A Theoretical Study on Pd-catalyzed, Friedel-Crafts Intermolecular Acylation: Does Generated In Situ Aroyl Triflate Act as A Reactive Electrophile to Functionalize C–H Bond of Arenes?
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
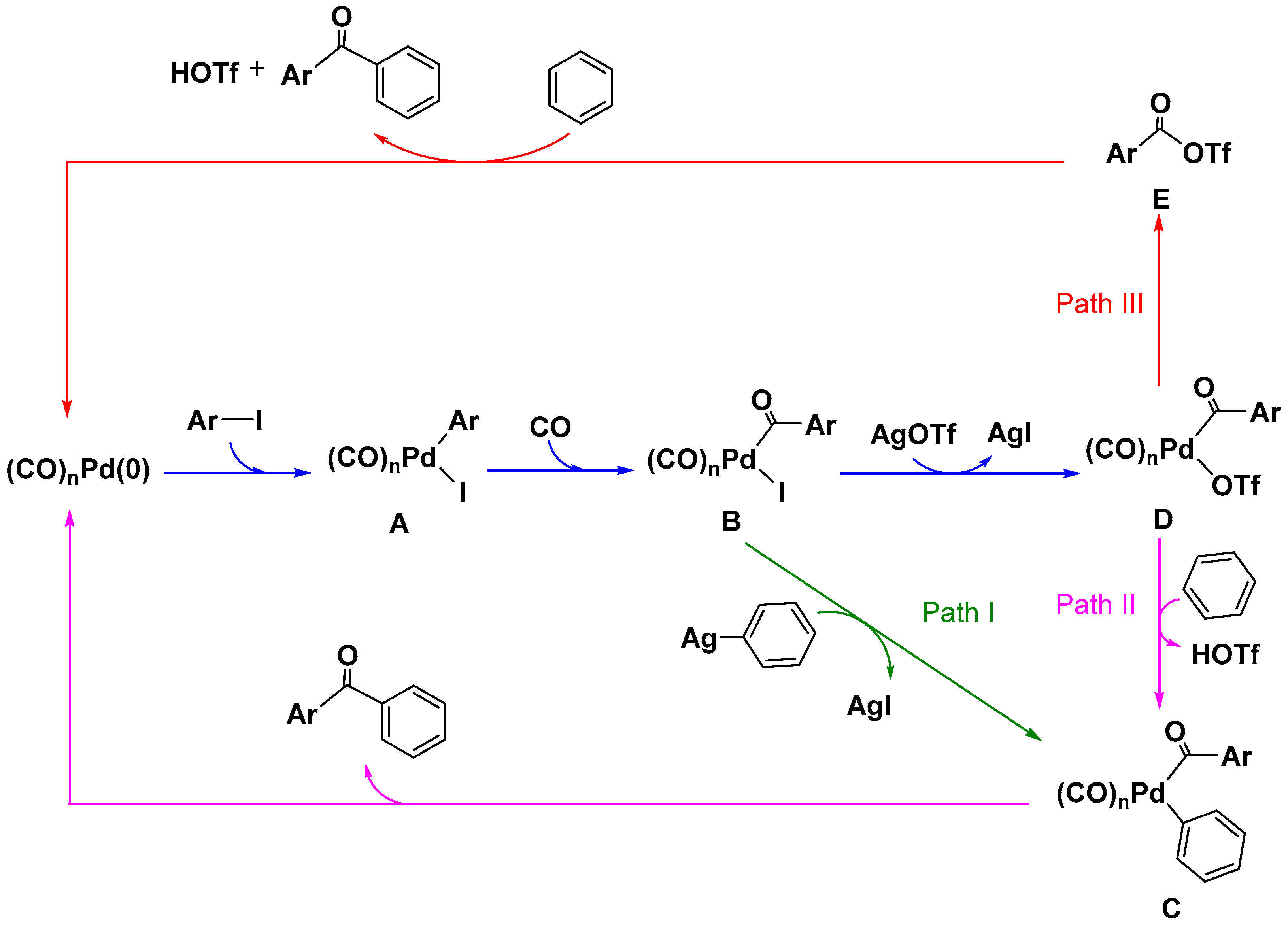
3.1. Mechanism
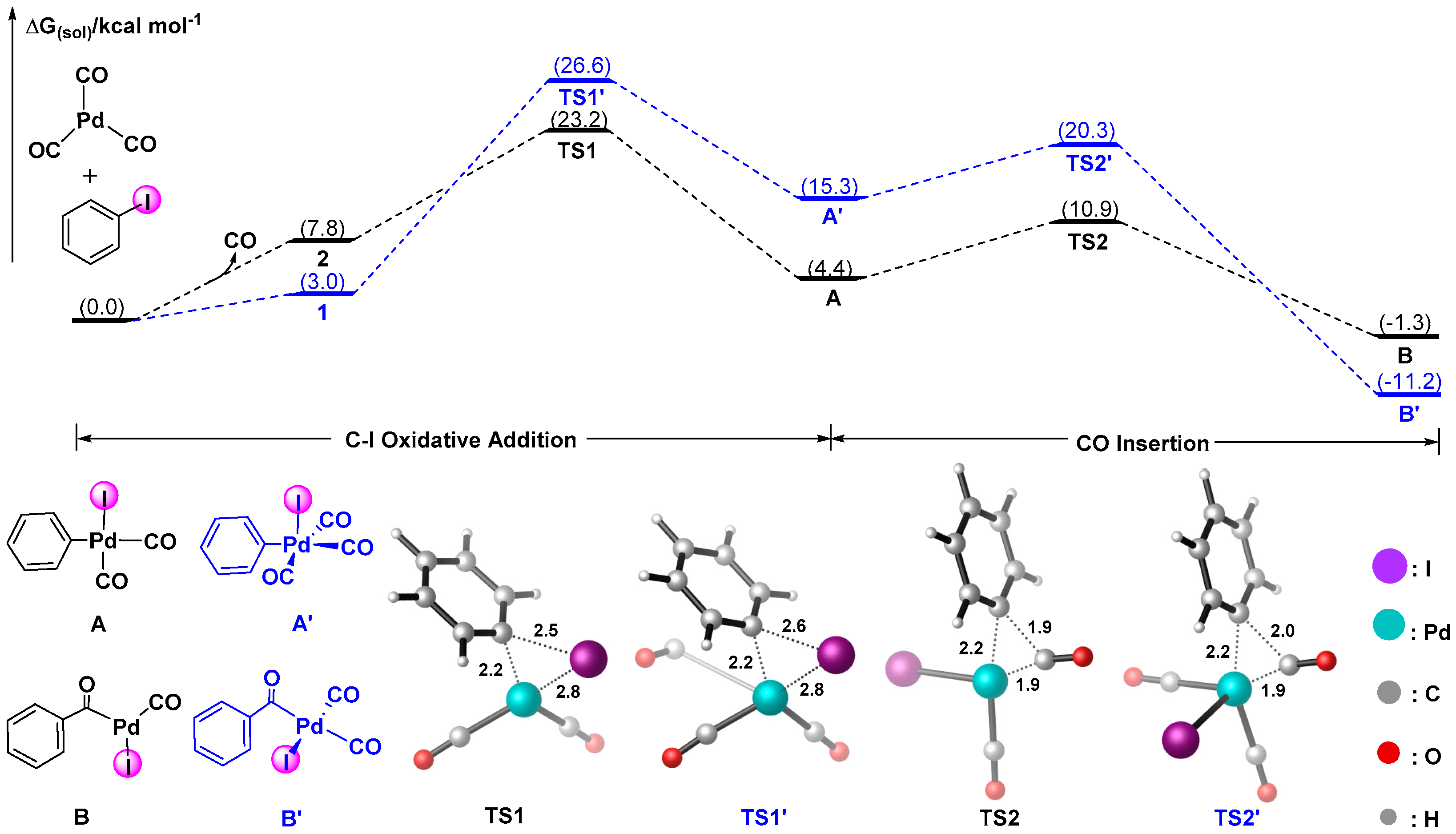
3.1.1. Mechanism of Forming Palladium-Aroyl Intermediate B from Reaction between (CO)nPd(0) and Aryl Iodide
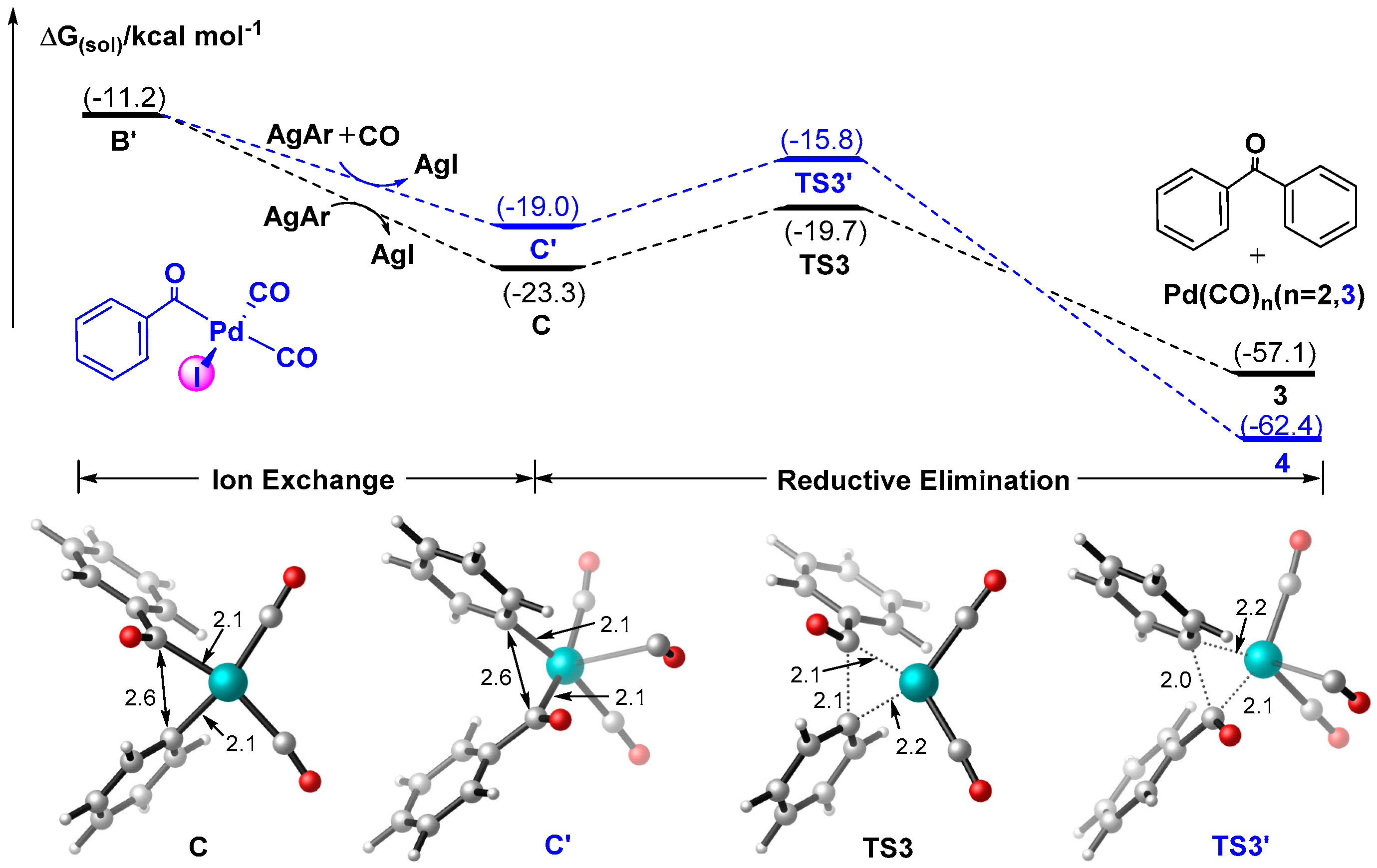
3.1.2. Mechanism for the Formation of Ketones Derived from Aromatic Silver
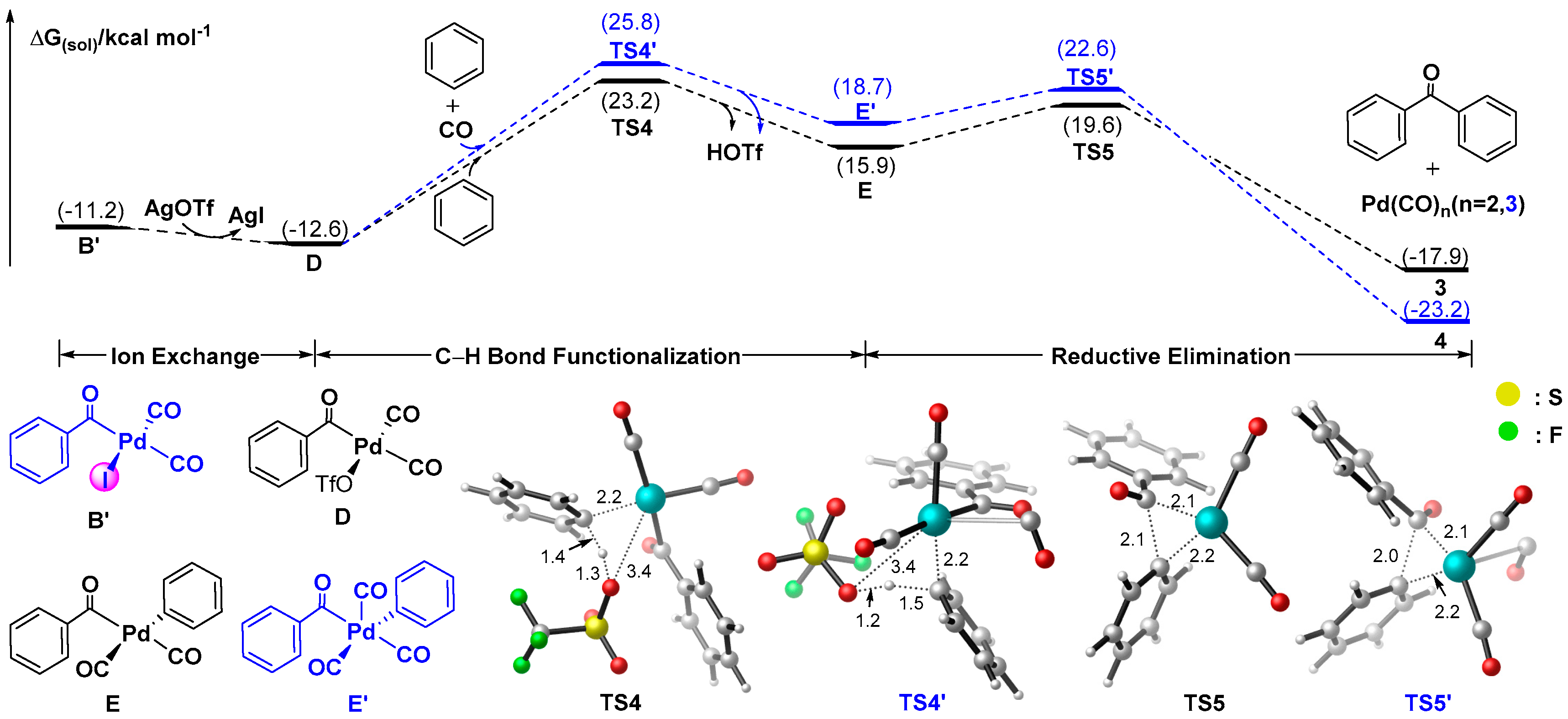
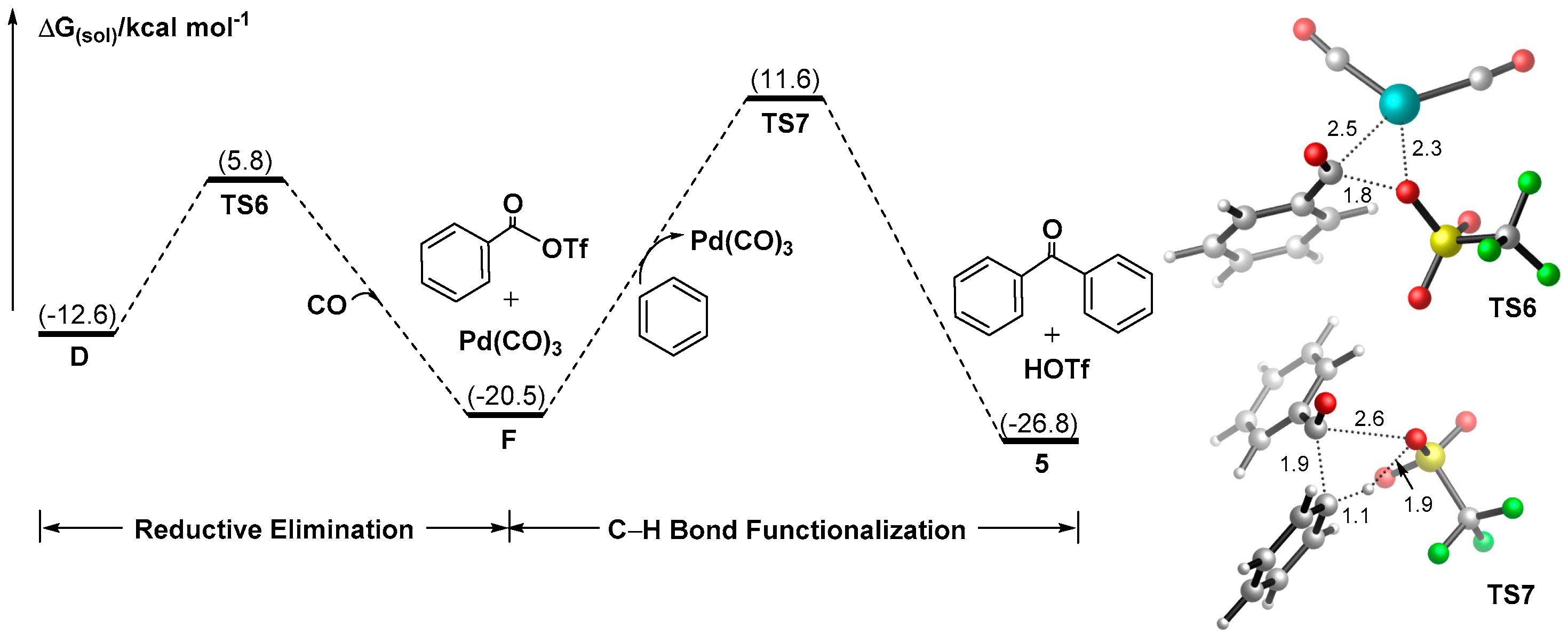
3.1.3. Mechanism for the Formation of Ketones Involving Palladate Benzene
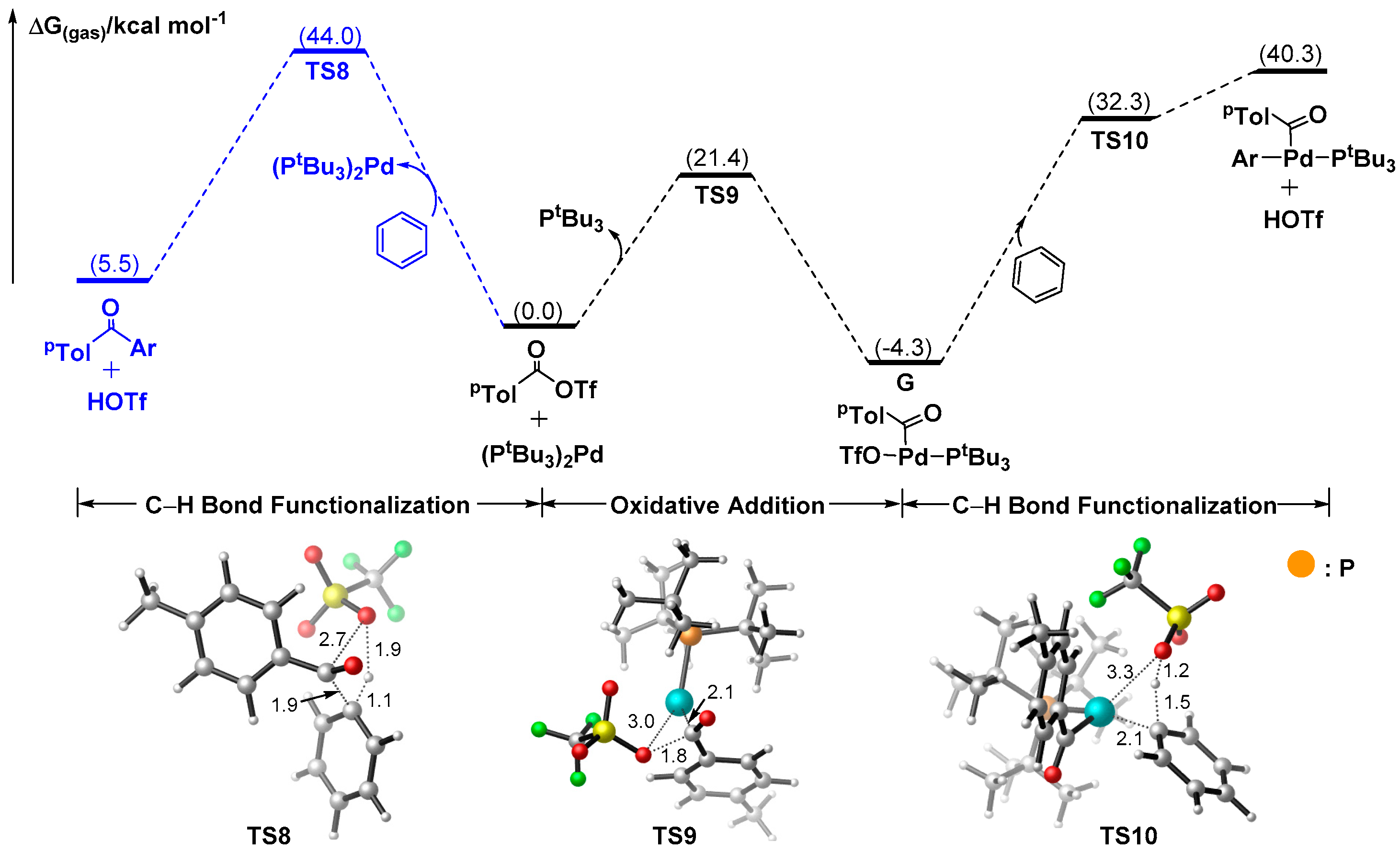
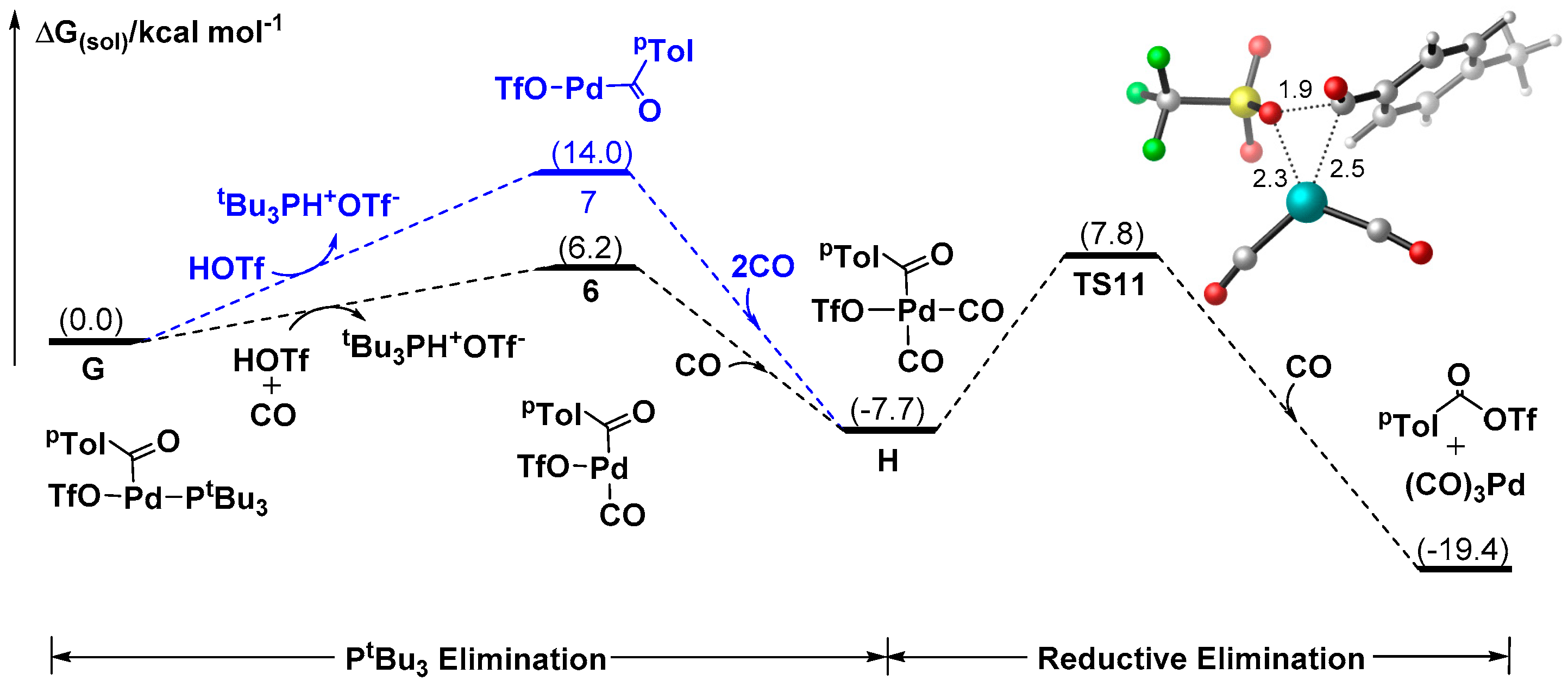
3.1.4. Mechanism for the Formation of Ketones Delivered by Aroyl Triflate
3.2. Why are Pd Salts Superior to the Ligated Pd Complexes in Catalytic Activities?
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hatano, B.; Kadokawa, J.I.; Tagaya, H. Disproportionation of diarylmethanol derivatives by using supercritical water. Tetrahedron Lett. 2002, 43, 5859–5861. [Google Scholar] [CrossRef]
- Dieter, R.K. Reaction of acyl chlorides with organometallic reagents: A banquet table of metals for ketone synthesis. Tetrahedron 1999, 55, 4177–4236. [Google Scholar] [CrossRef]
- Sharmoukh, W.; Ko, K.C.; Noh, C.; Lee, J.Y.; Son, S.U. Designed Synthesis of Multi–Electrochromic Systems Bearing Diaryl Ketone and Isophthalates. J. Org. Chem. 2010, 75, 6708–6711. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Evolution of C–H Bond Functionalization from Methane to Methodology. J. Am. Chem. Soc. 2016, 138, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.W.; Sanford, M.S. Palladium–Catalyzed Ligand–Directed C–H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef] [PubMed]
- Colby, D.A.; Bergman, R.G.; Ellman, J.A. Rhodium–Catalyzed C–C Bond Formation via Heteroatom–Directed C–H Bond Activation. Chem. Rev. 2010, 110, 624–655. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Engle, K.M.; Wang, D.H.; Yu, J.Q. Palladium(II)–Catalyzed C–H Activation/C–C Cross–Coupling Reactions: Versatility and Practicality. Angew. Chem. Int. Ed. 2009, 48, 5094–5115. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yuan, J.W.; Gao, M.; Tang, S.; Li, W.; Shi, R.Y.; Lei, A.W. Oxidative Coupling between Two Hydrocarbons: An Update of Recent C–H Functionalizations. Chem. Rev. 2015, 115, 12138–12204. [Google Scholar] [CrossRef]
- Cernak, T.; Dykstra, K.D.; Tyagarajan, S.; Vachal, P.; Krska, S.W. The medicinal chemist’s toolbox for late stage functionalization of drug–like molecules. Chem. Soc. Rev. 2016, 45, 546–576. [Google Scholar] [CrossRef]
- Olah, G.A. Friedel-Crafts and Related Reactions; Interscience Publishers-John Wiley & Sons, Inc.: London, UK; Beccles, UK, 1963; Volume 1. [Google Scholar]
- Kuhl, N.; Hopkinson, M.N.; Wencel–Delord, J.; Glorius, F. Beyond Directing Groups: Transition–Metal–Catalyzed C–H Activation of Simple Arenes. Angew. Chem. Int. Ed. 2012, 51, 10236–10254. [Google Scholar] [CrossRef]
- Hartwig, J.F.; Larsen, M.A. Undirected, Homogeneous C–H Bond Functionalization: Challenges and Opportunities. ACS Cent. Sci. 2016, 2, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Kinney, R.G.; Tjutrins, J.; Torres, G.M.; Liu, N.J.; Kulkarni, O.; Arndtsen, B.A. A general approach to intermolecular carbonylation of arene C–H bonds to ketones through catalytic aroyl triflate formation. Nat. Chem. 2018, 10, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density–functional thermochemistry. I. The effect of the exchange–only gradient correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Becke, A.D. Density–functional thermochemistry. II. The effect of the Perdew–Wang generalized–gradient correlation correction. J. Chem. Phys. 1992, 97, 9173–9177. [Google Scholar] [CrossRef]
- Becke, A.D. Density–functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle–Salvetti correlation–energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chem. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. Energy–adjusted ab initio pseudopotentials for the first row transition elements. J. Chem. Phys. 1987, 86, 866–872. [Google Scholar] [CrossRef]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy–adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chem. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical reactions–the IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.P.P.; Gomes, J.R.B.; Illas, F. Accounting for van der Waals interactions between adsorbates and surfaces in density functional theory based calculations: Selected examples. RSC Adv. 2013, 3, 13085–13100. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. A new local density functional for main–group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 2006, 125, 194101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06–class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self–consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Yang, Y.F.; Cheng, G.J.; Liu, P.; Leow, D.; Sun, T.Y.; Chen, P.; Zhang, X.; Yu, J.Q.; Wu, Y.D.; Houk, K.N. Palladium–Catalyzed Meta–Selective C–H Bond Activation with a Nitrile–Containing Template: Computational Study on Mechanism and Origins of Selectivity. J. Am. Chem. Soc. 2014, 136, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Dang, Z.M.; Yu, H.Z. Density functional theory investigation on Pd–catalyzed cross–coupling of azoles with aryl thioethers. Org. Biomol. Chem. 2016, 14, 4499–4506. [Google Scholar] [CrossRef]
- Anand, M.; Sunoj, R.B.; Schaefer, H.F. Palladium–Silver Cooperativity in an Aryl Amination Reaction through C–H Functionalization. ACS Catal. 2016, 6, 696–708. [Google Scholar] [CrossRef]
- Zhou, M.J.; Yang, T.L.; Dang, L. Theoretical Studies on Palladium–Mediated Enantioselective C–H Iodination. J. Org. Chem. 2016, 81, 1006–1020. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Ling, B.; Bi, S. Mechanistic investigation into Et3N C–H activation and chemoselectivity by Pd–Catalyzed intramolecular heck reaction of N–Vinylacetamides. J. Organomet. Chem. 2017, 827, 56–66. [Google Scholar] [CrossRef]
- Legault, C.Y. CYLView, 1.0b; Université de Sherbrooke: Sherbrooke, QC, Canada, 2009; Available online: http://www.cylview.org (accessed on 2 December 2018).
- Lotz, M.D.; Camasso, N.M.; Canty, A.J.; Sanford, M.S. Role of Silver Salts in Palladium–Catalyzed Arene and Heteroarene C–H Functionalization Reactions. Organometallics 2017, 36, 165–171. [Google Scholar] [CrossRef]
- Whitaker, D.; Burés, J.; Larrosa, I. Ag(I)–Catalyzed C–H Activation: The Role of the Ag(I) Salt in Pd/Ag–Mediated C–H Arylation of Electron–Deficient Arenes. J. Am. Chem. Soc. 2016, 138, 8384–8387. [Google Scholar] [CrossRef] [PubMed]
- Joost, M.; Zeineddine, A.; Estevez, L.; Ladeira, S.M.; Miqueu, K.; Amgoune, A.; Bourissou, D. Facile Oxidative Addition of Aryl Iodides to Gold(I) by Ligand Design: Bending Turns on Reactivity. J. Am. Chem. Soc. 2014, 136, 14654–14657. [Google Scholar] [CrossRef] [PubMed]
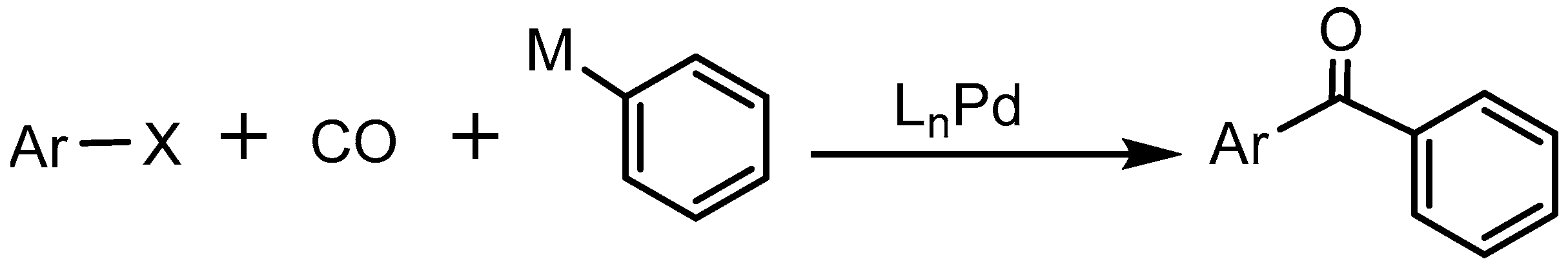


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, R.; Tian, Y.; Li, N.; Bai, J.; Yan, H.; Yang, W.; Guo, Z.; Li, Y. A Theoretical Study on Pd-catalyzed, Friedel-Crafts Intermolecular Acylation: Does Generated In Situ Aroyl Triflate Act as A Reactive Electrophile to Functionalize C–H Bond of Arenes? Catalysts 2019, 9, 141. https://doi.org/10.3390/catal9020141
Chang R, Tian Y, Li N, Bai J, Yan H, Yang W, Guo Z, Li Y. A Theoretical Study on Pd-catalyzed, Friedel-Crafts Intermolecular Acylation: Does Generated In Situ Aroyl Triflate Act as A Reactive Electrophile to Functionalize C–H Bond of Arenes? Catalysts. 2019; 9(2):141. https://doi.org/10.3390/catal9020141
Chicago/Turabian StyleChang, Rong, Ye Tian, Niu Li, Jin Bai, Huimin Yan, Wenjing Yang, Zhen Guo, and Yanrong Li. 2019. "A Theoretical Study on Pd-catalyzed, Friedel-Crafts Intermolecular Acylation: Does Generated In Situ Aroyl Triflate Act as A Reactive Electrophile to Functionalize C–H Bond of Arenes?" Catalysts 9, no. 2: 141. https://doi.org/10.3390/catal9020141
APA StyleChang, R., Tian, Y., Li, N., Bai, J., Yan, H., Yang, W., Guo, Z., & Li, Y. (2019). A Theoretical Study on Pd-catalyzed, Friedel-Crafts Intermolecular Acylation: Does Generated In Situ Aroyl Triflate Act as A Reactive Electrophile to Functionalize C–H Bond of Arenes? Catalysts, 9(2), 141. https://doi.org/10.3390/catal9020141



