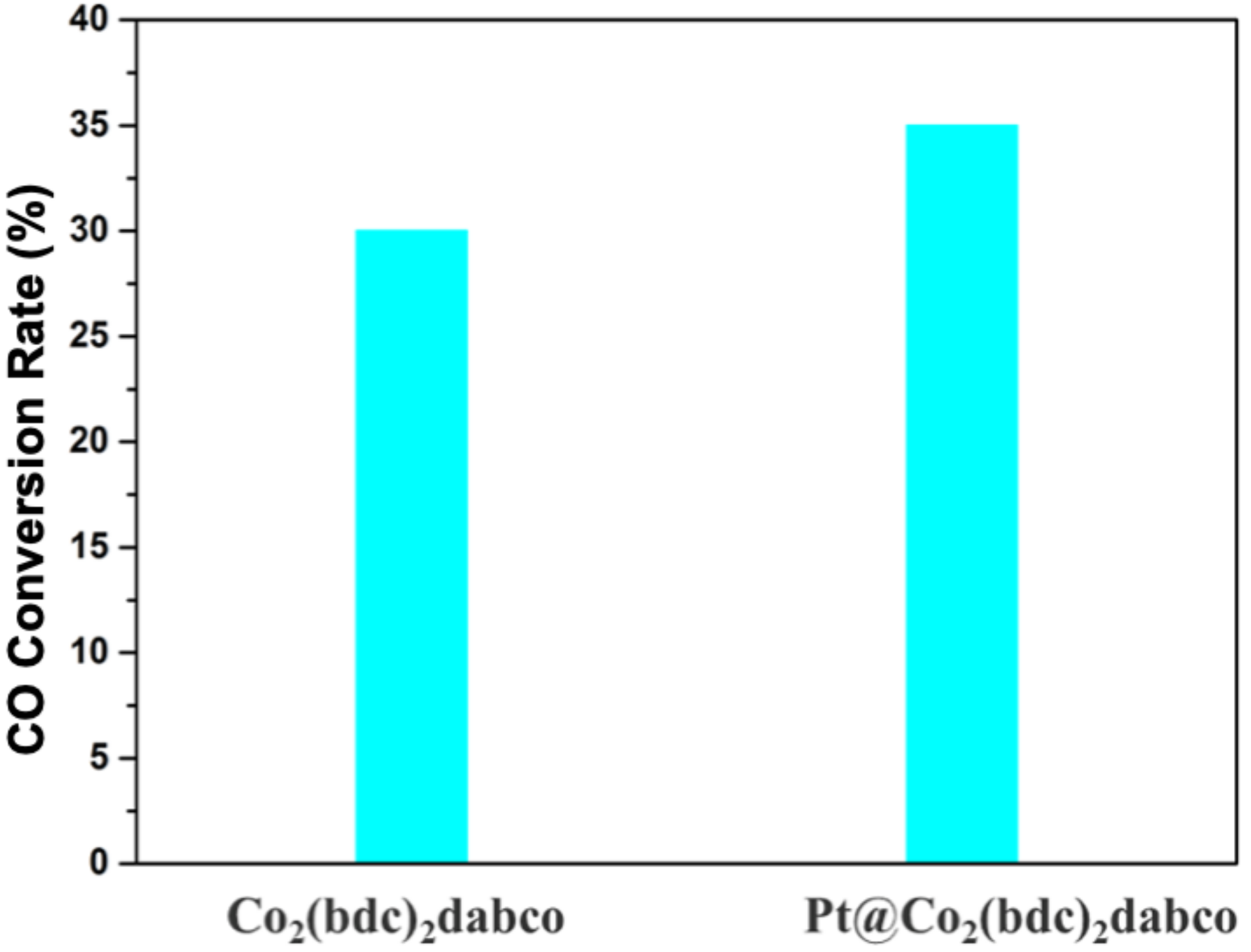
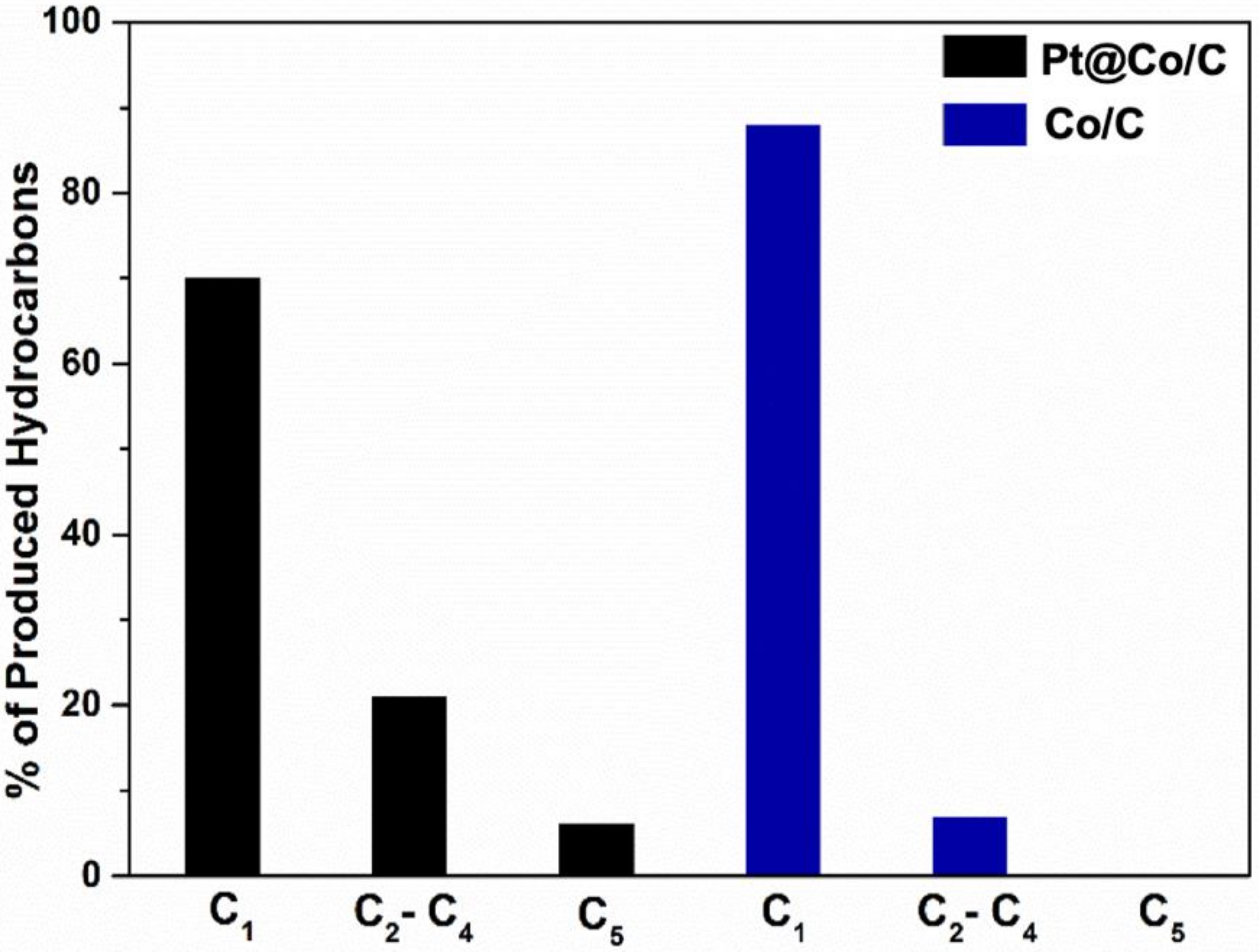
3.2. Fisher-Tropsch Reaction Using Cobalt-Based Catalysts
3.2.1. Study of the FTS Mechanism
3.2.2. Catalyst Stability Test
3.3. Comparison with the Literature
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Instruments and Experimental Conditions
4.3. Synthesis of M2(bdc)2(dabco)
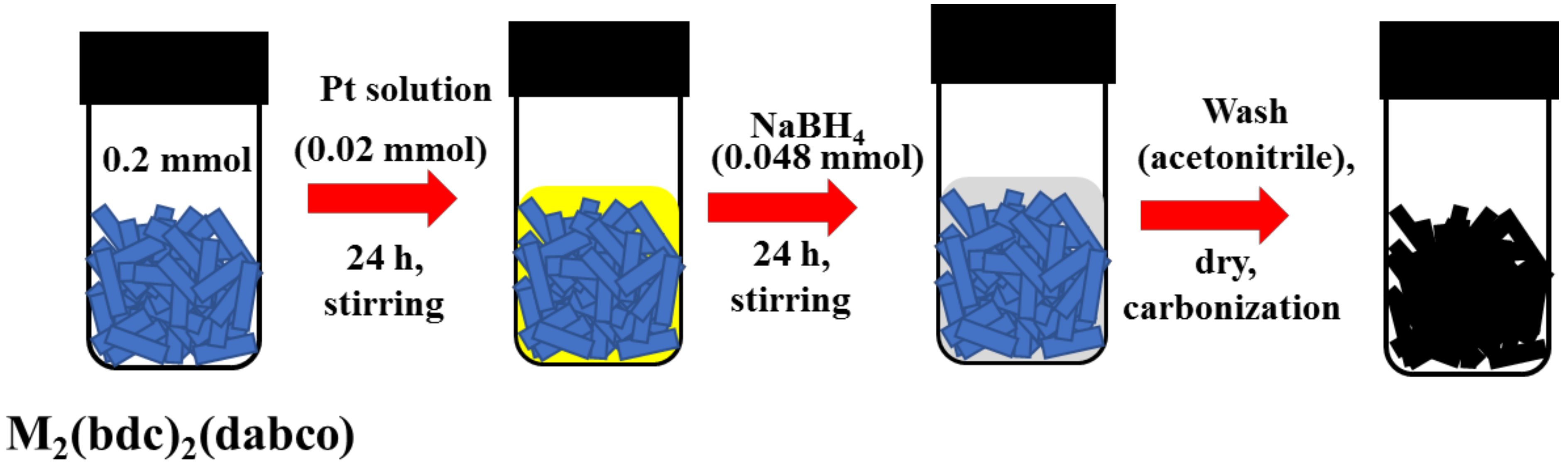
4.4. Synthesis of Pt@M2(bdc)2(dabco)
4.5. Synthesis of Pt-doped M/C Catalysts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Comm. 2017, 8, 15174–15178. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Tropsch, H. On the Direct Synthesis of Petroleum Hydrocarbons at Ordinary Pressure. (First Communication). Dtsch. Chem. Ges. 1926, 59, 830–831. [Google Scholar] [CrossRef]
- Yao, Y.; Hildebrandt, D.; Glasser, D.; Liu, X. Fischer-Tropsch Synthesis Using H2/CO/CO2 Syngas Mixtures over a Cobalt Catalyst. Ind. Eng. Chem. Res. 2010, 49, 11061–11066. [Google Scholar] [CrossRef]
- Bai, L.; Deng, S.; Dong, G.; Liren, C.; Hongwei, X.; Yongwang, L. Research Status and Industrial Prospects of Coal Indirect Liquefaction Technology. Chem. Ind. Eng. Prog. 2003, 22, 441–447. [Google Scholar]
- Fischer, F.; Tropsch, H. Uber die Herstellung synthetischer olgemische (Synthol) durch Aufbau aus Kohlenoxyd und Wasserstoff. Brennst. Chem. 1923, 4, 276–285. [Google Scholar]
- Beaumont, S.K. Recent developments in the application of nanomaterials to understanding molecular level processes in cobalt catalysed Fischer–Tropsch synthesis. Phys. Chem. Chem. Phys. 2014, 16, 5034–5043. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhang, S.; Zhang, Q.; Wang, Y. Ruthenium nanoparticles supported on carbon nanotubes as efficient catalysts for selective conversion of synthesis gas to diesel fuel. Angew. Chem. Int. Ed. 2009, 48, 2565–2568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; He, Y.R.; Cao, D.B.; Wen, X.D.; Xiang, H.W.; Li, Y.W.; Wang, J.G.; Jiao, H.J. High coverage adsorption and co-adsorption of CO and H2 on Ru (0001) from DFT and thermodynamics. Phys. Chem. Chem. Phys. 2015, 17, 19446–19456. [Google Scholar]
- Xie, J.X.; Yang, J.A.; Dugulan, I.; Holmen, A.; Chen, D.; Jong, K.P.D.; Louwerse, M.J. Size and promoter effects in supported iron Fischer–Tropsch catalysts: Insights from experiment and theory. ACS Catal. 2016, 6, 3147–3157. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; Bitter, J.H.; Davidian, T.; Ruitenbeek, M.; Dugulan, A.I.; Jong, K.P.D. Iron particle size effects for direct production of lower olefins from synthesis gas. J. Am. Chem. Soc. 2012, 134, 16207–16215. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, H.B.; Hou, Y.L.; Ma, D. Fe5C2 nanoparticles: A facile bromide-induced synthesis and as an active phase for Fischer–Tropsch synthesis. J. Am. Chem. Soc. 2012, 134, 15814–15821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Deng, W.; Wang, Y. Recent advances in understanding the key catalyst factors for Fischer-Tropsch synthesis. J. Energy Chem. 2013, 22, 27–38. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, J.; Wang, Y. Development of novel catalysts for Fischer–Tropsch synthesis: Tuning the product selectivity. ChemCatChem 2010, 2, 1030–1058. [Google Scholar] [CrossRef]
- Shi, L.; Li, D.; Hou, B.; Wang, Y.; Sun, Y. The modification of SiO2 by various organic groups and its influence on the properties of cobalt-based catalysts for Fischer–Tropsch synthesis. Fuel Process Technol. 2010, 91, 394–398. [Google Scholar] [CrossRef]
- Zhang, Y.; Hanayama, K.; Tsubaki, N. The surface modification effects of silica support by organic solvents for Fischer–Tropsch synthesis catalysts. Catal. Commun. 2006, 7, 251–254. [Google Scholar] [CrossRef]
- Chen, W.; Lin, T.; Dai, Y.; An, Y.; Yu, F.; Zhong, L.; Li, S.; Sun, Y. Recent advances in the investigation of nanoeffects of Fischer-Tropsch catalysts. Catal. Today 2018, 311, 8–22. [Google Scholar] [CrossRef]
- Park, J.C.; Kwon, J.I.; Kang, S.W.; Chun, D.H.; Jung, H.; Lee, H.T.; Yang, J.I. Highly productive cobalt nanoparticles supported on mesocellular silica foam for the Fischer–Tropsch reaction. New J. Chem. 2016, 40, 9586–9592. [Google Scholar] [CrossRef]
- Chu, W.; Chernavskii, P.A.; Gengembre, L.; Pankina, G.A.; Fongarland, P.; Khodakov, A.Y. Cobalt species in promoted cobalt alumina-supported Fischer–Tropsch catalysts. J. Catal. 2007, 252, 215–230. [Google Scholar] [CrossRef]
- Pirola, C.; Scavini, M.; Galli, F.; Vitali, S.; Comazzi, A.; Manenti, F.; Ghigna, P. Fischer–Tropsch synthesis: EXAFS study of Ru and Pt bimetallic Co based catalysts. Fuel 2014, 132, 62–70. [Google Scholar] [CrossRef]
- Beaumont, S.K.; Alayoglu, S.; Specht, C.; Michalak, W.D.; Pushkarev, V.V.; Guo, J.; Kruse, N.; Somorjai, G.A. Combining in situ NEXAFS spectroscopy and CO2 methanation kinetics to study Pt and Co nanoparticle catalysts reveals key insights into the role of platinum in promoted cobalt catalysis. J. Am. Chem. Soc. 2014, 136, 9898–9901. [Google Scholar] [CrossRef]
- Kaye, S.S.; Dailly, A.; Yaghi, O.M.; Long, J.R. Impact of preparation and handling on the hydrogen storage properties of Zn4O (1, 4-benzenedicarboxylate)3(MOF-5). J. Am. Chem. Soc. 2007, 129, 14176–14177. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Mallat, T.; Krumeich, F.; Baiker, A. Copper-based metal-organic framework for the facile ring-opening of epoxides. J. Catal. 2008, 257, 390–395. [Google Scholar] [CrossRef]
- Ma, D.; Li, B.; Zhou, X.; Zhou, Q.; Liu, K.; Zeng, G.; Li, G.; Shi, Z.; Feng, S. A dual functional MOF as a luminescent sensor for quantitatively detecting the concentration of nitrobenzene and temperature. Chem. Commun. 2013, 49, 8964–8966. [Google Scholar]
- Bux, H.; Chmelik, C.; Krishna, R.; Caro, J. Ethene/ethane separation by the MOF membrane ZIF-8: Molecular correlation of permeation, adsorption, diffusion. J. Membr. Sci. 2011, 369, 284–289. [Google Scholar] [CrossRef]
- Alezi, D.; Belmabkhout, Y.; Suyetin, M.; Bhatt, P.M.; Weseliński, Ł.J.; Solovyeva, V.; Adil, K.; Spanopoulos, I.; Trikalitis, P.N.; Emwas, A.H.; et al. MOF crystal chemistry paving the way to gas storage needs: Aluminum-based soc-MOF for CH4, O2, and CO2 storage. J. Am. Chem. Soc. 2015, 137, 133081–133318. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, S.; Asghari, A.; Tavasoli, A. Enhancement of performance and stability of Graphene nano sheets supported cobalt catalyst in Fischer–Tropsch synthesis using Graphene functionalization. Chem. Eng. Res. Des. 2017, 199, 198–208. [Google Scholar] [CrossRef]
- Wezendonk, T.A.; Sun, X.; Dugulan, A.I.; van Hoof, A.J.; Hensen, E.J.; Kapteijn, F.; Gascon, J. Controlled formation of iron carbides and their performance in Fischer-Tropsch synthesis. J. Catal. 2018, 362, 106–117. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Q.; Oyunkhand, E.; Ding, S.; Yamane, N.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Nitrogen-rich mesoporous carbon supported iron catalyst with superior activity for Fischer-Tropsch synthesis. Carbon 2018, 130, 304–314. [Google Scholar] [CrossRef]
- Bahome, M.C.; Jewell, L.L.; Hildebrandt, D.; Glasser, D.; Coville, N.J. Fischer–Tropsch synthesis over iron catalysts supported on carbon nanotubes. Appl. Catal. A-Gen 2005, 287, 60–67. [Google Scholar] [CrossRef]
- Das, R.; Pachfule, P.; Banerjee, R.; Poddar, P. Metal and metal oxide nanoparticle synthesis from metal organic frameworks (MOFs): Finding the border of metal and metal oxides. Nanoscale 2012, 4, 591–599. [Google Scholar] [CrossRef]
- Bai, C.H.; Yao, X.F.; Li, Y.W. Easy access to amides through aldehydic C2H bond functionalization catalyzed by heterogeneous Co-based catalysts. ACS Catal. 2015, 5, 884–891. [Google Scholar] [CrossRef]
- Long, J.L.; Zhou, Y.; Li, Y.W. Transfer hydrogenation of unsaturated bonds in the absence of base additives catalyzed by a cobalt-based heterogeneous catalyst. Chem. Commun. 2015, 51, 2331–2334. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Wang, C.M.; Wu, Z.Y.; Xiong, Y.J.; Xu, Q.; Yu, S.H.; Jiang, H.L. From bimetallic metal-organic framework to porous carbon: High surface area and multicomponent active dopants for excellent electrocatalysis. Adv. Mater. 2015, 27, 5010–5016. [Google Scholar] [CrossRef] [PubMed]
- Enache, D.I.; Rebours, B.; Roy-Auberger, M.; Revel, R.J. In situ XRD study of the influence of thermal treatment on the characteristics and the catalytic properties of cobalt-based Fischer–Tropsch catalysts. J. Catal. 2002, 205, 346–353. [Google Scholar] [CrossRef]
- Sadeqzadeh, M.; Karaca, H.; Safonova, O.V.; Fongarland, P.; Chambrey, S.; Roussel, P.; Griboval-Constant, A.; Lacroix, M.; Curulla-Ferre, D.; Luck, F.; et al. Identification of the active species in the working alumina-supported cobalt catalyst under various conditions of Fischer–Tropsch synthesis. Catal. Today 2011, 164, 62–67. [Google Scholar] [CrossRef]
- Liu, J.X.; Su, H.Y.; Sun, D.P.; Zhang, B.Y.; Li, W.X. Crystallographic dependence of CO activation on cobalt catalysts: HCP versus FCC. J. Am. Chem. Soc. 2013, 135, 16284–16287. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Li, Z.; Li, Y. Highly active and selective Co-based Fischer–Tropsch catalysts derived from metal–organic frameworks. AIChE J. 2017, 63, 2935–2944. [Google Scholar] [CrossRef]
- Lee, Y.W.; Hwang, E.T.; Kwak, D.H.; Park, K.W. Preparation and characterization of PtIr alloy dendritic nanostructures with superior electrochemical activity and stability in oxygen reduction and ethanol oxidation reactions. Catal. Sci. Technol. 2016, 6, 569–576. [Google Scholar] [CrossRef]
- Zheng, J.N.; Lv, J.J.; Li, S.S.; Xue, M.W.; Wang, A.J.; Feng, J.J. One-pot synthesis of reduced graphene oxide supported hollow Ag@Pt core–shell nanospheres with enhanced electrocatalytic activity for ethylene glycol oxidation. J. Mater. Chem. A 2016, 2, 3445–3451. [Google Scholar] [CrossRef]
- Woo, H.; Kim, E.; Kim, J.H.; Yun, S.W.; Park, J.C.; Kim, Y.T.; Park, K.H. Shape and Composition Control of Monodisperse Hybrid Pt-CoO Nanocrystals by Controlling the Reaction Kinetics with Additives. Sci. Rep. 2017, 7, 3851. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534–540. [Google Scholar] [CrossRef] [PubMed]
- De La Pena O’Shea, V.A.; De La Piscina, P.R.; Homs, N.; Aromi, G.; Fierro, J.L.G. Development of hexagonal closed-packed cobalt nanoparticles stable at high temperature. Chem. Mater. 2009, 21, 5637–5643. [Google Scholar] [CrossRef]
- Kitakami, O.; Sato, H.; Shimada, Y.; Sato, F.; Tanaka, M. Size effect on the crystal phase of cobalt fine particles. Phys. Rev. B 1997, 56, 13849–13845. [Google Scholar] [CrossRef]
- Puntes, V.F.; Zanchet, D.; Erdonmez, C.K.; Alivisatos, A.P. Synthesis of hcp-Co nanodisks. J. Am. Chem. Soc. 2002, 124, 12874–12880. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Tavasoli, A.; Mortazavi, Y.; Karimi, A. Cobalt supported on Graphene–A promising novel Fischer–Tropsch synthesis catalyst. Appl. Catal. A-Gen. 2015, 499, 188–196. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, J.; Mastai, Y.; Felner, I.; Gedanken, A. Preparation and characteristics of carbon nanotubes filled with cobalt. Chem. Mater. 2000, 12, 2205–2211. [Google Scholar] [CrossRef]
- Guerret-Piecourt, C.; Le Bouar, Y.; Lolseau, A.; Pascard, H. Relation between metal electronic structure and morphology of metal compounds inside carbon nanotubes. Nature 1994, 372, 761–765. [Google Scholar] [CrossRef]
- Van de Loosdrecht, J.; Botes, F.G.; Ciobica, I.M.; Ferreira, A.C.; Gibson, P.; Moodley, D.J.; Saib, A.M.; Visagie, J.L.; Weststrate, C.J.; Niemantsverdriet, J.W. Fischer-Tropsch synthesis: Catalysts and chemistry. In Comprehensive Inorganic Chemistry II: From Elements to Applications; Elsevier: Amsterdam, The Netherlands, 2013; Volume 7, pp. 525–557. [Google Scholar]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the development of novel cobalt Fischer−Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef]
- Diehl, F.; Khodakov, A.Y. Promotion of cobalt Fischer-Tropsch catalysts with noble metals: A review. Oil Gas Sci. Technol. 2009, 64, 11–24. [Google Scholar] [CrossRef]
- Oosterbeek, H. Bridging the pressure and material gap in heterogeneous catalysis: Cobalt Fischer–Tropsch catalysts from surface science to industrial application. Phys. Chem. Chem. Phys. 2007, 9, 3570–3576. [Google Scholar] [CrossRef]
- Jahangiri, H.; Bennett, J.; Mahjoubi, P.; Wilson, K.; Gu, S. A review of advanced catalyst development for Fischer–Tropsch synthesis of hydrocarbons from biomass derived syn-gas. Catal. Sci Technol. 2014, 4, 2210–2229. [Google Scholar] [CrossRef]
- Yang, J.; Ma, W.; Chen, D.; Holmen, A.; Davis, B.H. Fischer–Tropsch synthesis: A review of the effect of CO conversion on methane selectivity. Appl. Catal. A-Gen. 2014, 470, 250–260. [Google Scholar] [CrossRef]
- Santos, V.P.; Wezendonk, T.A.; Jaen, J.J.D.; Dugulan, A.I.; Nasalevich, M.A.; Islam, H.U.; Chojecki, A.; Sartipi, S.; Sun, X.; Hakeem, A.A.; et al. Metal organic framework-mediated synthesis of highly active and stable Fischer-Tropsch catalysts. Nat. Comm. 2015, 6, 6451–6455. [Google Scholar] [CrossRef]
- Wezendonk, T.A.; Santos, V.P.; Nasalevich, M.A.; Warringa, Q.S.; Dugulan, A.I.; Chojecki, A.; Koeken, A.C.; Ruitenbeek, M.; Meima, G.; Islam, H.U.; et al. Elucidating the nature of Fe species during pyrolysis of the Fe-BTC MOF into highly active and stable Fischer–Tropsch catalysts. ACS Catal. 2016, 6, 3236–3247. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Eliseev, O.L.; Kazantsev, R.V.; Chernyshev, V.V.; Davydov, P.E.; Saifutdinov, B.R.; Lapidus, A.L.; Kustov, L.M. Fischer–Tropsch synthesis over MOF-supported cobalt catalysts (Co@MIL-53 (Al)). Dalton Trans. 2016, 45, 12006–12014. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Yang, C.; Guo, W.; Xu, Y.; Liang, Z.; Ma, D.; Zou, R. Highly dispersed Co-based Fischer–Tropsch synthesis catalysts from metal–organic frameworks. J. Mater. Chem. A 2017, 5, 8081–8086. [Google Scholar] [CrossRef]
- Cadman, L.K.; Bristow, J.K.; Stubbs, N.E.; Tiana, D.; Mahon, M.F.; Walsh, A.; Burrows, A.D. Compositional control of pore geometry in multivariate metal–organic frameworks: An experimental and computational study. Dalton Trans. 2016, 45, 4316–4326. [Google Scholar] [CrossRef]




| Co: Pt: NaBH4 | 10:1:0.6 | 10:1:1.2 | 10:1:2.4 | 20:1:0.6 | 20:1:1.2 | 20:1:2.4 |
|---|---|---|---|---|---|---|
| FCC % | 69% | 49% | 12% | 72.4% | 65% | 14% |
| HCP % | 31% | 51% | 88% | 27.6% | 35% | 86% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, A.; Kim, E.; Choi, Y.N.; Lee, J.; Venkateswarlu, S.; Yoon, M. Phase Controlled Synthesis of Pt Doped Co Nanoparticle Composites Using a Metal-Organic Framework for Fischer–Tropsch Catalysis. Catalysts 2019, 9, 156. https://doi.org/10.3390/catal9020156
Panda A, Kim E, Choi YN, Lee J, Venkateswarlu S, Yoon M. Phase Controlled Synthesis of Pt Doped Co Nanoparticle Composites Using a Metal-Organic Framework for Fischer–Tropsch Catalysis. Catalysts. 2019; 9(2):156. https://doi.org/10.3390/catal9020156
Chicago/Turabian StylePanda, Atanu, Euisoo Kim, Yong Nam Choi, Jihyun Lee, Sada Venkateswarlu, and Minyoung Yoon. 2019. "Phase Controlled Synthesis of Pt Doped Co Nanoparticle Composites Using a Metal-Organic Framework for Fischer–Tropsch Catalysis" Catalysts 9, no. 2: 156. https://doi.org/10.3390/catal9020156
APA StylePanda, A., Kim, E., Choi, Y. N., Lee, J., Venkateswarlu, S., & Yoon, M. (2019). Phase Controlled Synthesis of Pt Doped Co Nanoparticle Composites Using a Metal-Organic Framework for Fischer–Tropsch Catalysis. Catalysts, 9(2), 156. https://doi.org/10.3390/catal9020156





