Nitrogen and Cobalt Co-Coped Carbon Materials Derived from Biomass Chitin as High-Performance Electrocatalyst for Aluminum-Air Batteries
Abstract
1. Introduction
2. Results and Discussion
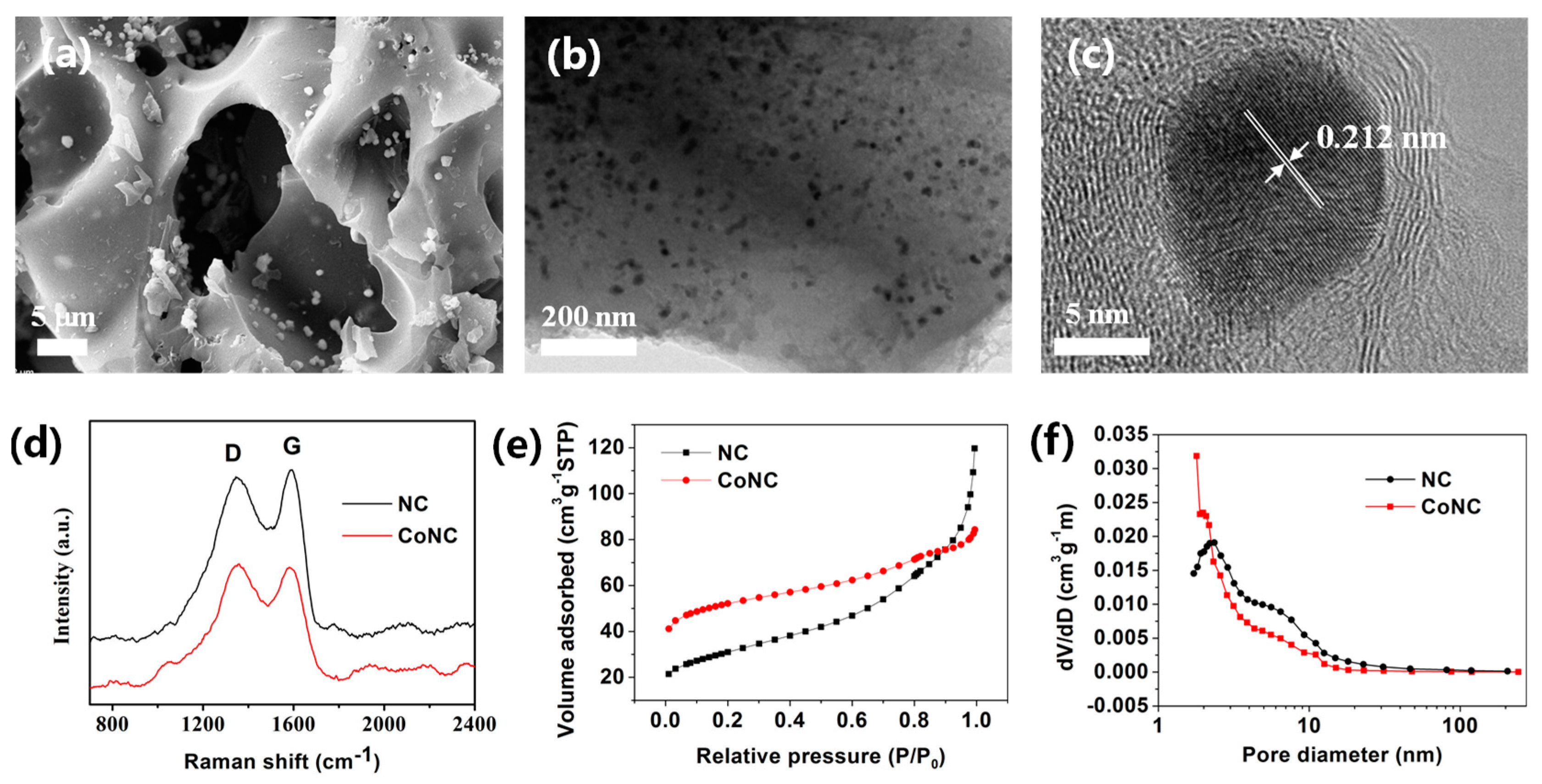
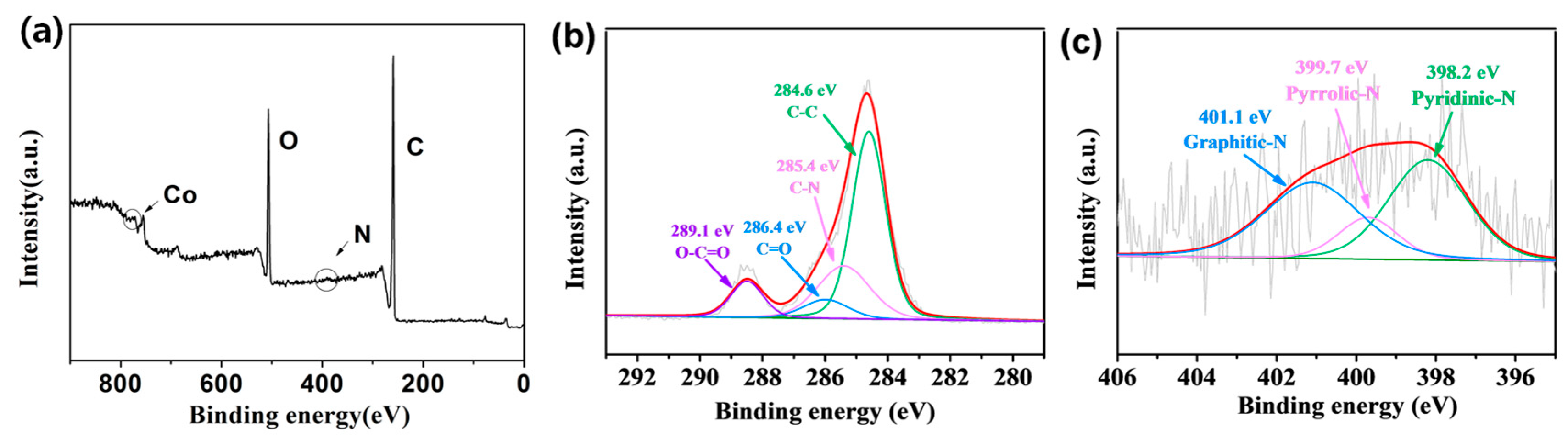
2.1. Morphological and Structural Characterization
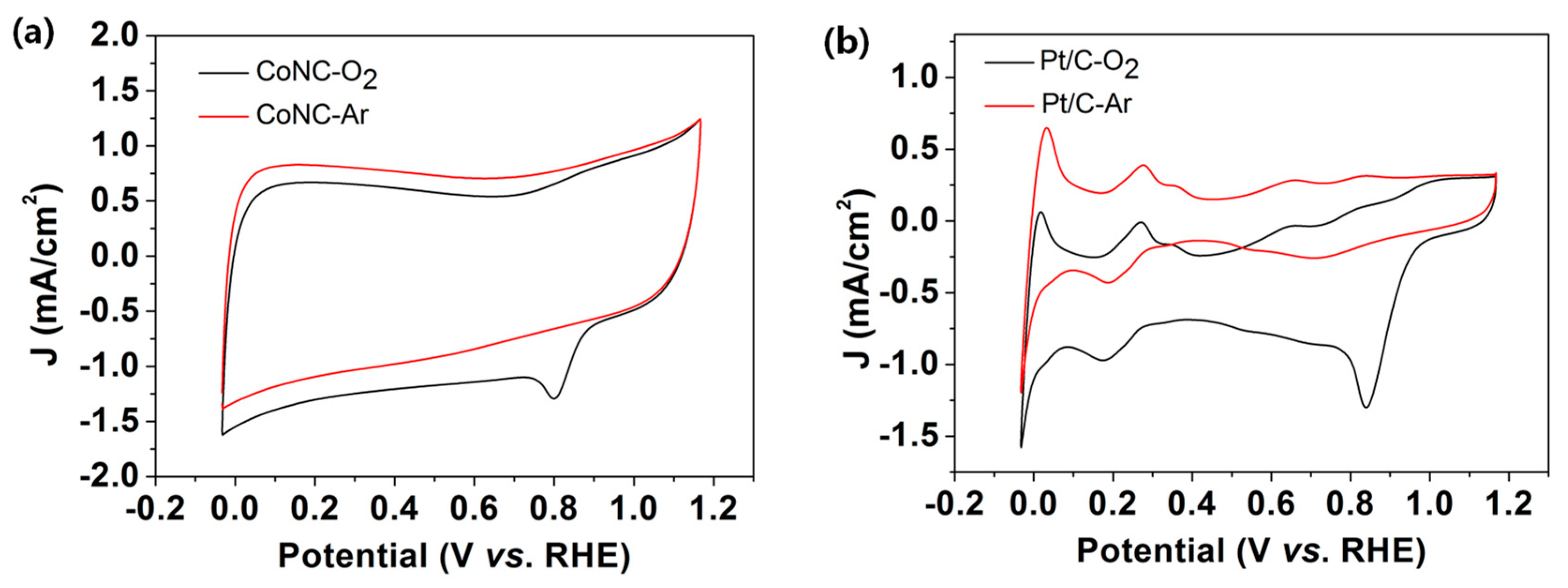
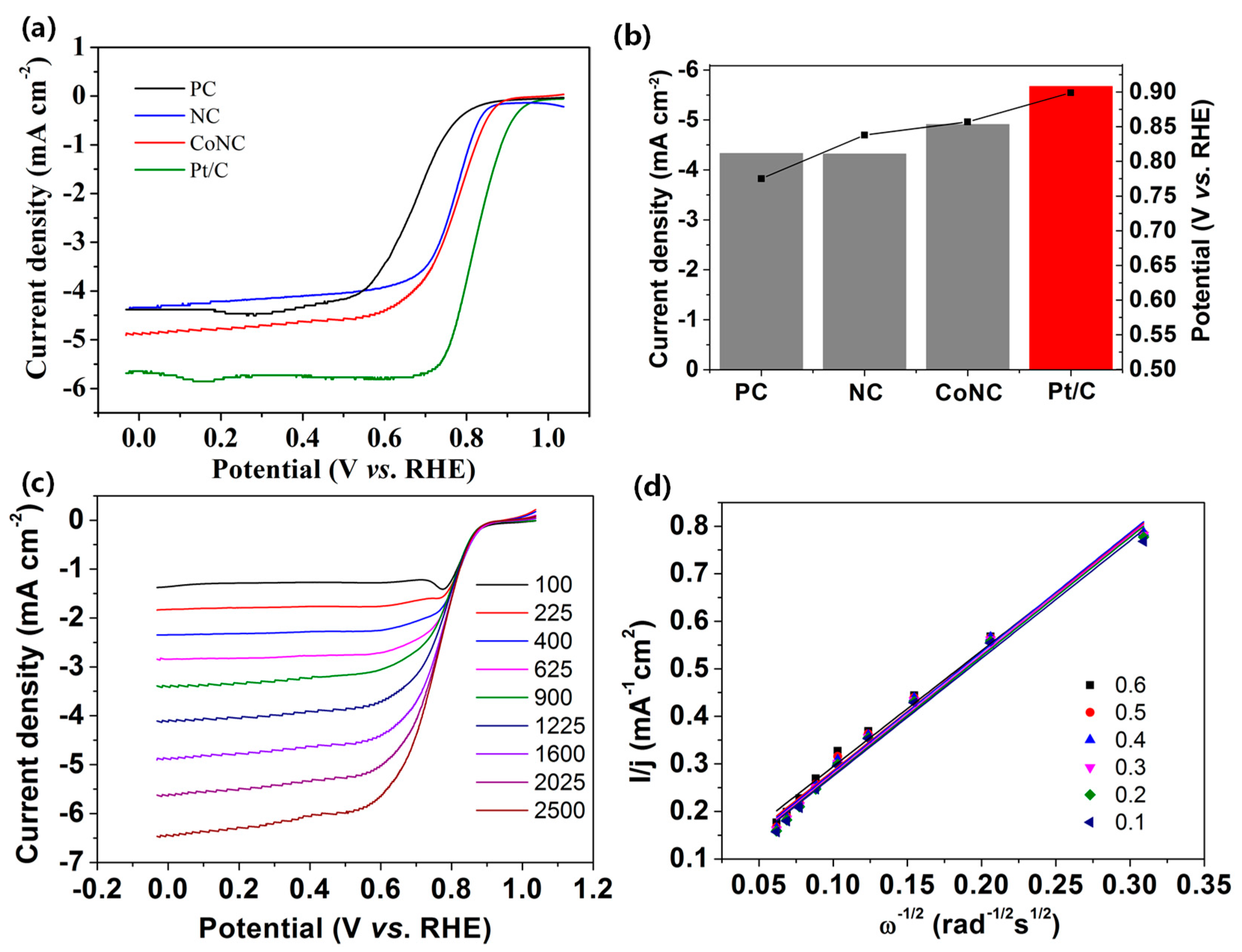
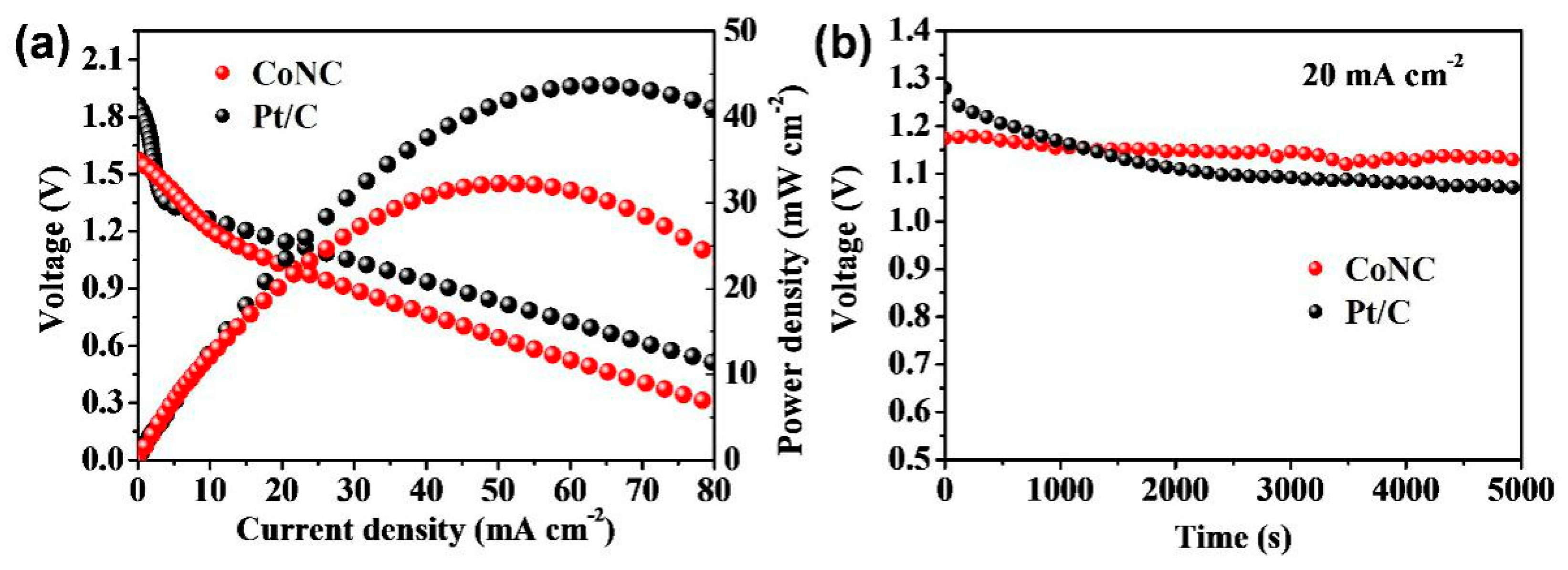
2.2. Electrocatalytic Characteristics and Active Sites
3. Experimental Section
3.1. Preparation of Co and N Co-Doped Biocarbon
3.2. Physicochemical Characterization
3.3. Electrochemical Measurements
3.4. Al-Air Battery Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Z.N.; Li, Z.Y.; Ma, J.; Dong, X.; Ku, W.; Wang, M.; Sun, H.; Liang, S.; Lu, G.L. Nitrogen and cobalt-doped porous biocarbon materials derived from corn stover as efficient electrocatalysts for aluminum-air batteries. Energy 2018, 162, 453–459. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Li, C.; Liu, J.Y.; Wang, Y.G.; Xia, Y.Y. A Long-Life Lithium-Air Battery in Ambient Air with a Polymer Electrolyte Containing a Redox Mediator. Angew. Chem. Int. Ed. 2017, 56, 7505–7509. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.; Liu, H.; Xia, B.Y. Advanced Architectures and Relatives of Air Electrodes in Zn-Air Batteries. Adv. Sci. (Weinh.) 2018, 5, 1700691. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Park, M.; Cho, J. Advanced Technologies for High-Energy Aluminum-Air Batteries. Adv. Mater. 2019, 31, 1804784. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Chen, M.J.; Cullen, D.A.; Hwang, S.; Wang, M.Y.; Li, B.Y.; Liu, K.X.; Karakalos, S.; Lucero, M.; Zhang, H.G.; et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 2018, 1, 935–945. [Google Scholar] [CrossRef]
- Yang, D.J.; Zhang, L.J.; Yan, X.C.; Yao, X.D. Recent Progress in Oxygen Electrocatalysts for Zinc-Air Batteries. Small Methods 2017, 1, 1700209. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Liu, H.; Liao, M. Novel Porous Nitrogen Doped Graphene/Carbon Black Composites as Efficient Oxygen Reduction Reaction Electrocatalyst for Power Generation in Microbial Fuel Cell. Nanomaterials 2019, 9, 836. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, M.G.; Lu, L.L.; Barkholtz, H.M.; Li, Y.P.; Wang, Y.; Jiang, L.H.; Wu, Z.J.; Liu, D.J.; Zhuang, L.; et al. High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun. 2017, 8, 15938. [Google Scholar] [CrossRef]
- Liu, H.Y.; Qin, J.Q.; Zhao, S.Q.; Gao, Z.M.; Fu, Q.; Song, Y.J. Two-dimensional circular platinum nanodendrites toward efficient oxygen reduction reaction and methanol oxidation reaction. Electrochem. Commun. 2019, 98, 53–57. [Google Scholar] [CrossRef]
- Li, Y.; Xia, X.H. Study on the Mechanism and Kinetics of Oxygen Reduction Reaction on 3D Porous Platinum Film Constructed Using Colloidal Crystal Template. J. Nanosci. Nanotech. 2016, 16, 12388–12393. [Google Scholar] [CrossRef]
- Lange, K.; Schulz-Ruhtenberg, M.; Caro, J. Platinum Electrodes for Oxygen Reduction Catalysis Designed by Ultrashort Pulse Laser Structuring. Chemelectrochem 2017, 4, 570–576. [Google Scholar] [CrossRef]
- Oh, T.; Kim, K.; Kim, J. Controllable active sites and facile synthesis of cobalt nanoparticle embedded in nitrogen and sulfur co-doped carbon nanotubes as efficient bifunctional electrocatalysts for oxygen reduction and evolution reactions. J. Energy Chem. 2019, 38, 60–67. [Google Scholar] [CrossRef]
- Kang, Y.S.; Choi, D.; Park, H.-Y.; Yoo, S.J. Tuning the surface structure of PtCo nanocatalysts with high activity and stability toward oxygen reduction. J. Ind. Eng. Chem. 2019, 78, 448–454. [Google Scholar] [CrossRef]
- Kim, S.; Kato, S.; Ishizaki, T.; Li, O.L.; Kang, J. Transition Metal (Fe, Co, Ni) Nanoparticles on Selective Amino-N-Doped Carbon as High-Performance Oxygen Reduction Reaction Electrocatalyst. Nanomaterials 2019, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xiao, C.H.; Xiang, Y.; Dong, B.T.; Ding, S.J.; Tang, Y.H. Nitrogen-Doped Graphene Quantum Dots Anchored on Thermally Reduced Graphene Oxide as an Electrocatalyst for the Oxygen Reduction Reaction. Chemelectrochem 2016, 3, 864–870. [Google Scholar] [CrossRef]
- Su, C.Y.; Cheng, H.; Li, W.; Liu, Z.Q.; Li, N.; Hou, Z.F.; Bai, F.Q.; Zhang, H.X.; Ma, T.Y. Atomic Modulation of FeCo-Nitrogen-Carbon Bifunctional Oxygen Electrodes for Rechargeable and Flexible All-Solid-State Zinc-Air Battery. Adv. Energy Mater. 2017, 7, 1602242. [Google Scholar] [CrossRef]
- Zhu, J.W.; Li, W.Q.; Li, S.H.; Zhang, J.; Zhou, H.; Zhang, C.T.; Zhang, J.A.; Mu, S.C. Defective N/S-Codoped 3D Cheese-Like Porous Carbon Nanomaterial toward Efficient Oxygen Reduction and Zn-Air Batteries. Small 2018, 14, 1800563. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, K.X.; Qiu, Y.F.; Liu, X.Z.; Cao, C.Y.; Feng, Y.J.; Hu, P.A. Nitrogen and sulfur co-doped porous carbon derived from bio-waste as a promising electrocatalyst for zinc-air battery. Energy 2018, 143, 43–55. [Google Scholar] [CrossRef]
- Wang, G.H.; Deng, Y.J.; Yu, J.N.; Zheng, L.; Du, L.; Song, H.Y.; Liao, S.J. From Chlorella to Nestlike Framework Constructed with Doped Carbon Nanotubes: A Biomass-Derived, High-Performance, Bifunctional Oxygen Reduction/Evolution Catalyst. ACS Appl. Mater. Interface 2017, 9, 32168–32178. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Gao, X.J.; Dou, M.L.; Ji, J.; Wang, F. Biomass Derived N-Doped Porous Carbon Supported Single Fe Atoms as Superior Electrocatalysts for Oxygen Reduction. Small 2017, 13, 1604290. [Google Scholar] [CrossRef]
- Xu, L.N.; Fan, H.; Huang, L.X.; Xia, J.L.; Li, S.H.; Li, M.; Ding, H.Y.; Huang, K. Chrysanthemum-derived N and S co-doped porous carbon for efficient oxygen reduction reaction and aluminum-air battery. Electrochim. Acta 2017, 239, 1–9. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.F.; Ma, N.; Liu, H.T.; Xia, Y.Z.; Chen, C.M.; Yang, D.J.; Yao, X.D. Scalable and Cost-Effective Synthesis of Highly Efficient Fe2N-Based Oxygen Reduction Catalyst Derived from Seaweed Biomass. Small 2016, 12, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zhu, Y.; Lu, L.; Xu, K.; Wang, H.; Jin, Y.; Ren, Z.J.; Liu, Z.; Zhang, W. Iron-rich nanoparticle encapsulated, nitrogen doped porous carbon materials as efficient cathode electrocatalyst for microbial fuel cells. J. Power Sources 2016, 315, 302–307. [Google Scholar] [CrossRef]
- Lu, G.; Zhu, Y.; Xu, K.; Jin, Y.; Ren, Z.J.; Liu, Z.; Zhang, W. Metallated porphyrin based porous organic polymers as efficient electrocatalysts. Nanoscale 2015, 7, 18271–18277. [Google Scholar] [CrossRef]
- Yan, L.; Yu, J.; Houston, J.; Flores, N.; Luo, H. Biomass Derived Porous Nitrogen doped Carbon for Electrochemical Devices. Green Energy Environ. 2017, 2, 84–99. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, D.B.; Gan, J.; Duan, X.Z.; Zang, K.T.; Ronning, M.; Song, L.; Luo, J.; Chen, D. Sustainable and Atomically Dispersed Iron Electrocatalysts Derived from Nitrogen- and Phosphorus-Modified Woody Biomass for Efficient Oxygen Reduction. Adv. Mater. Interfaces 2019, 6, 1801623. [Google Scholar] [CrossRef]
- Wu, D.Y.; Zhu, C.; Shi, Y.T.; Jing, H.Y.; Hu, J.W.; Song, X.D.; Si, D.H.; Liang, S.X.; Hao, C. Biomass-Derived Multilayer-Graphene-Encapsulated Cobalt Nanoparticles as Efficient Electrocatalyst for Versatile Renewable Energy Applications. ACS Sustain. Chem. Eng. 2019, 7, 1137–1145. [Google Scholar] [CrossRef]
- Wei, Q.L.; Yang, X.H.; Zhang, G.X.; Wang, D.N.; Zuin, L.; Banham, D.; Yang, L.J.; Ye, S.Y.; Wang, Y.L.; Mohamedi, M.; et al. An active and robust Si-Fe/N/C catalyst derived from waste reed for oxygen reduction. Appl. Catal. B Environ. 2018, 237, 85–93. [Google Scholar] [CrossRef]
- Yuan, H.; Deng, L.; Cai, X.; Zhou, S.; Chen, Y.; Yuan, Y. Nitrogen-doped carbon sheets derived from chitin as non-metal bifunctional electrocatalysts for oxygen reduction and evolution. RSC Adv. 2015, 5, 56121–56129. [Google Scholar] [CrossRef]
- Bo, W.; Li, S.; Wu, X.; Liu, J.; Jing, C. Biomass chitin-derived honeycomb-like nitrogen-doped carbon/graphene nanosheet networks for applications in efficient oxygen reduction and robust lithium storage. J. Mater. Chem. A 2016, 4. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, L.; Li, Z.; Gao, G.; Zhong, W.; Zhang, P.; Gong, Y.; Deng, L. Chitin-derived porous carbon loaded with Co, N and S with enhanced performance towards electrocatalytic oxygen reduction, oxygen evolution, and hydrogen evolution reactions. Electrochim. Acta 2019, 304, 350–359. [Google Scholar] [CrossRef]
- Borghei, M.; Laocharoen, N.; Kibena-Poldsepp, E.; Johansson, L.S.; Campbell, J.; Kauppinen, E.; Tammeveski, K.; Rojas, O.J. Porous N,P-doped carbon from coconut shells with high electrocatalytic activity for oxygen reduction: Alternative to Pt-C for alkaline fuel cells. Appl. Catal. B Environ. 2017, 204, 394–402. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Zhao, X.S.; Tian, X.N.; Luo, L.J.; Fang, J.H.; Gao, H.Q.; Jiang, Z.J. Hydrothermal Synthesis of Boron and Nitrogen Codoped Hollow Graphene Microspheres with Enhanced Electrocatalytic Activity for Oxygen Reduction Reaction. ACS Appl. Mater. Interface 2015, 7, 19398–19407. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.L.; Zhong, H.X.; Bao, D.; Yan, J.M.; Zhang, X.B. In Situ Coupling of Strung Co4N and Intertwined N-C Fibers toward Free-Standing Bifunctional Cathode for Robust, Efficient, and Flexible Zn Air-Batteries. J. Am. Chem. Soc. 2016, 138, 10226–10231. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gao, X.; Wei, X.; Wang, X.; Li, Y.; Wu, T.; Guo, J.; Gu, Q.; Wu, W.D.; Chen, X.D. Directly anchoring Fe 3 C nanoclusters and FeN x sites in ordered mesoporous nitrogen-doped graphitic carbons to boost electrocatalytic oxygen reduction. Carbon 2017, 121, 143–153. [Google Scholar] [CrossRef]
- Li, C.L.; Wu, M.C.; Liu, R. High-performance bifunctional oxygen electrocatalysts for zinc-air batteries over mesoporous Fe/Co-N-C nanofibers with embedding FeCo alloy nanoparticles. Appl. Catal. B Environ. 2019, 244, 150–158. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Tian, S.; Wang, M.; Sun, H.; Liang, S.; Chang, Z.; Lu, G. Conversion of peanut biomass into electrocatalysts with vitamin B12 for oxygen reduction reaction in Zn-air battery. Int. J. Hydrogen Energy 2019, 44, 11788–11796. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Wang, G.; Dai, B.; Yu, F.; Tian, Z.; Guo, X. Enhanced Oxygen Reduction Reaction by In Situ Anchoring Fe2N Nanoparticles on Nitrogen-Doped Pomelo Peel-Derived Carbon. Nanomaterials 2017, 7, 404. [Google Scholar] [CrossRef]
- Lu, G.; Li, Z.; Fan, W.; Wang, M.; Yang, S.; Li, J.; Chang, Z.; Sun, H.; Liang, S.; Liu, Z. Sponge-like N-doped carbon materials with Co-based nanoparticles derived from biomass as highly efficient electrocatalysts for the oxygen reduction reaction in alkaline media. RSC Adv. 2019, 9, 4843–4848. [Google Scholar] [CrossRef]
- Lu, J.; Zeng, Y.; Ma, X.; Wang, H.; Gao, L.; Zhong, H.; Meng, Q. Cobalt Nanoparticles Embedded into N-Doped Carbon from Metal Organic Frameworks as Highly Active Electrocatalyst for Oxygen Evolution Reaction. Polymers 2019, 11, 828. [Google Scholar] [CrossRef]
- Stosevski, I.; Krstic, J.; Milikic, J.; Sljukic, B.; Kacarevic-Popovic, Z.; Mentus, S.; Miljanic, S. Radiolitically synthesized nano Ag/C catalysts for oxygen reduction and borohydride oxidation reactions in alkaline media, for potential applications in fuel cells. Energy 2016, 101, 79–90. [Google Scholar] [CrossRef]
- Zhang, J.W.; Xu, D.; Wang, C.C.; Guo, J.N.; Yan, F. Rational Design of Fe1-xS/Fe3O4/Nitrogen and Sulfur-Doped Porous Carbon with Enhanced Oxygen Reduction Reaction Catalytic Activity. Adv. Mater. Interfaces 2018, 5, 1701461. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Ma, J.; Yang, H.; Lu, G.; Yang, S.; Chang, Z. Nitrogen and Cobalt Co-Coped Carbon Materials Derived from Biomass Chitin as High-Performance Electrocatalyst for Aluminum-Air Batteries. Catalysts 2019, 9, 954. https://doi.org/10.3390/catal9110954
Wang M, Ma J, Yang H, Lu G, Yang S, Chang Z. Nitrogen and Cobalt Co-Coped Carbon Materials Derived from Biomass Chitin as High-Performance Electrocatalyst for Aluminum-Air Batteries. Catalysts. 2019; 9(11):954. https://doi.org/10.3390/catal9110954
Chicago/Turabian StyleWang, Mi, Jian Ma, Haoqi Yang, Guolong Lu, Shuchen Yang, and Zhiyong Chang. 2019. "Nitrogen and Cobalt Co-Coped Carbon Materials Derived from Biomass Chitin as High-Performance Electrocatalyst for Aluminum-Air Batteries" Catalysts 9, no. 11: 954. https://doi.org/10.3390/catal9110954
APA StyleWang, M., Ma, J., Yang, H., Lu, G., Yang, S., & Chang, Z. (2019). Nitrogen and Cobalt Co-Coped Carbon Materials Derived from Biomass Chitin as High-Performance Electrocatalyst for Aluminum-Air Batteries. Catalysts, 9(11), 954. https://doi.org/10.3390/catal9110954




