Novel Cobalt Complex as an Efficient Catalyst for Converting CO2 into Cyclic Carbonates under Mild Conditions
Abstract
1. Introduction
2. Results and Discussion
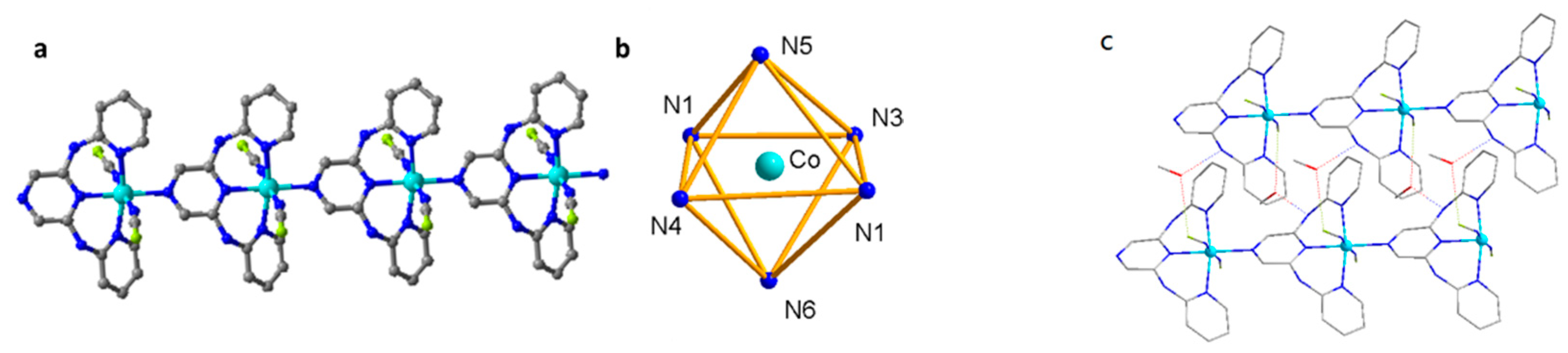
2.1. Structure and Characterization
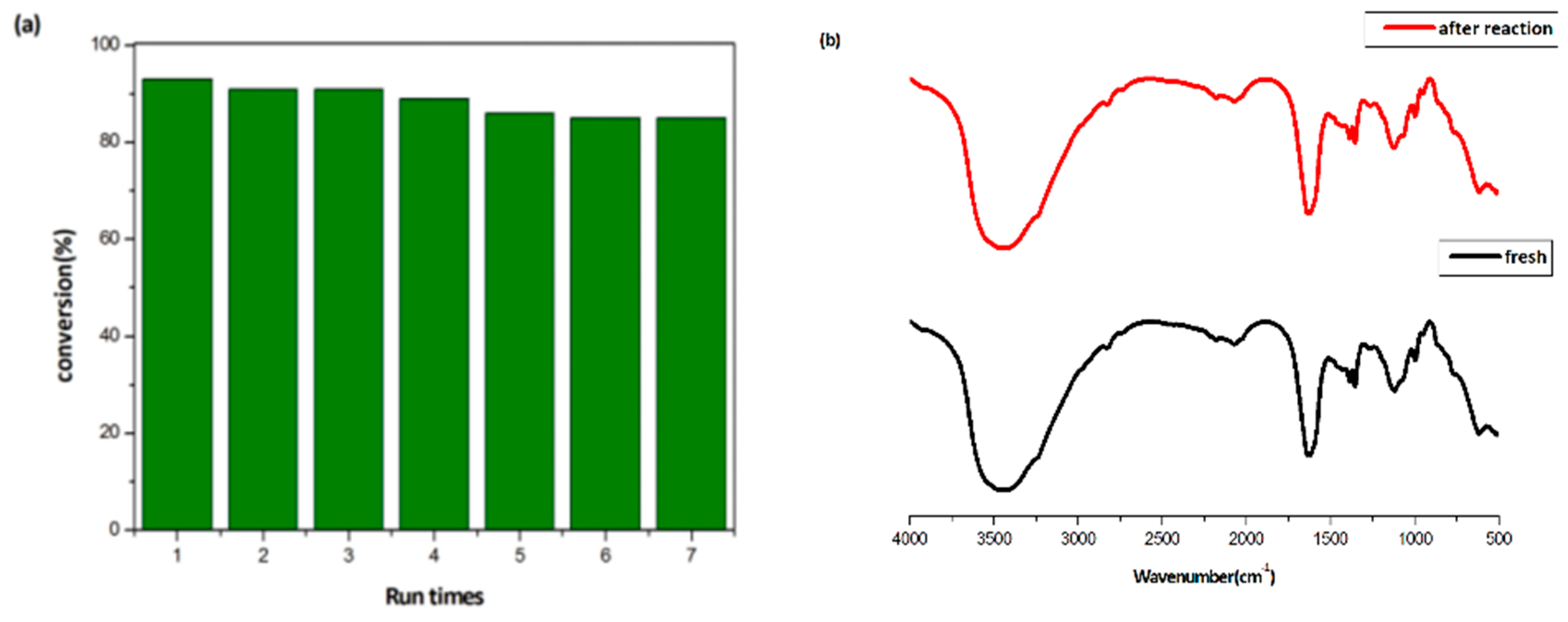
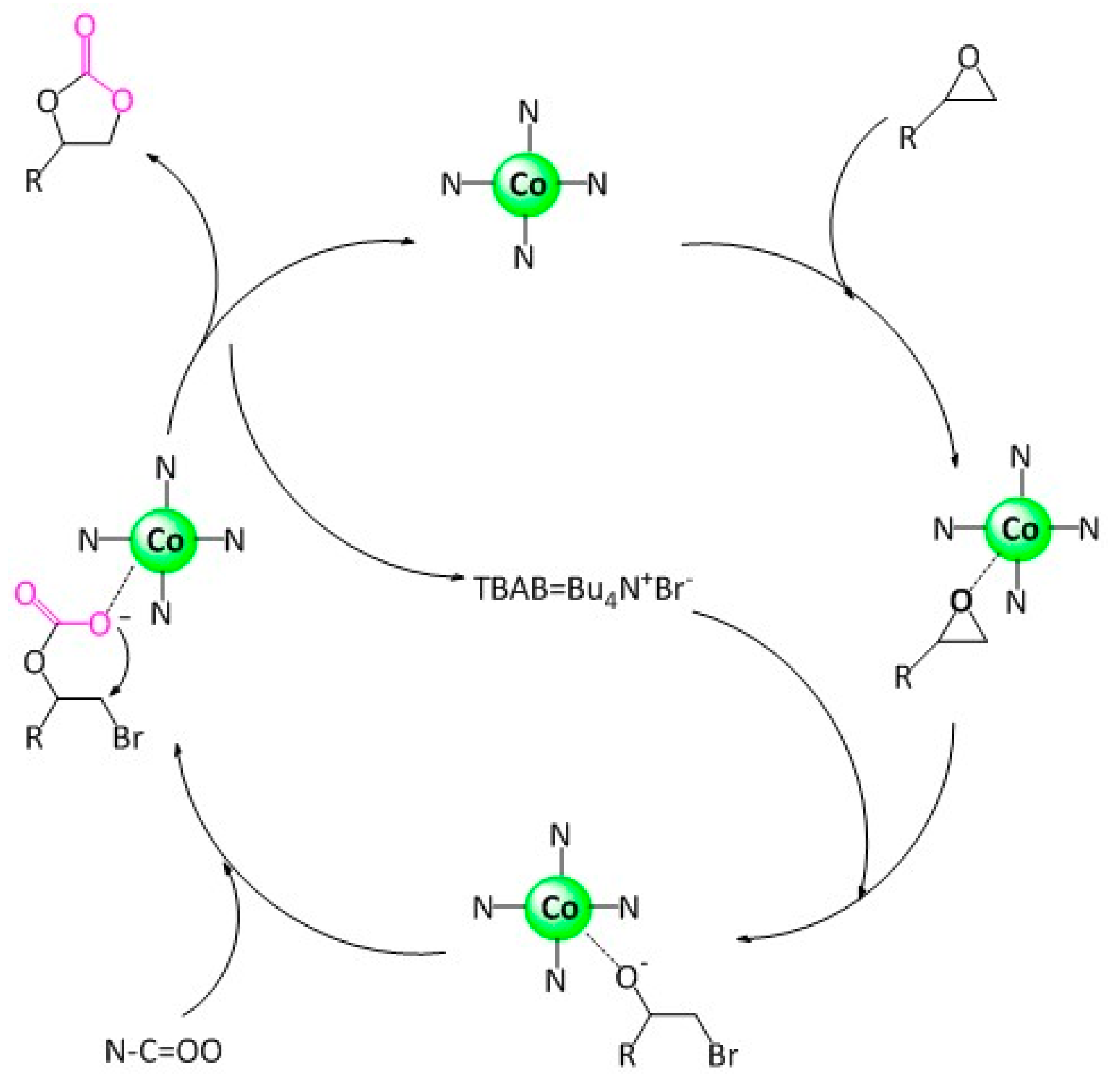
2.2. Catalytic Activity for CO2 Fixation
3. Materials and Methods
3.1. Materials and Characterizations
3.2. Synthesis of Complex 2
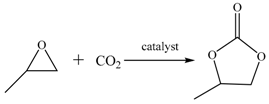
3.3. Catalytic Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gao, L.; Feng, J. A one-step strategy for thermally and mechanically reinforced pseudo-interpenetrating poly (propylene carbonate) networks by terpolymerization of CO2, propylene oxide and pyromellitic dianhydride. J. Mater. Chem. A 2013, 1, 3556–3560. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Lin, L.; Xiao, M.; Meng, Y. Semi-crystalline terpolymers with varying chain sequence structures derived from CO2, cyclohexene oxide and ε-caprolactone: One-step synthesis catalyzed by tri-zinc complexes. Polym. Chem. 2015, 6, 1533–1540. [Google Scholar] [CrossRef]
- Kember, M.R.; Buchard, A.; Williams, C.K. Feature Article Catalysts for CO2/epoxide copolymerisation. Chem. Commun. 2011, 47, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Park, J.; Byun, J.; Jung, Y.; Yavuz, C.T. Highly Efficient Catalytic Cyclic Carbonate Formation by Pyridyl Salicylimines. ACS Appl. Mater. Interfaces 2018, 10, 9478–9484. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xie, Y.F.; Wang, C.; Li, S.J.; Wei, D.H.; Li, M.; Dai, B. Cooperative Multifunctional Organocatalysts for Ambient Conversion of Carbon Dioxide into Cyclic Carbonates. ACS Catal. 2018, 8, 9945–9957. [Google Scholar] [CrossRef]
- Taherimehr, M.; Sertã, J.P.C.C.; Kleij, A.W.; Whiteoak, C.J.; Pescarmona, P.P. New Iron Pyridylamino-Bis (Phenolate) Catalyst for Converting CO2 into Cyclic Carbonates and Cross-Linked Polycarbonates. ChemSusChem 2015, 8, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.A.; Gómez, C.F.; Paninho, A.B.; Nunes, A.V.M.; Mahmudov, K.T.; Najdanovic-Visak, V.; Nunes da Ponte, M. Cyclic carbonate synthesis from CO2 and epoxides using zinc(II) complexes of arylhydrazones of β-diketones. J. Catal. 2016, 335, 135–140. [Google Scholar] [CrossRef]
- Peng, J.; Geng, Y.; Yang, H.J.; He, W.; Wei, Z.; Yang, J.; Guo, C.Y. Efficient solvent-free fixation of CO2 into cyclic carbonates catalyzed by Bi(III) porphyrin/TBAI at atmospheric pressure. Mol. Catal. 2017, 432, 37–46. [Google Scholar] [CrossRef]
- Sengupta, M.; Bag, A.; Ghosh, S.; Mondal, P.; Bordoloi, A.; Islam, S.M. CuxOy@COF: An efficient heterogeneous catalyst system for CO2 cycloadditions under ambient conditions. J. CO2 Util. 2019, 34, 533–542. [Google Scholar] [CrossRef]
- Sudakar, P.; Sivanesan, D.; Yoon, S. Copolymerization of Epichlorohydrin and CO2 Using Zinc Glutarate: An Additional Application of ZnGA in Polycarbonate Synthesis. Macromol. Rapid Commun. 2016, 37, 788–793. [Google Scholar] [CrossRef]
- Zhang, W.H.; He, P.P.; Wu, S.; Xu, J.; Li, Y.; Zhang, G.; Wei, X.Y. Graphene oxide grafted hydroxyl-functionalized ionic liquid: A highly efficient catalyst for cycloaddition of CO2 with epoxides. Appl. Catal. A Gen. 2016, 509, 111–117. [Google Scholar] [CrossRef]
- Liu, M.; Lan, J.; Liang, L.; Sun, J.; Arai, M. Heterogeneous catalytic conversion of CO2 and epoxides to cyclic carbonates over multifunctional tri-s-triazine terminal-linked ionic liquids. J. Catal. 2017, 347, 138–147. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Kodama, K.; Hirose, T. Poly (4-vinylphenol)/tetra-n-butylammonium iodide: Efficient organocatalytic system for synthesis of cyclic carbonates from CO2 and epoxides. J. Appl. Polym. Sci. 2017, 134, 45189–45196. [Google Scholar] [CrossRef]
- Wu, X.; Chen, C.; Guo, Z.; North, M.; Whitwood, A.C. Metal and halide free catalyst for the synthesis of cyclic carbonates from epoxides and carbon dioxide. ACS Catal. 2019, 9, 1895–1906. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, G.; Ansari, A.; Kureshy, R.I.; Khan, N.H. A nitrogen rich polymer as an organo-catalyst for cycloaddition of CO2 to epoxides and its application for the synthesis of polyurethane. Sustain. Energy Fuels 2017, 1, 1620–1629. [Google Scholar]
- Milani, J.L.S.; Oliveira, I.S.; Santos, P.A.D.; Valdo, A.K.S.M.; Martins, F.T.; Cangussu, D.; Chagas, R.P.D. Chemical fixation of carbon dioxide to cyclic carbonates catalyzed by zinc (II) complex bearing 1,2-disubstituted benzimidazole ligand. Chin. J. Catal. 2018, 39, 245–249. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Y.B.; Cao, R. Metal–organic frameworks and porous organic polymers for sustainable fixation of carbon dioxide into cyclic carbonates. Coord. Chem. Rev. 2019, 378, 32–65. [Google Scholar] [CrossRef]
- José, A.C.; Javier, M.; Felipe, C.M.; María, P.C.; Juan, F.B.; Julián, R.L.; Antonio, O.; Agustín, L.S.; Juan, T. Development of hydroxy-containing imidazole organocatalysts for CO2 fixation into cyclic carbonates. Catal. Sci. Technol. 2018, 8, 1981–1987. [Google Scholar]
- Stößer, T.; Li, C.; Unruangsri, J.; Saini, P.K.; Sablong, R.J.; Meier, M.A.R.; Koning, C. Bio-derived polymers for coating applications: Comparing poly (limonene carbonate) and poly (cyclohexadiene carbonate). Polym. Chem. 2017, 8, 6099–6113. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, B.; Ding, H.; Chen, J.; Duan, Z. Polycarbonates derived from propylene oxide, CO2, and 4-vinyl cyclohexene oxides terpolymerization catalyzed by bifunctional salcyCoIIINO3 complex and its post-polymerization modification. Polymer 2017, 129, 5–11. [Google Scholar] [CrossRef]
- Chang, T.; Jin, L.; Jing, H. Bifunctional Chiral Catalyst for the Synthesis of Chiral Cyclic Carbonates from Carbon Dioxide and Epoxides. ChemCatChem 2009, 1, 379–384. [Google Scholar] [CrossRef]
- Ismayilov, R.H.; Wang, W.Z.; Lee, G.H.; Wang, R.R.; Liu, I.C.P.; Yeh, C.Y.; Peng, S.M. New versatile ligand family, pyrazine-modulated oligo-α-pyridylamino ligands, from coordination polymer to extended metal atom chains. Dalton Trans. 2007, 27, 2898–2912. [Google Scholar] [CrossRef] [PubMed]
- Khattak, Z.A.; Younus, K.H.A.; Ahmad, N.; Ullah, H.; Suleman, S.; Hossain, M.S.; Verpoort, F. Highly active dinuclear cobalt complexes for solvent-free cycloaddition of CO2 to epoxides at ambient pressure. Chem. Commun. 2019, 55, 8274–8277. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; He, L.N.; Zhou, Y.B. An efficient and recyclable tetraoxo-coordinated zinc catalyst for the cycloaddition of epoxides with carbon dioxide at atmospheric pressure. Green Chem. 2016, 18, 226–231. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, R.; Xu, Q.; Jiang, J.; Zhou, X.; Ji, H. Charged Metalloporphyrin Polymers for Cooperative Synthesis of Cyclic Carbonates from CO2 under Ambient Conditions. ChemSusChem 2017, 10, 2534–2537. [Google Scholar] [CrossRef]
- Ema, T.; Miyazaki, Y.; Shimonishi, J.; Maeda, C.; Hasegawa, J.Y. Bifunctional Porphyrin Catalysts for the Synthesis of Cyclic Carbonates from Epoxides and CO2: Structural Optimization and Mechanistic Study. J. Am. Chem. Soc. 2014, 136, 15270–15279. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, T.T.; Yang, R.X.; Huang, N.Y.; Zou, K.; Deng, W.Q. Efficient Fixation of CO2 by a Zinc-Coordinated Conjugated Microporous Polymer. ChemSusChem 2014, 7, 2110–2114. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, R.L.; Han, Q.X.; Xu, C.; Chen, W.M.; Yang, H.; Gao, G.S.; Qin, W.W.; Liu, W.S. Amide-functionalized heterometallic helicate cages as highly efficient catalysts for CO2 conversion under mild conditions. Green Chem. 2018, 20, 5311–5317. [Google Scholar] [CrossRef]
- Gao, G.; Wang, L.; Zhang, R.; Xu, C.; Yang, H.; Liu, W. Hexanuclear 3d–4f complexes as efficient catalysts for converting CO2 into cyclic carbonates. Dalton Trans. 2019, 48, 3941–3945. [Google Scholar] [CrossRef]
- Hu, J.; Ma, J.; Liu, H.; Qian, Q.; Xie, C.; Han, B. Dual-ionic liquid system: An efficient catalyst for chemical fixation of CO2 to cyclic carbonates under mild conditions. Green Chem. 2018, 20, 2990–2996. [Google Scholar] [CrossRef]
- Guo, Z.; Cai, X.; Xie, J.; Wang, X.; Zhou, Y.; Wang, J. Hydroxyl-Exchanged Nanoporous Ionic Copolymer toward Low-Temperature Cycloaddition of Atmospheric Carbon Dioxide into Carbonates. ACS Appl. Mater. Interfaces 2016, 8, 12812–12821. [Google Scholar] [CrossRef] [PubMed]
- Karamé, I.; Zaher, S.; Eid, N.; Christ, L. New zinc/tetradentate N4 ligand complexes: Efficient catalysts for solvent-free preparation of cyclic carbonates by CO2/epoxide coupling. Mol. Catal. 2018, 456, 87–95. [Google Scholar] [CrossRef]
- Xia, L.; Wang, W.Z.; Liu, S.; Jia, X.G.; Zhang, Y.H.; Li, L.L.; Wu, Y.; Su, B.Y.; Geng, S.B.; Fan, W. New Coordination Complexes Based on 2,6-bis [1-(phenylimino)ethyl Ligand: Effective Catalysts for the Synthesis of Propylene Carbonates from Carbon Dioxide and Epoxides. Molecules 2018, 23, 2304. [Google Scholar] [CrossRef] [PubMed]

| Entry | Cat | Co-cat | T(°C) | P(bar) | T(h) | Conversions b (%) | TOF c (h−1) |
|---|---|---|---|---|---|---|---|
| 1 | - | - | 90 | 10 | 1 | - | - |
| 2 | 1 | - | 90 | 10 | 1 | 9 | 360 |
| 3 | 2 | - | 90 | 10 | 1 | 16 | 640 |
| 4 | - | TBAB | 90 | 10 | 1 | 25 | - |
| 5 | 2 | TBAB | 90 | 10 | 1 | 89 | 3560 |
| 6 | 2 | TBAB | 80 | 10 | 1 | 79 | 3160 |
| 7 | 2 | TBAB | 70 | 10 | 1 | 63 | 2520 |
| 8 | 2 | TBAB | 60 | 10 | 1 | 45 | 1800 |
| 9 | 2 | TBAB | 90 | 15 | 1 | 95 | 3800 |
| 10 | 2 | TBAB | 90 | 8 | 1 | 84 | 3360 |
| 11 | 2 | TBAB | 90 | 6 | 1 | 56 | 2240 |
| 12 | 2 | DMAP | 90 | 10 | 1 | 71 | 1840 |
| 13 d | 2 | TBAB | 90 | 10 | 1 | 75 | 3000 |
| 14 e | 2 | TBAB | 90 | 10 | 1 | 62 | 2480 |
| 15 f | 2 | TBAB | 90 | 10 | 1 | 86 | 3440 |
| 16 g | 2 | TBAB | 90 | 10 | 1 | 83 | 3320 |
| 17 h | - | TBAB | 120 | 10 | 1 | 19 | - |
| 18 h | 2 | TBAB | 120 | 10 | 1 | 73 | 29,200 |
| Cat. | Co-Cat. | Catalyst/Epoxide (Mole Ratio) | P (MPa) | T (°C) | Time (h) | Conversions (%) | TOF (h−1) | Ref |
|---|---|---|---|---|---|---|---|---|
| Co-Salen | DMAP (1) | (Epichlorohydrin) 1:200000 | 0.1 | 120 | 3 | 5 | 3333 | [23] |
| Zn(OPO)2 | TBAB (0.9) | (propylene epoxide) 1:40000 | 3 | 120 | 1 | 46 | 18,400 | [24] |
| Al-iPOP-2 | - | (propylene oxide) 1:10000 | 1 | 100 | 4 | 97 | 7600 | [25] |
| Mg-porphyrin | - | (1,2-Epoxyhexane) 1:33333 | 1.7 | 120 | 1 | 36 | 12,000 | [26] |
| Zn-CMP | TBAB (0.9) | (propylene oxide) 1:25000 | 3 | 120 | 1 | 29 | 11,600 | [27] |
| 2 | TBAB | 1:40000 | 1 | 120 | 1 | 73 | 29,200 | This work |
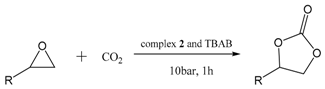
| Entry | Substrate | Product | Conversion (%) | TOF (h−1) |
|---|---|---|---|---|
| 1 | 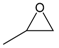 | 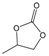 | 89 | 3560 |
| 2 | 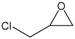 | 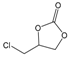 | 99 | 3960 |
| 3 | 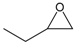 | 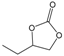 | 98 | 3920 |
| 4 | 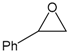 | 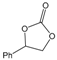 | 97 | 3880 |
| 5 | 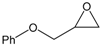 | 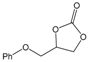 | 75 | 3000 |
| 6 | 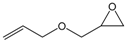 | 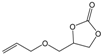 | 99 | 3960 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, W.; Wang, W.-Z.; Wang, L.; Jia, X.-G.; Li, L.-L.; Xiao, T.-C.; Edwards, P.P. Novel Cobalt Complex as an Efficient Catalyst for Converting CO2 into Cyclic Carbonates under Mild Conditions. Catalysts 2019, 9, 951. https://doi.org/10.3390/catal9110951
Fan W, Wang W-Z, Wang L, Jia X-G, Li L-L, Xiao T-C, Edwards PP. Novel Cobalt Complex as an Efficient Catalyst for Converting CO2 into Cyclic Carbonates under Mild Conditions. Catalysts. 2019; 9(11):951. https://doi.org/10.3390/catal9110951
Chicago/Turabian StyleFan, Wei, Wen-Zhen Wang, Li Wang, Xin-Gang Jia, Lei-Lei Li, Tian-Cun Xiao, and Peter P. Edwards. 2019. "Novel Cobalt Complex as an Efficient Catalyst for Converting CO2 into Cyclic Carbonates under Mild Conditions" Catalysts 9, no. 11: 951. https://doi.org/10.3390/catal9110951
APA StyleFan, W., Wang, W.-Z., Wang, L., Jia, X.-G., Li, L.-L., Xiao, T.-C., & Edwards, P. P. (2019). Novel Cobalt Complex as an Efficient Catalyst for Converting CO2 into Cyclic Carbonates under Mild Conditions. Catalysts, 9(11), 951. https://doi.org/10.3390/catal9110951






