Size-Controlled Synthesis of Pt Particles on TiO2 Surface: Physicochemical Characteristic and Photocatalytic Activity
Abstract
:1. Introduction
2. Results
2.1. Structural, Textural and Surface Characteristic of TiO2 Photocatalysts
2.2. Morphology of Pt-TiO2 Photocatalysts Obtained by the Wet-Impregnation and Microemulsion Method (Me)
2.3. Photocatalytic Activity and Property-Activity Correlations for Pt-TiO2 Samples
3. Discussion
4. Materials and Methods
4.1. Preparation of TiO2 and Pt-TiO2 Photocatalysts
4.2. Preparation of Pt-TiO2 Photocatalysts
4.3. Characterization of Photocatalysts
4.4. Measurements of Photocatalytic Activity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tryba, B. Immobilization of TiO2 and Fe-C-TiO2 photocatalysts on the cotton material for application in a flow photocatalytic reactor for decomposition of phenol in water. J. Hazard. Mater. 2008, 151, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Choi, W.; Kim, S.H.; Hong, S.B. Selective photocatalytic degradation of aquatic pollutants by titania encapsulated into FAU-type zeolites. J. Hazard. Mater. 2011, 188, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Benoit-Marquié, F.; Wilkenhöner, U.; Simon, V.; Braun, A.M.; Oliveros, E.; Maurette, M.T. VOC photodegradation at the gas–solid interface of a TiO2 photocatalyst Part I: 1-butanol and 1-butylamine. J. Photochem. Photobiol. A Chem. 2000, 132, 225–232. [Google Scholar] [CrossRef]
- Lu, N.; Yu, H.T.; Su, Y.; Wu, Y. Water absorption and photocatalytic activity of TiO2 in a scrubber system for odor control at varying pH. Sep. Purif. Technol. 2012, 90, 196–203. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Wei, Z.; Wysocka, I.; Szweda, P.; Kowalska, E. The effect of nanoparticles size on photocatalytic and antimicrobial properties of Ag-Pt/TiO2 photocatalysts. Appl. Surf. Sci. 2015, 353, 317–325. [Google Scholar] [CrossRef]
- Folli, A.; Pade, C.; Hansen, T.B.; de Marco, T.; Macphee, D.E. TiO2 photocatalysis in cementitious systems: Insights into self-cleaning and depollution chemistry. Cem. Concr. Res. 2012, 42, 539–548. [Google Scholar] [CrossRef]
- Zhu, M.; Han, M.; Zhu, C.; Hu, L.; Huang, H.; Liu, Y.; Kang, Z. Strong coupling effect at the interface of cobalt phosphate-carbon dots boost photocatalytic water splitting. J. Colloid Interface Sci. 2018, 530, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Razalia, M.H.; Yusoff, M. Highly efficient CuO loaded TiO2 nanotube photocatalyst for CO2 photoconversion. Mater. Lett. 2018, 221, 168–171. [Google Scholar] [CrossRef]
- Sugishita, N.; Kuroda, Y.; Ohtani, B. Preparation of decahedral anatase titania particles with high-level photocatalytic activity. Catal. Today 2011, 164, 391–394. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Decahedral-shaped anatase titania photocatalyst particles: Synthesis in a newly developed coaxial-flow gas-phase reactor. Chem. Eng. J. 2016, 289, 502–512. [Google Scholar] [CrossRef] [Green Version]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Jurek, A.; Klein, M.; Hupka, J. Enhanced visible light photocatalytic activity of Pt/I-TiO2 in a slurry system and supported on glass packing. Sep. Purif. Technol. 2017, 189, 246–252. [Google Scholar] [CrossRef]
- Roberts, P.H.; Thomas, K.V. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, S.W.; Keulemans, M.; Goris, B.; Blommaerts, N.; Bals, S.; Martens, J.A.; Lenaerts, S. Plasmonic ‘rainbow’ photocatalyst with broadband solar light response for environmental applications. Appl. Catal. B Environ. 2016, 188, 147–153. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Endo, M.; Colbeau-Justin, C.; Ohtani, B.; Kowalska, E. Silver-modified octahedral anatase particles as plasmonic photocatalyst. Catal. Today 2018, 310, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Nahar, S.; Hasan, M.R.; Kadhum, A.A.H.; Hasan, H.A.; Zain, M.F.M. Photocatalytic degradation of organic pollutants over visible light active plasmonic Ag nanoparticle loaded Ag2SO3 photocatalysts. J. Photochem. Photobiol. A Chem. 2019, 375, 191–200. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A. Progress, challenge and perspective of bimetallic TiO2-based photocatalysts. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Chen, H.W.; Ku, Y.; Kuo, Y.L. Effect of Pt/TiO2 characteristics on temporal behavior of o-cresol decomposition by visible light-induced photocatalysis. Water Res. 2007, 41, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Jurek, A.; Hupka, J. Preparation and characterization of Pt/Pd-modified titanium dioxide nanoparticles for visible light irradiation. Catal. Today 2013, 230, 181–187. [Google Scholar] [CrossRef]
- Litke, A.; Frei, H.; Hensen, E.J.M.; Hofmann, J.P. Interfacial charge transfer in Pt-loaded TiO2 P25 photocatalysts studied by in-situ diffuse reflectance FTIR spectroscopy of adsorbed CO. J. Photochem. Photobiol. A Chem. 2019, 370, 84–88. [Google Scholar] [CrossRef]
- Xiong, Z.; Lei, Z.; Chen, X.; Gong, B.; Zhao, Y.; Zhang, J.; Zheng, C.; Wu, J.C.S. CO2 photocatalytic reduction over Pt deposited TiO2 nanocrystals with coexposed {101} and {001} facets: Effect of deposition method and Pt precursors. Catal. Commun. 2017, 96, 1–5. [Google Scholar] [CrossRef]
- Sun, B.; Vorontsov, A.V.; Smirniotis, P.G. Role of platinum deposited on TiO2 in phenol photocatalytic oxidation. Langmuir 2003, 19, 3151–3156. [Google Scholar] [CrossRef]
- Wu, S.; Tan, X.; Liu, K.; Lei, J.; Wang, L.; Zhang, J. TiO2 (B) nanotubes with ultrathin shell for highly efficient photocatalytic fixation of nitrogeni. Catal. Today 2018, 335, 214–220. [Google Scholar] [CrossRef]
- Kowalska, E.; Wei, Z.; Karabiyik, B.; Herissan, A.; Janczarek, M.; Endo, M.; Markowska-Szczupak, A.; Remita, H.; Ohtani, B. Silver-modified titania with enhanced photocatalytic and antimicrobial properties under UV and visible light irradiation. Catal. Today 2015, 252, 136–142. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Poyraz, A.S.; Kuo, C.H.; Miao, R.; Meng, Y.; Chen, S.Y.; Jiang, T.; Wenos, C.; Suib, S.L. Crystalline mixed phase (anatase/rutile) mesoporous titanium dioxides for visible light photocatalytic activity. Chem. Mater. 2015, 27, 6–17. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Andino, J.M.; Li, Y. Bicrystalline TiO2 with controllable anatase-brookite phase content for enhanced CO2 photoreduction to fuels. J. Mater. Chem. A 2013, 1, 8209–8216. [Google Scholar] [CrossRef]
- Kitchens, C.L.; McLeod, M.C.; Roberts, C.B. Chloride ion effects on synthesis and directed assembly of copper nanoparticles in liquid and compressed alkane microemulsions. Langmuir 2005, 21, 5166–5173. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, C.J.; Ge, Q. Effect of surface oxygen vacancy on Pt cluster adsorption and growth on the defective anatase TiO2 (101) surface. J. Phys. Chem. C 2007, 111, 16397–16404. [Google Scholar] [CrossRef]
- Cueto, M.; Piedrahita, M.; Caro, C.; Martínez-Haya, B.; Sanz, M.; Oujja, M.; Castillejo, M. Platinum nanoparticles as photoactive substrates for mass spectrometry and spectroscopy sensors. J. Phys. Chem. C 2014, 118, 11432–11439. [Google Scholar] [CrossRef]
- Jung, S.; Shuford, K.L.; Park, S. Optical property of a colloidal solution of platinum and palladium nanorods: Localized surface plasmon resonance. J. Phys. Chem. C 2011, 115, 19049–19053. [Google Scholar] [CrossRef]
- Kowalska, E.; Abea, R.; Ohtani, B. Visible light-induced photocatalytic reaction of gold-modified titanium(IV) oxide particles: Action spectrum analysis. Chem. Commun. 2009, 2, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Galhenage, R.P.; Yan, H.; Tenney, S.A.; Park, N.; Henkelman, G.; Albrecht, P.; Mullins, D.R.; Chen, D.A. Understanding the nucleation and growth of metals on TiO2: Co compared to Au, Ni, and Pt. J. Phys. Chem. C 2013, 117, 7191–7201. [Google Scholar] [CrossRef]
- Campbell, C.T. Ultrathin metal films and particles on oxide surfaces: Structural, electronic and chemisorptive properties. Surf. Sci. Rep. 1997, 27, 1–111. [Google Scholar] [CrossRef]


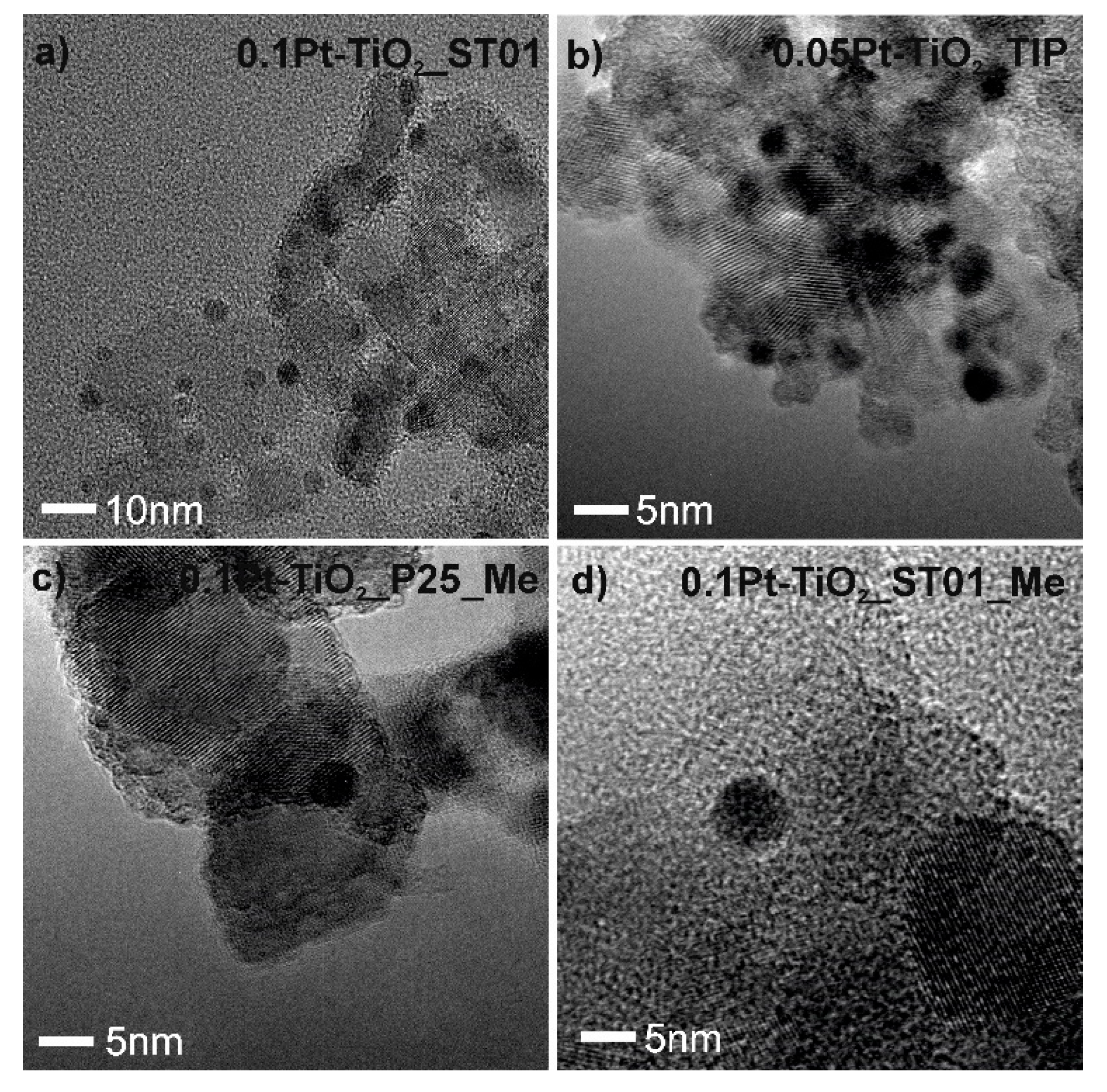
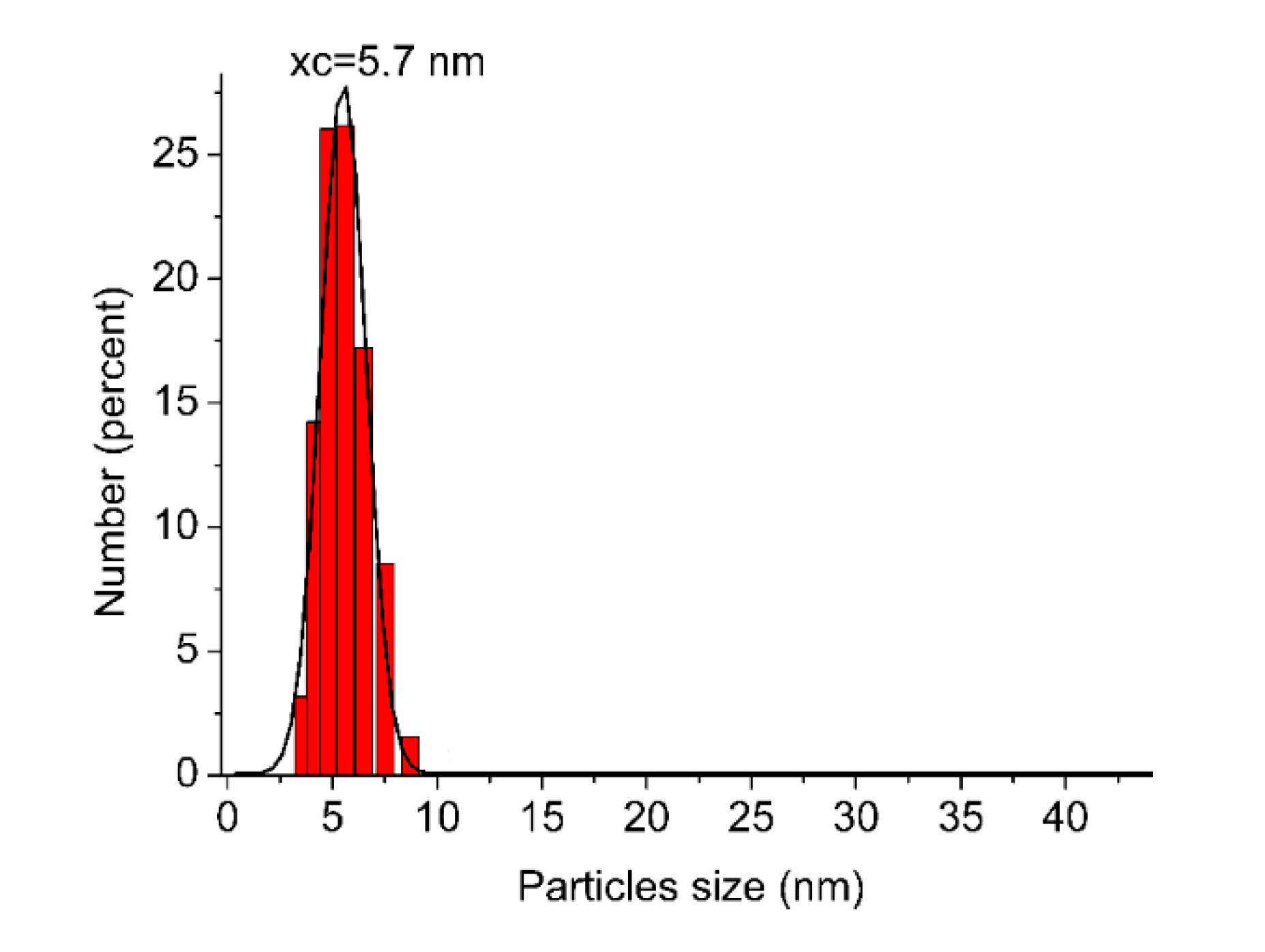

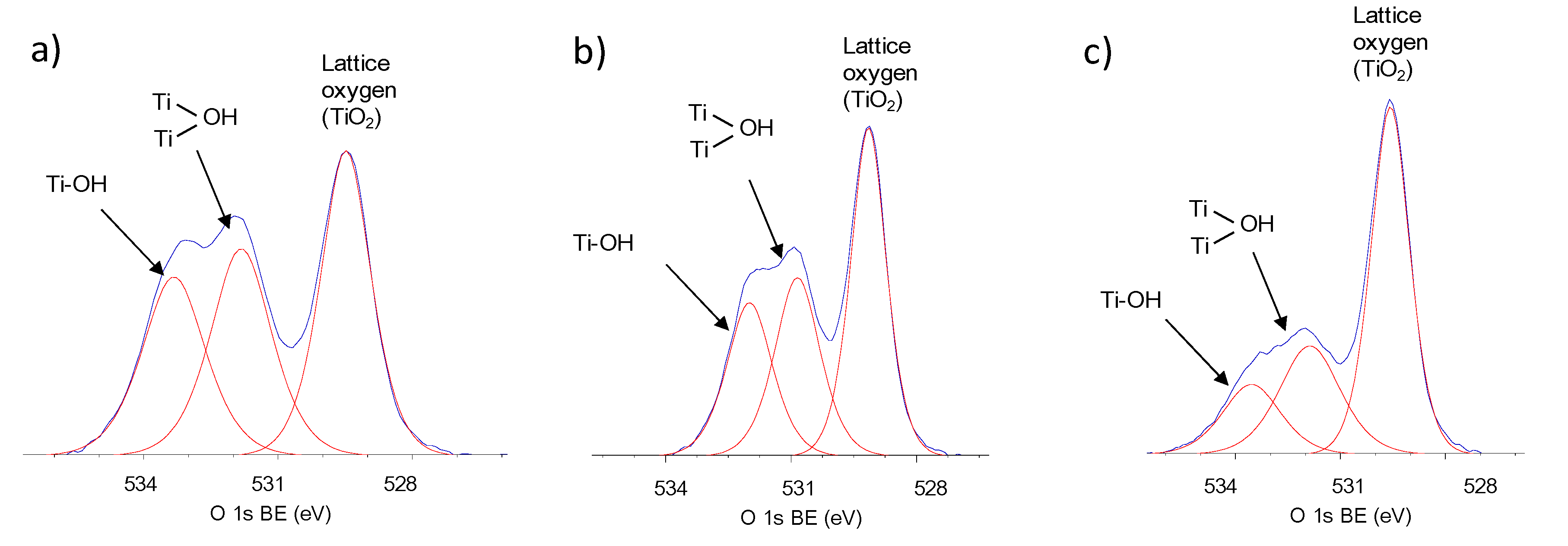
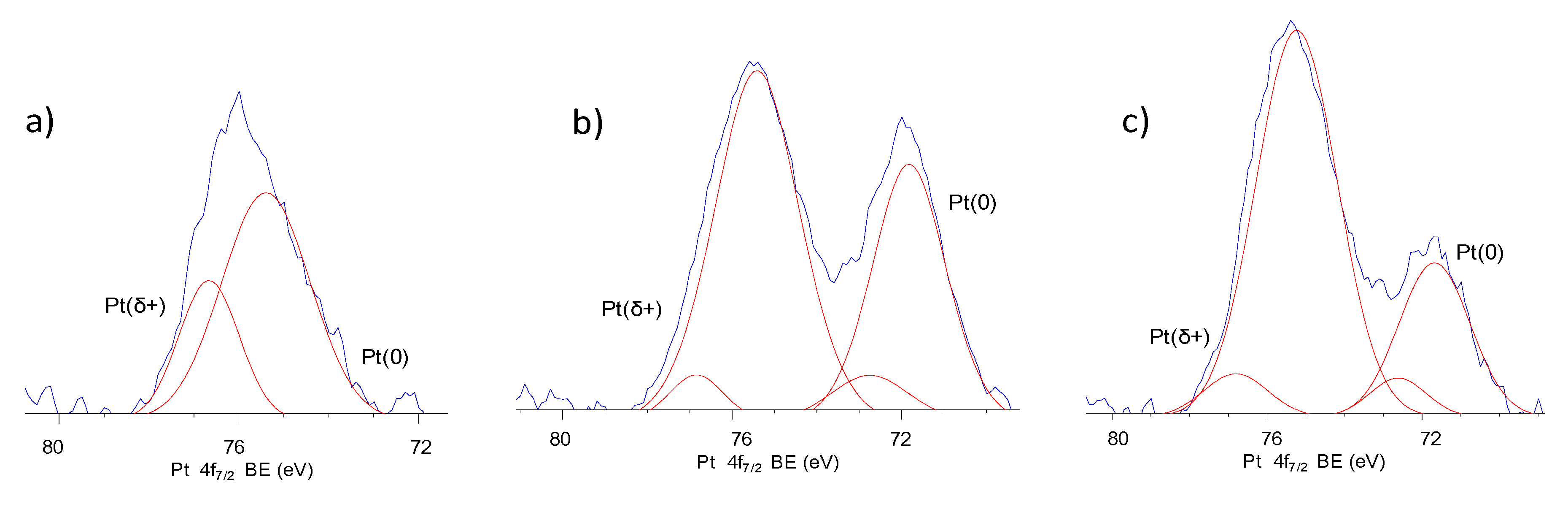
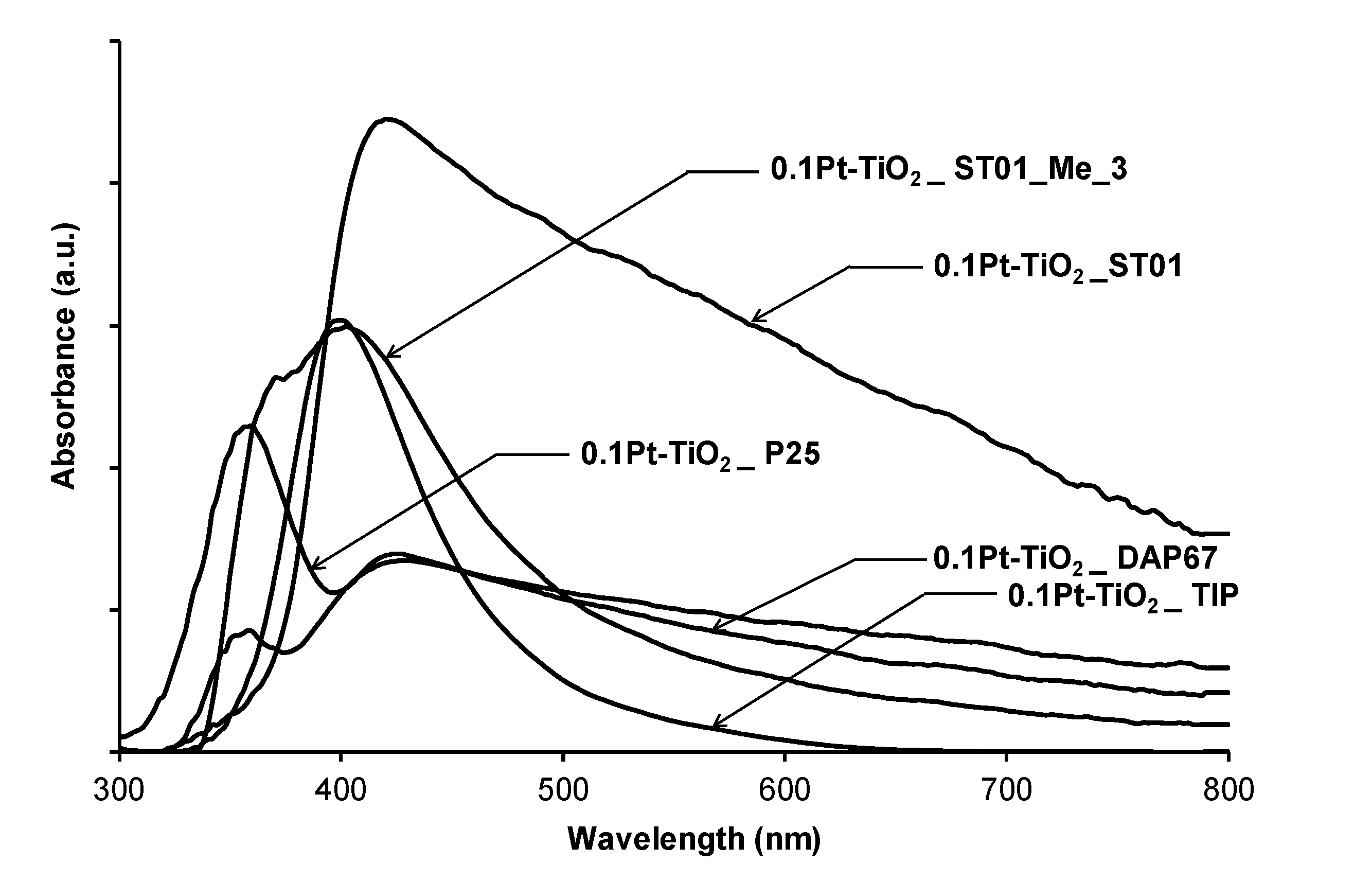
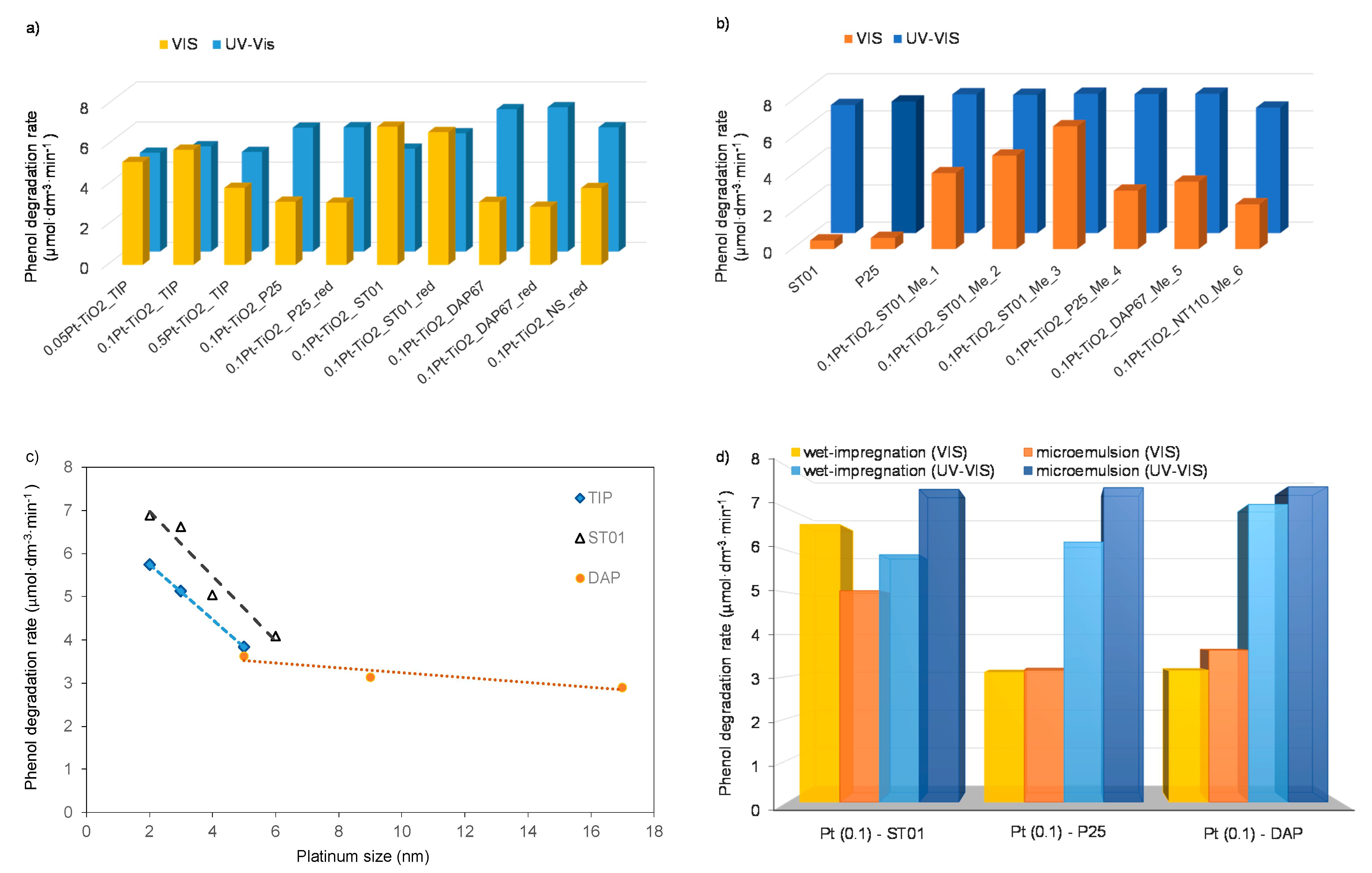
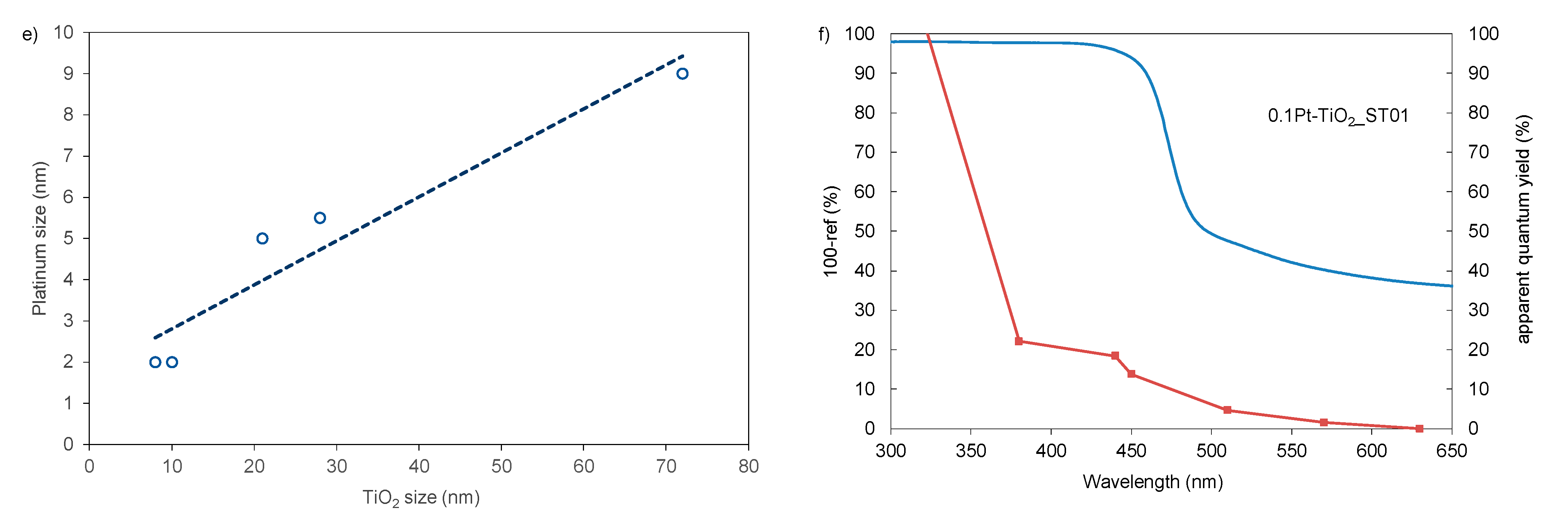
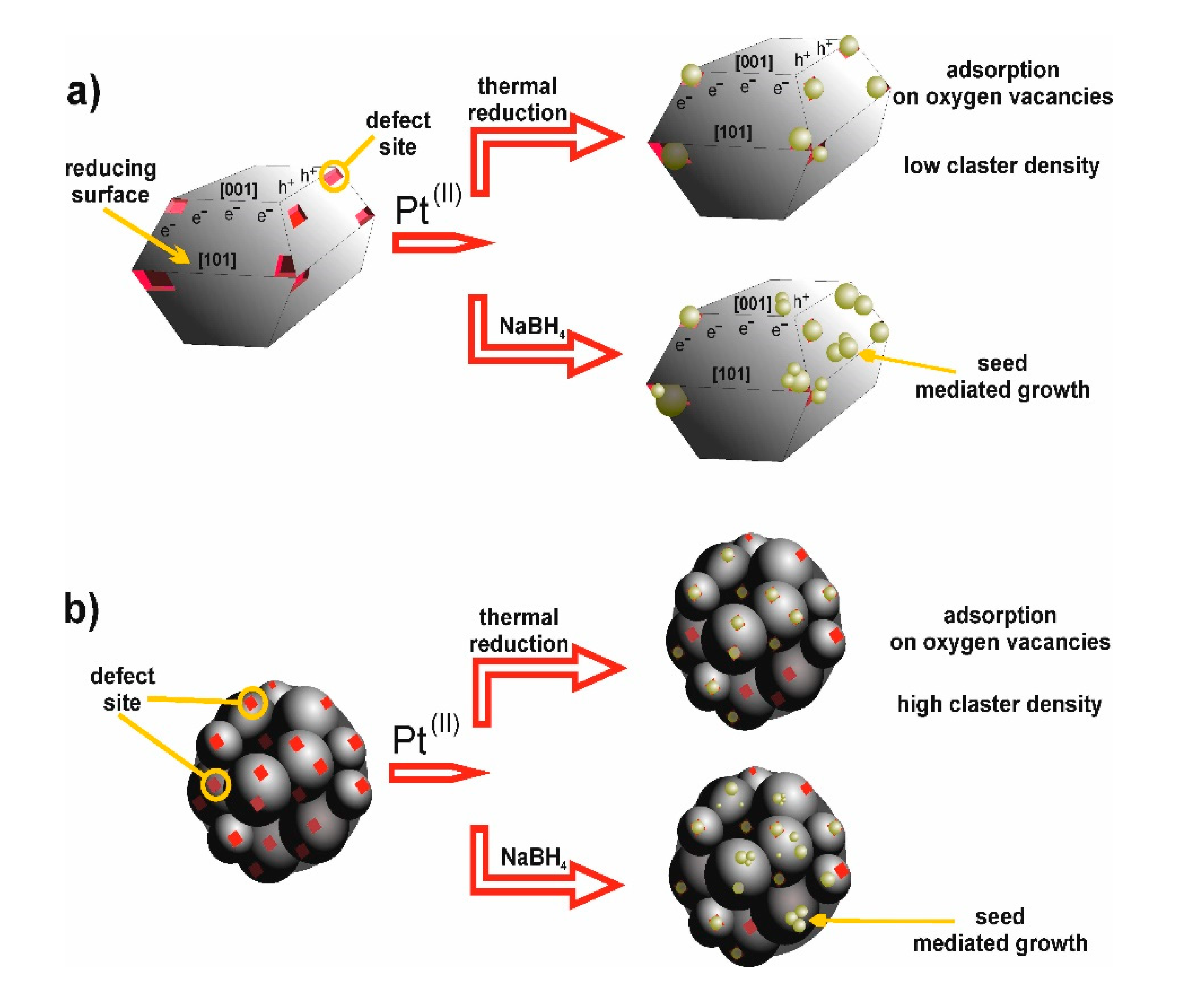
| Sample No. | TiO2 Source | Crystallinity (%) | Crystallite Size (nm) | BET (m2·g−1) | Phenol Degradation Rate (μmol·dm−3·min−1) | |
|---|---|---|---|---|---|---|
| a | r | |||||
| TiO2_TIP | TIP | 79 | 10 | - | 81 | 4.29 |
| TiO2_TBT | TBT | 85 | 6 | - | 190 | 6.80 |
| TiO2_P25 | P25 | 87 | 28 | 33 | 55 | 6.75 |
| TiO2_ST01 | ST01 | 93 | 8 | - | 250 | 6.12 |
| * TiO2_NT_350 | P25 | 67 | 11 | 4 | 375 | 5.79 |
| TiO2_NT_450 | P25 | 77 | 11 | 2 | 375 | 5.87 |
| TiO2_NT_550 | P25 | 81 | 13 | 12 | 210 | 5.82 |
| ** TiO2_NS | TIP | 81 | 5 | - | 290 | 5.20 |
| *** TiO2_DAP67 | TiCl4 | 94 | 67 | - | 16 | 7.82 |
| TiO2_DAP55 | TiCl4 | n.a. | 55 | - | 20 | 6.43 |
| TiO2_DAP10 | TiCl4 | n.a. | 10 | - | 180 | 6.33 |
| Photocatalyst | TiO2 Source | Amount of Platinum Precursor | Reducing Agent | Crystallite Size (nm) | Phenol Degradation Rate (μmol·dm−3·min−1) | |||
|---|---|---|---|---|---|---|---|---|
| Anatase (101) | Rutile (110) | Pt (111) | Under UV-Vis | Under Vis λ > 420 nm | ||||
| 0.05Pt-TiO2_TIP | TIP | 0.05 | temperature | 7 | - | 3 | 4.95 | 5.13 |
| 0.1Pt-TiO2_TIP | TIP | 0.1 | temperature | 8 | - | 2 | 5.25 | 5.74 |
| 0.5Pt-TiO2_TIP | TIP | 0.5 | temperature | 8 | - | 5 | 4.97 | 3.84 |
| 0.1Pt-TiO2_P25 | P25 | 0.1 | temperature | 21.5 | 29 | 5 | 6.18 | 3.15 |
| 0.1Pt-TiO2_P25_red | P25 | 0.1 | NaBH4 | 20 | 33 | 6 | 6.20 | 3.10 |
| 0.1Pt-TiO2_ST01 | ST01 | 0.1 | temperature | 10 | - | 2 | 5.13 | 6.89 |
| 0.1Pt-TiO2_ST01_red | ST01 | 0.1 | NaBH4 | 14 | - | 3 | 5.90 | 6.62 |
| 0.1Pt-TiO2_DAP67 | DAP * | 0.1 | temperature | 72 | - | 9 | 7.10 | 3.14 |
| 0.1Pt-TiO2_DAP67_red | DAP * | 0.1 | NaBH4 | 72 | - | 17 | 7.20 | 2.90 |
| 0.1Pt-TiO2_NS_red | sphere | 0.1 | NaBH4 | 28 | 5.5 | 6.20 | 3.83 | |
| Photocatalyst | TiO2 Source | Crystallite Size (nm) | Phenol Degradation Rate (μmol·dm−3·min−1) | |||
|---|---|---|---|---|---|---|
| Anatase (101) | Rutile (110) | Pt (111) | Under UV-Vis | Under Vis λ > 420 nm | ||
| 0.1Pt-TiO2_ST01_Me_1 | ST01 | 12 | - | 6 | 7.49 | 4.09 |
| 0.1Pt-TiO2_ST01_Me_2 | ST01 | 13 | - | 4 | 7.46 | 5.04 |
| * 0.1Pt-TiO2_ST01_Me_3 | ST01 | 13 | - | 3 | 7.52 | 6.62 |
| 0.1Pt-TiO2_P25_Me_4 | P25 | 20 | 29 | 5 | 7.50 | 3.15 |
| 0.1Pt-TiO2_DAP67_Me_5 | DAP67 | 91 | 158 | 5 | 7.52 | 3.63 |
| 0.1Pt-TiO2_NT110_Me_6 | NT110 | 15 | - | 6.5 | 6.77 | 2.41 |
| Sample Labeling | TiO2 Source | Ti 2p3/2 (%) | Content (at.%) | Ratio | |||
|---|---|---|---|---|---|---|---|
| Ti4+ | Ti3+ | Ti | O | Pt | O/Ti | ||
| 0.1Pt-TiO2_ST01 | ST01 | 98.4 | 1.6 | 12.8 | 87.0 | 0.2 | 6.8 |
| 0.1Pt-TiO2_ST01_Me_3 | ST01 | 98.6 | 1.4 | 13.3 | 87.6 | 0.1 | 6.5 |
| 0.1Pt-TiO2_P25_Me_4 | P25 | 99.4 | 0.6 | 20.7 | 79.1 | 0.2 | 3.8 |
| 0.1Pt-TiO2_DAP67 | DAP | 99.1 | 0.9 | 26.7 | 73.0 | 0.3 | 2.7 |
| 0.1Pt-TiO2_DAP67_Me_5 | DAP | 99.0 | 1.0 | 27.9 | 71.7 | 0.4 | 2.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska-Jurek, A.; Wei, Z.; Janczarek, M.; Wysocka, I.; Kowalska, E. Size-Controlled Synthesis of Pt Particles on TiO2 Surface: Physicochemical Characteristic and Photocatalytic Activity. Catalysts 2019, 9, 940. https://doi.org/10.3390/catal9110940
Zielińska-Jurek A, Wei Z, Janczarek M, Wysocka I, Kowalska E. Size-Controlled Synthesis of Pt Particles on TiO2 Surface: Physicochemical Characteristic and Photocatalytic Activity. Catalysts. 2019; 9(11):940. https://doi.org/10.3390/catal9110940
Chicago/Turabian StyleZielińska-Jurek, Anna, Zhishun Wei, Marcin Janczarek, Izabela Wysocka, and Ewa Kowalska. 2019. "Size-Controlled Synthesis of Pt Particles on TiO2 Surface: Physicochemical Characteristic and Photocatalytic Activity" Catalysts 9, no. 11: 940. https://doi.org/10.3390/catal9110940






