Hydrogen Production from Ammonia Borane over PtNi Alloy Nanoparticles Immobilized on Graphite Carbon Nitride
Abstract
1. Introduction
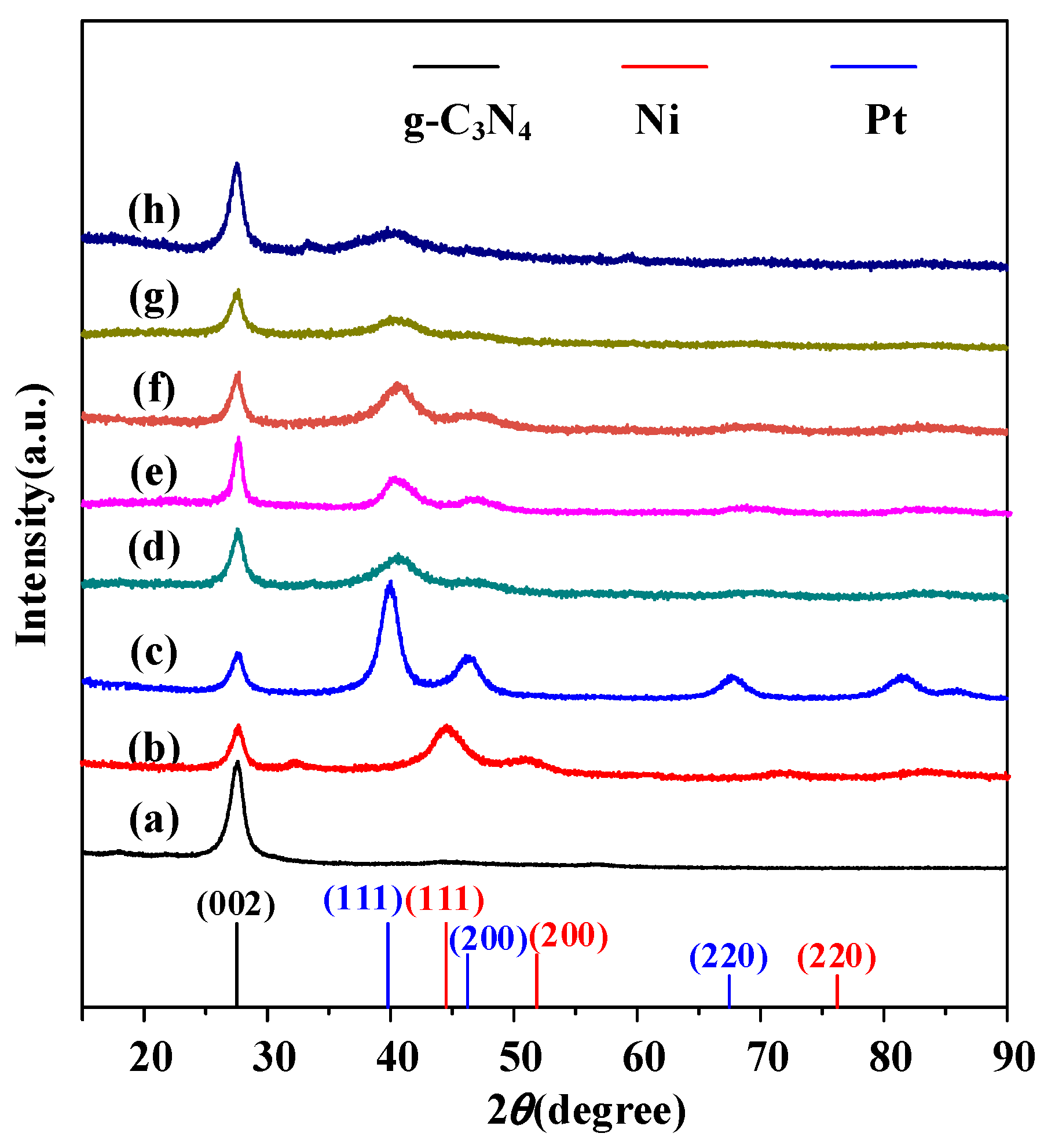
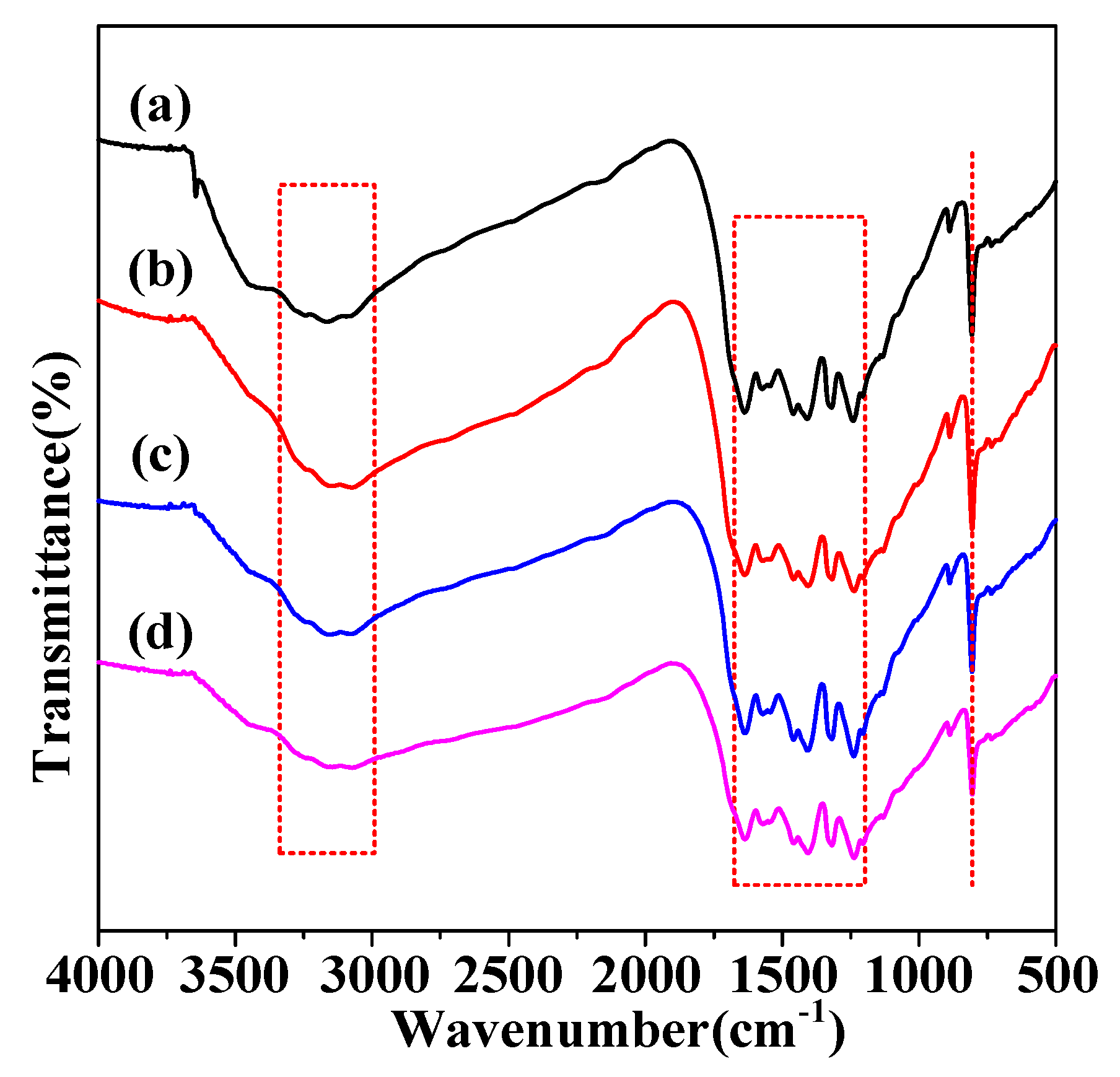
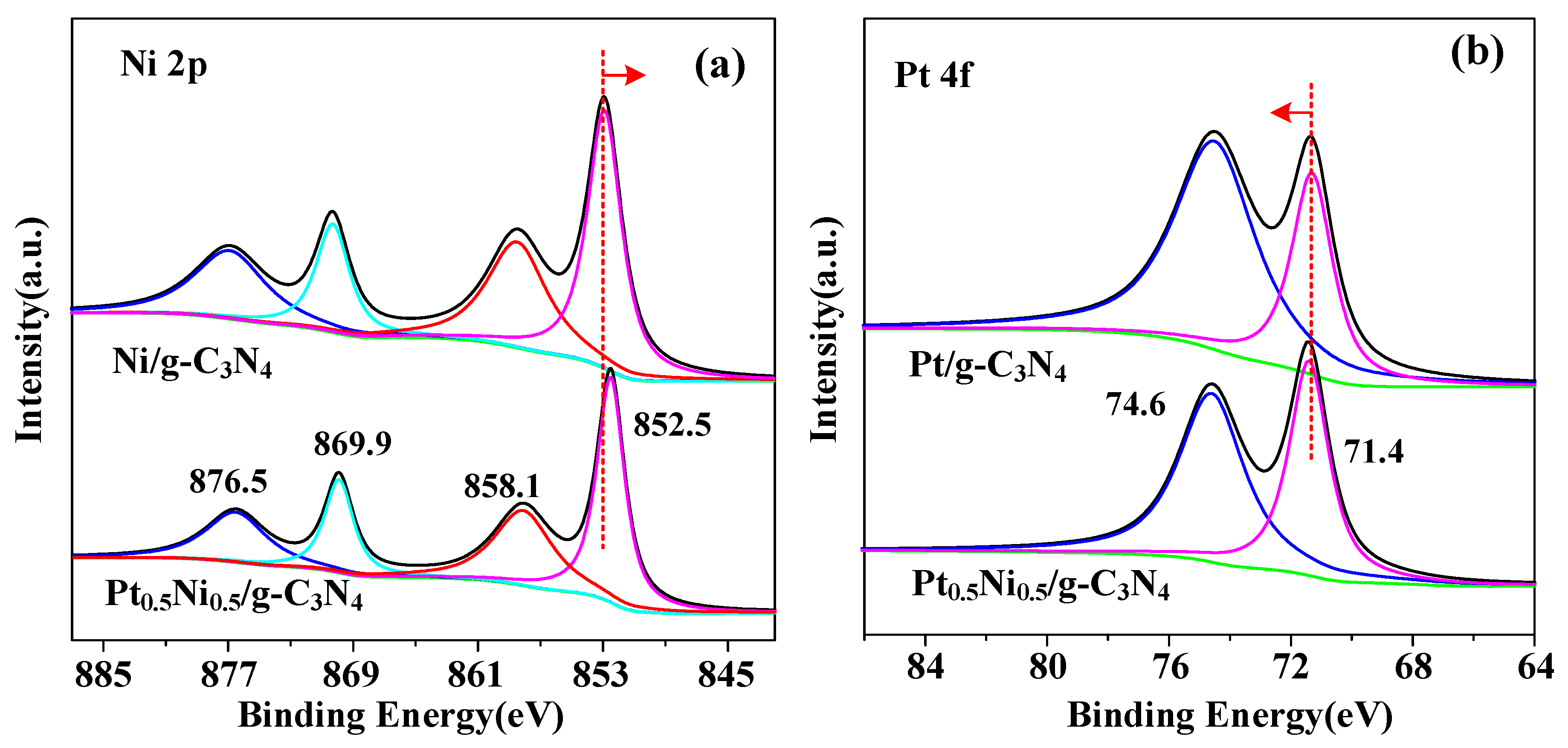
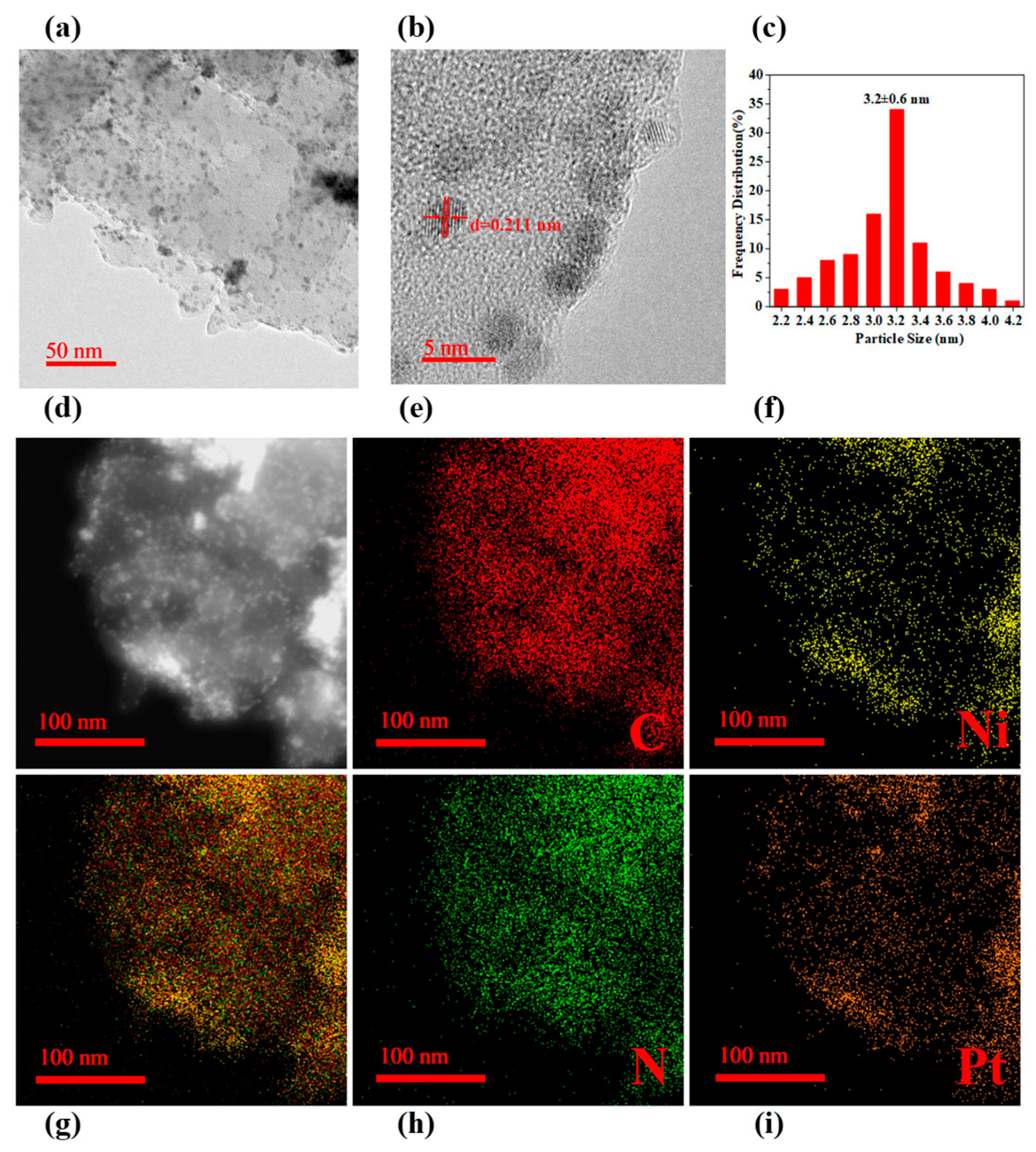
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Synthesis of g-C3N4
3.3. Synthesis of PtNi/g-C3N4
3.4. Characterization
3.5. Catalytic Activity Measurement
3.6. Stability Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Graetz, J. New approaches to hydrogen storage. Chem. Soc. Rev. 2009, 38, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.B.; Tang, Z.W.; Sun, D.L.; Ouyang, L.Z.; Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- He, T.; Pachfule, P.; Wu, H.; Xu, Q.; Chen, P. Hydrogen carriers. Nat. Rev. Mater. 2016, 1, 16059. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Q.T.; Yuan, J.Z.; Zhao, X.; Gao, G.H. Highly Dispersed Bimetallic Nanoparticles Supported on Titanium Carbides for Remarkable Hydrogen Release from Hydrous Hydrazine. ChemCatChem 2018, 10, 2200–2204. [Google Scholar] [CrossRef]
- Yadav, M.; Xu, Q. Liquid-phase chemical hydrogen storage materials. Energy Environ. Sci. 2012, 5, 9698–9725. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Mater. Sustain. Energy 2011, 265–270. [Google Scholar]
- Yao, F.; Li, X.; Wan, C.; Xu, L.X.; An, Y.; Ye, M.F.; Lei, Z. Highly efficient hydrogen release from formic acid using a graphitic carbon nitride-supported AgPd nanoparticle catalyst. Appl. Surf. Sci. 2017, 426, 605–611. [Google Scholar] [CrossRef]
- Staubitz, A.; Robertson, A.P.M.; Manners, I. Ammonia-Borane and related compounds as dihydrogen sources. Chem. Rev. 2010, 110, 4079–4124. [Google Scholar] [CrossRef]
- Zhou, Y.-H.; Wang, S.Q.; Zhang, Z.Y.; Williams, N.; Cheng, Y.; Gu, J. Hollow Nickel–Cobalt Layered Double Hydroxide Supported Palladium Catalysts with Superior Hydrogen Evolution Activity for Hydrolysis of Ammonia Borane. ChemCatChem 2018, 10, 3206–3213. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wang, A.J.; Zhang, L.; Fang, K.M.; Wu, L.J.; Feng, J.J. Melamine-assisted solvothermal synthesis of PtNi nanodentrites as highly efficient and durable electrocatalyst for hydrogen evolution reaction. J. Colloid Interface Sci. 2018, 531, 578–584. [Google Scholar] [CrossRef]
- Wan, C.; Yao, F.; Li, X.; Hu, K.; Ye, M.F.; Xu, L.X.; An, Y. Bimetallic AgPd Nanoparticles Immobilized on Amine-Functionalized SBA-15 as Efficient Catalysts for Hydrogen Generation from Formic Acid. ChemistrySelect 2016, 1, 6907–6913. [Google Scholar] [CrossRef]
- Chen, J.M.; Lu, Z.H.; Yao, Q.L.; Feng, G.; Luo, Y. Complete dehydrogenation of N2H4BH3 with Ni-MCr2O3 (M = Pt, Rh, and Ir) hybrid nanoparticles. J. Mater. Chem. A 2018, 6, 20746–20752. [Google Scholar] [CrossRef]
- Zhang, L.T.; Cai, Z.L.; Yao, Z.D.; Ji, L.; Sun, Z.; Yan, N.H.; Zhang, B.Y.; Xiao, B.B.; Du, J.; Zhu, X.Q.; et al. A striking catalytic effect of facile synthesized ZrMn2 nanoparticles on the de/rehydrogenation properties of MgH2. J. Mater. Chem. A 2019, 7, 5626–5634. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özçifçi, Z.; Tabak, A. Regular Article Noble metal nanoparticles supported on activated carbon: Highly recyclable catalysts in hydrogen generation from the hydrolysis of ammonia borane. J. Colloid Interface Sci. 2019, 546, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.P.; Jiang, R.; Li, Q.; Zeng, F.N.; Zheng, X.; Xu, Z.M.; Chen, C.H.; Peng, J. The hydrolysis of ammonia borane catalyzed by NiCoP/OPC-300 nanocatalysts: High selectivity and efficiency, and mechanism. Green Chem. 2019, 21, 850–860. [Google Scholar] [CrossRef]
- Li, J.; Li, F.; Liao, J.; Liu, Q.; Li, H. Cu0.4Co0.6MoO4 Nanorods Supported on Graphitic Carbon Nitride as a Highly Active Catalyst for the Hydrolytic Dehydrogenation of Ammonia Borane. Catalysts 2019, 9, 714. [Google Scholar] [CrossRef]
- Torres, D.A.; Garcia, M.N.; Mori, K.; Kuwahara, Y.; Yamashita, H. Nitrogen-doped carbon materials as a promising platform toward the efficient catalysis for hydrogen generation. Appl. Catal. A Gen. 2019, 571, 25–41. [Google Scholar] [CrossRef]
- Bandaru, S.; English, N.J.; Phillips, A.D.; MacElroy, J.M.D. Exploring Promising Catalysts for Chemical Hydrogen Storage in Ammonia Borane: A Density Functional Theory Study. Catalysts 2017, 7, 140. [Google Scholar] [CrossRef]
- Wei, Z.H.; Liu, Y.; Peng, Z.K.; Song, H.Q.; Liu, Z.Y.; Liu, B.Z.; Li, B.J.; Yang, B.; Lu, S.Y. Cobalt-Ruthenium Nanoalloys Parceled in Porous Nitrogen-Doped Graphene as Highly Efficient Difunctional Catalysts for Hydrogen Evolution Reaction and Hydrolysis of Ammonia Borane. ACS Sustain. Chem. Eng. 2019, 7, 7014–7023. [Google Scholar] [CrossRef]
- Chen, W.Y.; Wang, Z.J.; Duan, X.Z.; Qian, G.; Chen, D.; Zhou, X.G. Structural and kinetic insights into Pt/CNT catalysts during hydrogen generation from ammonia borane. Chem. Eng. Sci. 2018, 192, 1242–1251. [Google Scholar] [CrossRef]
- Xu, C.L.; Wang, H.; Wang, Q.; Wang, Y.; Zhang, Y.; Fan, G.Y. Ruthenium coordinated with triphenylphosphine-hyper-crosslinked polymer: An efficient catalyst for hydrogen evolution reaction and hydrolysis of ammonia borane. Appl. Surf. Sci. 2019, 466, 193–201. [Google Scholar] [CrossRef]
- Zhang, J.K.; Chen, W.Y.; Ge, H.B.; Chen, C.Q.; Yan, W.J.; Gao, Z.; Gan, J.; Zhang, B.Y.; Duan, X.Z.; Qin, Y. Synergistic effects in atomic-layer-deposited PtCox/CNTs catalysts enhancing hydrolytic dehydrogenation of ammonia borane. Appl. Catal. B 2018, 235, 256–263. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Guan, H.J.; Zhao, Y.F.; Yang, J.H.; Zhang, B. Preparation of bimetallic Cu-Co nanocatalysts on poly (diallyldimethylammonium chloride) functionalized halloysite nanotubes for hydrolytic dehydrogenation of ammonia borane. Appl. Surf. Sci. 2018, 427, 106–113. [Google Scholar] [CrossRef]
- Hou, C.C.; Li, Q.; Wang, C.J.; Peng, C.Y.; Chen, Q.Q.; Ye, H.F.; Fu, W.F.; Che, C.M.; López, N.; Chen, Y. Ternary Ni–Co–P nanoparticles as noble-metal-free catalysts to boost the hydrolytic dehydrogenation of ammonia-borane. Energy Environ. Sci. 2017, 10, 1770–1776. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, R.Y.; Wang, Y.; Feng, J.W.; Li, C.C.; Chen, G.Z. Effect of LDH composition on the catalytic activity of Ru/LDH for the hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2019, 44, 14820–14830. [Google Scholar] [CrossRef]
- Özhava, D.; Özkar, S. Nanoceria supported rhodium (0) nanoparticles as catalyst for hydrogen generation from methanolysis of ammonia borane. Appl. Catal. B 2018, 237, 1012–1020. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özkar, S. Ammonia borane as hydrogen storage materials. Int. J. Hydrogen Energy 2018, 43, 18592–18606. [Google Scholar] [CrossRef]
- Wen, M.; Wu, Q.N.; Peng, J.; Wu, Q.S.; Wang, C.X. Fabrication of Pt-loaded NiCo nanochains with superior catalytic dehydrogenation activity. J. Colloid Interface Sci. 2014, 416, 220–226. [Google Scholar] [CrossRef]
- Wan, C.; Cheng, D.G.; Chen, F.Q.; Zhan, X.L. Fabrication of CeO2 nanotube supported Pt catalyst encapsulated with silica for high and stable performance. Chem. Commun. 2015, 51, 9785–9788. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Ma, Y.Y.; Zhang, H.; Gao, J.; Nie, Y.T.; Sun, X.H. Aqueous solution synthesis of Pt-M (M = Fe, Co, Ni) bimetallic nanoparticles and their catalysis for the hydrolytic dehydrogenation of ammonia borane. ACS Appl. Mater. Interfaces 2014, 6, 12429–12435. [Google Scholar] [CrossRef]
- Du, Y.S.; Su, J.; Luo, W.; Cheng, G.Z. Graphene-Supported Nickel−Platinum Nanoparticles as Efficient Catalyst for Hydrogen Generation from Hydrous Hydrazine at Room Temperature. ACS Appl. Mater. Interfaces 2015, 7, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.H.; Feng, K.; Wang, Y.; Lv, X.X.; Zheng, H.C.; Ma, Y.Y.; Yan, W.S.; Sun, X.H.; Zhong, J. PtxNi10−xO nanoparticles supported on N-doped graphene oxide with a synergetic effect for highly efficient hydrolysis of ammonia borane. Catal. Sci. Technol. 2017, 7, 5135–5142. [Google Scholar] [CrossRef]
- Xia, Y.; Ye, J.R.; Cheng, D.G.; Chen, F.Q.; Zhan, X.L. Identification of a flattened Pd–Ce oxide cluster as a highly efficient catalyst for low-temperature CO oxidation. Catal. Sci. Technol. 2018, 8, 5137–5147. [Google Scholar] [CrossRef]
- Huang, X.Y.; Zhu, X.Y.; Zhang, X.F.; Zhang, L.; Feng, J.J.; Wang, A.J. Simple solvothermal synthesis of uniform Pt66Ni34 nanoflowers as advanced electrocatalyst to significantly boost the catalytic activity and durability of hydrogen evolution reaction. Electrochim. Acta 2018, 271, 397–405. [Google Scholar] [CrossRef]
- Chen, X.L.; Zhang, H.X.; Huang, X.Y.; Feng, J.J.; Han, D.M.; Zhang, L.; Chen, J.R.; Wang, A.J. Facile solvothermal fabrication of Pt47Ni53 nanopolyhedrons for greatly boosting electrocatalytic performances for oxygen reduction and hydrogen evolution. J. Colloid Interface Sci. 2018, 525, 260–268. [Google Scholar] [CrossRef]
- Karaca, T.; Sevim, M.; Metin, Ö. Facile Synthesis of Monodisperse Copper–Platinum Alloy Nanoparticles and Their Superb Catalysis in the Hydrolytic Dehydrogenation of Ammonia Borane and Hydrazine Borane. ChemCatChem 2017, 9, 4185–4190. [Google Scholar] [CrossRef]
- Zhang, H.M.; Ke, D.D.; Cheng, L.N.; Feng, X.L.; Hou, X.W.; Wang, J.; Lia, Y.; Han, S.M. CoPt-Co hybrid supported on amino modified SiO2 nanospheres as a high performance catalyst for hydrogen generation from ammonia borane. Sci. Mater. 2019, 29, 1–9. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Qi, L.; Yang, H.X.; Xu, C.X. Hierarchical nanoporous platinum–copper alloy nanoflowers as highly active catalysts for the hydrolytic dehydrogenation of ammonia borane. J. Colloid Interface Sci. 2018, 513, 258–265. [Google Scholar] [CrossRef]
- Ge, Y.Z.; Ye, W.Y.; Shah, Z.H.; Lin, X.J.; Lu, R.W.; Zhang, S.F. PtNi/NiO Clusters Coated by Hollow Sillica: Novel Design for Highly Efficient Hydrogen Production from Ammonia–Borane. ACS Appl. Mater. Interfaces 2017, 9, 3749–3756. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Xu, C.X. Nanoporous PtCo/Co3O4 composites with high catalytic activities toward hydrolytic dehydrogenation of ammonia borane. J. Colloid Interface Sci. 2017, 508, 542–550. [Google Scholar] [CrossRef]
- Chen, W.Y.; Ji, J.; Feng, X.; Duan, X.Z.; Qian, G.; Li, P.; Zhou, X.G.; Chen, D.; Yuan, W.K. Mechanistic Insight into Size-Dependent Activity and Durability in Pt/CNT Catalyzed Hydrolytic Dehydrogenation of Ammonia Borane. J. Am. Chem. Soc. 2014, 136, 16736–16739. [Google Scholar] [CrossRef] [PubMed]
- Kamegawa, T.; Nakaue, T. Complete hydrogen release from aqueous ammonia-borane over a platinum-loaded titanium dioxide photocatalyst. Chem. Commun. 2015, 51, 16802–16805. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Y.; Xu, L.X.; Du, L.; An, Y.; Wan, C. Palladium supported on carbon nanotubes as a high-performance catalyst for the dehydrogenation of dodecahydro-N-ethylcarbazole. Catalysts 2018, 8, 638. [Google Scholar] [CrossRef]
- Ke, D.D.; Wang, J.; Zhang, H.M.; Li, Y.; Zhang, L.; Zhao, X.; Han, S.M. Fabrication of Pt-Co NPs supported on nanoporous graphene as high-efficient catalyst for hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2017, 42, 26617–26625. [Google Scholar] [CrossRef]
- Wang, C.L.; Tuninetti, J.; Wang, Z.; Zhang, C.; Ciganda, R.; Salmon, L.; Moya, S.; Ruiz, J.; Astruc, D. Hydrolysis of Ammonia-Borane over Ni/ZIF-8 Nanocatalyst: High Efficiency, Mechanism, and Controlled Hydrogen Release. J. Am. Chem. Soc. 2017, 139, 11610–11615. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, Z.H.; Luo, Y.; Zou, A.H.; Yao, Q.L.; Chen, X.S. Mesoporous Carbon Nitride Supported Pd and Pd-Ni Nanoparticles as Highly Efficient Catalyst for Catalytic Hydrolysis of NH3BH3. ChemCatChem 2018, 10, 1620–1626. [Google Scholar] [CrossRef]
- Han, C.H.; Meng, P.; Waclawik, E.R.; Zhang, C.; Li, X.H.; Yang, H.Q.; Antonietti, M.; Xu, J.S. Palladium/Graphitic Carbon Nitride (g-C3N4) Stabilized Emulsion Microreactor as a Store for Hydrogen from Ammonia Borane for Use in Alkene Hydrogenation. Angew. Chem. Int. Ed. 2018, 57, 14857–14861. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Guo, L.T.; Cai, Y.Y.; Ge, J.M.; Zhang, Y.N.; Gong, L.H.; Li, X.H.; Wang, K.X.; Ren, Q.Z.; Su, J.; Chen, J.S. Multifunctional Au−Co@CN Nanocatalyst for Highly Efficient Hydrolysis of Ammonia Borane. ACS Catal. 2015, 5, 388–392. [Google Scholar] [CrossRef]
- Lu, R.; Hu, M.; Xu, C.L.; Wang, Y.; Zhang, Y.; Xu, B.; Gao, D.J.; Bi, J.; Fan, G.Y. Hydrogen evolution from hydrolysis of ammonia borane catalyzed by Rh/g-C3N4 under mild conditions. Int. J. Hydrogen Energy 2018, 43, 7038–7045. [Google Scholar] [CrossRef]
- Wang, X.C.; Blechert, S.; Antonietti, M. Polymeric Graphitic Carbon Nitride for Heterogeneous Photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Xu, L.X.; Liu, N.; Hong, B.; Cui, P.; Cheng, D.G.; Chen, F.Q.; An, Y.; Wan, C. Nickel–platinum nanoparticles immobilized on graphitic carbon nitride as highly efficient catalyst for hydrogen release from hydrous hydrazine. RSC Adv. 2016, 6, 31687–31691. [Google Scholar] [CrossRef]
- Fu, F.Y.; Wang, C.L.; Wang, Q.; Martinez-Villacorta, A.M. Highly Selective and Sharp Volcano-type Synergistic Ni2Pt@ZIF-8-Catalyzed Hydrogen Evolution from Ammonia Borane Hydrolysis. J. Am. Chem. Soc. 2018, 140, 10034–10042. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.L.; Hu, X.P.; Ren, H.; Xu, P.F.; Yu, R.B.; Chen, J.; Xing, X.R. Magnetic Ni and Ni/Pt hollow nanospheres and their catalytic activities for hydrolysis of ammonia borane. J. Mater. Chem. A 2014, 2, 18171–18176. [Google Scholar] [CrossRef]
- Li, Z.; He, T.; Matsumura, D.J.; Miao, S.; Wu, A.A.; Liu, L.; Wu, G.T.; Chen, P. Atomically Dispersed Pt on the Surface of Ni Particles: Synthesis and Catalytic Function in Hydrogen Generation from Aqueous Ammonia–Borane. ACS Catal. 2017, 7, 6762–6769. [Google Scholar] [CrossRef]
- Wan, C.; Zhu, M.Y.; Du, L.; Xu, L.X.; Ye, M.F.; An, Y. Highly efficient aerobic oxidation of tetralin to alpha-tetralone over MnOx-CoOy/γ-Al2O3 catalysts. Catal. Commun. 2019, 125, 87–92. [Google Scholar] [CrossRef]
- Yao, Q.L.; Lu, Z.H.; Huang, W.; Chen, X.S.; Zhu, J. High Pt-like activity of the Ni–Mo/graphene catalyst for hydrogen evolution from hydrolysis of ammonia borane. J. Mater. Chem. A 2016, 4, 8579–8583. [Google Scholar] [CrossRef]
- Wan, C.; Sun, L.; Xu, L.X.; Cheng, D.G.; Chen, F.Q.; Zhan, X.L.; Yang, Y.R. Novel NiPt alloy nanoparticle decorated 2D layered g-C3N4 nanosheets: A highly efficient catalyst for hydrogen generation from hydrous hydrazine. J. Mater. Chem. A 2019, 7, 8798–8804. [Google Scholar] [CrossRef]
- Aranishi, K.; Singh, A.K.; Xu, Q. Dendrimer-encapsulated bimetallic Pt-Ni nanoparticles as highly efficient catalysts for hydrogen generation from chemical hydrogen storage materials. ChemCatChem 2013, 5, 2248–2252. [Google Scholar] [CrossRef]
- Yang, X.J.; Cheng, F.Y.; Liang, J.; Tao, Z.L.; Chen, J. Carbon-supported Ni1-x@Ptx (x = 0.32, 0.43, 0.60, 0.67, and 0.80) core-shell nanoparticles as catalysts for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2011, 36, 1984–1990. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Liu, L.; Lu, S.; Xu, L.X.; An, Y.; Wan, C. Facile Fabrication of NiPt/CNTs as an Efficient Catalyst for Hydrogen Production from Hydrous Hydrazine. ChemistrySelect 2019, 4, 10494–10500. [Google Scholar] [CrossRef]
- Hu, Y.J.; Wang, Y.Q.; Lu, Z.H.; Chen, X.S.; Xiong, L.H. Core–shell nanospheres Pt@SiO2 for catalytic hydrogen production. Appl. Surf. Sci. 2015, 341, 185–189. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Xu, C.X. Nanoporous PtRu alloys with unique catalytic activity toward hydrolytic dehydrogenation of ammonia borane. Chem. Asian J. 2016, 11, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Hu, K.; Luo, W.; Cheng, G.Z. RuCu nanoparticles supported on graphene: A highly efficient catalyst for hydrolysis of ammonia borane. J. Alloy. Compd. 2014, 590, 241–246. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.P.; Song, S.Y.; Zhang, H.J. Graphene oxide induced formation of Pt–CeO2 hybrid nanoflowers with tunable CeO2 thickness for catalytic hydrolysis of ammonia borane. Chem. Eur. J. 2013, 19, 8082–8086. [Google Scholar] [CrossRef]
- Rachiero, G.P.; Demirci, U.B.; Miele, P. Bimetallic RuCo and RuCu catalysts supported on γ–Al2O3 a comparative study of their activity in hydrolysis of ammonia-borane. Int. J. Hydrogen Energy 2011, 36, 7051–7065. [Google Scholar] [CrossRef]
- Yang, X.J.; Cheng, F.Y.; Liang, J.; Tao, Z.L.; Chen, J. PtxNi1-x nanoparticles as catalysts for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2009, 34, 8785–8791. [Google Scholar] [CrossRef]
- Rakap, M. Poly(N–vinyl–2–pyrrolidone)–stabilized palladium–platinum nanoparticles–catalyzed hydrolysis of ammonia borane for hydrogen generation. J. Power Sources 2015, 276, 320–327. [Google Scholar] [CrossRef]
- Kalidendi, S.B.; Sanyal, U.; Jagirdar, B.R. Nanostructured Cu and Cu@Cu2O core shell catalysts for hydrogen generation from ammonia–borane. Phys. Chem. Chem. Phys. 2008, 10, 5870–5874. [Google Scholar] [CrossRef]
- Ma, H.; Na, C. Isokinetic Temperature and Size-Controlled Activation of Ruthenium-Catalyzed Ammonia Borane Hydrolysis. ACS Catal. 2015, 5, 1726–1735. [Google Scholar] [CrossRef]
- Gao, M.Y.; Yang, W.W.; Yu, Y.S. Monodisperse PtCu alloy nanoparticles as highly efficient catalysts for the hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2018, 43, 14293–14300. [Google Scholar] [CrossRef]
- Xu, D.; Wang, W.D.; Tian, M.; Dong, Z.P. Immobilization of Pt nanoparticles in hollow mesoporous silica nanocapsules: An aggregation- and leaching-resistant catalyst. J. Colloid Interface Sci. 2018, 516, 407–415. [Google Scholar] [CrossRef] [PubMed]

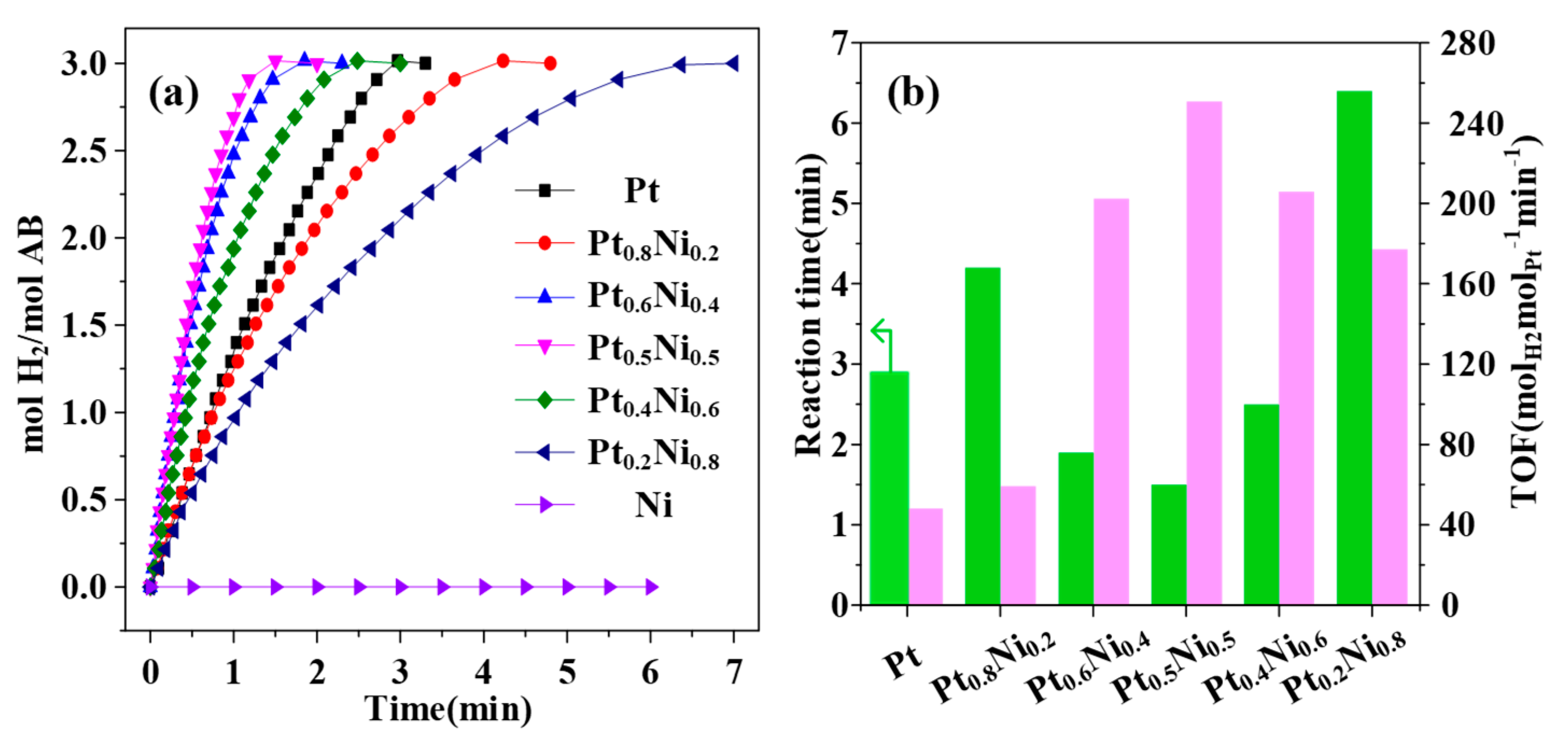


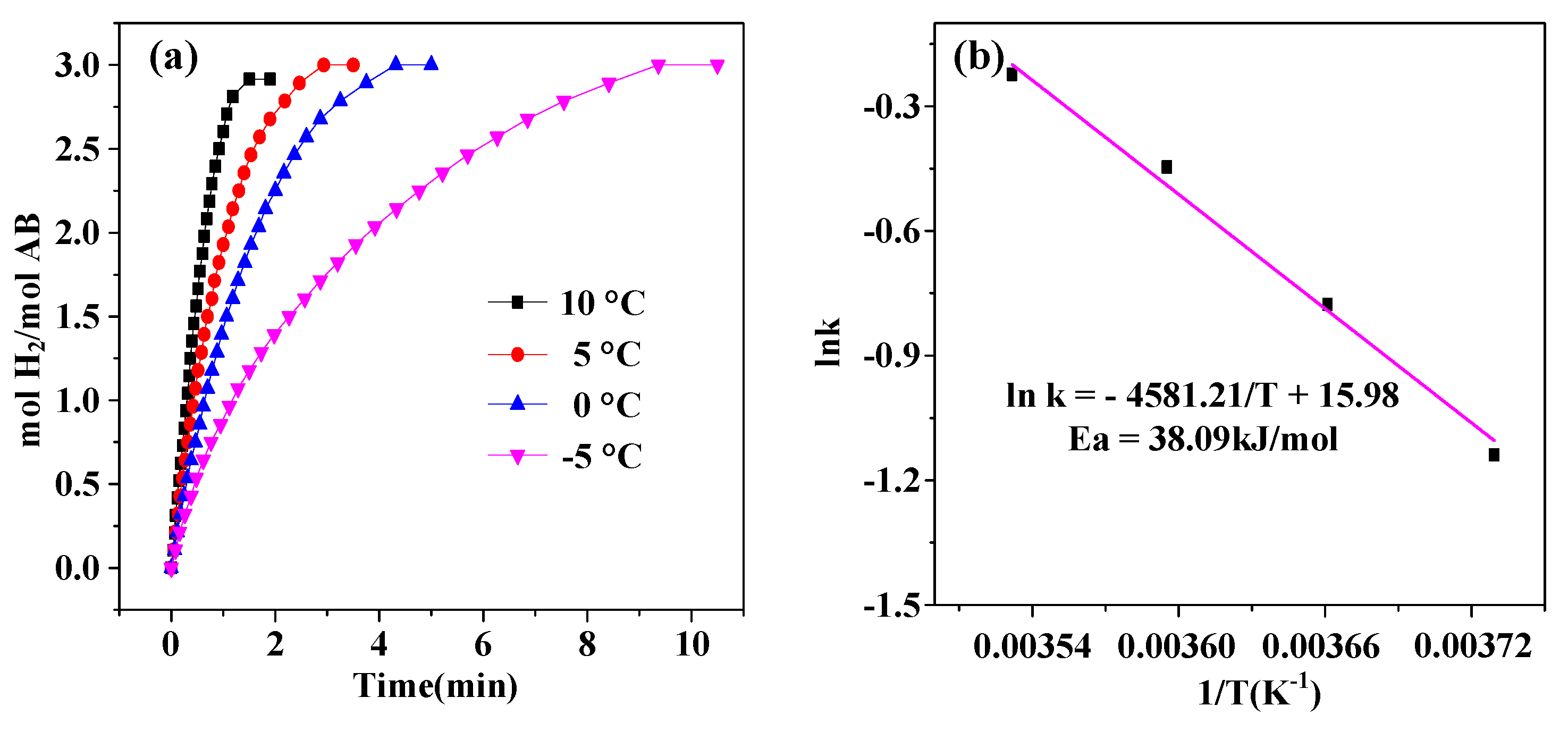

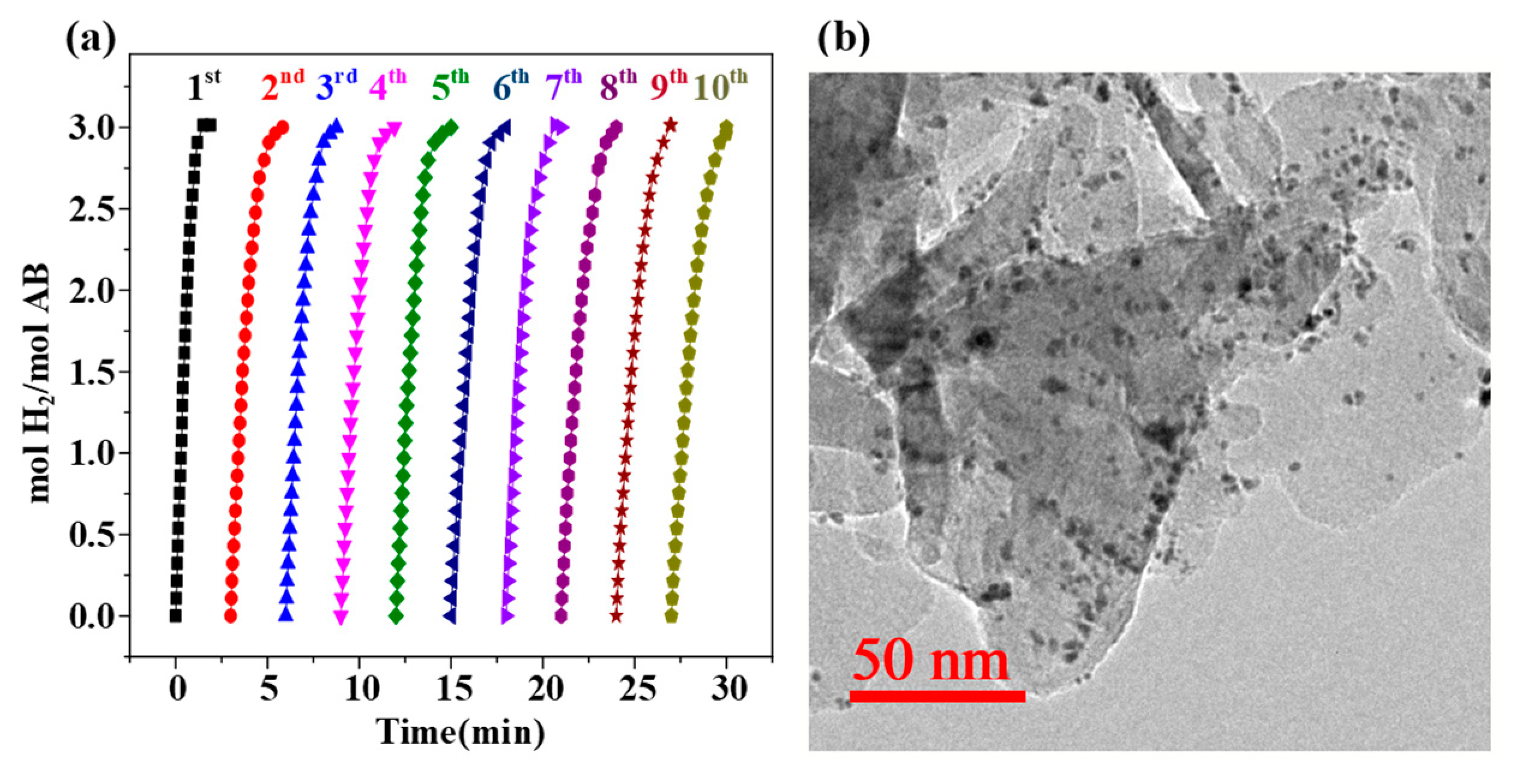
| Catalysts | TOF (molH2min−1mol−1M) M = Pt, Ru, Ag | Ea (kJ mol−1) | Refs. |
|---|---|---|---|
| Pt0.5Ni0.5/g-C3N4 | 250.8 | 38.09 | This work |
| NP–Pt40Co60 composite | 131 | 38.8 | 40 |
| Pt@SiO2 | 158 | 53.6 | 62 |
| PtRu | 59.6 | 38.9 | 63 |
| RuCu/graphene | 135 | 30.89 | 64 |
| Pt/CeO2/RGO | 48 | - | 65 |
| RuCo(1:1)/γ–Al2O3 | 32.9 | 47 | 66 |
| Pt0.65Ni0.35 | 44.3 | 39.0 | 67 |
| hnp–Pt35Cu65 | 108 | 40.5 | 38 |
| Pd–Pt@PVP NPs | 125 | 51.7 | 68 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Xiao, X.; Wu, Y.; An, Y.; Xu, L.; Wan, C. Hydrogen Production from Ammonia Borane over PtNi Alloy Nanoparticles Immobilized on Graphite Carbon Nitride. Catalysts 2019, 9, 1009. https://doi.org/10.3390/catal9121009
Zhang M, Xiao X, Wu Y, An Y, Xu L, Wan C. Hydrogen Production from Ammonia Borane over PtNi Alloy Nanoparticles Immobilized on Graphite Carbon Nitride. Catalysts. 2019; 9(12):1009. https://doi.org/10.3390/catal9121009
Chicago/Turabian StyleZhang, Mingya, Xue Xiao, Yan Wu, Yue An, Lixin Xu, and Chao Wan. 2019. "Hydrogen Production from Ammonia Borane over PtNi Alloy Nanoparticles Immobilized on Graphite Carbon Nitride" Catalysts 9, no. 12: 1009. https://doi.org/10.3390/catal9121009
APA StyleZhang, M., Xiao, X., Wu, Y., An, Y., Xu, L., & Wan, C. (2019). Hydrogen Production from Ammonia Borane over PtNi Alloy Nanoparticles Immobilized on Graphite Carbon Nitride. Catalysts, 9(12), 1009. https://doi.org/10.3390/catal9121009







