Abstract
In recent years, defective TiO2-based composite nanomaterials have received much attention in the field of photocatalysis. In this work, TiB2 was used as a precursor to successfully prepare Ti3+ defective TiO2 (TiO2-B) with a truncated bipyramidal structure by a one-step method. Then, the SnS2 nanosheets were assembled onto the as-prepared TiO2-B through simple hydrothermal reaction. TiO2-B exhibits strong visible light absorption properties, but the recombination rate of the photo-generated electron-hole pair was high and does not exhibit ideal photocatalytic performance. Upon introducing SnS2, the heterojunction catalyst SnS2-Ti3+ defective TiO2 (SnS2/TiO2-B) not only possesses the strong light absorption from UV to visible light region, the lowest photo-generated charge recombination rate but also achieves a more negative conduction band potential than the reduction potential of CO2 to CO, and thereby, exhibits the significantly enhanced selectivity and yield of CO in photocatalytic CO2 reduction. Notably, SnS2/TiO2-B produces CO at a rate of 58 µmol·h−1·g−1 with CO selectivity of 96.3% under visible light irradiation, which is 2 and 19 times greater than those of alone TiO2-B and SnS2, respectively. Finally, a plausible photocatalytic mechanism on SnS2/TiO2-B was proposed that the electron transfer between TiO2 and SnS2 follows the Z-scheme mode. Our results present an effective way to gain highly efficient TiO2 based photocatalysts for CO2 reduction by combining different modification methods of TiO2 and make full use of the synergistic effects.
1. Introduction
In modern society, the energy crisis and global warming have been two serious issues for human beings to face. As it is known, most of the energy demands are fulfilled through the combustion of fossil fuels, and then the excessive utilization of fossil fuels cause the ever-increasing emission of greenhouse gas CO2, a major contributor to global warming [1,2]. As one of the sustainable “green” approaches towards solving above issues, photocatalytic reduction of CO2 into high value-added chemicals has remarkable advantages in terms of utilizing renewable energy and reducing CO2 emissions simultaneously [3].
Titanium dioxide (TiO2), as a typical semiconductor, has gained lots of interest due to its environmental friendliness, low cost, resistance to light corrosion, and high stability [4,5,6]. However, it has drawbacks in terms of the ineffective utilization of visible light and low quantum efficiency due to its wide band gap (3.2 eV) and high recombination of photo-generated electron-hole pairs [7]. Therefore, various strategies have been explored to boost the efficiency of TiO2 for photocatalytic CO2 reduction, including controlling TiO2 crystals facets [8], introducing oxygen vacancies and Ti3+ defects [9] and building heterostructures with other semiconductors [10]. From the previous studies, it has been found that single modification route can only improve a specific property of TiO2, which is far from the requirements of a photocatalyst for achieving the efficient and selective reduction of CO2. Therefore, it could be an effective way to fabricate highly efficient TiO2 based photocatalysts by combining different modification methods to make full use of the synergistic effects.
As for the advantage of the faceted TiO2, it is generally accepted that different TiO2 facets have quite different surface adsorption properties and surface electronic structures, which determine the adsorption of CO2 and H2O, the light absorption and redox potentials of the excited charges, in turn affecting the photocatalytic activity. It has been reported that the photocatalytic activities for CO2 reduction on different anatase TiO2 facets are in the order of {010} > {101} > {001} facets [8,11]. In addition, the faceted TiO2 with co-exposed facets of {101} and {001} could favor the separation and transfer of the photo-generated charge carriers due to the heterojunction between different facets [12]. However, the photocatalytic activities of the faceted TiO2 are still too low, especially under visible light irradiation. As thus, most studies focus on the further modifications of the faceted TiO2 to enhance the visible light absorption and promote photo-generated charge separation. One useful way is the introduction of oxygen vacancies and the corresponding Ti3+ ions into TiO2 [13,14,15]. As reported, the remarkably enhanced and selective photoreduction of CO2 could be obtained on an oxygen-deficient TiO2 with co-exposed {101} and {001} facets [16]. Moreover, except the ability to improve visible light absorption, oxygen vacancies could enhance the chemisorption of CO2 and further mediate the electron transfer to the adsorbed CO2 to form the key mediate CO2, which is the initial and crucial step in CO2 reduction, but unfavorable on the perfect TiO2 surfaces [17]. Another way to modify the faceted TiO2 is to construct a TiO2-semiconductor heterojunction, especially with a semiconductor with strong visible light absorption, which could act as a photosensitizer to promote visible-light-driven photocatalytic CO2 reduction [18,19]. Based on the individual role of each modification method, it could be expected that a TiO2-based photocatalyst with features of co-exposed {101} and {001} facets, oxygen vacancies and a heterojunction with a narrow band gap semiconductor, demonstrates a significantly high activity and selectivity towards visible-light-driven photocatalytic CO2 reduction.
Regarding the preparation of defective TiO2 materials, post-processes are usually applied through hydrogen thermal treatment or chemical reduction with NaHB4 [20,21]. Recently, a facile one-step method was reported to synthesize defective anatase TiO2 with co-exposed {101} and {001} facets using TiB2 as a precursor, which exhibited the strong visible light absorption due to its large amount of Ti3+ defects [22,23]. Besides, in respect of the combination of a semiconductor, two-dimensional tin disulfide (SnS2) nanosheets are a good candidate because of its narrow band gap, easy synthesis, and easy formation of heterojunctions with other materials [24,25,26]. Zhang et al. reported that a hierarchical nanostructured SnS2/TiO2 photocatalyst exhibited excellent visible-light photocatalytic activity in degradation of organic dyes due to the photo-synergistic effects [27].
In our present work, we report a facile approach to synthesize a heterojunction catalyst combing the Ti3+ defective TiO2 nanoplates and SnS2 nanosheets for enhancing the photocatalytic activity and selectivity towards CO2 reduction. Firstly, TiB2 was used as a precursor to successfully prepare Ti3+ defective TiO2 with a truncated bi-pyramidal structure by a one-step method. Then, the SnS2 nanosheets were assembled onto the as-prepared TiO2 through simple hydrothermal reaction. In the photocatalytic CO2 reduction under visible light irradiation, the as-prepared heterojunction catalyst SnS2-Ti3+ defective TiO2 (SnS2/TiO2-B) exhibited superior photocatalytic activity and selectivity to CO compared with catalysts Ti3+ defective TiO2 (TiO2-B), SnS2, and SnS2-TiO2 without Ti3+ defects (SnS2/TiO2-W). The excellent photocatalytic performance of SnS2/TiO2-B is ascribed to its enhanced absorption of visible light, the reduced recombination rate of photo-generated charge carriers and more negative conduction band potential than the reduction potential of CO2 to CO. Our work demonstrates that it is effective to gain highly efficient TiO2 based photocatalysts for CO2 reduction through combining different modification of faceted TiO2 and make full use of the synergistic effects.
2. Results and Discussion
2.1. Synthesis and Characterization
The preparation route of SnS2/TiO2-B is illustrated in Figure 1. A certain amount of HF aqueous solution is mixed with the sonicated TiB2 suspension. Then a hydrothermal reaction of the mixture is conducted in a 100 mL Teflon-lined autoclave at 180 °C for 12 h. After the reaction, the dark blue Ti3+ defective TiO2 (TiO2-B) is obtained. Then TiO2-B is mixed with an aqueous solution of Poly (sodium 4-styrenesulfonate) (PSS) for 20 min. The treated TiO2-B is then dispersed in deionized water in a 100 mL Teflon tube. After adding an appropriate amount of SnCI4·5H2O, thioacetamide (TAA) and acetic acid, the hydrothermal reaction is performed at 160 °C for 12 h. Finally, a heterojunction photocatalyst SnS2/TiO2-B is obtained.
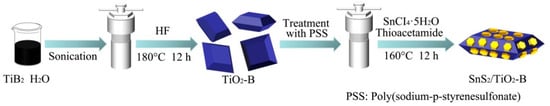
Figure 1.
Schematic illustration for the preparation of SnS2/TiO2-B.
The X-ray diffraction (XRD) patterns of SnS2, TiO2-B, and SnS2/TiO2-B are shown in Figure 2. The XRD peaks of SnS2 and TiO2-B are indexed to pure hexagonal phase SnS2 (berndtite-2H, JCPDS No. 01-089-2358) and tetragonal phase TiO2 (anatase, JCPDS No. 01-078-2486), respectively [28]. The sample SnS2/TiO2-B shows the characteristic peaks of both TiO2-B and SnS2, demonstrating that SnS2 is successfully assembled onto the TiO2-B. However, the peak intensities corresponding to SnS2 are very weak, which is ascribed to the low content of SnS2 in SnS2/TiO2-B (7.6 wt.% based on the elemental mapping result) [29]. Besides, the control samples TiO2-W, SnS2/TiO2-W obtained through calcination treatment of TiO2-B, SnS2/TiO2-B in muffle are also characterized and the results are shown in Figure S1. The peaks of TiO2-W are identical to that of TiO2-B, indicating that calcination treatment does not change the crystal structure. SnS2/TiO2-W also contains peaks of TiO2-W and SnS2, indicating the successful combination of SnS2 and TiO2-W.
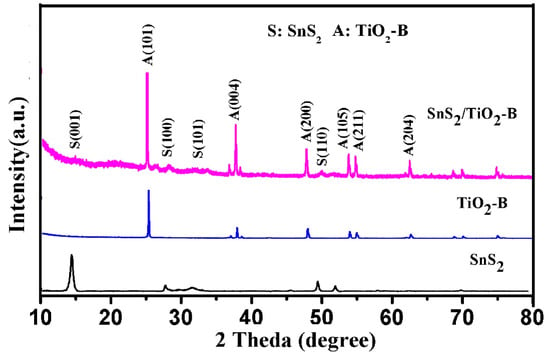
Figure 2.
XRD patterns of SnS2/TiO2-B, TiO2-B, and SnS2.
Figure 3 shows the SEM images of the as-prepared samples TiO2-B, TiO2-W, SnS2/TiO2-B, and SnS2/TiO2-W. From Figure 3a, it can be seen that TiO2-B presents a nanoplate structure with a size of about 500 to 700 nm wide and a thickness of about 80 nm. In addition, the enlarged SEM image (Figure S2a) of TiO2-B crystals reveals a truncated bipyramidal structure, in which there are the co-exposed {001} and {101} facets according to the literature [22,30,31,32]. Figure 3b shows the SEM image of TiO2-W. It indicates that the nanoplate morphology remains substantially intact after calcination except some flakes forming on the surface. Besides, the optical photographs of TiO2-B before and after calcination are compared as shown in Figure S2, a significant color change from black blue to white can be observed after calcination in an oxygen atmosphere, meaning that TiO2-W could hardly absorb visible light due to the absence of Ti3+ defects [13]. In addition, it is found that the mass of TiO2-W increased by about 5 wt.% compared with that of TiO2-B, indicating the oxidation reaction of the defective TiO2-B, which could be as evidence that TiO2-B possesses the oxygen vacancy due to partial reduced state Ti ions. After loading SnS2 (Figure 3c,d), both SnS2/TiO2-B and SnS2/TiO2-W present the coating layers consisting of numerous nanosheets with a size of approximately 70 to 100 nm, indicating the successful assembling of SnS2 on TiO2-B and TiO2-W, respectively.
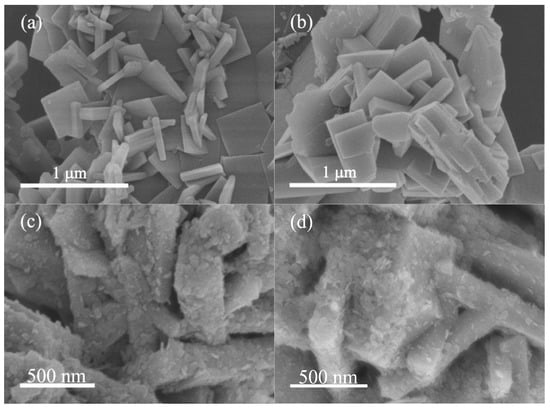
Figure 3.
(a) SEM images of TiO2-B, (b) TiO2-W, (c) SnS2/TiO2-B, (d) SnS2/TiO2-W.
Figure 4a shows the typical TEM images of SnS2/TiO2-B. From Figure 4a, it can be seen that SnS2 nanosheets grow flatly or vertically on the TiO2-B the surface. Furthermore, a high-resolution TEM image of SnS2 nanosheets (Figure 4b) displays the inter-planar distance of 0.31 nm, which is consistent with that of SnS2 {100} plane [33]. The selected area electron diffraction (SEAD) pattern (Figure 4c) reveals the presence of both TiO2 and SnS2 crystals in the heterostructure SnS2/TiO2-B, and a single-crystalline TiO2-B with co-exposed {101} and {001} facets [12,27,34]. In addition, the magnified HRTEM image (Figure 4d) of the interface region clearly displays the interplanar distances of 0.35 and 0.59 nm, corresponding to the {101} plane of TiO2 and the {001} plane of SnS2, respectively. What’s more, the TEM elemental mapping of SnS2/TiO2-B has been performed to identify the spatial distributions of elements Ti, O, Sn and S, as shown in Figure 4e–i. It is indicated that the elements Sn, S evenly distribute on a support consisting of densely distributed Ti, O elements. The above results clearly demonstrate that the TiO2-B nanoplate surfaces are coated by SnS2 and the interface heterojunction forms between TiO2-B and SnS2. The tight heterojunction structure of SnS2/TiO2-B would benefit the interfacial charge transfer spatially, and thereby increase the photocatalytic activity [35].
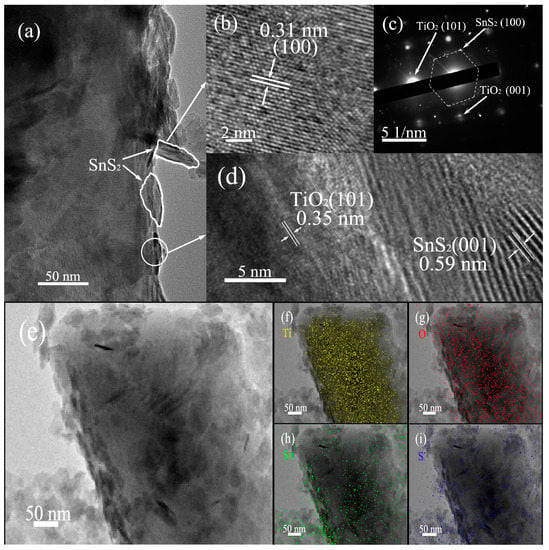
Figure 4.
(a) TEM image of a SnS2/TiO2-B nanoplate, (b) HRTEM image of SnS2 nanosheets and (c) The corresponding SAED pattern, (d) HRTEM image at interface region of SnS2/TiO2-B, (e–i) The elemental mapping images of SnS2/TiO2-B.
The surface compositions and chemical states of the as-prepared samples have been investigated by XPS. As seen in Figure S3a,b, the full spectra of TiO2-B and SnS2/TiO2-B indicate the presence of elements Ti and O, while SnS2/TiO2-B also displays the existence of S and Sn elements, which are consistent with the above elemental mapping results. Figure 5a shows the comparison of the Ti 2p core-level spectra of samples TiO2-B, TiO2-W, SnS2/TiO2-W and SnS2/TiO2-B. For samples TiO2-B and TiO2-W, two peaks at 458.9 and 464.5 eV are assigned to Ti (IV) 2p3/2 and Ti (IV) 2p1/2, and another two at 458.2 and 463.9 eV are assigned to Ti (III) 2p3/2 and Ti (III) 2p1/2, respectively [35,36]. Obviously, the intensities of two Ti (III) 2p peaks decrease significantly for sample TiO2-W, indicating that most of Ti3+ ions in TiO2-B are oxidized to Ti4+ ions during calcination. Therefore, it is reasonable to use TiO2-W as a control sample without Ti3+ defects, and then to explore the role of Ti3+defects in TiO2-B for photocatalytic reduction of CO2. Furthermore, as for SnS2/TiO2-B and SnS2/TiO2-W, the four peaks of Ti 2p all shift to higher binding energy (BE), and the positive shift is even significant for SnS2/TiO2-B, which demonstrates stronger interface interaction between TiO2-B and SnS2. Figure 5b displays the O 1s core-level spectra for SnS2/TiO2-B and SnS2/TiO2-W. The three peaks at the binding energy of 530.4, 531.5 and 532.8 eV are attributed to the Ti−O bond, Ti-OH group and the oxygen vacancy (OVS) in the vicinity of Ti3+, respectively [35]. Notably, SnS2/TiO2-B exhibits higher peak intensity corresponding to OVS than that of SnS2/TiO2-W, indicating more oxygen vacancies exist in SnS2/TiO2-B. This is consistent with the above analysis for Ti 2p spectra. In respect of the spectra of Sn 3d shown in Figure 5c, both pure SnS2 and SnS2/TiO2-B show two peaks corresponding to the Sn 3d5/2 and Sn 3d3/2 of Sn(IV) [27,37,38]. Besides, the S 2p core-level spectra of single SnS2 and SnS2/TiO2-B are given in Figure 5d. It can be seen that the S 2p3/2 and S 2p1/2 peaks for SnS2 are centered at around 161.3 and 162.5 eV, respectively, which agree with the reference values of S–Sn bond in SnS2 [37]. In contrast, it is observed that binding energies of S 2p3/2 and S 2p1/2 of SnS2/TiO2-B display the negative shift of 0.3 eV comparing with that of pure SnS2, which might be caused by the interface interaction between TiO2-B and SnS2. Based on the above XPS results, it is clearly demonstrated that TiO2-B prepared by TiB2 possesses a large number of the reduced states Ti (III) along with oxygen vacancies, which are maintained well in the composite SnS2/TiO2-B. In addition, for SnS2/TiO2-B, the strong electronic interaction between TiO2-B and SnS2 due to the formation of heterojunction interfaces, could provide an effective channel for the separation and transfer of charge carriers [27,28,39,40].
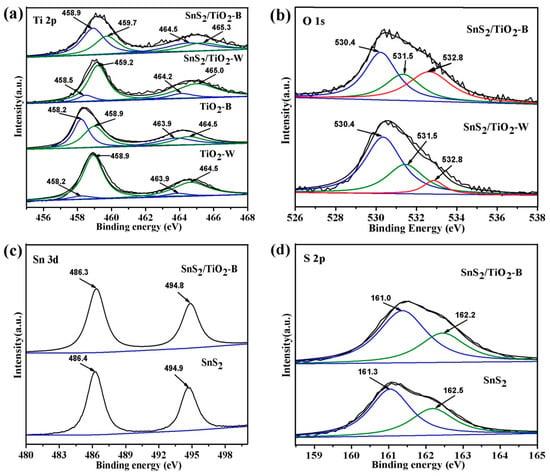
Figure 5.
XPS spectra of the as-prepared products: (a) Ti 2p core-level spectra, (b) O 1s core-level spectra, (c) Sn 3d core-level spectra, (d) S 2p core-level spectra.
The optical properties of the as-prepared samples have also been investigated. UV−vis absorption spectra are shown in Figure 6a. TiO2-W exhibits a typical absorption band of the commercial TiO2 with the absorption edge around 400 nm. In contrast, TiO2−B shows relatively strong absorption in the UV light range along with strong absorption in visible light region starting from 400 nm, which is in agreement with the photo-absorption profiles of the reported Ti3+ defective TiO2 [41]. As reported, the Ti3+ defects form an extra state below the conduction band of TiO2 with the largest state density at about 0.8 eV, which could extend the photo-response of TiO2 to visible light range. Moreover, SnS2, a typical semiconductor with visible light response, shows the light-absorbing ability nearly in the entire UV−vis region [42]. Accordingly, the composite samples with SnS2, both SnS2/TiO2-W and SnS2/TiO2-B display the stronger absorption in the visible light region. Worthy of note, SnS2/TiO2-B exhibits the most remarkable optical response in the visible light region due to the cooperative contribution to the photo-absorption by SnS2 and TiO2-B, which is desirable for achieving high efficient utilization of visible light.
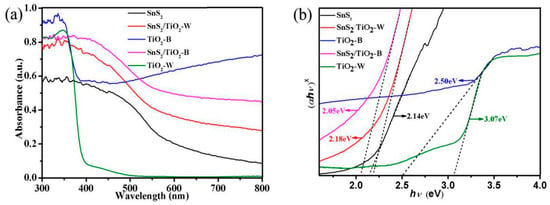
Figure 6.
(a) UV-Vis absorption spectra of the as-prepared samples, (b) The band gaps converted from UV−vis absorption spectra of the as-prepared samples.
The band gaps of the as-prepared samples have been calculated according to the following formula: [43]
where α, h, ν, B, and Eg are the absorption coefficient, discrete photon energy, a constant and band gap, respectively. Herein, the value of x depends on the characteristics of the transition in a semiconductor: 2 for SnS2 and 1/2 for TiO2 based on the definition of direct transition semiconductor (x = 2) and indirect transition semiconductor (x = 1/2), respectively. Taking hν as the abscissa axis and (αhν)x as the ordinate axis, the corresponding plots are obtained. As shown in Figure 6b, the band gap values of SnS2 and TiO2-W are evaluated to be 2.14 and 3.07 eV, respectively, which are consistent with the reported values [27,44]. In comparison with TiO2-W, TiO2-B shows much narrower the band gap of 2.50 eV, owing to the presence of Ti3+ state lying below the conduction band of TiO2. What’s more, both SnS2/TiO2-B and SnS2/TiO2-W present even much narrower band gaps of 2.05 and 2.18 eV, respectively. The narrowest band gap of SnS2/TiO2-B among these test samples should be ascribed to the synergistic effects resulting from both high-density state of Ti3+ and the formation of heterojunction structure between SnS2/TiO2-B, which would improve the photocatalytic activity under visible light irradiation.
(αhν)x = B (hν − Eg )
The Mott−Schottky measurements have been performed to calculate the energy band positions (ECB for conduction band and EVB for valence band) of samples SnS2/TiO2-B, SnS2/TiO2-W, TiO2-B, TiO2-W, and SnS2. The conduction band potential of a sample can be calculated through the intersection value of the linear part of its MS curve and the abscissa axis [45]. As shown in Figure 7a–e, the slopes of the MS curves for all samples are positive, indicating that they are n-type semiconductors. By fitting the obtained MS curves, the ECB values of SnS2/TiO2-B, SnS2/TiO2-W, TiO2-B, TiO2-W, and SnS2 are evaluated to be −1.21, −0.78, −0.59, −0.70, −0.90 eV, respectively. Furthermore, the valence band positions of samples can be estimated by the formula (EVB = Eg + ECB) [46]. Accordingly, the EVB values of SnS2/TiO2-B, SnS2/TiO2-W, TiO2-B, TiO2-W, and SnS2 are obtained as 0.84, 1.40, 2.00, 2.37, 1.24 eV, respectively. Based on the above results, the band structures of these samples are summarized in Figure 7f. It is indicated that the conduction band potentials of all samples are located above CO2/CO redox potential, which mean that after the electrons in the valence band of the semiconductor are excited by the photons to the conduction band, the electrons on the conduction band can transition to the CO2 molecules adsorbed on the surface of the catalyst to carry out the reduction reaction [45,47]. However, from a kinetics point of view, it is, in fact, critical to providing an overpotential, that is, there should be substantial margins over the reduction potential of CO2. Without an overpotential, even a good catalyst would not ensure a high rate of reaction [38]. Obviously, among these samples, SnS2/TiO2-B shows the highest conduction band potential and enough wide band gap spanning the range of the reduction and oxidation potentials, suggesting its strongest reduction ability to convert CO2 to CO under light irradiation [48].
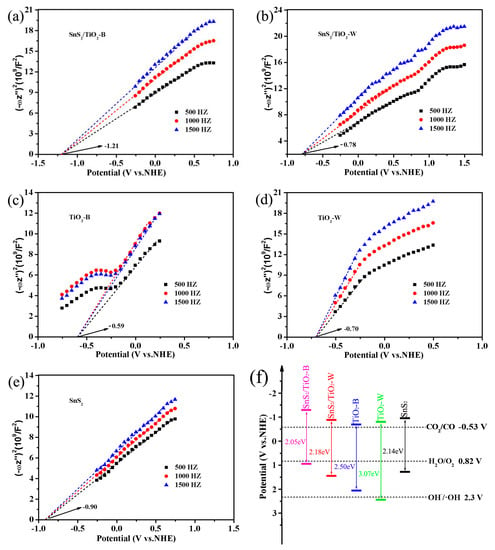
Figure 7.
(a–e) Mott−Schottky curves of the as-prepared samples, (f) Band positions and the band gap energies of the as-prepared samples and the redox potentials of CO2/CO, H2O/O2, and OH−/∙OH.
Moreover, the photoluminescence (PL) spectra were applied to evaluate the separation and recombination of the photo-generated charge carriers, which is an important factor for an efficient photocatalyst. Figure 8a depicts the PL spectra of SnS2/TiO2-B, SnS2/TiO2-W, TiO2-B, TiO2-W and SnS2 with an excitation wavelength λ = 370 nm. As shown in Figure 8a, TiO2-B, SnS2 and TiO2-W exhibit high PL intensity due to the quick recombination of the electron-hole pairs, which might lead to the lower photocatalytic activity despite having the strong light absorption. According to the literature, Ti3+ defects or oxygen vacancies are likely to be the photo-generated electron-hole recombination centers [49,50,51]. Therefore, it is believed that the high recombination rate for TiO2-B is caused by the presence of a large amount of Ti3+ defects in the bulk TiO2-B. Generally, the introduction of another semiconductor is an effective approach by constructing the interfacial heterojunction [14]. When their band gap edges match, an electron is preferentially transferred to the semiconductor with the lower CB edge, while a hole moves in the opposite direction to a higher VB level, thereby the recombination rate decreases [38]. Accordingly, upon introducing SnS2, it is obvious that both SnS2/TiO2-B and SnS2/TiO2-W show the decreased PL intensity, which confirms that the alignment of the band gap edges between SnS2 and TiO2-B or TiO2-W, thereby suppressing the recombination of electron-hole pairs [29]. Notably, SnS2/TiO2-B displays the lowest intensity among samples, which suggests its outstanding ability to separate and transfer the electrons and holes. In addition, the PL peaks of SnS2/TiO2-W and SnS2/TiO2-B move to the longer wavelength direction between the PL peaks of TiO2-W, TiO2-B, and SnS2, indicating an extension in the absorption range of light through forming a heterojunction [52]. Furthermore, the photocurrent (Iph) responses were also measured to investigate the property of the separation and transfer of the photoinduced charge carriers. As shown in Figure 8b, the photocurrent of SnS2/TiO2-B is as high as about 14 and 7 times of those of TiO2-B and SnS2 alone, respectively. The higher photocurrent demonstrates that the decoration of SnS2 on TiO2-B is able to facilitate the generation of more electron−hole pairs by extending the light absorption range for harvesting more light. In addition, the energetically favorable band alignment between SnS2 and TiO2-B is beneficial for promoting efficient interfacial charge transfer.
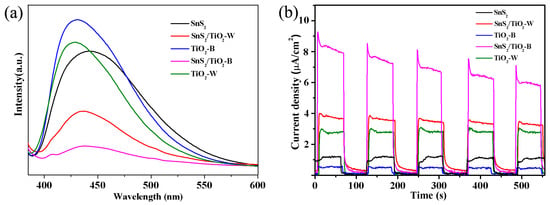
Figure 8.
(a) The photoluminescence spectrum of the as-prepared samples, (b) Photocurrent responses of different samples.
Consequently, the above results demonstrate that combination of SnS2 and TiO2-B achieves an optimal band gap, which is not only narrow enough for obtaining strong absorption throughout UV and visible light region but also wide enough for spanning the range of the reduction and oxidation potentials relevant to the photocatalytic reduction of CO2 and oxidation of H2O. In addition, the low recombination rate of electron-hole pairs by interface heterojunction and enough overpotential for the reduction of CO2 to CO are achieved as well. All these advantages could enable it the excellent photocatalytic performance in CO2 reduction.
2.2. Photocatalytic Activity
The photocatalytic CO2 reduction reaction was carried out in a 270 mL Pyrex reactor containing 20 mL N, N-dimethylformamide (DMF) and 10 mL ultrapure water. All samples were tested for 4 h under simulated solar light and visible light irradiation, respectively. The gaseous products obtained are analyzed by GC. The results indicate that the main product from CO2 reduction is CO, while hydrogen is in a negligible amount and no CH4 or other gases are detected. Figure 9a exhibits the CO production profile with reaction time under simulated solar light irradiation. It can be seen that SnS2 shows the lowest CO amount during entire reaction period due to the quick recombination of electron-hole pairs as indicated in Figure 8a. While TiO2-W, SnS2/TiO2-W show improved CO production than SnS2, in agreement with the order of the photocurrent responses shown in Figure 8b. Herein, it is believed that the unique truncated bipyramidal structure of TiO2 with the co-exposed {101} and {001} facets play an essential role in enhancing reduction of CO2, possibly through promoting the absorption and activation of CO2, or the improved separation of electro-hole pairs [12]. By contrast, TiO2-B shows higher CO production than TiO2-W, SnS2/TiO2-W, and SnS2, although TiO2-B displays the lowest photocurrent and highest PL intensity. It is suggested that Ti3+ defects play a dominant role in increase CO production. Generally, the efficiency of CO2 photocatalytic reduction mainly depends on the chemisorption and activation of CO2, light-harvesting, charge separation, and transfer. It should be noted that the chemisorption and activation of CO2 is the initial and crucial step in CO2 photocatalytic reduction. Moreover, it has been reported that oxygen vacancies could enhance the chemisorption of CO2, and mediate the electron transfer to the adsorbed CO2 to form the key mediate CO2-, thereby promoting the reduction of CO2 [17]. Upon introducing SnS2 for improving the light-harvesting and separation of electron-hole pairs, the photocatalytic properties of SnS2/TiO2-B exhibit the highest CO production among samples. For comparison, the CO amount obtained the same reaction was performed for a catalyst of physical mixed TiO2-B and SnS2 (SnS2+TiO2-B). Obviously, the produced CO amount is much lower than that of SnS2/TiO2-B, indicating the important role of the tight interfacial heterojunction in improving the photocatalytic performance. As for the results obtained under visible light irradiation as shown in Figure 9b, the order of the photocatalytic activity does not follow the same trend under simulated solar light conditions. Based on CO production, the catalysts can be classified into three distinctive groups. For SnS2/TiO2-W, SnS2, and TiO2-W, they hardly produce CO. As for TiO2-B and (SnS2+TiO2-B), they show similar level of CO production, suggesting the main contribution to photocatalytic activity arising from Ti3+ defective TiO2. Remarkably, SnS2/TiO2-B presents an exclusively highest CO production than others, which demonstrates its high photocatalytic efficiency solely driven by visible-light. Furthermore, the differences in CO production rates between simulated solar light and visible light irradiation are illustrated for each catalyst, as shown in Figure 9c. It is clear that the catalysts with TiO2-B component all show significantly higher activity than TiO2-W samples, revealing that Ti3+ defects play the most essential role in the reduction of CO2. In addition, as shown in Table S1 (Entry 6–9), the control experiments performed under the conditions: under N2 flow without CO2 (Entry 6), without light irradiation (Entry 7), only using water without DMF (Entry 8), and only using DMF without water under N2 flow (Entry 9) were conducted to identify the carbon source of the produced CO. A few amounts of CO and relatively enhanced amounts of H2 are observed when using water alone, and not-detected amounts of CO is observed in other control experiments. These results demonstrate that the detected CO product is through the photocatalytic reduction of CO2, not other sources in system. Moreover, the stability test of SnS2/TiO2-B was conducted under simulated solar light irradiation and shown in Figure 9d. There is no significant reduction of CO production in the consecutive four cycles, indicating its good stability.
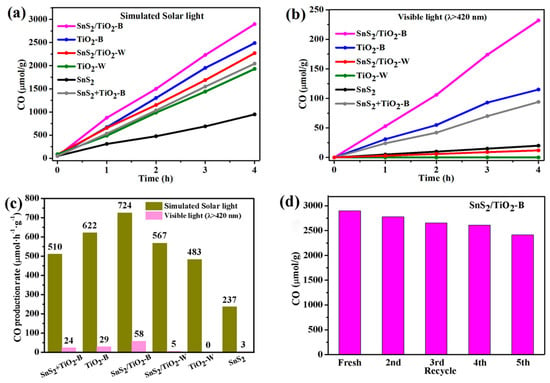
Figure 9.
CO production over different photocatalysts (a) under simulated solar light and (b) visible light (λ > 420 nm) irradiation, (c) Comparison of average CO production rates of all samples and (d) Stability test of SnS2/TiO2-B under simulated solar light irradiation.
2.3. Photocatalytic Mechanism
Based on our results and the reported literature [38,53,54,55,56], a plausible mechanism of photocatalytic CO2 reduction on SnS2/TiO2-B is presented in Figure 10. The CB and VB potential TiO2-B are −0.59 and 2.00 eV, respectively. The Ti3+ defects in TiO2-B cause a sub-band between VB and CB of TiO2, this sub-band act as a drawing board when photoelectrons are excited to improve the light-harvesting property [53]. Under the radiation of light, the electrons on the VB of TiO2-B can jump directly to the CB (path (1)), or indirectly transferred to the CB through the Ti3+ sub-band (path (2)). However, in TiO2-W, due to the absence of Ti3+ state, the electrons can only directly reach to the CB through (path (1)), which is why TiO2-W does not absorb visible light (Figure 6a). However, this sub-band also might provide recombination centers of photo-generated electro-hole pairs, thus increasing the recombination rate, evidenced by PL spectrum and photocurrent responses. By forming a heterojunction with SnS2, due to the higher CB edge at −0.90 eV of SnS2 and the lower VB edge at 1.24 eV, electron transfer between TiO2 and SnS2 may follow two mechanisms (traditional type-II and Z-scheme) [54]. For traditional type-II mechanism, under the radiation of light, SnS2 and TiO2 will transfer photo-generated electrons from the higher HOMO level of SnS2 to the lower CB of TiO2 and photo-induced holes from the lower VB of TiO2 to the higher LUMO of SnS2. In contrast, for Z-scheme shown in Figure 10, the electrons on the VB of TiO2-B and SnS2 are excited to the respective CB, along with the formed holes on VB. The electrons on the CB of TiO2-B tend to jump to the VB of SnS2 and recombine with holes. Finally, the electrons distributed on the CB of SnS2 and the holes distributed on the VB of TiO2-B. Different from the traditional type-II mechanism, the Z-scheme electron transfer mode can make the catalyst exhibit stronger redox ability in the photocatalytic process.
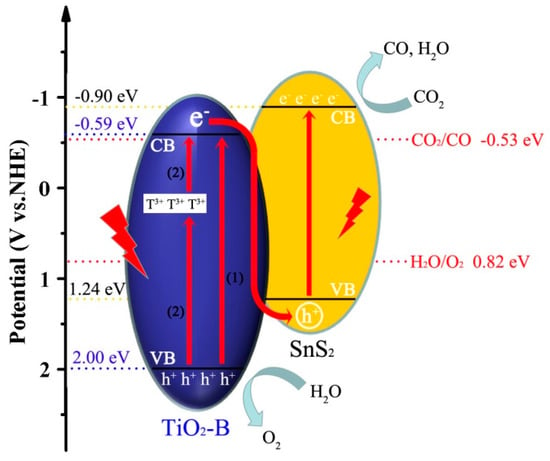
Figure 10.
Schematic diagram for interfacial photoexcited electron-hole pairs transfer process.
Herein, it is deluded that the photogenerated charge transfer between TiO2 and SnS2 can follow the Z-scheme mode based on the following two points: (1) In the case of the photo-generated electrons following the traditional type-II mode, the electrons transfer to the conduction band of TiO2-B (−0.59 eV) for the reduction reaction. However, the potential of −0.59 eV could not provide enough overpotential for CO2 reduction to CO (−0.53 eV). In other words, the reduction ability is too weak to initiate the reduction of CO2; (2) According to the XPS results in Figure 5, the significant increase in the binding energy of Ti 2p and the contrasting decrease in the binding energy of S 2p could support the transfer of electrons from TiO2 to SnS2 due to the formation of a heterojunction interfaces [38]. This Z-scheme charge transfer mode not only effectively inhibits the recombination of photo-generated electron-hole pairs, but also explains the strong redox capacity of the heterojunction catalyst SnS2/TiO2-B. What’s more, according to the electron transfer in the Z-scheme mode, CO2 reduction reaction would occur on SnS2 surface. As thus, the selective formation of CO while insignificant production of H2 on SnS2/TiO2-B might be reasonably illustrated by the cation–dipole interaction of the lattice Sn2+ with DMF leading to a cationic surface, which could prevent the adsorption of H+, consequently the generation H2 [55,56].
Overall, the improved photoactivity of SnS2/TiO2-B as compared with TiO2-B, SnS2 alone and the composite SnS2/TiO2-W toward CO2 reduction can be ascribed to the following factors. On one side, the coupling of SnS2 with TiO2-B achieves an optimal band gap size, which is relatively small to absorb light throughout UV and visible light range, and comparably large to span the range of the reduction and oxidation potentials relevant to the photocatalytic reduction of CO2 and oxidation of H2O. On another side, the efficient separation and transfer of electrons and holes due to the interface heterojunction and the more reductive conduction band potential toward the reduction of CO2 to CO are achieved as well.
3. Materials and Methods
3.1. Materials
N,N-dimethylformamide (99.9%, Aladdin), Titanium boride (TiB2, 99.9% metals basis, 3–5 μm, Aladdin), Poly(sodium 4-styrenesulfonate) (PSS, average Mv ~ 70,000, powder, Aladdin), Hydrofluoric acid aqueous solution (HF, 40%, Shanghai Zhongqin Chemical Reagent Co., Ltd., Shanghai, China), Acetic acid (98.0%, Aladdin, Shanghai, China), Thioacetamide (TAA, 98.0%, Aladdin), Tin(IV) chloride pentahydrate(SnCI4·5H2O, 98.0%, J&K Technology Co., Ltd., Beijing, China). All the reagents were used as received without any further purification. High-purity water (18.25 MΩ·cm) was used in all the experiments.
3.2. Synthesis of Ti3+ Defective TiO2 Nanoplates (TiO2-B)
In a typical synthesis procedure, 0.5 g of TiB2 was dispersed in 10 mL of deionized water and was sonicated for 60 min. The resulting suspension was mixed with 8 mL of deionized water and 2 mL of HF aqueous solution under magnetic stirring at 700 rpm. The hydrothermal reaction of the mixture is performed in a 100 mL Teflon-lined autoclave at 180 °C for 12 h. After the reaction, the resulting solid powder was collected by centrifugation and then washed with deionized water three times. Finally, it was dried at 60 °C under vacuum overnight. The obtained dark blue sample was denoted as TiO2-B. For comparison, TiO2-W as a control sample without Ti3+ defect was prepared by calcining TiO2-B at 600 °C for 8 h in muffle furnace.
3.3. Synthesis of SnS2/TiO2-B Photocatalyst
A total of 0.15 g of as-prepared TiO2-B was added to 20 mL aqueous solution of Poly (sodium 4-styrenesulfonate) of 0.3%, and the mixture was sonicated ultrasonication for 20 min. The solid was recovered by centrifugation and washed three times with deionized water, then dispersed in 20 mL deionized water. Next, 0.086 g SnCI4·5H2O, 0.092 g thioacetamide (TAA) and 1 mL acetic acid were added under stirring. Five minutes later, the resulting suspension was transferred into a 100 mL Teflon-lined autoclave and heated at 160 °C for 12 h. After the reaction, the resulting solid powders were collected by centrifugation and washed with deionized water three times and finally dried at 60 °C under vacuum overnight. The resultant sample was denoted as SnS2/TiO2-B. Using TiO2-W as a substrate, the control sample SnS2/TiO2-W was obtained. In the absence of the TiO2, we prepared the sample SnS2 nanosheets.
3.4. Characterization
The X-ray diffraction (XRD, Rigaku D/MAX-2500 diffractometer, Tokyo, Japan) with Cu Kα radiation was recorded to determine the crystal structure of the samples. The scanning electron microscopy (SEM, Hitachi S-4800, Tokyo, Japan) was performed to analyze the morphology and structure of materials. High-resolution TEM was carried out on a Tecnai G2 F-20 transmission electron microscope. A UV-vis spectrophotometer (Instant Spec BWS003 spectrometers, Shanghai, China) was used to obtain the optical absorbance spectra of the samples. The surface composition and chemical states were analyzed by X-ray photoelectron spectroscopy (XPS) on a PHI 1600 (PerkinElmer, Waltham, MA, USA) analyzer with X-ray excitation provided by an Al Kα X-ray source, and all the XPS spectra were calibrated by C1s binding energy which is 284.6 eV. Steady-state photoluminescence (PL) spectra were measured using a Horiba JobinYvon Fluorolog 3–21 with excitation at 370 nm. Photoelectrochemical measurements were recorded with an Autolab 302N Electrochemical System. An Ag/AgCl and a Pt plate were used as the reference electrode and the counter electrode, respectively. A glassy carbon electrode (GCE, 1.0 cm × 1.0 cm) coated with catalyst was used as a working electrode. Na2SO4 (0.5 M) was used as electrolyte solution. Photocurrent curves of the samples were collected as switching the light source every 60 s at a potential of 0.2 V.
3.5. Photocatalytic CO2 Reduction Measurements
The photocatalytic CO2 reduction reaction was carried out in a 270 mL Pyrex reactor under ultraviolet-visible irradiation (300 W Xe lamp), visible light was obtained with a 400 nm long-pass filter. In brief, 30 mg catalyst was dispersed into 30 mL reaction suspension containing 20 mL N, N-dimethylformamide and 10 mL ultrapure water. Prior to the illumination, high purity CO2 was bubbled through the reactor for 30 min, allowing the reactor to be filled with CO2 (1 bar) while venting the air. The reaction temperature was maintained at 25 °C with a cooling water circulator. The gases were analyzed using gas chromatography equipped with a thermal conductive detector (TCD) and a TDX-01 packed molecular sieves column.
4. Conclusions
In summary, we have successfully synthesized dark blue TiO2 nanoplates containing a large amount of Ti3+ defects. This method is simple, safe and energy-efficient compared to the post-treatment for doping Ti3+ defect into TiO2. After characterization, we found that Ti3+ defects help to increase the absorption of visible light. However, a large number of Ti3+ defects also inhibit the separation of photo-generated electron-hole pairs, which is not conducive to the photocatalytic reaction. Upon introducing SnS2, the heterojunction catalyst SnS2/TiO2-B not only possesses the strong light absorption from UV to visible light region, the lowest photo-generated charge recombination rate but also achieves a more negative conduction band potential than the reduction potential of CO2 to CO, and thereby exhibits the significantly enhanced selectivity and yield of CO in photocatalytic CO2 reduction. Notably, SnS2/TiO2-B produces CO at a rate of 58 µmol·h−1·g−1 under visible light irradiation, which is 2 and 19 times greater than those of alone TiO2-B and SnS2, respectively. Finally, a plausible photocatalytic mechanism on SnS2/TiO2-B was proposed that the electron transfer between TiO2 and SnS2 follows the Z-scheme mechanism. Our results present an effective way to gain highly efficient TiO2 based photocatalysts for CO2 reduction by combining different modification methods of TiO2 and make full use of the synergistic effects.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/11/927/s1, Figure S1: XRD patterns of SnS2/TiO2-W, TiO2-W and SnS2, Figure S2: SEM images of TiO2-B (a), optical photos of TiO2-B (b), TiO2-W (c), Figure S3: XPS full spectrum of (a) TiO2-B and (b) SnS2/TiO2-B, Table S1: Series of control experiments.
Author Contributions
Conceptualization, H.W.; Data curation, A.H., M.L., S.Z. and H.W.; Formal analysis, A.H.; Funding acquisition, H.W., Q.G., X.Z.; Investigation, A.H.; Methodology, A.H., M.L. and S.Z.; Project administration, H.W.; Resources, H.W.; Supervision, H.W. and J.H.; Validation, H.W.; Visualization, A.H.; Writing—Original draft, A.H.; Writing—Review editing, H.W.
Funding
This research was funded by National Natural Science Foundation of China (Grant No. 21576204 and 21206117).
Acknowledgments
We are grateful to the analysis and test center of Tianjin University for providing XRD, SEM, XPS characterizations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, L.; Duan, S.; Jin, P.; She, H.; Huang, J.; Lei, Z.; Zhang, T.; Wang, Q. Anchored Cu(II) tetra(4-carboxylphenyl)porphyrin to P25 (TiO2) for efficient photocatalytic ability in CO2 reduction. Appl. Catal. B 2018, 239, 599–608. [Google Scholar] [CrossRef]
- Tan, L.; Ong, W.; Chai, S.; Mohamed, A.R. Visible-light-activated oxygen-rich TiO2 as next generation photocatalyst: Importance of annealing temperature on the photoactivity toward reduction of carbon dioxide. Chem. Eng. J. 2016, 283, 1254–1263. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef]
- Guo, X.L.; Kuang, M.; Li, F.; Liu, X.Y.; Zhang, Y.X.; Dong, F.; Losic, D. Engineering of three dimensional (3-D) diatom@TiO2@MnO2 composites with enhanced supercapacitor performance. Electrochim. Acta 2016, 190, 159–167. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, W.; Lv, X.; Sun, Y.; Dong, F.; Zhang, Y. Noble metal-free Bi nanoparticles supported on TiO2 with plasmon-enhanced visible light photocatalytic air purification. Environ. Sci. Nano 2016, 3, 1306–1317. [Google Scholar] [CrossRef]
- Wang, Q.; Lian, J.; Bai, Y.; Hui, J.; Zhong, J.; Li, J.; An, N.; Yu, J.; Wang, F. Photocatalytic activity of hydrogen production from water over TiO2 with different crystal structures. Mater. Sci. Semicond. Process. 2015, 40, 418–423. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, T.; Andino, J.M.; Li, Y. Copper and iodine co-modified TiO2 nanoparticles for improved activity of CO2 photoreduction with water vapor. Appl. Catal. B 2012, 123, 257–264. [Google Scholar] [CrossRef]
- Pan, J.; Liu, G.; Lu, G.Q.M.; Cheng, H. On the true photoreactivity order of {001}, {010}, and {101} facets of anatase TiO2 crystals. Angew. Chem. Int. Ed. 2011, 50, 2133–2137. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photo-electrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Ye, L.; Mao, J.; Peng, T.; Zan, L.; Zhang, Y. Opposite photocatalytic activity orders of low-index facets of anatase TiO2 for liquid phase dye degradation and gaseous phase CO2 photoreduction. Phys. Chem. Chem. Phys. 2014, 16, 15675–15680. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2 reduction activity of anatase TiO2 by co-exposed {001} and {101} facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef]
- Wang, P.; Jia, C.; Li, J.; Yang, P. Ti3+-doped TiO2 (B)/anatase spheres prepared using thioglycolic acid towards super photocatalysis performance. J. Alloy. Compd. 2019, 780, 660–670. [Google Scholar] [CrossRef]
- Liu, X.; Du, G.; Li, M. True photoreactivity origin of Ti3+-Doped anatase TiO2 crystals with respectively dominated exposed {001}, {101}, and {100} facets. ACS Omega 2019, 4, 14902–14912. [Google Scholar] [CrossRef]
- Umer, M.; Tahir, M.; Azam, M.U.; Tahir, B.; Musaab, M. Self-doped Ti3+ mediated TiO2/In2O3/SWCNTs heterojunction composite under acidic/basic heat medium for boosting visible light induced H2 evolution. Int. J. Hydrog. Energy 2019, 44, 13466–13479. [Google Scholar] [CrossRef]
- Fang, W.; Khrouz, L.; Zhou, Y.; Shen, B.; Dong, C.; Xing, M.; Mishra, S.; Daniele, S.; Zhang, J. Reduced {001}-TiO2−x photocatalysts: Noble-metal-free CO2 photoreduction for selective CH4 evolution. Phys. Chem. Chem. Phys. 2017, 19, 13875–13881. [Google Scholar] [CrossRef] [PubMed]
- Pipornpong, W.; Wanbayor, R.; Ruangpornvisuti, V. Adsorption CO2 on the perfect and oxygen vacancy defect surfaces of anatase TiO2 and its photocatalytic mechanism of conversion to CO. Appl. Surf. Sci. 2011, 257, 10322–10328. [Google Scholar] [CrossRef]
- Janbandhu, S.Y.; Joshi, A.; Munishwar, S.R.; Gedam, R.S. CdS/TiO2 heterojunction in glass matrix: Synthesis, characterization, and application as an improved photocatalyst. Appl. Surf. Sci. 2019, 497, 143758–143769. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Nengzi, L.; Li, B.; Gou, J.; Cheng, X. Synthesis of SnS/TiO2 nano-tube arrays photoelectrode and its high photoelectrocatalytic performance for elimination of 2,4,6-trichlorophenol. Sep. Purif. Technol. 2019, 228, 115742–115750. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.; Fu, X.; Zhang, N.; Xu, Y. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Miller, J.T.; Li, Y. Mechanistic study of CO2 photoreduction with H2O on Cu/TiO2 nanocomposites by in situ X-ray absorption and infrared spectroscopies. J. Phys. Chem. C 2016, 121, 490–499. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Wang, X.; Cheng, L.; Lu, H.; Wang, L.; Lu, G.Q.M.; Cheng, H. Enhanced photoactivity of oxygen-deficient anatase TiO2 sheets with dominant {001} facets. J. Phys. Chem. C 2009, 113, 21784–21788. [Google Scholar] [CrossRef]
- Zhao, J.; Xing, W.; Li, Y.; Lu, K. Solvothermal synthesis and visible light absorption of anatase TiO2. Mater. Lett. 2015, 145, 332–335. [Google Scholar] [CrossRef]
- Huo, Y.; Yang, Y.; Dai, K.; Zhang, J. Construction of 2D/2D porous graphitic C3N4/SnS2 composite as a direct Z-scheme system for efficient visible photocatalytic activity. Appl. Surf. Sci. 2019, 481, 1260–1269. [Google Scholar] [CrossRef]
- Song, Y.; Gu, J.; Xia, K.; Yi, J.; Chen, H.; She, X.; Chen, Z.; Ding, C.; Li, H.; Xu, H. Construction of 2D SnS2/g-C3N4 Z-scheme composite with superior visible-light photocatalytic performance. Appl. Surf. Sci. 2019, 467, 56–64. [Google Scholar] [CrossRef]
- Su, Y.; Wang, C.; Zhang, Y.; Yang, Z.; Dionysiou, D.D. Design and preparation of SnO2/SnS2/conjugated polyvinyl chloride derivative ternary composite with enhanced visible-light photocatalytic activity. Mater. Res. Bull. 2019, 118, 110524. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, C.; Li, X.; Sun, Y.; Zhang, M.; Mu, J.; Zhang, P.; Guo, Z.; Liu, Y. Hierarchical assembly of ultrathin hexagonal SnS2 nanosheets onto electrospun TiO2 nanofibers: Enhanced photocatalytic activity based on photoinduced interfacial charge transfer. Nanoscale 2013, 5, 606–618. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Li, J.; Xu, H.Y. One-step in situ solvothermal synthesis of SnS2/TiO2 nanocomposites with high performance in visible light-driven photocatalytic reduction of aqueous Cr (VI). Appl. Catal. B 2012, 123, 18–26. [Google Scholar] [CrossRef]
- She, H.; Zhou, H.; Li, L.; Zhao, Z.; Jiang, M.; Huang, J.; Wang, L.; Wang, Q. Construction of a two-dimensional composite derived from TiO2 and SnS2 for enhanced photocatalytic reduction of CO2 into CH4. ACS Sustain. Chem. Eng. 2018, 7, 650–659. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Sun, C.; Cheng, L.; Wang, L.; Lu, G.Q.M.; Cheng, H. Titania polymorphs derived from crystalline titanium diboride. CrystEngComm 2009, 11, 2677–2682. [Google Scholar] [CrossRef]
- Chiesa, M.; Livraghi, S.; Giamello, E.; Albanese, E.; Pacchioni, G. Ferromagnetic interactions in highly stable, partially reduced TiO2; the S = 2 State in anatase. Angew. Chem. Int. Ed. 2017, 56, 2604–2607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, J.; Zhang, M.; Yuan, Q.; Dong, B. Ultrathin hexagonal SnS2 nanosheets coupled with g-C3N4 nanosheets as 2D/2D heterojunction photocatalysts toward high photocatalytic activity. Appl. Catal. B 2015, 163, 298–305. [Google Scholar] [CrossRef]
- Gordon, T.R.; Cargnello, M.; Paik, T.; Mangolini, F.; Weber, R.T.; Fornasiero, P.; Murray, C.B. Nonaqueous synthesis of TiO2 nanocrystals using TiF4 to engineer morphology, oxygen vacancy concentration, and photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 6751–6761. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, P.; Wu, J.; Hou, J.; Bai, H.; Liu, Z. Effective protect of oxygen vacancies in carbon layer coated black TiO2−x/CNNS hetero-junction photocatalyst. Chem. Eng. J. 2019, 359, 58–68. [Google Scholar] [CrossRef]
- Shen, L.; Xing, Z.; Zou, J.; Li, Z.; Wu, X.; Zhang, Y.; Zhu, Q.; Yang, S.; Zhou, W. Black TiO2 nanobelts/g-C3N4 nanosheets laminated heterojunctions with efficient visible-light-driven photocatalytic performance. Sci. Rep. 2017, 7, 41978–41988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Li, J.; Zhang, M.; Dionysiou, D.D. Size-tunable hydrothermal synthesis of SnS2 nanocrystals with high performance in visible light-driven photocatalytic reduction of aqueous Cr (VI). Environ. Sci. Technol. 2011, 45, 9324–9331. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Zhao, J.; Wang, H.; Zhu, X.; Han, J.; Liu, X. Plasmonic AuPd-based Mott-Schottky photocatalyst for synergistically enhanced hydrogen evolution from formic acid and aldehyde. Appl. Catal. B 2019, 252, 24–32. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, C.; Li, X.; Wang, C.; Zhang, M.; Liu, Y. Electrospun nanofibers of p-type NiO/n-type ZnO heterojunctions with enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2010, 2, 2915–2923. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, L.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K. Ag/ZnO heterostructure nanocrystals: Synthesis, characterization, and photocatalysis. Inorg. Chem. 2007, 46, 6980–6986. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhao, Z.; Li, S.; Su, Y.; Huang, L.; Shao, N.; Liu, F.; Bu, Y.; Zhang, H.; Zhang, Z. Role of SnS2 in 2D–2D SnS2/TiO2 nanosheet heterojunctions for photocatalytic hydrogen evolution. ACS Appl. Nano Mater. 2019, 2, 2144–2151. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis and physical properties of the semiconducting electrode WO2. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Pian, X.; Lin, B.; Chen, Y.; Kuang, J.; Zhang, K.; Fu, L. Pillared nanocomposite TiO2/Bi-Doped hexaniobate with visible-light photocatalytic activity. J. Phys. Chem. C 2011, 115, 6531–6539. [Google Scholar] [CrossRef]
- Liang, H.; Acharjya, A.; Anito, D.A.; Vogl, S.; Wang, T.; Thomas, A.; Han, B. Rhenium-metalated polypyridine-based porous polycarbazoles for visible-light CO2 photoreduction. ACS Catal. 2019, 9, 3959–3968. [Google Scholar] [CrossRef]
- Lu, J.; Li, D.; Chai, Y.; Li, L.; Li, M.; Zhang, Y.; Liang, J. Rational design and preparation of nanoheterostructures based on zinc titanate for solar-driven photocatalytic conversion of CO2 to valuable fuels. Appl. Catal. B 2019, 256, 117800. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]
- Xing, M.; Zhou, Y.; Dong, C.; Cai, L.; Zeng, L.; Shen, B.; Pan, L.; Dong, C.; Chai, Y.; Zhang, J.; et al. Modulation of the reduction potential of TiO2-x by fluorination for efficient and selective CH4 generation from CO2 photoreduction. Nano Lett. 2018, 18, 3384–3390. [Google Scholar] [CrossRef]
- Wang, J.; Tafen, D.N.; Lewis, J.P.; Hong, Z.; Manivannan, A.; Zhi, M.; Li, M.; Wu, N. Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts. J. Am. Chem. Soc. 2009, 131, 12290–12297. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, N.; Fu, X.; Xu, Y. Selective oxidation of benzyl alcohol over TiO2 nanosheets with exposed {001} facets: Catalyst deactivation and regeneration. Appl. Catal. A 2013, 453, 181–187. [Google Scholar] [CrossRef]
- Xie, T.; Lin, J. Origin of photocatalytic deactivation of TiO2 film coated on ceramic substrate. J. Phys. Chem. C 2007, 111, 9968–9974. [Google Scholar] [CrossRef]
- Qin, J.; Wang, S.; Ren, H.; Hou, Y.; Wang, X. Photocatalytic reduction of CO2 by graphitic carbon nitride polymers derived from urea and barbituric acid. Appl. Catal. B 2015, 179, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Guan, Z.; Li, Q.; He, C.; Yang, J. Synergistic effect of surface and bulk single-electron-trapped oxygen vacancy of TiO2 in the photocatalytic reduction of CO2. Appl. Catal. B 2017, 206, 300–307. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, X.; Wang, C.; Xu, J.; Du, X.; Li, L. Ti3+ defect mediated g-C3N4/TiO2 Z-scheme system for enhanced photocatalytic redox performance. Appl. Surf. Sci. 2018, 448, 288–296. [Google Scholar] [CrossRef]
- Fujiwar, H.; Hosokawa, H.; Murakoshi, K.; Wada, Y.; Yanagida, S. Effect of surface structures on photocatalytic CO2 reduction using quantized CdS nanocrystallites1. J. Phys. Chem. B 1997, 101, 8270–8278. [Google Scholar] [CrossRef]
- Liu, B.; Torimoto, T.; Yoneyama, H. Photocatalytic reduction of CO2 using surface-modified CdS photocatalysts in organic solvents. J. Photochem. Photobiol. A 1998, 113, 93–97. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).