Lipase Catalyzed Acidolysis for Efficient Synthesis of Phospholipids Enriched with Isomerically Pure cis-9,trans-11 and trans-10,cis-12 Conjugated Linoleic Acid
Abstract
1. Introduction
2. Results and Discussion
2.1. Specificity of Lipases—Choice of Phospholipid Substrate for Acidolysis
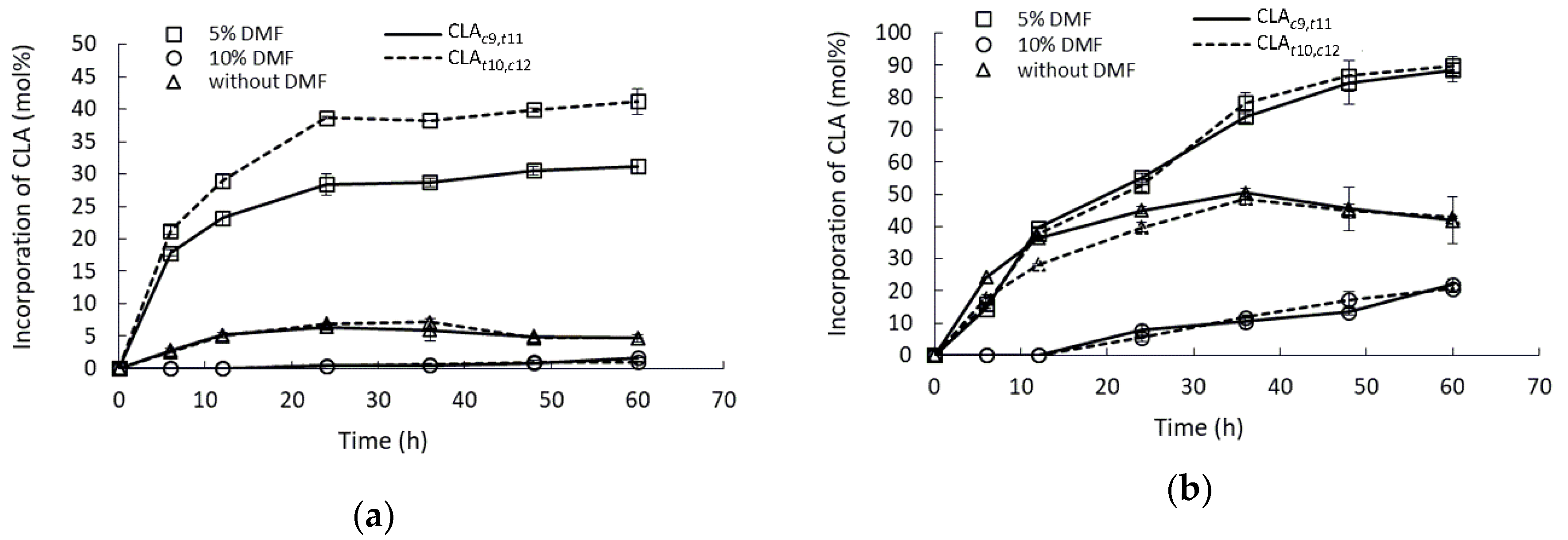
2.2. Effect of Mixing Conditions
2.3. Lipases Selectivity to Conjugated Linoleic Acid Isomers
2.4. Effect of Water Activity
2.5. Effect of Water-Mimicking Co-Solvent
2.6. Discussion of Developed Method with the Literature
3. Materials and Methods
3.1. Substrates and Enzymes for Enzymatic Reactions
3.2. Chemicals
3.3. Analytical Methods
3.3.1. Thin-Layer Chromatography (TLC)
3.3.2. High Pressure Chromatography (HPLC)
3.3.3. Gas Chromatography
3.3.4. Water Content
3.4. Lipase-Catalyzed Acidolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Schneider, M. Phospholipids. In Lipid Technologies and Applications, 1st ed.; Gunstone, F.D., Padley, F.B., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 51–78. [Google Scholar]
- Guo, Z.; Vikbjerg, A.F.; Xu, X. Enzymatic modification of phospholipids for functional applications and human nutrition. Biotechnol. Adv. 2005, 23, 203–259. [Google Scholar] [CrossRef]
- Dahan, A.; Markovic, M.; Epstein, S.; Cohen, N.; Zimmermann, E.M.; Aponick, A.; Ben-Shabat, S. Phospholipid-drug conjugates as a novel oral drug targeting approach for the treatment of inflammatory bowel disease. Eur. J. Pharm. Sci. 2017, 108, 78–85. [Google Scholar] [CrossRef]
- Kłobucki, M.; Urbaniak, A.; Grudniewska, A.; Kocbach, B.; Maciejewska, G.; Kiełbowicz, G.; Ugorski, M.; Wawrzeńczyk, C. Syntheses and cytotoxicity of phosphatidylcholines containing ibuprofen or naproxen moieties. Sci. Rep. 2019, 9, 220. [Google Scholar] [CrossRef]
- Rychlicka, M.; Niezgoda, N.; Gliszczyńska, A. Lipase-catalyzed acidolysis of egg-yolk phosphatidylcholine with citronellic acid. New insight into synthesis of isoprenoid-phospholipids. Molecules 2018, 23, 314. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska, A.; Niezgoda, N.; Gładkowski, W.; Czarnecka, M.; Świtalska, M.; Wietrzyk, J. Synthesis and biological evaluation of novel phosphatidylcholine analogues containing monoterpene acids as potent antiproliferative agents. PLoS ONE 2016, 11, e0157278. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska, A.; Niezgoda, N.; Gładkowski, W.; Świtalska, M.; Wietrzyk, J. Isoprenoid-phospholipid conjugates as potential therapeutic agents: Synthesis, characterization and antiproliferative studies. PLoS ONE 2017, 12, e0172238. [Google Scholar] [CrossRef] [PubMed]
- Kaki, S.S.; Balakrishna, M.; Prasad, R.B.N. Enzymatic synthesis and characterization of 1-lipoyl-2-palmitoyl phosphatidylcholine: A novel phospholipid containing lipoic acid. Eur. J. Lipid Sci. Technol. 2014, 116, 1347–1353. [Google Scholar] [CrossRef]
- Czarnecka, M.; Świtalska, M.; Wietrzyk, J.; Maciejewska, G.; Gliszczyńska, A. Synthesis, characterization, and in vitro cancer cell growth inhibition evaluation of novel phosphatidylcholines with anisic and veratric acids. Molecules 2018, 23, 2022. [Google Scholar] [CrossRef]
- Czarnecka, M.; Świtalska, M.; Wietrzyk, J.; Maciejewska, G.; Gliszczyńska, A. Synthesis and biological evaluation of phosphatidylcholines with cinnamic and 3-methoxycinnamic acids with potent antiproliferative activity. RSC Adv. 2018, 8, 35744–35752. [Google Scholar] [CrossRef]
- Markovic, M.; Ben-Shabat, S.; Keinan, S.; Aponick, A. Molecular modeling-guided design of phospholipid-based prodrugs. Int. J. Mol. Sci. 2019, 20, 2210. [Google Scholar] [CrossRef]
- Damnjanović, J.; Iwasaki, Y. Enzymatic modification of phospholipids. Prog. Food Biotechnol. 2018, 4, 81–130. [Google Scholar]
- Ang, X.; Chen, H.; Xiang, J.Q.; Wei, F.; Quek, S.Y. Preparation and functionality of lipase-catalysed structured phospholipid–A review. Trends Food Sci. Technol. 2019, 88, 373–383. [Google Scholar] [CrossRef]
- Gładkowski, W.; Kiełbowicz, G.; Chojnacka, A.; Gil, M.; Trziszka, T.; Dobrzański, Z.; Wawrzeńczyk, C. Fatty acid composition of egg yolk phospholipid fractions following feed supplementation of Lohmann Brown hens with humic-fat preparations. Food Chem. 2011, 126, 1013–1018. [Google Scholar] [CrossRef]
- Chojnacka, A.; Gładkowski, W. Production of structured phosphatidylcholine with high content of myristic acid by lipase-catalyzed acidolysis and interesterification. Catalysts 2018, 8, 281. [Google Scholar] [CrossRef]
- Ochoa-Flores, A.A.; Hernández-Becerra, J.A.; Cavazos-Garduño, A.; Vernon-Carter, E.J.; García, H.S. Optimization of the Synthesis of Structured Phosphatidylcholine with Medium Chain Fatty Acid. J. Oleo Sci. 2017, 66, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S. Enzymatic Synthesis and Characterization of Modified Phospholipids using Decanoic Acid. Int. J. Biotechnol. Biochem. 2017, 13, 31–38. [Google Scholar]
- Li, D.; Qin, X.; Wang, W.; Li, Z.; Yang, B.; Wang, Y. Synthesis of DHA/EPA-rich phosphatidylcholine by immobilized phospholipase A1: Effect of water addition and vacuum condition. Bioprocess Biosyst. Eng. 2016, 39, 1305–1314. [Google Scholar] [CrossRef]
- Marsaoui, N.; Naghmouchi, K.; Baah, J.; Raies, A.; Laplante, S. Incorportation of ethyl esters of EPA and DHA in soybean lecithin using Rhizomucor miehei lipase: Effect of additives and solvent-free conditions. Appl. Biochem. Biotechnol. 2015, 176, 938–946. [Google Scholar] [CrossRef]
- Chojnacka, A.; Gładkowski, W.; Grudniewska, A. Lipase-catalyzed transesterification of egg-yolk phophatidylcholine with concentrate of n-3 polyunsaturated fatty acids from cod liver oil. Molecules 2017, 22, 1771. [Google Scholar] [CrossRef]
- Xu, X.; Vikbjerg, A.F.; Guo, Z.; Zhang, L.; Acharya, A.K. Chapter 3–Enzymatic modification of phospholipids and related polar lipids. In Phospholipid Technology and Applications; Gunstone, F.D., Ed.; Woodhead Publishing: Philadelphia, PA, USA, 2012; pp. 41–82. [Google Scholar]
- Chojnacka, A.; Gładkowski, W.; Kiełbowicz, G.; Wawrzeńczyk, C. Enzymatic enrichment of egg-yolk phosphatidylcholine with α-linolenic acid. Biotechnol. Lett. 2009, 31, 705–709. [Google Scholar] [CrossRef]
- Doig, S.D.; Diks, R.M.M. Toolbox for exchanging constituent fatty acids in lecithins. Eur. J. Lipid Sci. Technol. 2003, 105, 359–367. [Google Scholar] [CrossRef]
- Adlercreutz, D.; Wehtje, E. An enzymatic method for the synthesis of mixed-acid phosphatidylcholine. J. Am. Oil Chem. Soc. 2004, 81, 553–557. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hosokawa, M.; Miyashita, K. Production of phosphatidylcholine containing conjugated linoleic acid mediated by phospholipase A2. J. Mol. Catal. B Enzym. 2006, 41, 92–96. [Google Scholar] [CrossRef][Green Version]
- Adlercreutz, P.; Virto, C.; Persson, M.; Vaz, S.; Adlercreutz, D.; Svensson, I.; Wehtje, E. Enzymatic conversions of polar lipids. Principles, problems and solutions. J. Mol. Catal.-B Enzym. 2001, 11, 173–178. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Juárez, M. Trans fatty acids and conjugated linoleic acid in food: Origin and biological properties. Nutr. Hosp. 2019, 36, 479–486. [Google Scholar]
- Koba, K.; Yanagita, T. Health benefits of conjugated linoleic acid (CLA). Obes. Res. Clin. Pract. 2014, 8, e525–e532. [Google Scholar] [CrossRef]
- Niezgoda, N.; Mituła, P.; Wawrzeńczyk, C. Preparation of conjugated linoleic acid (CLA) isomers. Przem. Chem. 2011, 90, 949–957. [Google Scholar]
- Peng, L.; Xu, X.; Mu, H.; Høy, C.E.; Adler-Nissen, J. Production of structured phospholipids by lipase-catalyzed acidolysis: Optimization using response surface methodology. Enzym. Microb. Technol. 2002, 31, 523–532. [Google Scholar] [CrossRef]
- Hossen, M.; Hernandez, E. Enzyme-catalyzed synthesis of structured phospholipids with conjugated linoleic acid. Eur. J. Lipid Sci. Technol. 2005, 107, 730–736. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Mu, H.; Xu, X. Synthesis of structured phospholipids by immobilized phospholipase A2 catalyzed acidolysis. J. Biotechnol. 2007, 128, 545–554. [Google Scholar] [CrossRef]
- Liu, L.P.; Wang, W.F.; Wang, Y.H.; Yang, B.; Ning, Z.X. Study on enzymatic synthesis of phospholipids with conjugated linoleic acid. Sci. Technol. Food Ind. 2011, 7. [Google Scholar]
- Hong, S.I.; Kim, Y.; Kim, C.T.; Kim, I.H. Enzymatic synthesis of lysophosphatidylcholine containing CLA from sn-glycero-3-phosphatidylcholine (GPC) under vacuum. Food Chem. 2011, 129, 1–6. [Google Scholar] [CrossRef]
- Baeza-Jiménez, R.; Noriega-Rodríguez, J.A.; García, H.S.; Otero, C. Structured phosphatidylcholine with elevated content of conjugated linoleic acid: Optimization by response surface methodology. Eur. J. Lipid Sci. Technol. 2012, 114, 1261–1267. [Google Scholar] [CrossRef]
- Baeza-Jiménez, R.; González-Rodríguez, J.; Kim, I.-H.; García, H.S.; Otero, C. Use of immobilized phospholipase A1-catalyzed acidolysis for the production of structured phosphatidylcholine with an elevated conjugated linoleic acid content. Grasas Aceites 2012, 63, 44–52. [Google Scholar]
- Zeng, C.; Luo, R.; Yang, B.; Wang, Y. Synthesis of structured phospholipids with conjugated linoleic acid by lipase-catalyzed acidolysis: Optimization using response surface methodology. China Oils Fats 2012, 12. [Google Scholar]
- Li, D.; Wang, W.; Zhang, L.; Liu, N.; Faiza, M.; Tan, C.P.; Yang, B.; Lan, D.; Wang, Y. Synthesis of CLA-Rich Lysophosphatidylcholine by Immobilized MAS1-H108A-Catalyzed Esterification: Effects of the Parameters and Monitoring of the Reaction Process. Eur. J. Lipid Sci. Technol. 2018, 120, 1–7. [Google Scholar] [CrossRef]
- Niezgoda, N.; Gliszczyńska, A.; Gładkowski, W.; Chojnacka, A.; Kiełbowicz, G.; Wawrzeńczyk, C. Lipase-catalyzed enrichment of egg yolk phosphatidylcholine with conjugated linoleic acid. J. Mol. Catal. B Enzym. 2016, 123, 14–22. [Google Scholar] [CrossRef]
- Niezgoda, N.; Gliszczyńska, A.; Gładkowski, W.; Chojnacka, A.; Kiełbowicz, G.; Wawrzeńczyk, C. Production of concentrates of CLA obtained from sunflower and safflower and their application to the lipase-catalyzed acidolysis of egg yolk phosphatidylcholine. Eur. J. Lipid Sci. Technol. 2016, 118, 1566–1578. [Google Scholar] [CrossRef]
- Verdasco-Martín, C.M.; Corchado-Lopo, C.; Fernández-Lafuente, R.; Otero, C. Prolongation of secondary drying step of phospholipid lyophilization greately improves acidolysis reactions catalyzed by immobilized lecitase ultra. Enzym. Microb. Technol. 2019, 132, 109388. [Google Scholar] [CrossRef]
- Verdasco-Martín, C.M.; Corchado-Lopo, C.; Fernández-Lafuente, R.; Otero, C. Rapid and high yield production of phospholipids enriched in CLA via acidolysis: The critical role of the enzyme immobilization protocol. Food Chem. 2019, 296, 123–131. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.; Kim, Y.J.; Park, Y. Conjugated linoleic acid: Potential health benefits as a functional food ingredient. Annu. Rev. Food Sci. Technol. 2016, 7, 221–244. [Google Scholar] [CrossRef]
- Ke, X.-Y.; Zhao, B.-J.; Zhao, X.; Wang, Y.; Huang, Y.; Chen, X.-M.; Zhao, B.-X.; Zhao, S.-S.; Zhang, X.; Zhang, Q. The therapeutic efficacy of conjugated linoleic acid–Paclitaxel on glioma in the rat. Biomaterials 2010, 31, 5855–5864. [Google Scholar] [CrossRef]
- Guo, D.-D.; Hong, S.-H.; Jiang, H.-L.; Kim, J.-H.; Minai-Tehrani, A.; Kim, J.-E.; Shin, J.-Y.; Jiang, T.; Kim, Y.-K.; Choi, Y.-J.; et al. Synergistic effects of Akt1 shRNA and paclitaxel-incorporated conjugated linoleic acid-coupled poloxamer thermosensitive hydrogel on breast cancer. Biomaterials 2012, 33, 2272–2281. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.-M.; Wang, J.; Wang, J.; Feng, Q.; Gao, S.; Zhang, L.-R.; Zhang, Q. Enhanced anticancer activity of gemcitabine coupling with conjugated linoleic acid against human breast cancer in vitro and in vivo. Eur. J. Pharm. Biopharm. 2012, 82, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Niezgoda, N.; Mituła, P.; Kempińska, K.; Wietrzyk, J.; Wawrzeńczyk, C. Synthesis of Phosphatidylcholine with Conjugated Linoleic Acid and Studies on Its Cytotoxic Activity. Aust. J. Chem. 2013, 66, 354. [Google Scholar] [CrossRef]
- Niezgoda, N.; Gliszczyńska, A.; Gładkowski, W.; Kempińska, K.; Wietrzyk, J.; Wawrzeńczyk, C. Phosphatidylcholine with cis-9,trans-11 and trans-10,cis-12 Conjugated Linoleic Acid Isomers: Synthesis and Cytotoxic Studies. Aust. J. Chem. 2015, 68, 1065. [Google Scholar] [CrossRef][Green Version]
- Pucek, A.; Niezgoda, N.; Kulbacka, J.; Wawrzeńczyk, C.; Wilk, K.A. Phosphatidylcholine with conjugated linoleic acid in fabrication of novel lipid nanocarriers. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 377–388. [Google Scholar] [CrossRef]
- Churruca, I.; Fernández-Quintela, A.; Portillo, M.P. Conjugated linoleic acid isomers: Differences in metabolism and biological effects. BioFactors 2009, 35, 105–111. [Google Scholar] [CrossRef]
- Beppu, F.; Hosokawa, M.; Tanaka, L.; Kohno, H.; Tanaka, T.; Miyashita, K. Potent inhibitory effect of trans9,trans11 isomer of conjugated linoleic acid on the growth of human colon cancer cells. J. Nutr. Biochem. 2006, 17, 830–836. [Google Scholar] [CrossRef]
- Palombo, J.D.; Ganguly, A.; Bistrian, B.R.; Menard, M.P. The antiproliferative effects of biologically active isomers of conjugated linoleic acid on human colorectal and prostatic cancer cells. Cancer Lett. 2002, 177, 163–172. [Google Scholar] [CrossRef]
- Yamasaki, M.; Chujo, H.; Koga, Y.; Oishi, A.; Rikimaru, T.; Shimada, M.; Sugimachi, K.; Tachibana, H.; Yamada, K. Potent cytotoxic effect of the trans10,cis12 isomer of conjugated linoleic acid on rat hepatoma dRLh-84 cells. Cancer Lett. 2002, 188, 171–180. [Google Scholar] [CrossRef]
- Tanmahasamut, P.; Liu, J.; Hendry, L.B.; Sidell, N. Conjugated linoleic acid blocks estrogen signaling in human breast cancer cells. J. Nutr. 2004, 134, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.M.K.; Felder, M.; Ludwig, K.; Van Galder, H.R.; Anderson, M.L.; Kim, J.; Cook, M.E.; Kapur, A.K.; Patankar, M.S. Trans10,cis12 conjugated linoleic acid inhibits proliferation and migration of ovarian cancer cells by inducing ER stress, autophagy, and modulation of Src. PLoS ONE 2018, 13, e0189524. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Mu, H.; Xu, X. Elucidation of acyl migration during lipase-catalyzed production of structured phospholipids. J. Am. Oil Chem. Soc. 2006, 83, 609–614. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Mu, H.; Xu, X. Parameters affecting incorporation and by-product formation during the production of structured phospholipids by lipase-catalyzed acidolysis in solvent-free system. J. Mol. Catal. B Enzym. 2005, 36, 14–21. [Google Scholar] [CrossRef]
- Egger, D.; Wehtje, E.; Adlercreutz, P. Characterization and optimization of phospholipase A2 catalyzed synthesis of phosphatidylcholine. Biochim. Biophys. Acta-Protein Struct. Mol. Enzymol. 1997, 1343, 76–84. [Google Scholar] [CrossRef]
- Kiatsimkul, P.P.; Sutterlin, W.R.; Suppes, G.J. Selective hydrolysis of epoxidized soybean oil by commercially available lipases: Effects of epoxy group on the enzymatic hydrolysis. J. Mol. Catal. B Enzym. 2006, 41, 55–60. [Google Scholar] [CrossRef]
- Haraldsson, G.G.; Kristinsson, B. Separation of eicosapentaenoic acid and docosahexaenoic acid in fish oil by kinetic resolution using lipase. J. Am. Oil Chem. Soc. 1998, 75, 1551–1556. [Google Scholar] [CrossRef]
- Sugasini, D.; Subbaiah, P.V. Rate of acyl migration in lysophosphatidylcholine (LPC) is dependent upon the nature of the acyl group. Greater stability of sn-2 docosahexaenoyl LPC compared to the more saturated LPC species. PLoS ONE 2017, 12, e0187826. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Skoronski, E.; Padoin, N.; Soares, C.; Furigo, A., Jr. Stability of immobilized Rhizomucor miehei lipase for the synthesis of pentyl octanoate in a continuous packed bed bioreactor. Braz. J. Chem. Eng. 2014, 31, 633–641. [Google Scholar] [CrossRef]
- Mallin, H.; Muschiol, J.; Byström, E.; Bornscheuer, U.T. Efficient biocatalysis with immobilized enzymes or encapsulated whole cell microorganism by using the SpinChem reactor system. ChemCatChem 2013, 5, 3529–3532. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Mu, H.; Xu, X. Lipase-catalyzed acyl exchange of soybean phosphatidylcholine in n-hexane: A critical evaluation of both acyl incorporation and product recovery. Biotechnol. Prog. 2005, 21, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Ihsan, K.; Gokhan, D.A.; Ali, A.H. Fatty acid selectivity of lipases during acidolysis reaction between triolein and saturated fatty acids varying from caproic to behenic acids. J. Agric. Food Chem. 2009, 57, 7584–7590. [Google Scholar]
- Akanbi, T.O.; Adcock, J.L.; Barrow, C.J. Selective concentration of EPA and DHA using Thermomyces lanuginosus lipase is due to fatty acid selectivity and not regioselectivity. Food Chem. 2013, 138, 615–620. [Google Scholar]
- Hong, S.I.; Ma, N.; Kim, I.; Seo, J.; Kim, I.H. Lipase-catalyzed synthesis of capsiate analog using vanillyl alcohol and conjugated linoleic acid under vacuum system. Process Biochem. 2012, 47, 2317–2322. [Google Scholar] [CrossRef]
- Warwel, S.; Borgdorf, R. Substrate selectivity of lipases in the esterification of cis/trans-isomers and positional isomers of conjugated linoleic acid (CLA). Biotechnol. Lett. 2000, 22, 1151–1155. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, X.F.; Liang, Y.X.; Yang, B.; Zhang, S.H. Enzymatic fractionation of conjugated linoleic acid isomers by selective esterification. J. Mol. Catal. B Enzym. 2007, 46, 20–25. [Google Scholar] [CrossRef]
- Yi, D.; Sun, X.; Li, G.; Liu, F.; Lin, X.; Shen, J. Optimization of conjugated linoleic acid triglycerides via enzymatic esterification in no-solvent system. Chin. J. Oceanol. Limnol. 2009, 27, 574–577. [Google Scholar] [CrossRef]
- Yamauchi-Sato, Y.; Nagao, T.; Yamamoto, T.; Terai, T.; Sugihara, A.; Shimada, Y. Fractionation of conjugated linoleic acid isomers by selective hydrolysis with Candida rugosa lipase. J. Oleo Sci. 2003, 52, 367–374. [Google Scholar] [CrossRef]
- Ma, L.; Persson, M.; Adlercreutz, P. Water activity dependence of lipase catalysis in organic media explains successful transesterification reactions. Enzym. Microb. Technol. 2002, 31, 1024–1029. [Google Scholar] [CrossRef]
- Adlercreutz, P.; Lyberg, A.M.; Adlercreutz, D. Enzymatic fatty acid exchange in glycerophospholipids. Eur. J. Lipid Sci. Technol. 2003, 105, 638–645. [Google Scholar] [CrossRef]
- Svensson, I.; Adlercreutz, P.; Mattiasson, B. Lipase-catalyzed transesterification of phosphatidylcholine at controlled water activity. J. Am. Oil Chem. Soc. 1992, 69, 986–991. [Google Scholar] [CrossRef]
- Kim, I.H.; Garcia, H.S.; Hill, C.G. Synthesis of structured phosphatidylcholine containing n-3 PUFA residues via acidolysis mediated by immobilized phospholipase A1. J. Am. Oil Chem. Soc. 2010, 87, 1293–1299. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B. Lipase-catalyzed synthesis of lysophosphatidylcholine using organic cosolvent for in situ water activity control. J. Am. Oil Chem. Soc. 2000, 77, 791–792. [Google Scholar] [CrossRef]
- Hosokawa, M.; Takahashi, K.; Miyazaki, N.; Okamura, K.; Hatano, M. Application of water mimics on preparation of eicosapentaenoic and docosahexaenoic acids containing glycerolipids. J. Am. Oil Chem. Soc. 1995, 72, 421–425. [Google Scholar] [CrossRef]
- Hamid, T.H.; Rahman, R.N.Z.R.; Salleh, A.B.; Basri, M. The role of lid in protein-solvent interaction of the simulated solvent stable thermostable lipase from Bacillus strain 42 in water-solvent mixtures. Biotechnol. Biotechnol. Equip. 2009, 23, 1524–1530. [Google Scholar] [CrossRef]
- Hosokawa, M.; Takahashi, K.; Kikuchi, Y.; Hatano, M. Preparation of therapeutic phospholipids through porcine pancreatic phospholipase A2 -mediated esterification and lipozyme-mediated acidolysis. J. Am. Oil Chem. Soc. 1995, 72, 1287–1291. [Google Scholar] [CrossRef]
- Kamal, Z.; Yedavalli, P.; Deshmukh, M.V.; Rao, N.M. Lipase in aqueous-polar organic solvents: Activity, structure, and stability. Protein Sci. 2013, 22, 904–915. [Google Scholar] [CrossRef]
- Chen, H.; Meng, X.; Xu, X.; Liu, W.; Li, S. The molecular basis for lipase stereoselectivity. Appl. Microbiol. Biotechnol. 2018, 102, 3487–3495. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Fernandez-Lafuente, R. Lipase from Rhizomucor miehei as a biocatalyst in fats and oils modification. J. Mol. Catal. B Enzym. 2010, 66, 15–32. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, Y.Q.; Chen, W. Review of the roles of conjugated linoleic acid in health and disease. J. Funct. Foods 2015, 15, 314–325. [Google Scholar]
- Niezgoda, N.; Wawrzeńczyk, C. An efficient method for enzymatic purification of cis-9,trans-11 isomer of conjugated linoleic acid. J. Mol. Catal. B Enzym. 2014, 100, 40–48. [Google Scholar]
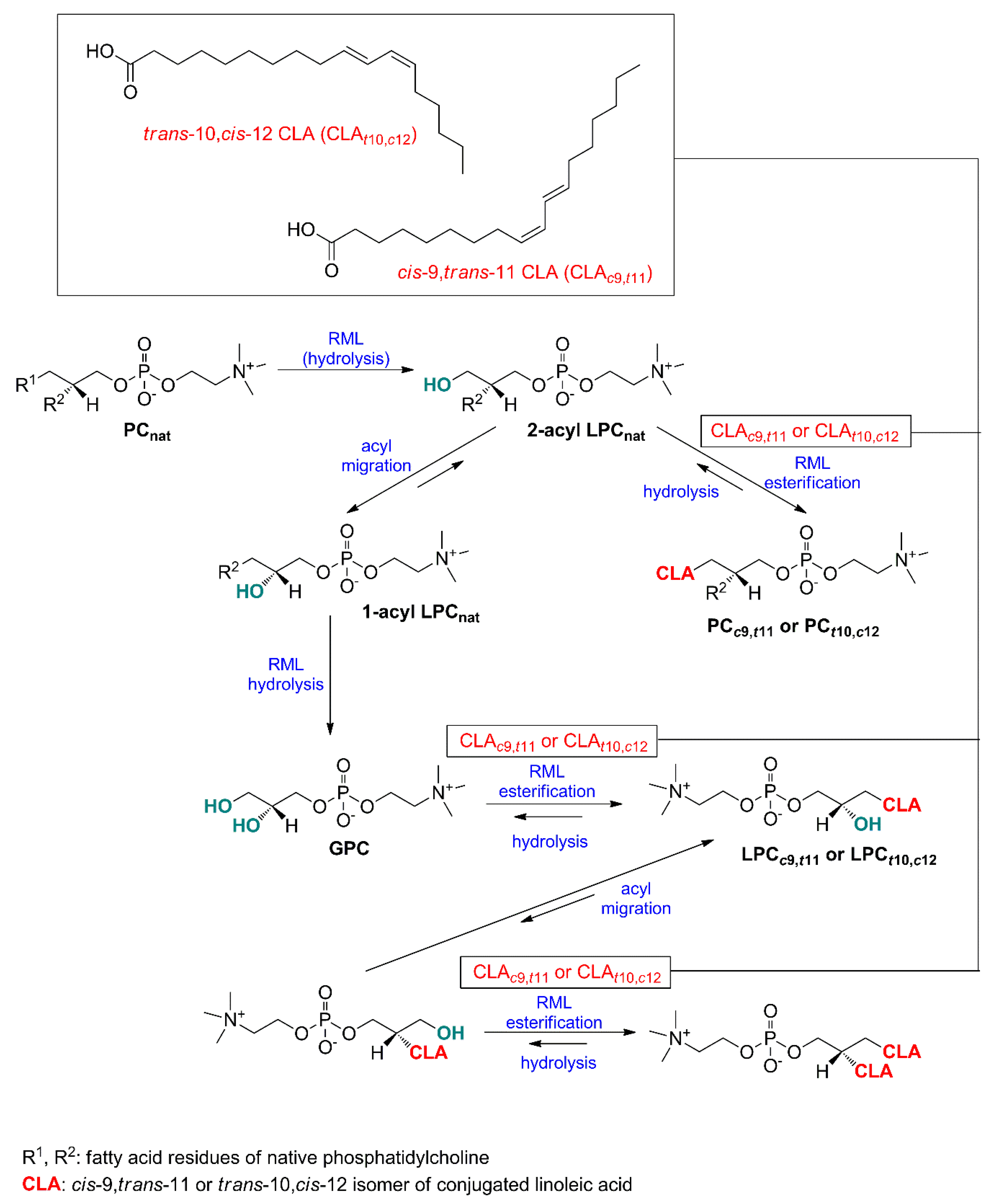
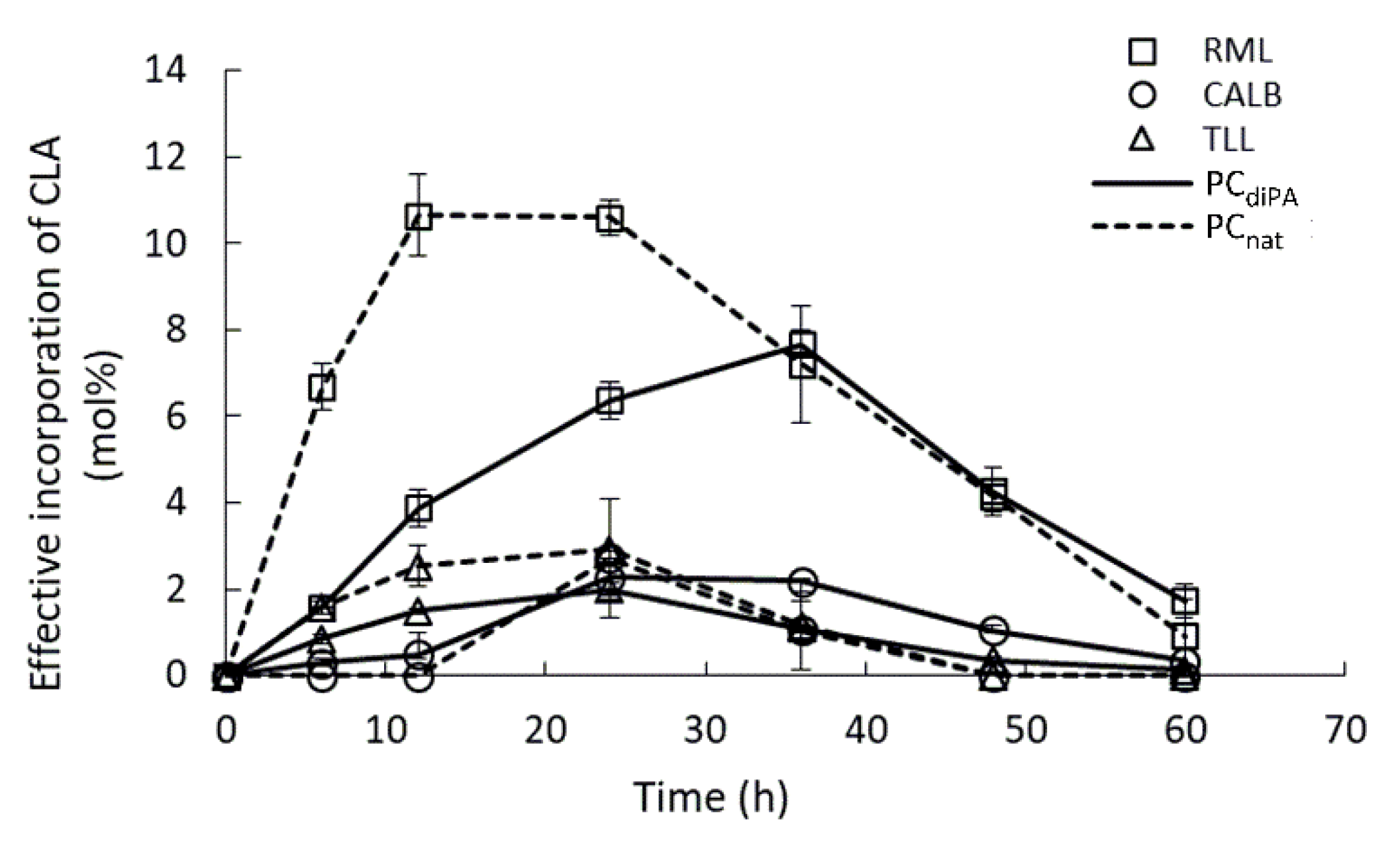

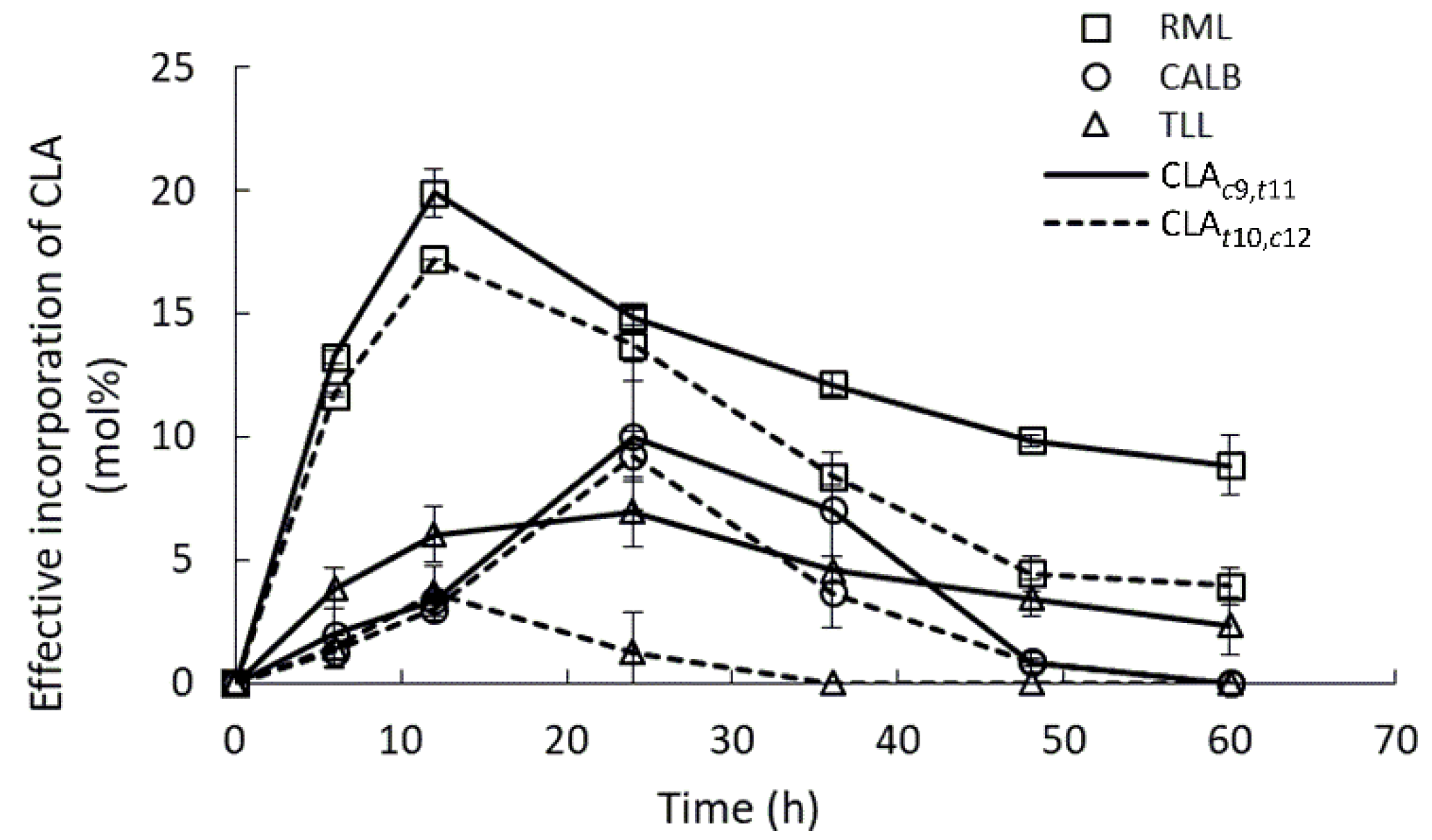
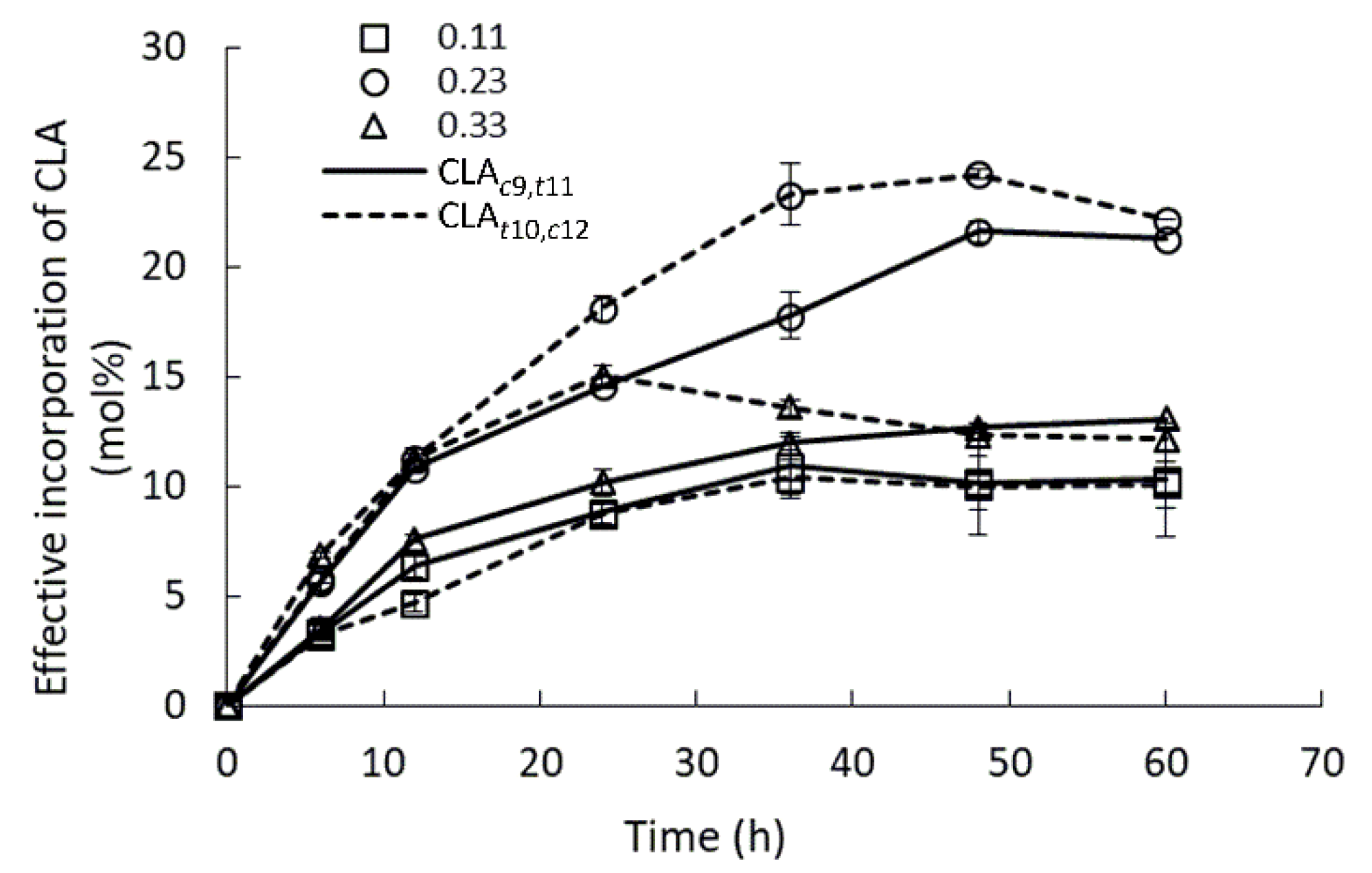


| Substrate/Reaction | Enzyme Load 1 | Substrate Molar Ratio | Parameters | INC (%)/Yield (%)/EINC (%) | Reference |
|---|---|---|---|---|---|
| Soy PL/acidolysis | 20% Lipozyme TL IM | 1/5.5 (PL/CLA 2) | Solvent free/ 60 °C/72 h | ca. 30% into PL/nd/nd | [30] |
| Soy PC/acidolysis | 7% Lipozyme RM IM | 1/5 (PC/CLA 3) | Hexane/40 °C/ 72 h | 16% into PC, 8.1% LPC/80.3% PC/EINC = 19% | [31] |
| Egg-yolk LPC/esterification | Freeze-dried Lecitase 10L 3.3 × 104 U (PLA2)/11 mg LPC | ca. 1/3 (LPC/CLA 4) | Glycerol with formamide/37 °C/6 h/albumin, CaCl2 | -/65% PC | [25] |
| Soy PC/acidolysis | 30% PLA2 immobilized on Amberlite XAD7 | 1/3 (PC/CLA 5) | Solvent free/ 45 °C/48 h/water addition | 30% into PC/21% PC, 74% LPC/EINC = 6% | [32] |
| Soy PL/transesterification | Lipozyme RM IM | PL/CLAEE | Solvent free/ 45 °C/48 h | 31% into PL/nd/nd | [33] |
| GPC/esterification | 10% Novozyme 435 | 1/50 (GPC/CLA 6) | Solvent free/ 40 °C/48 h | -/70% LPC | [34] |
| Soy PC/acidolysis | 15% Lecitase Ultra (PLA1) immobilized on Duolite A568 | 1/4 (PC/CLA 2) | Solvent free / 55 °C/24 h | 85.8% calculated on sn-1 of PC/12.6% PC/ EINC = 11% | [35] |
| Soy PC/acidolysis | 15% Lecitase Ultra (PLA1) immobilized on Duolite A568 | 1/4 (PC/CLA 2) | Solvent free/ 50 °C/24 h | 90.1% calculated on sn-1 of PC/nd/nd | [36] |
| Soy PC/acidolysis | 30% Lipozyme RM IM | 1/6 (PC/CLA 5) | Solvent free/ 48 °C/64 h | 24% into PL/nd/nd | [37] |
| GPC/esterification | 300 U/g GPC mutant lipase from marine Streptomyces sp. (MAS1-H108A) immobilized on ECR1030 resin | 1:40 (GPC/CLA 7) | Solvent free/ 55 °C/48 h | -/89% LPC, 4% PC | [38] |
| Egg-yolk PC/acidolysis | 24% Lipozyme RM IM | 1/8 (PC/CLA 8) | Heptane/45 °C/ 36 h/aw = 0.32, aw = 0.11 (after 12 h) | 33.8% into PC, 50.1% into LPC/39.5% PC, 25.3% LPC/EINC = 39% | [39] |
| Egg-yolk PC/acidolysis | 24% Lipozyme RM IM | 1/8 (PC/CLA 9) | Heptane/45 °C/ 36 h/aw = 0.32, aw = 0.11 (after 12 h) | 42–44% into PC, 62–65% into LPC/34–36% PC, 16–18% LPC/EINC = 42% | [40] |
| Soy PC/acidolysis | 15% Lecitase Ultra (PLA1) immobilized on Duolite A658 | 1/12 (PC/CLA 2) | Solvent free/50 °C /24 h/4 d lyophilized PC | 72% into PC/<1% LPC and GPC/EINC > 70% | [41] |
| Soy PC/acidolysis | 15% Lecitase Ultra (PLA1) immobilized on Lifetech™ ECR8804M | 1/12 (PC/CLA 2) | Solvent free/ 50 °C/2 h/4 d lyophilized PC | 74% into PC/<1% LPC and GPC/EINC > 70% | [42] |
| Lipase | Incorporation of CLA (mol %) | Phospholipids Distribution (mol %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Substrate | Substrate | |||||||||
| PCnat | PCdiPA | PCnat | PCdiPA | |||||||
| INCPC | INCLPC | INCPC | INCLPC | %PC | %LPC | %GPC | %PC | %LPC | %GPC | |
| RML | 12.8 ± 0.9 2 | 34.1 ± 1.1 6 | 5.3 ± 0.5 4 | 8.4 ± 0.3 9 | 35.8 ± 0.6 11 | 17.2 ± 0.8 16 | 47.1 ± 1.3 19 | 57.5 ± 0.2 13 | 31.3 ± 1.1 18 | 11.3 ± 1.2 21 |
| CALB | 3.8 ± 0.3 3 | 22.7 ± 3.9 7 | 2.6 ± 0.4 5 | 3.4 ± 0.4 10 | 38.2 ± 3.4 11,12 | 11.7 ± 0.6 20 | 50.1 ± 4.0 19 | 53.2 ± 1.0 14 | 30.9 ± 1.2 18 | 15.9 ± 2.2 22 |
| TLL | 5.9 ± 0.4 4 | 5.3 ± 0.5 8 | 3.1 ± 0.6 3,5 | nd | 37.1 ± 0.4 12 | 13.9 ± 0.3 17 | 49.0 ± 0.7 19 | 42.7 ± 0.5 15 | 30.6 ± 1.1 18 | 26.7 ± 1.5 23 |
| Lipase | Incorporation of CLA Isomers (mol%) | Phospholipids Distribution (mol%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Substrate | Substrate | |||||||||
| cis-9,trans-11 CLA | trans-10,cis-12 CLA | cis-9,trans-11 CLA | trans-10,cis-12 CLA | |||||||
| INCPC | INCLPC | INCPC | INCLPC | %PC | %LPC | %GPC | %PC | %LPC | %GPC | |
| RML | 29.8 ± 0.1 2 | 52.8 ± 1.5 7 | 34.8 ± 1.0 5 | 55.1 ± 1.3 7 | 27.0 ± 0.3 11 | 12.8 ± 0.1 15 | 60.3 ± 0.1 21 | 23.1 ± 2.8 13 | 9.1 ± 0.1 18 | 67.8 ± 2.9 24 |
| CALB | 12.5 ± 2.0 3 | 38.7 ± 15.0 8 | 12.4 ± 0.1 3 | 17.3 ± 1.3 9 | 30.6 ± 1.7 12 | 26.2 ± 0.1 16 | 43.2 ± 1.8 22 | 37.7 ± 0.2 14 | 23.4 ± 2.4 19 | 38.9 ± 2.1 25 |
| TLL | 8.8 ± 0.8 4 | 17.9 ± 0.5 9 | 1.4 ± 0.4 6 | 9.7 ± 1.0 10 | 29.5 ± 1.0 12 | 20.2 ± 0.7 17 | 50.3 ± 0.3 23 | 25.9 ± 4.5 11,13 | 10.6 ± 1.5 20 | 63.5 ± 6.0 21,24 |
| Lipase | Incorporation of CLA Isomers (mol %) | Phospholipids Distribution (mol %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Substrate | Substrate | |||||||||
| cis-9,trans-11 CLA | trans-10,cis-12 CLA | cis-9,trans-11 CLA | trans-10,cis-12 CLA | |||||||
| INCPC | INCLPC | INCPC | INCLPC | %PC | %LPC | %GPC | %PC | %LPC | %GPC | |
| 0.11 | 4.9 ± 0.1 2 | 45.5 ± 6.7 7 | 4.7 ± 0.4 2 | 44.7 ± 2.2 7 | 54.3 ± 0.6 10 | 16.2 ± 0.2 16 | 28.0 ± 1.7 20 | 72.6 ± 2.0 13 | 15.9 ± 0.5 16 | 11.6 ± 2.5 23 |
| 0.23 | 10.6 ± 0.2 3 | 85.9 ± 2.9 8 | 24.9 ± 1.2 5 | 87.5 ± 1.8 8 | 46.6 ± 1.1 11 | 20.0 ± 0.2 17 | 33.5 ± 1.2 21 | 63.3 ± 2.8 14 | 22.3 ± 0.9 18 | 14.4 ± 3.7 23 |
| 0.33 | 6.4 ± 0.1 4 | 24.4 ± 0.8 9 | 11.8 ± 0.3 6 | 42.0 ± 0.8 7 | 36.1 ± 0.5 12 | 15.5 ± 0.8 16 | 48.0 ± 0.8 22 | 18.3 ± 0.8 15 | 8.7 ± 0.3 19 | 73.6 ± 3.0 24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niezgoda, N.; Gliszczyńska, A. Lipase Catalyzed Acidolysis for Efficient Synthesis of Phospholipids Enriched with Isomerically Pure cis-9,trans-11 and trans-10,cis-12 Conjugated Linoleic Acid. Catalysts 2019, 9, 1012. https://doi.org/10.3390/catal9121012
Niezgoda N, Gliszczyńska A. Lipase Catalyzed Acidolysis for Efficient Synthesis of Phospholipids Enriched with Isomerically Pure cis-9,trans-11 and trans-10,cis-12 Conjugated Linoleic Acid. Catalysts. 2019; 9(12):1012. https://doi.org/10.3390/catal9121012
Chicago/Turabian StyleNiezgoda, Natalia, and Anna Gliszczyńska. 2019. "Lipase Catalyzed Acidolysis for Efficient Synthesis of Phospholipids Enriched with Isomerically Pure cis-9,trans-11 and trans-10,cis-12 Conjugated Linoleic Acid" Catalysts 9, no. 12: 1012. https://doi.org/10.3390/catal9121012
APA StyleNiezgoda, N., & Gliszczyńska, A. (2019). Lipase Catalyzed Acidolysis for Efficient Synthesis of Phospholipids Enriched with Isomerically Pure cis-9,trans-11 and trans-10,cis-12 Conjugated Linoleic Acid. Catalysts, 9(12), 1012. https://doi.org/10.3390/catal9121012






