Switchable Access to Amines and Imines from Reductive Coupling of Nitroarenes with Alcohols Catalyzed by Biomass-Derived Cobalt Nanoparticles
Abstract
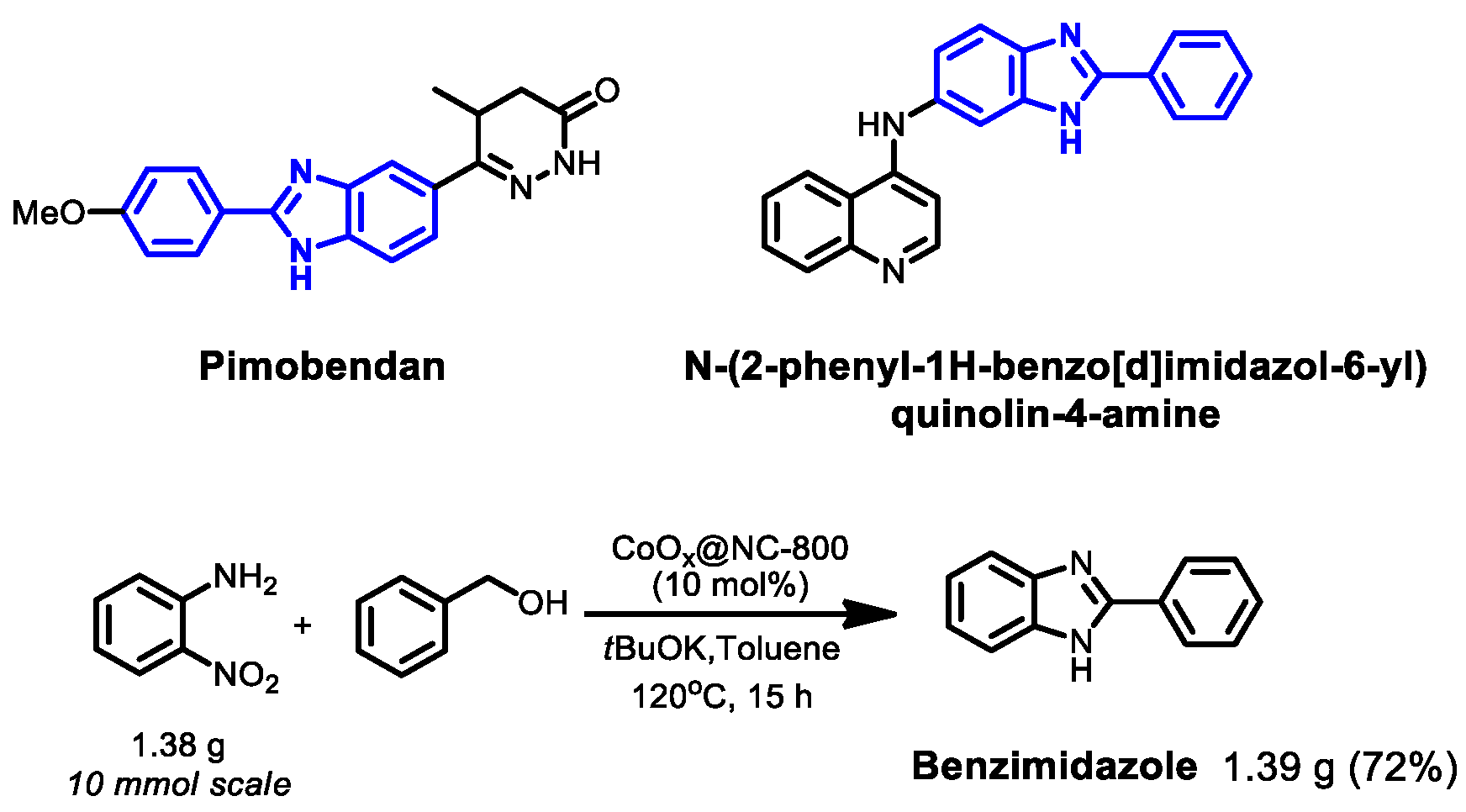
1. Introduction
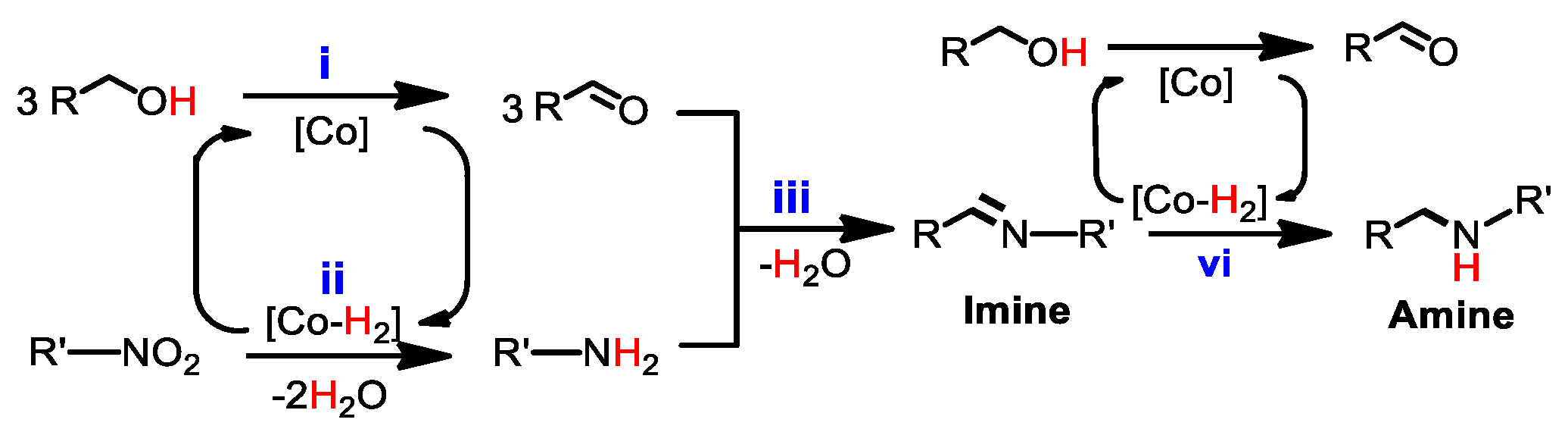
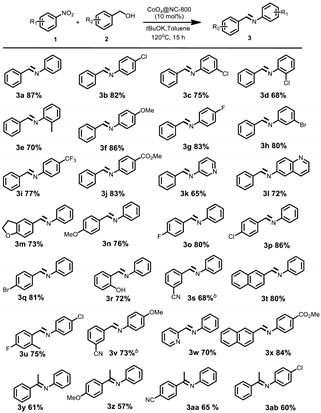
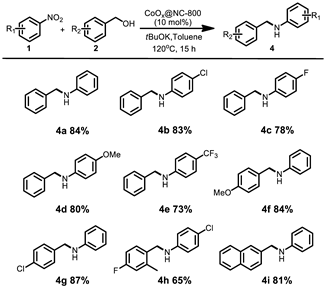
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Adams, J.P. Imines, Enamines and oximes. J. Chem. Soc. Perkin Trans. 2000, 1, 125–139. [Google Scholar] [CrossRef]
- Chen, B.; Wang, L.; Gao, S. Recent Advances in Aerobic Oxidation of Alcohols and Amines to Imines. ACS Catal. 2015, 5, 5851–5876. [Google Scholar] [CrossRef]
- Li, N.; Lang, X.; Ma, W.; Ji, H.; Chen, C.; Zhao, J. Selective aerobic oxidation of amines to imines by TiO2 photocatalysis in water. Chem. Commun. 2013, 49, 5034–5036. [Google Scholar] [CrossRef]
- Su, F.; Mathew, S.C.; Möhlmann, L.; Antonietti, M.; Wang, X.; Blechert, S. Aerobic oxidative coupling of amines by carbon nitride photocatalysis with visible light. Angew. Chem. Int. Ed. 2011, 50, 657–660. [Google Scholar] [CrossRef]
- Ho, H.-A.; Manna, K.; Sadow, A.D. Acceptorless photocatalytic dehydrogenation for alcohol decarbonylation and imine synthesis. Angew. Chem. Int. Ed. 2012, 51, 8607–8610. [Google Scholar] [CrossRef]
- Grirrane, A.; Corma, A.; Arcia, H. Highly active and selective gold catalysts for the aerobic oxidative condensation of benzylamines to imines and one-pot, two-step synthesis of secondary benzylamines. J. Catal. 2009, 264, 138–144. [Google Scholar] [CrossRef]
- Biswas, S.; Dutta, B.; Mullick, K.; Kuo, C.H.; Poyraz, A.S.; Suib, S.L. Aerobic oxidation of amines to imines by cesium-promoted mesoporous manganese oxide. ACS Catal. 2015, 5, 4394–4403. [Google Scholar] [CrossRef]
- Yuan, H.; Yoo, W.-J.; Miyamura, H.; Kobayashi, S. Discovery of a Metalloenzyme-like Cooperative Catalytic System of Metal Nanoclusters and Catechol Derivatives for the Aerobic Oxidation of Amines. J. Am. Chem. Soc. 2012, 134, 13970–13973. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Mizuno, N. Efficient heterogeneous aerobic oxidation of amines by a supported ruthenium catalyst. Angew. Chem. Int. Ed. 2003, 42, 1480–1483. [Google Scholar] [CrossRef]
- Gu, X.-Q.; Chen, W.; Morales-Morales, D.; Jensen, C.M. Dehydrogenation of secondary amines to imines catalyzed by an iridium PCP pincer complex: Initial aliphatic or direct amino dehydrogenation. J. Mol. Catal. A Chem. 2002, 189, 119–124. [Google Scholar] [CrossRef]
- Tamura, M.; Tomishige, K. Redox Properties of CeO2 at Low Temperature: The Direct Synthesis of Imines from Alcohol and Amine. Angew. Chem. Int. Ed. 2015, 54, 864–867. [Google Scholar] [CrossRef]
- Blackburn, L.; Taylor, R.J.K. In Situ Oxidation−Imine Formation−Reduction Routes from Alcohols to Amines. Org. Lett. 2001, 3, 1637–1639. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Zhang, Y. Copper-catalyzed highly efficient aerobic oxidative synthesis of imines from alcohols and amines. Green Chem. 2012, 14, 1016–1019. [Google Scholar] [CrossRef]
- Gnanapra-kasam, B.; Zhang, J.; Milstein, D. Direct Synthesis of Imines from Alcohols and Amines with Liberation of H2. Angew. Chem. Int. Ed. 2010, 49, 1468–1471. [Google Scholar] [CrossRef]
- Soul, J.-F.; Miyamura, H.; Kobayashi, S. Selective imine formation from alcohols and amines catalyzed by polymer incarcerated gold/palladium alloy nanoparticles with molecular oxygen as an oxidant. Chem. Commun. 2013, 49, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhuang, R.; Bao, L.; Tang, G.; Zhao, Y. KOH-mediated transition metal-free synthesis of imines from alcohols and amines. Green Chem. 2012, 14, 2384–2387. [Google Scholar] [CrossRef]
- Corma, A.; Navas, J.; Sabater, M.J. Advances in one-pot synthesis through borrowing hydrogen catalysis. Chem. Rev. 2018, 118, 1410–1459. [Google Scholar] [CrossRef] [PubMed]
- Quintard, A.; Rodriguez, J. A Step into an eco-Compatible Future: Iron- and Cobalt catalyzed Borrowing Hydrogen Transformation. ChemSusChem 2016, 9, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Reed-Berendt, B.G.; Polidano, K.; Morrill, L.C. Recent advances in homogeneous borrowing hydrogen catalysis using earth-abundant first row transition metals. Org. Biomol. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Shibuya, R.; Nakahara, Y.; Germain, N.; Ohshima, T.; Mashima, K. Platinum-Catalyzed Direct Amination of Allylic Alcohols with Aqueous Ammonia: Selective Synthesis of Primary Allylamines. Angew. Chem. Int. Ed. 2012, 51, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Hakki, A.; Dillert, R.; Bahnemann, D.W. Factors affecting the selectivity of the photocatalytic conversion of nitroaromatic compounds over TiO2 to valuable nitrogen-containing organic compounds. Phys. Chem. Chem. Phys. 2013, 15, 2992–3002. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Ramalingam, B.; Shan, S.P.; Seayad, A.M. An efficient palladium-catalyzed N-alkylation of amines using primary and secondary alcohols. ACS Catal. 2013, 3, 2536–2540. [Google Scholar] [CrossRef]
- Imm, S.; Bahn, S.; Neubert, L.; Neumann, H.; Beller, M. An Efficient and General Synthesis of Primary Amines by Ruthenium-Catalyzed Amination of Secondary Alcohols with Ammonia. Angew. Chem. Int. Ed. 2010, 49, 8126–8129. [Google Scholar] [CrossRef]
- Pingen, D.; Müller, C.; Vogt, D. Direct amination of secondary alcohols using ammonia. Angew. Chem. Int. Ed. 2010, 49, 8130–8133. [Google Scholar] [CrossRef]
- Imm, S.; Baehn, S.; Zhang, M.; Neubert, L.; Neumann, H.; Klasovsky, F.; Pfeffer, J.; Haas, T.; Beller, M. Improved Ruthenium-Catalyzed Amination of Alcohols with Ammonia: Synthesis of Diamines and Amino Esters. Angew. Chem. Int. Ed. 2011, 50, 7599–7603. [Google Scholar] [CrossRef]
- Cano, R.; Ramon, D.J.; Yus, M. Impregnated Ruthenium on Magnetite as a Recyclable Catalyst for the N-Alkylation of Amines, Sulfonamides, Sulfinamides, and Nitroarenes Using Alcohols as Electrophiles by a Hydrogen Autotransfer Process. J. Org. Chem. 2011, 76, 5547–5557. [Google Scholar] [CrossRef]
- Balaraman, E.; Srimani, D.; Diskin-Posner, Y.; Milstein, D.; Balaraman, E.; Srimani, D.; Diskin-Posner, Y.; Milstein, D. Direct synthesis of secondary amines from alcohols and ammonia catalyzed by a ruthenium pincer complex. Catal. Lett. 2015, 145, 139–144. [Google Scholar] [CrossRef]
- Marichev, K.O.; Takacs, J.M. Ruthenium-Catalyzed Amination of Secondary Alcohols Using Borrowing Hydrogen Methodology. ACS Catal. 2016, 6, 2205–2210. [Google Scholar] [CrossRef]
- Şahin, Z.; Gürbüz, N.; Özdemir, İ.; Şahin, O.; Büyükgüngör, O.; Achard, M.; Bruneau, C. N-Alkylation and N,C-Dialkylation of Amines with Alcohols in the Presence of Ruthenium Catalysts with Chelating N-Heterocyclic Carbene Ligands. Organometallics 2015, 34, 2296–2304. [Google Scholar] [CrossRef]
- Kawahara, R.; Fujita, K.-I.; Yamaguchi, R. Dehydrogenative Oxidation of Alcohols in Aqueous Media Using Water-Soluble and Reusable Cp*Ir Catalysts Bearing a Functional Bipyridine Ligand. J. Am. Chem. Soc. 2010, 132, 15108–15111. [Google Scholar] [CrossRef] [PubMed]
- Ruch, S.; Irrgang, T.; Kempe, R. New Iridium Catalysts for the Selective Alkylation of Amines by Alcohols under Mild Conditions and for the Synthesis of Quinolines by Acceptorless Dehydrogenative Condensation. Chem. Eur. J. 2014, 20, 13279–13285. [Google Scholar] [CrossRef]
- Michlik, S.; Kempe, R. New iridium catalysts for the efficient alkylation of anilines by alcohols under mild conditions. Chem. Eur. J. 2010, 16, 13193–13198. [Google Scholar] [CrossRef]
- Gunanathan, C.; Milstein, D. Selective Synthesis of Primary Amines Directly from Alcohols and Ammonia. Angew. Chem. Int. Ed. 2008, 47, 8661–8664. [Google Scholar] [CrossRef]
- Zou, Q.; Wang, C.; Smith, J.; Xue, D.; Xiao, J. Alkylation of Amines with Alcohols and Amines by a Single Catalyst under Mild Conditions. Chem. Eur. J. 2015, 21, 9656–9661. [Google Scholar] [CrossRef]
- Corma, A.; Navas, J.; Sabater, M.J. Coupling of Two Multistep Catalytic Cycles for the One-Pot Synthesis of Propargylamines from Alcohols and Primary Amines on a Nanoparticulated Gold Catalyst. Chem. Eur. J. 2012, 18, 14150–14156. [Google Scholar] [CrossRef]
- Yan, T.; Feringa, B.L.; Barta, K. Iron catalysed direct alkylation of amines with alcohols. Nat. Commun. 2014, 5, 5602–5609. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Foo, S.W.; Saito, S. Iron/Amino Acid Catalyzed Direct N-Alkylation of Amines with Alcohols. Angew. Chem. Int. Ed. 2011, 50, 3006–3009. [Google Scholar] [CrossRef]
- Pan, H.J.; Ng, T.W.; Zhao, Y. Iron-catalyzed amination of alcohols assisted by Lewis acid. Chem. Commun. 2015, 51, 11907–11910. [Google Scholar] [CrossRef]
- Yan, T.; Feringa, B.L.; Barta, K. Benzylamines via Iron-Catalyzed Direct Amination of Benzyl Alcohols. ACS Catal. 2016, 6, 381–388. [Google Scholar] [CrossRef]
- Mukherjee, A.; Nerush, A.; Leitus, G.; Shimon, L.J.W.; David, Y.B.; Jalapa, N.A.E.; Milstein, D. Manganese-Catalyzed Environmentally Benign Dehydrogenative Coupling of Alcohols and Amines to Form Aldimines and H2: A Catalytic and Mechanistic Study. J. Am. Chem. Soc. 2016, 138, 4298–4301. [Google Scholar] [CrossRef]
- Elangovan, S.; Neumann, J.; Sortais, J.-B.; Junge, K.; Darce, C.; Beller, M. Efficient and selective N-alkylation of amines with alcohols catalysed by manganese pincer complexes. Nat. Commun. 2016, 7, 12641–12649. [Google Scholar] [CrossRef]
- Rosler, S.; Ertl, M.; Irrgang, T.; Kempe, R. Cobalt-Catalyzed Alkylation of Aromatic Amines by Alcohols. Angew. Chem. Int. Ed. 2015, 54, 15046–15050. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zeng, S.; Wu, J.; Zheng, S.; Zhang, G. Cobalt-Catalyzed Synthesis of Aromatic, Aliphatic, and Cyclic Secondary Amines via a “Hydrogen-Borrowing” Strategy. ACS Catal. 2016, 6, 6546–6550. [Google Scholar] [CrossRef]
- Freitag, F.; Irrgang, T.; Kempe, R. Cobalt-Catalyzed Alkylation of Secondary Alcohols with Primary Alcohols via Borrowing Hydrogen/Hydrogen Autotransfer. Chem. Eur. J. 2017, 23, 12110–12113. [Google Scholar] [CrossRef] [PubMed]
- Midya, S.P.; Mondal, A.; Begum, A.; Balaraman, E. A Simple Cobalt (II) Chloride Catalyzed N-Alkylation of Amines with Alcohols. Synthesis 2017, 49, 3957–3961. [Google Scholar]
- Tang, L.; Sun, H.; Li, Y.; Zha, Z.; Wang, Z. Highly active and selective synthesis of imines from alcohols and amines or nitroarenes catalyzed by Pd/DNA in water with dehydrogenation. Green Chem. 2012, 14, 3423–3428. [Google Scholar] [CrossRef]
- Chen, J.; Huang, S.; Lin, J.; Su, W. Recyclable palladium catalyst for facile synthesis of imines from benzyl alcohols and nitroarenes. Appl. Catal. A 2014, 470, 1–7. [Google Scholar] [CrossRef]
- Lee, C.; Liu, S. Preparation of secondary and tertiary amines from nitroarenes and alcohols. Chem. Commun. 2011, 47, 6981–6983. [Google Scholar] [CrossRef] [PubMed]
- Sabater, S.; Mata, J.A.; Peris, E. Dual Catalysis with an IrIII–AuI Heterodimetallic Complex: Reduction of Nitroarenes by Transfer Hydrogenation using Primary Alcohols. Chem. Eur. J. 2012, 18, 6380–6385. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, C.; Shi, F.; Deng, Y.Q. Au/Ag–Mo nano-rods catalyzed reductive coupling of nitrobenzenes and alcohols using glycerol as the hydrogen source. Chem. Commun. 2012, 48, 9391–9393. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; He, L.; Liu, Y.-H.; Cao, Y.; He, H.-Y.; Fan, K.N. Direct One-Pot Reductive N-Alkylation of Nitroarenes by using Alcohols with Supported Gold Catalysts. Chem. Eur. J. 2011, 17, 7172–7177. [Google Scholar] [CrossRef]
- Song, T.; Ren, P.; Duan, Y.; Wang, Z.; Chen, X.; Yang, Y. Cobalt nanocomposites on N-doped hierarchical porous carbon for highly selective formation of anilines and imines from nitroarenes. Green Chem. 2018, 20, 4629–4637. [Google Scholar] [CrossRef]
- Wei, Z.Z.; Wang, J.S.; Mao, J.; Su, D.F.; Jin, H.Y.; Wang, Y.H.; Xu, F.; Li, H.R.; Wang, Y. In Situ-Generated Co0-Co3O4/N-Doped Carbon Nanotubes Hybrids as Efficient and Chemoselective Catalysts for Hydrogenation of Nitroarenes. ACS Catal. 2015, 5, 4783–4789. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.-M.; Radnik, J.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332. [Google Scholar] [CrossRef]
- Navarrete-Vázquez, G.; Hidalgo-Figueroa, S.; Torres-Piedra, M.; Vergara-Galicia, J.; Rivera-Leyva, J.C.; Estrada-Soto, S.; León-Rivera, I.; Aguilar-Guardarrama, B.; Rios-Gómez, Y.; Villalobos-Molina, R.; et al. Synthesis, vasorelaxant activity and antihypertensive effect of benzo [d] imidazole derivatives. Bioorgan. Med. Chem. 2010, 18, 3985–3991. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wu, T.-T.; Wang, Z.; Xue, J.-Y.; Xu, Y.-G. Discovery of N-(2-phenyl-1H-benzo[d]imidazol-5-yl) quinolin-4-amine derivatives as novel VEGFR-2 kinase inhibitors. Eur. J. Med. Chem. 2014, 84, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Das, U.K.; Ben-David, Y.; Diskin-Posner, Y.; Milstein, D. N-Substituted Hydrazones by Manganese-Catalyzed Coupling of Alcohols with Hydrazine: Borrowing Hydrogen and Acceptorless Dehydrogenation in One System. Angew. Chem. Int. Ed. 2018, 57, 2179–2182. [Google Scholar] [CrossRef]


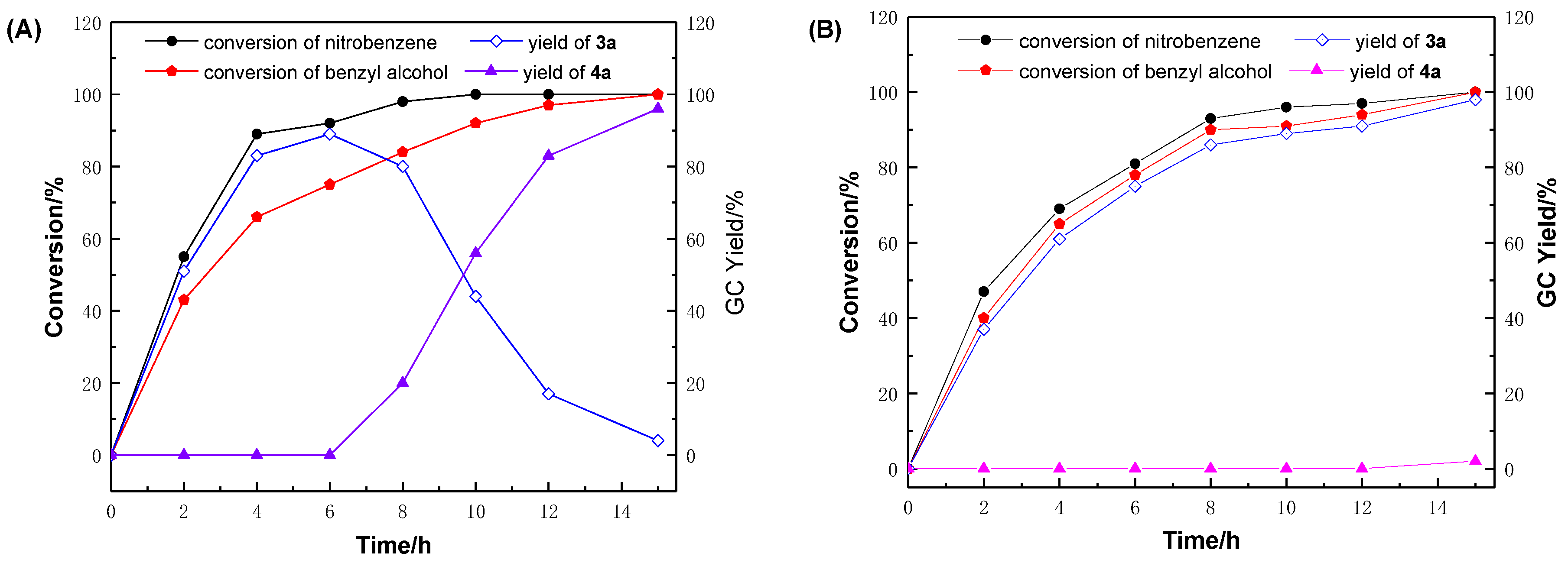
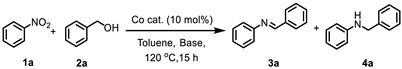
| Entry | Catalyst | Base (equiv.) | Conv. (%) b | Selectivity (%) b | TON h | |
|---|---|---|---|---|---|---|
| 3a | 4a | |||||
| 1 | CoOx@NC-800 | Na2CO3 (3.0) | 0 | 0 | 0 | 0 |
| 2 | CoOx@NC-800 | NaOH (3.0) | 21 | 81 | 19 | 2.1 |
| 3 | CoOx@NC-800 | KHMDS (3.0) | 100 | 57 | 43 | 10 |
| 4 | CoOx@NC-800 | KOH (3.0) | 27 | 78 | 22 | 2.7 |
| 5 | CoOx@NC-800 | - | 0 | 0 | 0 | 0 |
| 6 | CoOx@NC-800 | tBuOK (1.0) | 34 | 100 | 0 | 3.4 |
| 7 | CoOx@NC-800 | tBuOK (2.0) | 63 | 98 e | 2 | 6.3 |
| 8 | CoOx@NC-800 | tBuOK (3.0) | 100 | 98(87) e | 2 | 10 |
| 9 c | CoOx@NC-800 | tBuOK (4.0) | 100 | 20 | 80(72) e | 10 |
| 10 d | CoOx@NC-800 | tBuOK (3.0) | 100 | 95(86) e | 5 | 10 |
| 11 d | CoOx@NC-800 | tBuOK (4.0) | 100 | 4 | 96(84) e | 10 |
| 12 | CoOx@NC-800 | tBuOK (4.0) | 100 | 2 | 98 | 10 |
| 13 | CoOx@C-800 f | tBuOK (3.0) | 98 | 35 | 63 | 9.8 |
| 14 | tBuOK (3.0) | 47 | 45 | 40 | 4.7 | |
| 15 | CoCl2 | tBuOK (3.0) | 25 | 73 | 27 | 2.5 |
| 16 | Co3O4 | tBuOK (3.0) | 57 | 63 | 37 | 5.7 |
| 17 | Nano Co3O4 g | tBuOK (3.0) | 64 | 58 | 42 | 6.4 |
 |
 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Duan, Y.; Chen, X.; Yang, Y. Switchable Access to Amines and Imines from Reductive Coupling of Nitroarenes with Alcohols Catalyzed by Biomass-Derived Cobalt Nanoparticles. Catalysts 2019, 9, 116. https://doi.org/10.3390/catal9020116
Song T, Duan Y, Chen X, Yang Y. Switchable Access to Amines and Imines from Reductive Coupling of Nitroarenes with Alcohols Catalyzed by Biomass-Derived Cobalt Nanoparticles. Catalysts. 2019; 9(2):116. https://doi.org/10.3390/catal9020116
Chicago/Turabian StyleSong, Tao, Yanan Duan, Xiufang Chen, and Yong Yang. 2019. "Switchable Access to Amines and Imines from Reductive Coupling of Nitroarenes with Alcohols Catalyzed by Biomass-Derived Cobalt Nanoparticles" Catalysts 9, no. 2: 116. https://doi.org/10.3390/catal9020116
APA StyleSong, T., Duan, Y., Chen, X., & Yang, Y. (2019). Switchable Access to Amines and Imines from Reductive Coupling of Nitroarenes with Alcohols Catalyzed by Biomass-Derived Cobalt Nanoparticles. Catalysts, 9(2), 116. https://doi.org/10.3390/catal9020116






