Biochar-Supported FeS/Fe3O4 Composite for Catalyzed Fenton-Type Degradation of Ciprofloxacin
Abstract
:1. Introduction
2. Results and Discussion
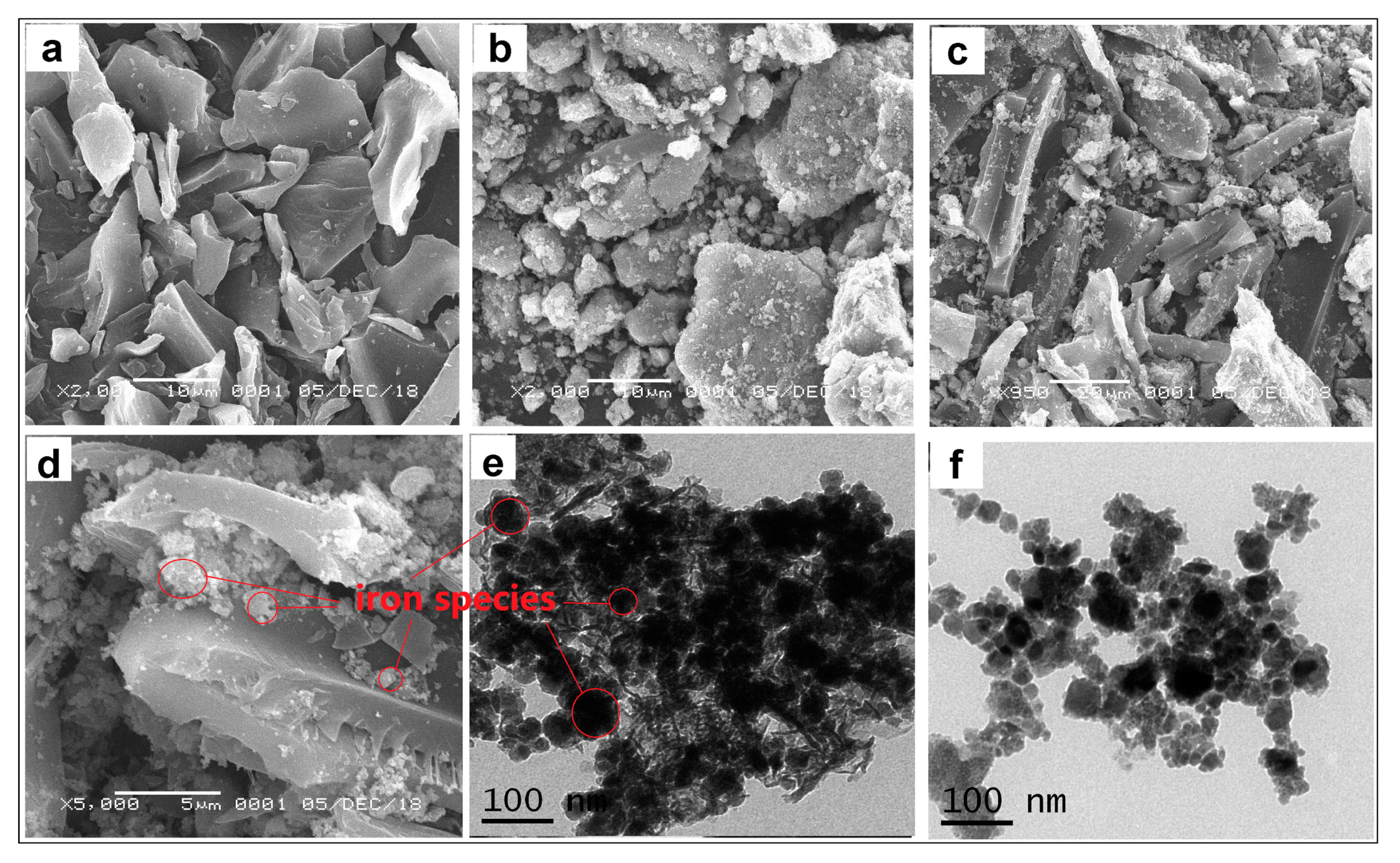
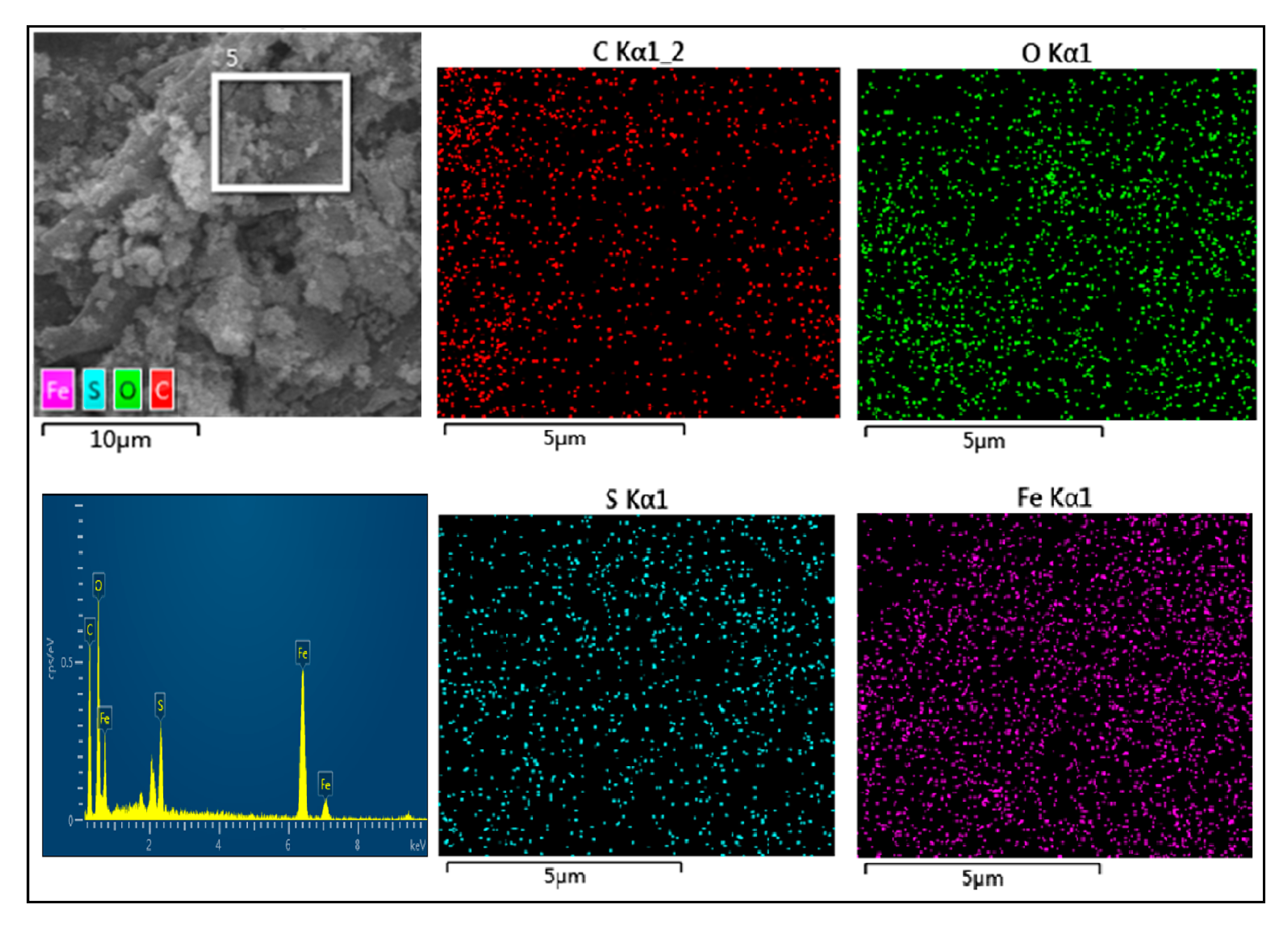
2.1. Characterizations of the Biochar-Supported Composite
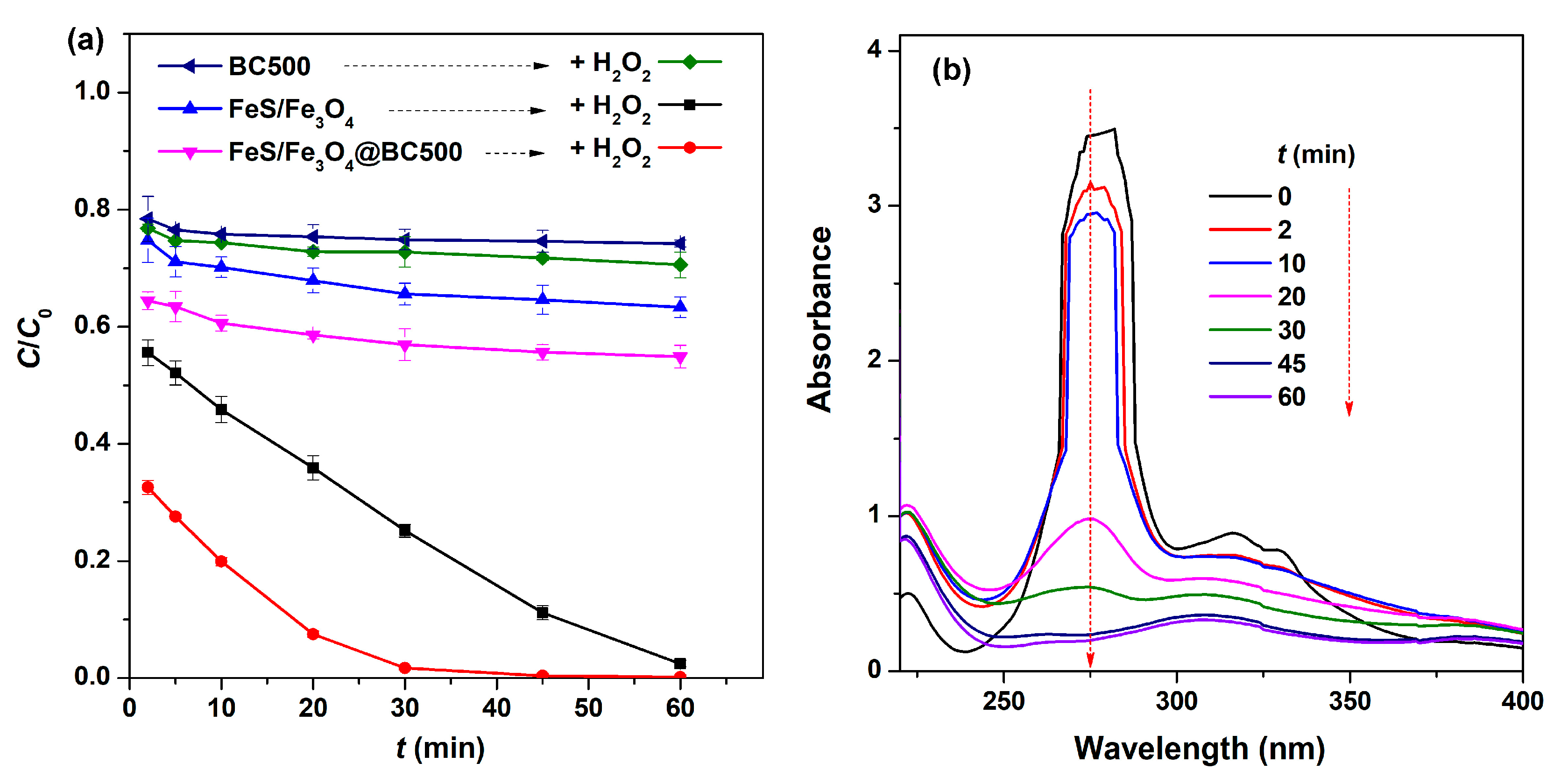
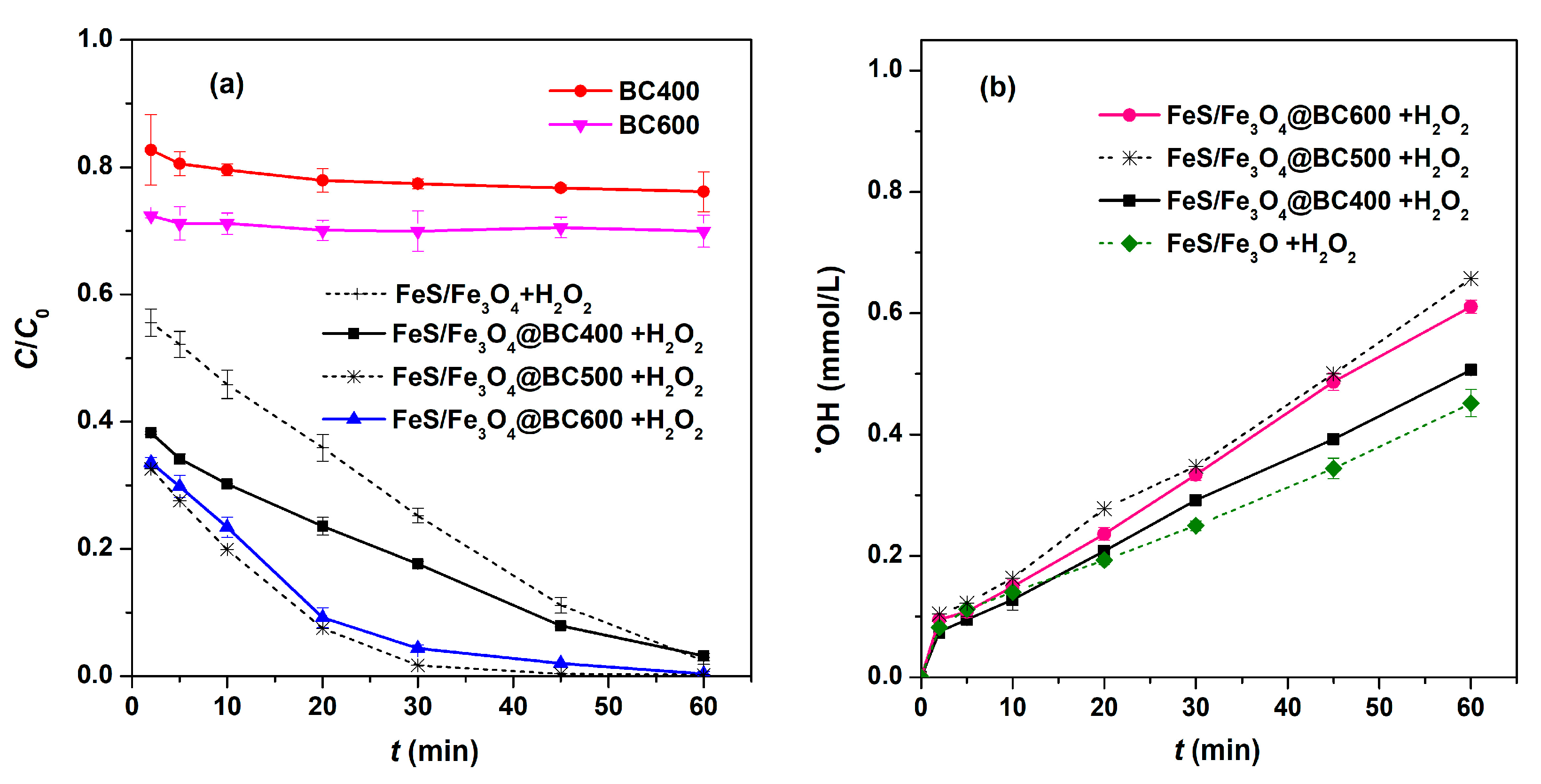
2.2. Comparison of Various Catalysis Systems for Removal of Ciprofloxacin
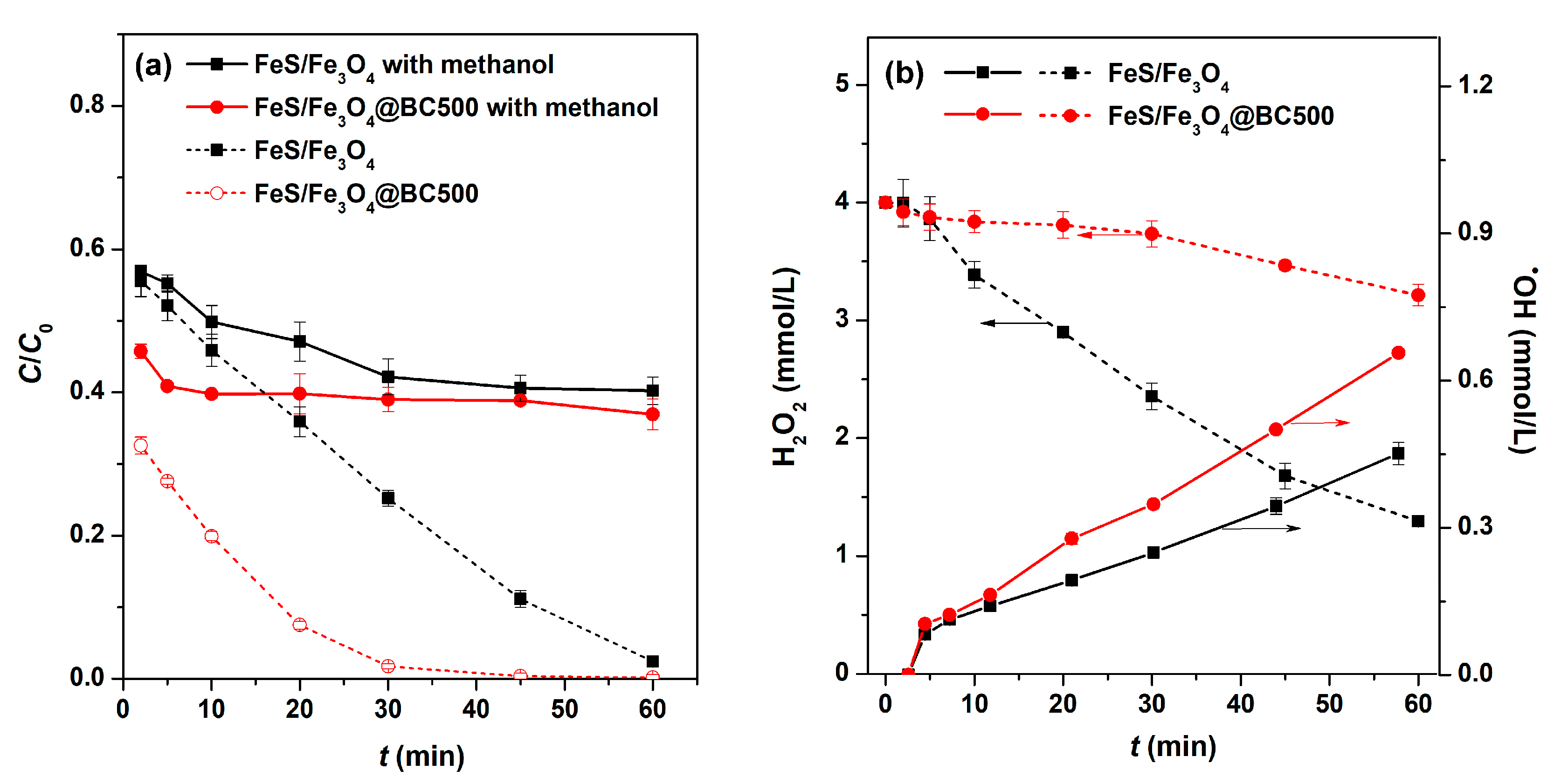
2.3. Investigation about the Reactive Species
2.4. The Biochar’s Influence to the Removal of Ciprofloxacin
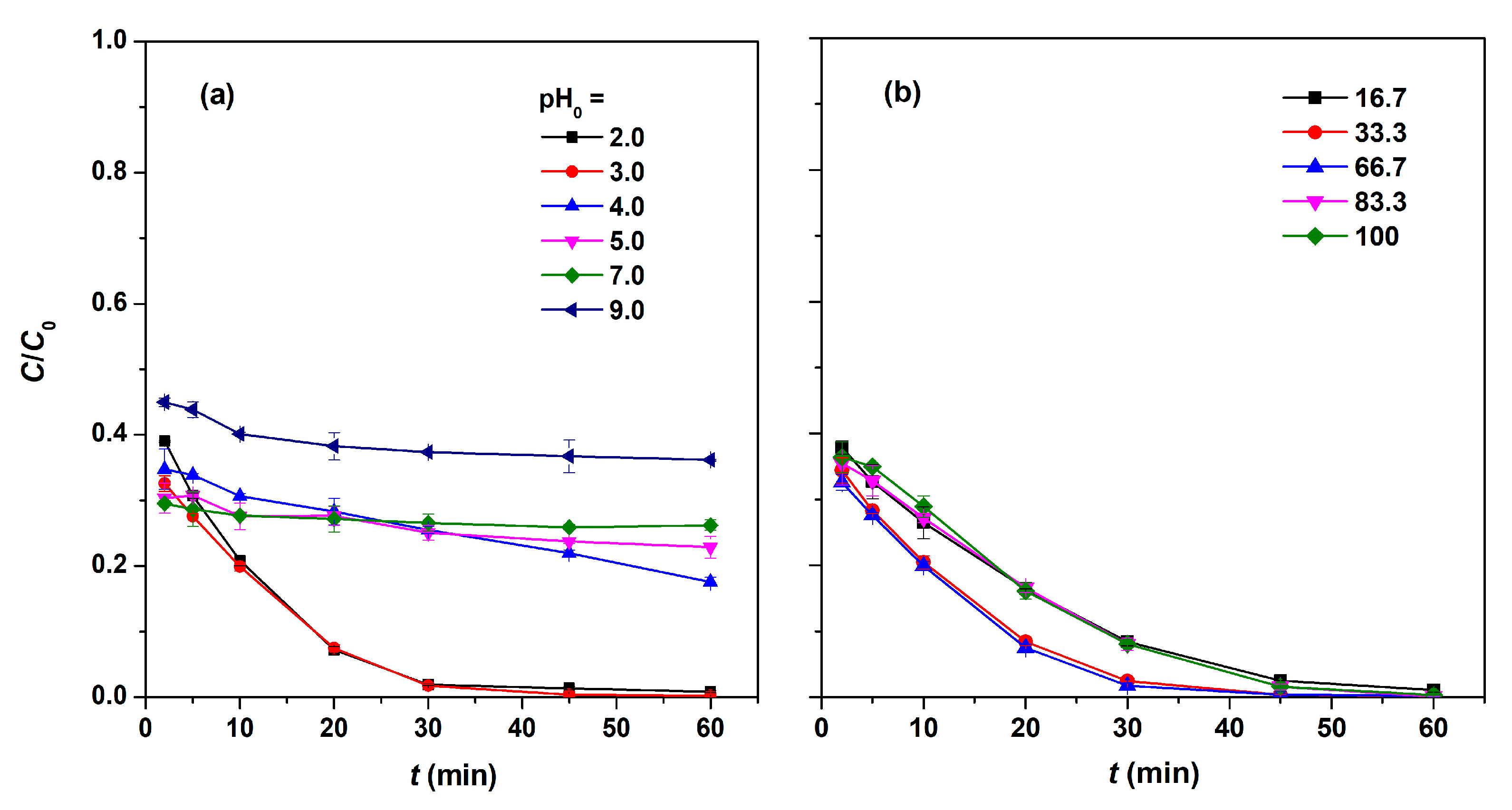
2.5. Influence of Reaction Conditions
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Biochar-Supported FeS/Fe3O4 Composites
3.3. Characterizations of the Biochar and Composites
3.4. Degradation Experiments
3.5. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, X.; Chen, B.; Zhu, L. Quantification of chemical states, dissociation constants and contents of oxygen-containing groups on the surface of biochars produced at different temperatures. Environ. Sci. Technol. 2014, 49, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lee, S.S.; Rajapaksha, A.U.; Vithanage, M.; Zhang, M.; Cho, J.S.; Lee, S.-E.; Ok, Y.S. Trichloroethylene adsorption by pine needle biochars produced at various pyrolysis temperatures. Bioresour. Technol. 2013, 143, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, C.; Li, J.; Xie, B.; Lü, J.; Li, Y. Thermal treatment of biochar in the air/nitrogen atmosphere for developed mesoporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2018, 263, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, X.; Zhao, L.; Wang, H.; Yu, H.; Gao, B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. 2013, 20, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhu, X.; Shi, L.; Li, J.; Li, S.; Lü, J.; Li, Y. Efficient removal of lead from solution by celery-derived biochars rich in alkaline minerals. Bioresour. Technol. 2017, 235, 185–192. [Google Scholar] [CrossRef]
- Outsiou, A.; Frontistis, Z.; Ribeiro, R.S.; Antonopoulou, M.; Konstantinou, I.K.; Silva, A.M.; Faria, J.L.; Gomes, H.T.; Mantzavinos, D. Activation of sodium persulfate by magnetic carbon xerogels (CX/CoFe) for the oxidation of bisphenol A: Process variables effects, matrix effects and reaction pathways. Water Res. 2017, 124, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Soares, O.S.G.P.; Rodrigues, C.S.; Madeira, L.M.; Pereira, M.F.R. Heterogeneous Fenton-Like degradation of p-nitrophenol over tailored carbon-based materials. Catalysts 2019, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Gao, B.; Zimmerman, A.R.; Chen, H.; Zhang, M.; Cao, X. Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. Bioresour. Technol. 2014, 152, 538–542. [Google Scholar] [CrossRef]
- Diao, Z.H.; Du, J.J.; Jiang, D.; Kong, L.J.; Huo, W.Y.; Liu, C.M.; Wu, Q.H.; Xu, X.R. Insights into the simultaneous removal of Cr6+ and Pb2+ by a novel sewage sludge-derived biochar immobilized nanoscale zero valent iron: Coexistence effect and mechanism. Sci. Total Environ. 2018, 642, 505–515. [Google Scholar] [CrossRef]
- Yan, J.; Han, L.; Gao, W.; Xue, S.; Chen, M. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour. Technol. 2015, 175, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, Y.; Yang, Y.; Han, Y.; Wang, T.; Chen, J.; Tsang, D.C. Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J. Hazard. Mater. 2020, 383, 121240. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Diao, Z.H.; Huo, W.Y.; Kong, L.J.; Du, J.J. Simultaneous removal of Cu2+ and bisphenol A by a novel biochar-supported zero valent iron from aqueous solution: Synthesis, reactivity and mechanism. Environ. Pollut. 2018, 239, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Dong, H.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zhang, L.; Fan, C. Nanoscale zero-valent iron/biochar composite as an activator for Fenton-like removal of sulfamethazine. Sep. Purif. Technol. 2018, 202, 130–137. [Google Scholar] [CrossRef]
- Mao, Q.; Zhou, Y.; Yang, Y.; Zhang, J.; Liang, L.; Wang, H.; Luo, S.; Luo, L.; Jeyakumar, P.; Ok, Y.S.; et al. Experimental and theoretical aspects of biochar-supported nanoscale zero-valent iron activating H2O2 for ciprofloxacin removal from aqueous solution. J. Hazard. Mater. 2019, 380, 120848. [Google Scholar] [CrossRef]
- Fang, G.; Gao, J.; Liu, C.; Dionysiou, D.D.; Wang, Y.; Zhou, D. Key role of persistent free radicals in hydrogen peroxide activation by biochar: Implications to organic contaminant degradation. Environ. Sci. Technol. 2014, 48, 1902–1910. [Google Scholar] [CrossRef]
- Fang, G.; Liu, C.; Gao, J.; Dionysiou, D.D.; Zhou, D. Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ. Sci. Technol. 2015, 49, 5645–5653. [Google Scholar] [CrossRef]
- Kemmou, L.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catal. Today 2018, 313, 128–133. [Google Scholar] [CrossRef]
- Magioglou, E.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Activation of persulfate by biochars from valorized olive stones for the degradation of sulfamethoxazole. Catalysts 2019, 9, 419. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Thines, K.R.; Abdullah, E.C.; Mubarak, N.M.; Ruthiraan, M. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review. Renew. Sustain. Energy Rev. 2017, 67, 257–276. [Google Scholar] [CrossRef]
- Ouyang, D.; Yan, J.; Qian, L.; Chen, Y.; Han, L.; Su, A.; Zhang, W.; Ni, H.; Chen, M. Degradation of 1, 4-dioxane by biochar supported nano magnetite particles activating persulfate. Chemosphere 2017, 184, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.D.; Chen, C.W.; Hung, C.M. Synthesis of magnetic biochar from bamboo biomass to activate persulfate for the removal of polycyclic aromatic hydrocarbons in marine sediments. Bioresour. Technol. 2017, 245, 188–195. [Google Scholar] [CrossRef]
- Dong, C.D.; Chen, C.W.; Kao, C.M.; Chien, C.C.; Hung, C.M. Wood-biochar-supported magnetite nanoparticles for remediation of PAH-contaminated estuary sediment. Catalysts 2018, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Yi, Y.; Tu, G.; Zhao, D.; Tsang, P.E.; Fang, Z. Pyrolysis of different biomass pre-impregnated with steel pickling waste liquor to prepare magnetic biochars and their use for the degradation of metronidazole. Bioresour. Technol. 2019, 289, 121613. [Google Scholar] [CrossRef]
- Yi, Y.; Tu, G.; Tsang, P.E.; Fang, Z. Insight into the influence of pyrolysis temperature on Fenton-like catalytic performance of magnetic biochar. Chem. Eng. J. 2020, 380, 122518. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Hung, C.M.; Nguyen, T.B.; Chang, J.H.; Wang, T.H.; Wu, C.H.; Lin, Y.L.; Chen, C.W.; Dong, C.D. Efficient heterogeneous activation of persulfate by iron-modified biochar for removal of antibiotic from aqueous solution: A case study of tetracycline removal. Catalysts 2019, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, Y.; Ai, Z.; Zhang, L. Hydrothermal synthesis of FeS2 as a high-efficiency Fenton reagent to degrade alachlor via superoxide-mediated Fe(II)/Fe(III) cycle. ACS Appl. Mater. Interfaces 2015, 7, 28534–28544. [Google Scholar] [CrossRef]
- Diao, Z.H.; Liu, J.J.; Hu, Y.X.; Kong, L.J.; Jiang, D.; Xu, X.R. Comparative study of Rhodamine B degradation by the systems pyrite/H2O2 and pyrite/persulfate: Reactivity, stability, products and mechanism. Sep. Purif. Technol. 2017, 184, 374–383. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Y.; Liu, Y.; Luo, C.; Wu, D. Enhanced degradation of chloramphenicol at alkaline conditions by S(-II) assisted heterogeneous Fenton-like reactions using pyrite. Chemosphere 2017, 188, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Z.; Yang, Z.; Yang, Q.; Li, B.; Bai, Z. Heterogeneous Fenton-like catalytic degradation of 2,4-dichlorophenoxyacetic acid in water with FeS. Chem. Eng. J. 2015, 273, 481–489. [Google Scholar] [CrossRef]
- Yuan, Y.; Tao, H.; Fan, J.; Ma, L. Degradation of p-chloroaniline by persulfate activated with ferrous sulfide ore particles. Chem. Eng. J. 2015, 268, 38–46. [Google Scholar] [CrossRef]
- Gong, Y.; Tang, J.; Zhao, D. Application of iron sulfide particles for groundwater and soil remediation: A review. Water Res. 2016, 89, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Tang, J.; Huang, Y.; Gai, L.; Zeng, E.Y.; Liber, K.; Gong, Y. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem. Eng. J. 2017, 322, 516–524. [Google Scholar] [CrossRef]
- Lyu, H.; Zhao, H.; Tang, J.; Gong, Y.; Huang, Y.; Wu, Q.; Gao, B. Immobilization of hexavalent chromium in contaminated soils using biochar supported nanoscale iron sulfide composite. Chemosphere 2018, 194, 360–369. [Google Scholar] [CrossRef]
- Yahya, M.S.; Oturan, N.; El Kacemi, K.; El Karbane, M.; Aravindakumar, C.T.; Oturan, M.A. Oxidative degradation study on antimicrobial agent ciprofloxacin by electro-Fenton process: Kinetics and oxidation products. Chemosphere 2014, 117, 447–454. [Google Scholar] [CrossRef]
- Diao, Z.H.; Xu, X.R.; Jiang, D.; Li, G.; Liu, J.J.; Kong, L.J.; Zuo, L.Z. Enhanced catalytic degradation of ciprofloxacin with FeS2/SiO2 microspheres as heterogeneous Fenton catalyst: Kinetics, reaction pathways and mechanism. J. Hazard. Mater. 2017, 327, 108–115. [Google Scholar] [CrossRef]
- Hassani, A.; Karaca, M.; Karaca, S.; Khataee, A.; Açışlı, Ö.; Yılmaz, B. Preparation of magnetite nanoparticles by high-energy planetary ball mill and its application for ciprofloxacin degradation through heterogeneous Fenton process. J. Environ. Manag. 2018, 211, 53–62. [Google Scholar] [CrossRef]
- Huang, D.; Luo, H.; Zhang, C.; Zeng, G.; Lai, C.; Cheng, M.; Wang, R.; Deng, R.; Xue, W.; Gong, X.; et al. Nonnegligible role of biomass types and its compositions on the formation of persistent free radicals in biochar: Insight into the influences on Fenton-like process. Chem. Eng. J. 2019, 361, 353–363. [Google Scholar] [CrossRef]
- Li, J.; Pan, L.; Yu, G.; Xie, S.; Li, C.; Lai, D.; Li, Z.; You, F.; Wang, Y. The synthesis of heterogeneous Fenton-like catalyst using sewage sludge biochar and its application for ciprofloxacin degradation. Sci. Total Environ. 2019, 654, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Yuan, S.; Liao, P.; Zhang, P. Oxidizing impact induced by mackinawite (FeS) nanoparticles at oxic conditions due to production of hydroxyl radicals. Environ. Sci. Technol. 2016, 50, 11646–11653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yuan, S.; Liao, P. Mechanisms of hydroxyl radical production from abiotic oxidation of pyrite under acidic conditions. Geochim. Cosmochim. Acta 2016, 172, 444–457. [Google Scholar] [CrossRef]
- Ardo, S.G.; Nélieu, S.; Ona-Nguema, G.; Delarue, G.; Brest, J.; Pironin, E.; Morin, G. Oxidative degradation of nalidixic acid by nano-magnetite via Fe2+/O2-mediated reactions. Environ. Sci. Technol. 2015, 49, 4506–4514. [Google Scholar] [CrossRef]
- Rakshit, S.; Sarkar, D.; Elzinga, E.J.; Punamiya, P.; Datta, R. Mechanisms of ciprofloxacin removal by nano-sized magnetite. J. Hazard. Mater. 2013, 246–247, 221–226. [Google Scholar] [CrossRef]
- Gupta, A.; Garg, A. Degradation of ciprofloxacin using Fenton’s oxidation: Effect of operating parameters, identification of oxidized by-products and toxicity assessment. Chemosphere 2018, 193, 1181–1188. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Yu, B.; Wu, R.; Mai, J.; Wang, R.; Chen, L.; Yang, S.T. Preparation of Fe3O4/TiO2/C nanocomposites and their application in Fenton-like catalysis for dye decoloration. Catalysts 2016, 6, 146. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tran, H.P.; Hussain, I.; Zhong, Y.; Huang, S. Degradation of p-chloroaniline by pyrite in aqueous solutions. Chem. Eng. J. 2015, 279, 396–401. [Google Scholar] [CrossRef]
- Zhu, X.; Li, J.; Xie, B.; Feng, D.; Li, Y. Accelerating effects of biochar for pyrite-catalyzed Fenton-like oxidation of herbicide 2,4-D. Chem. Eng. J. 2020, 123605. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, L.; An, T. Hydrothermal carbon-mediated Fenton-like reaction mechanism in the degradation of alachlor: Direct electron transfer from hydrothermal carbon to Fe (III). ACS Appl. Mater. Interfaces 2017, 9, 17115–17124. [Google Scholar] [CrossRef]
- Chen, R.; Pignatello, J.J. Role of quinone intermediates as electron shuttles in Fenton and photoassisted Fenton oxidations of aromatic compounds. Environ. Sci. Technol. 1997, 31, 2399–2406. [Google Scholar] [CrossRef]
- Fabbri, D.; Rombolà, A.G.; Torri, C.; Spokas, K.A. Determination of polycyclic aromatic hydrocarbons in biochar and biochar amended soil. J. Anal. Appl. Pyrolysis 2013, 103, 60–67. [Google Scholar] [CrossRef]
- Zhao, X.; Qin, L.; Gatheru Waigi, M.; Cheng, P.; Yang, B.; Wang, J.; Ling, W. Removal of bound PAH residues in contaminated soils by Fenton oxidation. Catalysts 2019, 9, 619. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Pan, B.; Li, H.; Liao, S.; Zhang, D.; Wu, M.; Xing, B. Degradation of p-nitrophenol on biochars: Role of persistent free radicals. Environ. Sci. Technol. 2015, 50, 694–700. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Wu, Y.; Zheng, M. A comparison of biochars from lignin, cellulose and wood as the sorbent to an aromatic pollutant. J. Hazard. Mater. 2014, 280, 450–457. [Google Scholar] [CrossRef]
- Tong, M.; Yuan, S.; Ma, S.; Jin, M.; Liu, D.; Cheng, D.; Liu, X.; Gan, Y.; Wang, Y. Production of abundant hydroxyl radicals from oxygenation of subsurface sediments. Environ. Sci. Technol. 2016, 50, 214–221. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhu, X.; Feng, D.; Hodge, A.K.; Hu, L.; Lü, J.; Li, J. Biochar-Supported FeS/Fe3O4 Composite for Catalyzed Fenton-Type Degradation of Ciprofloxacin. Catalysts 2019, 9, 1062. https://doi.org/10.3390/catal9121062
Wang Y, Zhu X, Feng D, Hodge AK, Hu L, Lü J, Li J. Biochar-Supported FeS/Fe3O4 Composite for Catalyzed Fenton-Type Degradation of Ciprofloxacin. Catalysts. 2019; 9(12):1062. https://doi.org/10.3390/catal9121062
Chicago/Turabian StyleWang, Yue, Xiaoxiao Zhu, Dongqing Feng, Anthony K. Hodge, Liujiang Hu, Jinhong Lü, and Jianfa Li. 2019. "Biochar-Supported FeS/Fe3O4 Composite for Catalyzed Fenton-Type Degradation of Ciprofloxacin" Catalysts 9, no. 12: 1062. https://doi.org/10.3390/catal9121062
APA StyleWang, Y., Zhu, X., Feng, D., Hodge, A. K., Hu, L., Lü, J., & Li, J. (2019). Biochar-Supported FeS/Fe3O4 Composite for Catalyzed Fenton-Type Degradation of Ciprofloxacin. Catalysts, 9(12), 1062. https://doi.org/10.3390/catal9121062




