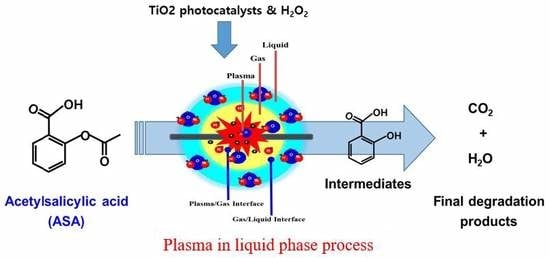
Assessment of Degradation Behavior for Acetylsalicylic Acid Using a Plasma in Liquid Process
Abstract
:1. Introduction
2. Results and Discussion
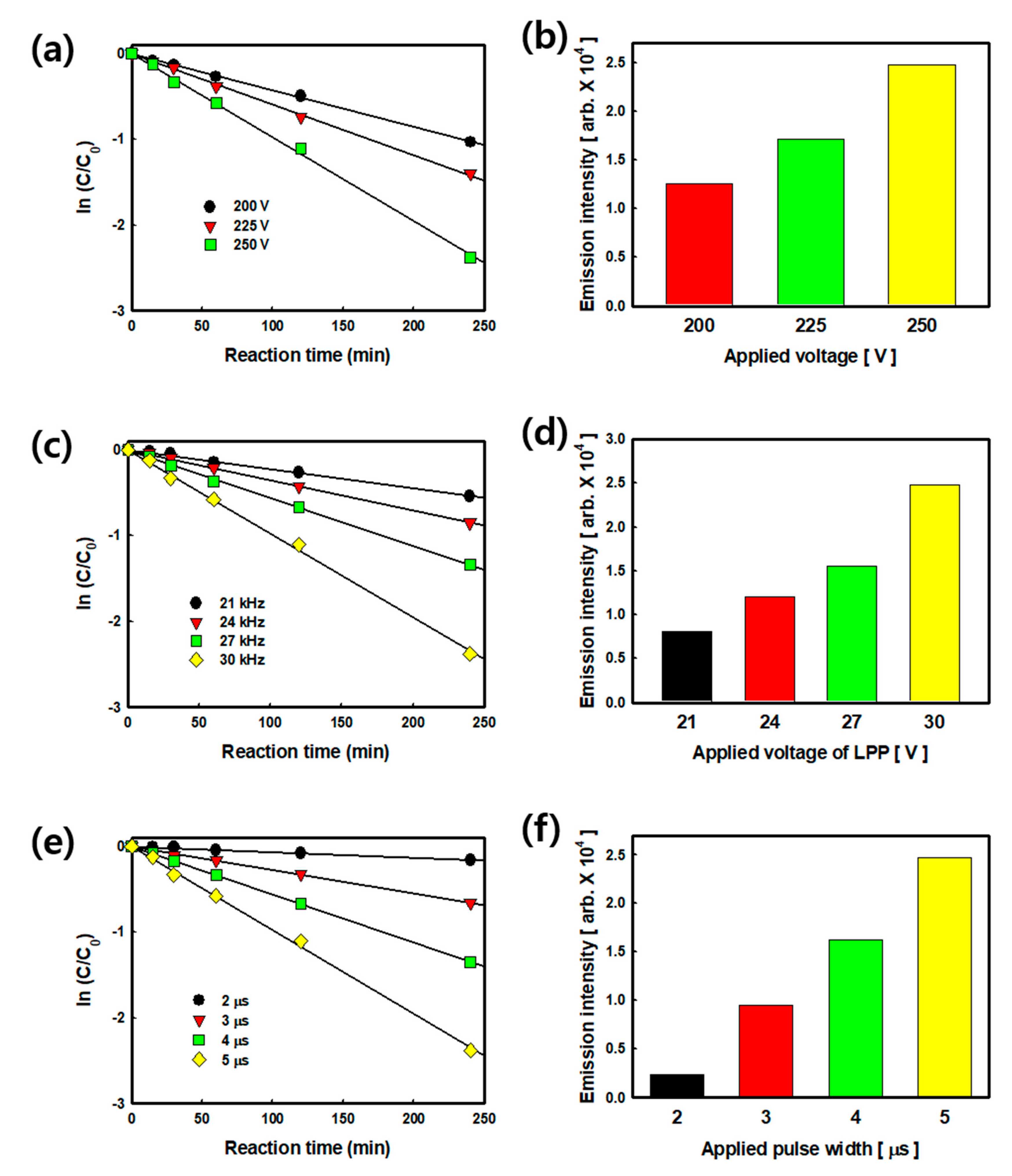
2.1. Effect of the Plasma Operating Conditions
2.2. Effect of H2O2
2.3. Effect of TiO2 Photocatalyst
2.4. Effect of a Combination of Reaction Processes
2.5. Degradation Reaction Pathway of ASA by the PiLP
3. Materials and Methods
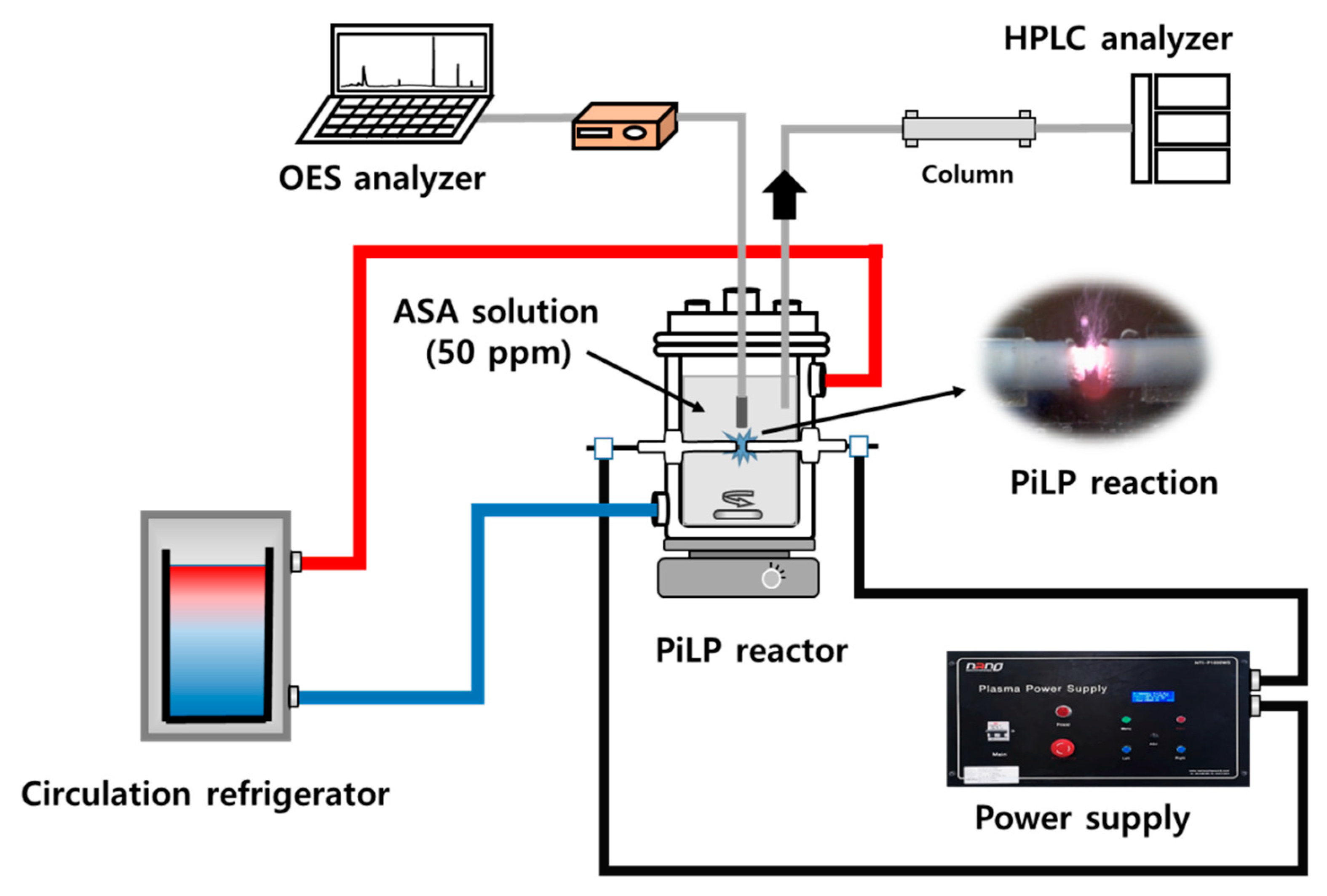
3.1. Materials and Equipment
3.2. Experimental Procedure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.; Aparicio, I.; Alonso, E.; Callejón, M. Simultaneous determination of pharmaceutically active compounds in wastewater samples by solid phase extraction and high-performance liquid chromatography with diode array and fluorescence detectors. Anal. Chim. Acta 2005, 550, 116–122. [Google Scholar] [CrossRef]
- Adebayo, G.I.; Williams, J.; Healy, S. Aspirin esterase activity-Evidence for skewed distribution in healthy volunteers. Eur. J. Int. Med. 2007, 18, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Calza, P.; Sakkas, V.A.; Medana, C.; Baiocchi, C.; Dimou, A.; Pelizzetti, E.; Albanis, T. Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions. Appl. Catal. B Environ. 2006, 67, 197–205. [Google Scholar] [CrossRef]
- Agunbiade, F.O.; Moodley, B. Occurrence and distribution pattern of acidic pharmaceuticals in surface water, wastewater, and sediment of the Msunduzi River, Kwazulu-Natal, South Africa. Environ. Toxicol. Chem. 2016, 35, 36–46. [Google Scholar] [CrossRef]
- Na, S.; Jinhua, C.; Cui, M.; Khim, J. Sonophotolytic diethyl phthalate (DEP) degradation with UVC or VUV irradiation. Ultrason. Sonochem. 2012, 19, 1094–1098. [Google Scholar] [CrossRef]
- Yang, G.C.C.; Huang, S.C.; Wang, C.L.; Jen, Y.S. Degradation of phthalate esters and acetaminophen in river sediments using the electrokinetic process integrated with a novel Fenton-like process catalyzed by nanoscale schwertmannite. Chemosphere 2016, 159, 282–292. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.H.; Park, Y.K.; Kim, S.J.; Seo, S.G.; Ki, S.J.; Jung, S.C. Photocatalytic reactions of 2,4-dichlorophenoxyacetic acid using a microwave-assisted photocatalysis system. Chem. Eng. J. 2015, 278, 259–264. [Google Scholar] [CrossRef]
- Ki, S.J.; Jeon, K.J.; Park, Y.K.; Jeong, S.; Lee, H.; Jung, S.C. Improving removal of 4-chlorophenol using a TiO2 photocatalytic system with microwave and ultraviolet radiation. Catal. Today 2017, 293–294, 15–22. [Google Scholar] [CrossRef]
- An, T.; Gao, Y.; Li, G.; Kamat, P.V.; Peller, J.; Joyce, M.V. Kinetics and Mechanism of •OH Mediated Degradation of Dimethyl Phthalate in Aqueous Solution: Experimental and Theoretical Studies. Environ. Sci. Technol. 2014, 48, 641–648. [Google Scholar] [CrossRef]
- Lee, D.J.; Park, Y.K.; Kim, S.J.; Lee, H.; Jung, S.C. Photo-catalytic destruction of ethylene using microwave discharge electrodeless lamp. Korean J. Chem. Eng. 2015, 32, 1188–1193. [Google Scholar] [CrossRef]
- Xu, B.; Gao, N.Y.; Sun, X.F.; Xia, S.J.; Rui, M.; Simonnot, M.O.; Causserand, C.; Zhao, J.F. Photochemical degradation of diethyl phthalate with UV/H2O2. J. Hazard. Mater. 2007, 139, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, S.H.; Park, Y.K.; Kim, B.H.; Kim, S.J.; Jung, S.C. Rapid destruction of the rhodamine B using TiO2 photocatalyst in the liquid phase plasma. Chem. Cent. J. 2013, 7, 156. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.H.; Cheong, C.J.; Kim, S.J.; Seo, S.G.; Park, Y.K.; Jung, S.C. Contribution of Dissolved Oxygen to Methyl Orange Decomposition by Liquid Phase Plasma Processes System. Ozone Sci. Eng. 2014, 36, 244–248. [Google Scholar] [CrossRef]
- Sato, M. Environmental and biotechnological applications of high-voltage pulsed discharges in water. Plasma Sour. Sci. Technol. 2008, 17, 024021–024027. [Google Scholar] [CrossRef]
- Reddy, P.M.K.; Subrahmanyam, C. Green Approach for Wastewater Treatment-Degradation and Mineralization of Aqueous Organic Pollutants by Discharge Plasma. Ind. Eng. Chem. Res. 2012, 51, 11097–11103. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. In-liquid plasma: A novel tool in the fabrication of nanomaterials and in the treatment of wastewaters. RSC Adv. 2017, 7, 47196–47218. [Google Scholar] [CrossRef]
- Sunka, P.; Babicky, V.; Clupek, M.; Lukes, P.; Simek, M.; Schmidt, J.; Cernak, M. Generation of chemically active species by electrical discharges in water. Plasma Sour. Sci. Technol. 1999, 8, 258–265. [Google Scholar] [CrossRef]
- Kang, J.; Li, O.L.; Saito, N. Synthesis of structure-controlled carbon nano spheres by solution plasma process. Carbon 2013, 60, 292–298. [Google Scholar] [CrossRef]
- Tantiplapol, T.; Singsawat, Y.; Narongsil, N.; Damrongsakkul, S.; Saito, N.; Prasertsung, I. Influences of solution plasma conditions on degradation rate and properties of chitosan. Innov. Food Sci. Emerg. Technol. 2015, 32, 116–120. [Google Scholar] [CrossRef]
- Hyun, K.; Ueno, T.; Saito, N. Synthesis of nitrogen-containing carbon by solution plasma in aniline with high-repetition frequency discharges. Jpn. J. Appl. Phys. 2016, 55, 01AE18. [Google Scholar] [CrossRef]
- Sugiarto, A.T.; Sato, M. Pulsed plasma processing of organic compounds in aqueous solution. Thin Solid Films 2001, 386, 295–299. [Google Scholar] [CrossRef]
- Alshamsi, F.A.; Albadwawi, A.S.; Alnuaimi, M.M.; Rauf, M.A.; Ashraf, S.S. Comparative efficiencies of the degradation of Crystal Violet using UV/hydrogen peroxide and Fenton’s reagent. Dyes Pigment. 2007, 74, 283–287. [Google Scholar] [CrossRef]
- Locke, B.R.; Sato, M.; Sunka, P.; Hoffman, M.R.; Chang, J.S. Electrohydraulic Discharge and Nonthermal Plasma for Water Treatment. Ind. Eng. Chem. Res. 2006, 45, 882–905. [Google Scholar] [CrossRef]
- Elliot, A.J.; McCracken, D.R. Estimation of Rate Constants for Near-diffusion-controlled Reactions in Water at High Temperatures. J. Chem. Soc. Faraday Trans. 1990, 86, 1539–1547. [Google Scholar] [CrossRef]
- Alnaizy, R.; Akgerman, A. Advanced oxidation of phenolic compounds. Adv. Environ. Res. 2000, 4, 233–244. [Google Scholar] [CrossRef]
- Cheng, H.H.; Chen, S.S.; Wu, Y.C.; Ho, D.L. Non-Thermal Plasma Technology for Degradation of Organic Compounds in Wastewater Control: A Critical Review. J. Environ. Eng. Manag. 2007, 17, 427–433. [Google Scholar]
- Hao, X.L.; Zhou, M.H.; Zhang, Y.; Lei, L.C. Enhanced degradation of organic pollutant 4-chlorophenol in water by non-thermal plasma process with TiO2. Plasma Chem. Plasma Process. 2006, 26, 455–468. [Google Scholar] [CrossRef]
- Mukherjee, D.; Ray, A.K.; Barghi, S. Mechanism of Acetyl Salicylic Acid (Aspirin) Degradation under Solar Light in Presence of a TiO2-Polymeric Film Photocatalyst. Processes 2016, 4, 13. [Google Scholar] [CrossRef]
- Li, L.; Ma, Q.; Wang, S.; Song, S.; Li, B.; Guo, R.; Cheng, X.; Cheng, Q. Photocatalytic Performance and Degradation Mechanism of Aspirin by TiO2 through Response Surface Methodology. Catalysts 2018, 8, 118. [Google Scholar] [CrossRef]
- Dai, Q.; Xia, Y.; Jiang, L.; Li, W.; Wang, J.; Chen, J. Enhanced Degradation of Aspirin by Electrochemical oxidation with Modified PbO2 Electrode and Hydrogen Peroxide. Int. J. Electrochem. Sci. 2012, 7, 12895–12906. [Google Scholar]
- Cui, Y.; Meng, Q.; Deng, X.; Ma, Q.; Zhang, H.; Cheng, X.; Li, X.; Xie, M.; Cheng, Q. Fabrication of platinum nano-crystallites decorated TiO2 nano-tube array photoelectrode and its enhanced photoelectrocatlytic performance for degradation of aspirin and mechanism. J. Ind. Eng. Chem. 2016, 43, 177–184. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Q.; Qin, F.; Dai, Q.; Wang, X. Fe doped CeO2 nanosheets as Fenton-like heterogeneous catalysts for degradation of salicylic acid. Chem. Eng. J. 2018, 333, 226–239. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bang, H.-J.; Lee, H.; Park, Y.-K.; Ha, H.-H.; Yu, Y.H.; Kim, B.-J.; Jung, S.-C. Assessment of Degradation Behavior for Acetylsalicylic Acid Using a Plasma in Liquid Process. Catalysts 2019, 9, 965. https://doi.org/10.3390/catal9110965
Bang H-J, Lee H, Park Y-K, Ha H-H, Yu YH, Kim B-J, Jung S-C. Assessment of Degradation Behavior for Acetylsalicylic Acid Using a Plasma in Liquid Process. Catalysts. 2019; 9(11):965. https://doi.org/10.3390/catal9110965
Chicago/Turabian StyleBang, Hye-Jin, Heon Lee, Young-Kwon Park, Hyung-Ho Ha, Young Hyun Yu, Byung-Joo Kim, and Sang-Chul Jung. 2019. "Assessment of Degradation Behavior for Acetylsalicylic Acid Using a Plasma in Liquid Process" Catalysts 9, no. 11: 965. https://doi.org/10.3390/catal9110965
APA StyleBang, H.-J., Lee, H., Park, Y.-K., Ha, H.-H., Yu, Y. H., Kim, B.-J., & Jung, S.-C. (2019). Assessment of Degradation Behavior for Acetylsalicylic Acid Using a Plasma in Liquid Process. Catalysts, 9(11), 965. https://doi.org/10.3390/catal9110965